4
Polymers
“Important things for easy life – through polymers.”
4.1 Introduction
In this modern world, polymers are an integral part in an individual‚s life. They have the most diverse structures and applications ranging from domestic articles to highly sophisticated instruments. These materials are used in almost all the fields like medicine, industry, agriculture, construction, etc. In recent days, these materials are used to prepare nanomaterials.
Human body is build up and functioning with different polymers like DNA, RNA, hormones, enzymes, proteins, lipids, phosponitrilic acids, etc. Most of the food materials we are eating are polymers like carbohydrates, starch, etc. In view of their importance, a proper understanding of polymeric materials is very essential.
The word polymer is derived from Greek word ‘poly‚, which means ‘many‚ and ‘meros‚ which means ‘units‚ (or) ‘parts‚. Polymers are macromolecules of high molecular masses built up by the linking together of a large number of small, repeated units by a covalent bond. The repeating unit present in the formation of a polymer is known as monomer. The chemical process leading to the formation of polymer is known as polymerization.
E.g.:

4.2 Degree of Polymerization
The size of the polymer molecule is decided by the number of repeating units present in it. The number of repeating units (n) in chain formed in a polymer is known as the “degree of polymerization.”

where, n is the degree of polymerization. It is different from polymer to polymer and can be 104 or more.
Mol. wt. of polymer = Mol. wt. of repeating unit × degree of polymerization
4.3 Classification of polymers
Polymeric materials can be classified into several ways:
4.3.1 Classification Depending on the Basis of Source
- Natural polymers: These polymers are isolated from natural material like animals and plants.
E.g.: Cotton, silk, wool, nucleic acids, proteins, starch, cellulose, natural rubber, etc.

- Synthetic polymers: Polymers synthesized from low molecular weight compounds are synthetic polymers.
E.g.: Polyethylene, polyvinyl chloride, nylon, terelene, etc.

4.3.2 Classification on the Basis of Composition
- Univalent or homopolymers: These are formed with the same monomer units.
E.g.: polyethylene, polystyrene, polyvinyl chloride, etc.
- Copolymers: These are formed with two or more different monomer units.
E.g.:

Copolymers are classified into four categories depending upon the nature of the distribution of different monomers in the polymer chain.
- Random copolymer: These are formed by the random arrangement of monomer units in the chain.

- Alternating copolymers: Monomer units in a copolymer molecule are arranged in alternate manner.

- Block copolymer: A block copolymer consists of one in which blocks of repeating units of one monomer alternate with blocks of another monomer.

- Graft copolymer: This copolymer consists of a linear polymer chain of one monomer to which side chain of different monomer has been grafted.

- Random copolymer: These are formed by the random arrangement of monomer units in the chain.
4.3.3 Classification on the Basis of Chemical Composition
- Organic polymers: A polymer whose backbone chain is essentially made of carbon atoms is termed as organic polymer.
E.g.: Polyethylene, polystyrene, polyvinyl chloride, etc.
- Inorganic polymers: Polymers that are formed by non carbon-carbon bonds.
E.g.: Polysilanes, polygermanes, etc.
4.3.4 Classification on the Basis of Structure
- Linear polymers: In which monomeric units are joined in the form of long, straight chains.
E.g.: Nylons, polyester, polyvinyl chloride, high-density polythene, etc.
- Branched chain polymers: These are mainly linear in nature but also possess some branches along the chain.
E.g.: Glycogen, amylopectin, low-density polythene, etc.
- Cross-linked polymer: These polymers adjacent linear chains are joined one to another at various positions by covalent bonds.
E.g.: Elastomers like rubber.
- Network polymers: Monomer units that are having trifunctional groups form three dimensional networks. In this, highly cross-linked polymer is also called network polymer.
E.g.: Bakelite, urea formaldehyde resin, silicones, etc.
4.3.5 Classification on the Basis of their Methods of Synthesis
- Addition polymers: Addition polymerization takes place in compounds containing reactive double bonds. Chain polymerization is characterized by a self-addition of the monomer molecules to each other very rapidly through a chain reaction.
E.g.: Polyethylene, polypropylene, polystyrene, polyvinyl chloride, etc.
- Condensation polymers: This type of polymerization is brought about by monomers containing two or more reactive functional groups condensing with each other to form a large condensed polymer and also loss of small molecules like H2O, NH3, HCl, etc.
E.g.: Polyester, nylon, polyamide, etc.
4.3.6 Classification Based on the Molecular Forces
- Thermoplastic polymers: These are linear, long chain polymers, which can be softened on heating and hardened on cooling reversibly. Thus they can be processed again and again.
E.g: Polythene, polypropylene, polyvinyl chloride, etc.
- Thermosetting polymers: These polymers during moulding get hardened, and once they have solidified, they cannot be softened. Such polymers during moulding acquire three-dimensional cross-linked structure with predominantly strong covalent bonds.
E.g.: Polyester, bakelite, urea formaldehyde, etc.
- Elastomer: These are rubber-like elastic polymers, which can be stretched to at least thrice its length, but it returns to its original shape and dimensions as soon as the stretching force is released.
- Fibers: Fibers are polymers whose chains are held by strong intermolecular forces like hydrogen bonding. They are crystalline in nature and of high tensile strength, due to strong intermolecular forces.
E.g.: Nylon, polyester, etc.
4.4 Types of polymerization
Polymerization is mainly of two types:
- Condensation polymerization (or) step polymerization
- Vinyl or addition polymerization (or) chain polymerization
4.4.1 Condensation Polymerization or Step Polymerization
Condensation is brought about by monomers containing two or more reactive functional groups condensing with each other to form large condensed polymer and also loss of small molecules like H2O, NH3, HCl, etc.
E.g.:
- Polyester: Condensation between dicarboxylic acid and diol gives polyester.

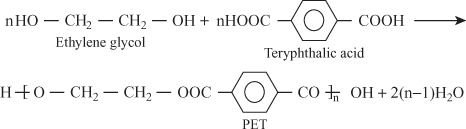
- Polyethylene terephthalate (PET):
- Polyamide: Condensation reaction between diamine and dicarboxylic acid gives polyamide.

- Nylon 66: Condensation reaction between hexamethylenediamine and adipic acid gives nylon 66.

Reaction Mechanism
- In this polymerization, the monomers should have minimum two reactive functional groups for polymerization.
- This polymerization proceeds by step-wise reaction between reactive functional groups. In this process, the first two monomer units condense to form dimer. This dimer reacts with another dimer to form tetramer or with monomer to form trimer. In this process, small units like NH3, H2O, HCl, etc., are also formed.
- The only one type of reaction between two functional groups is involved in polymer formation.
- The polymer formed still contains both the reactive functional groups at the end of the chain; hence it is active and not dead as in chain polymerization.
- Reaction between two monomers contains two active functional groups. They will give straight chain polymers; otherwise, monomers containing more than two functional groups form cross-linked polymer.
1. Formation of Polyester
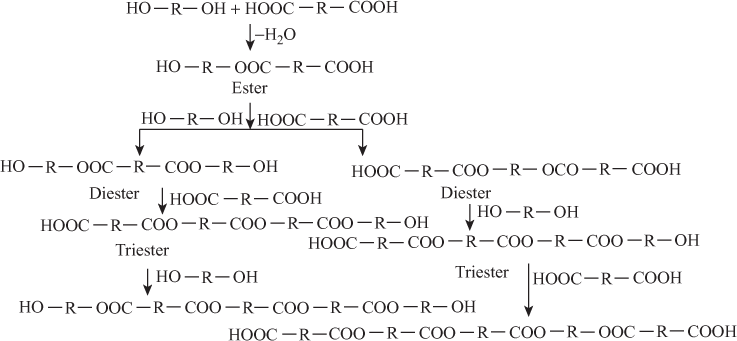
Above process continuous and form polymer.
2. Formation of Polyethylene Terephthalate

The above reaction continued and forms large polymer.
n molecules of ethylene glycol react with n molecules of terephthalic acid.

3. Formation of Nylon 66

The above reaction proceeds that n molecules of hexamethylene diamine that react with n molecules of adipic acid to form nylon 66.

4. Formation of Polyamide
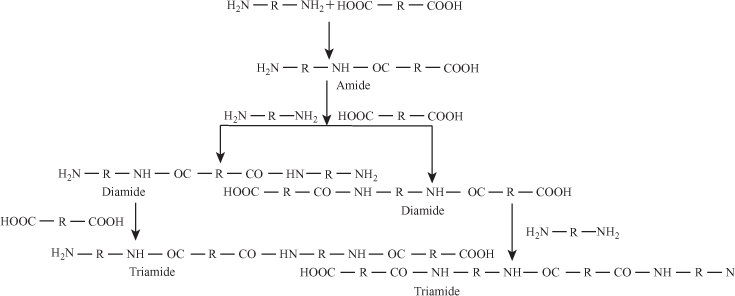
n molecules of diamine and n molecules of dicarboxylic acid react to form polyamide.
4.4.2 Addition or Vinyl or Chain Polymerization
Addition polymerization takes place in compounds containing reactive double bonds. Chain polymerization is characterized by a self-addition of the monomer molecules to each other very rapidly through a chain reaction. No byproduct like HCl, NH3, H2O, etc., is formed. This polymerization occurs in the presence of catalyst, light, or heat.
E.g.:
- In the presence of oxygen or ziegler-natta catalyst, ethylene gives addition polymer, i.e., polythene.

- In the presence of azo compounds, vinyl chloride gives polyvinyl chloride.

- In the presence of metal amide styrene gives polystyrene.

- In the presence of benzoyl peroxide acrylonitrile gives polyacrylonitrile.

Reaction Mechanism
In the addition polymerization, free radical, carbonium ion, or carbanium ions, act as active centers. Hence polymerization may occurs in
- Free radical
- Ionic (Cationic or Anionic)
- Coordination mechanisms.
In addition polymerization, mainly three steps are present:
- Chain initiation
- Chain propagation
- Chain termination
Free Radical Polymerization
The initiation of the polymer chain is brought about by free radicals produced by the decomposition of monomers; thus this reaction is polymerization.
The decomposition of the initiation to form free radicals can be induced by heat energy, light energy, or catalysts.
Three steps are included in free radical polymerization:
- Chain initiation
- Chain propagation
- Chain termination
- Chain initiation:
- In the presence of catalyst or heat or light, initiator converts into free radical.

Normally, H2O2, benzoyl peroxide, hydroperoxide, tertiary butyl peroxide, and azobisisobutyl nitriles (AIBN) act as initiators.
- Free radicals react with monomer unit to form new active-centered free radical.

- In the presence of catalyst or heat or light, initiator converts into free radical.
- Chain propagation:
- In this step, free radical attacks fresh monomer unit to form another free radical.

- This process continues till the monomer units are present in the reaction mixture. Finally, it forms chain propagate free radical.

Propagation reaction is very fast reaction; in this reaction, there is no middle product is formed.
- In this step, free radical attacks fresh monomer unit to form another free radical.
- Chain termination:
In this step, chain propagate polymer radical deactivates with coupling or disproportionation reaction to stop chain propagation and forms dead polymer.
- Coupling: Chain propagation free radicals meet to form polymer and terminate the reaction.

- Disproportination reaction: Hydrogen atom shift from one chain propagate free radical to another chain propagate free radical and ends the reaction.

E.g. 1, Polymerization of acrylonitrile in the presence of benzoyl peroxide
- Coupling: Chain propagation free radicals meet to form polymer and terminate the reaction.
Reaction Mechanism
- Chain initiation:
- In this step, benzoyl peroxide dissociates to form phenyl free radicals.

- Phenyl free radicals react with acrylonitrile and gives chain initiate free radical.

- In this step, benzoyl peroxide dissociates to form phenyl free radicals.
- Chain propagation:
- In this step, free radicals react with monomer units and give chain propagate free radical. This reaction continues till the monomer units present in reaction mixture.

- In this step, free radicals react with monomer units and give chain propagate free radical. This reaction continues till the monomer units present in reaction mixture.
- Chain termination:
- Chain terminates with coupling reaction.

E.g. 2, Polymerization of methyl methacrylate in the presence of azobis-isobutyl nitrile
- Chain terminates with coupling reaction.
Reaction Mechanism
- Chain initiation:
- In the presence of UV-rays, azobis-isobutyl nitrile homolysis and gives cyanopropyl radical.

- Cyanopropyl radicals react with methyl methacrylate monomer to form chain initiate free radical.

- In the presence of UV-rays, azobis-isobutyl nitrile homolysis and gives cyanopropyl radical.
- Chain propagation:
- In this step, chain initiate free radicals react with monomer units; finally, it gives chain propagate free radical.

- In this step, chain initiate free radicals react with monomer units; finally, it gives chain propagate free radical.
- Chain termination:
- It terminates with coupling reaction.

- It terminates with coupling reaction.
Cationic Addition Polymerization
The following are the important points in reaction mechanism:
- In this polymerization, carbonium ion acts as chain initiator.
- BF3, H2SO4, AlCl3 like lewis acids act as catalyst, water, carbonic acid etc., act as co-catalysts.
- This polymerization monomer acts as an electron donor and reacts with catalysts to form carbonium ion. This carbonium ion reacts with monomer units and propagates the reaction.
E.g. Cationic polymerization of isobutylene in the presence of BF3

Reaction Mechanism
- Chain initiation:
- Carbonium ion is formed due to the following reactions.


- Carbonium ion is formed due to the following reactions.
- Chain propagation:
- Monomer molecules react one by one with carbonium ion and propagate the chain to form addition polymer.

- Monomer molecules react one by one with carbonium ion and propagate the chain to form addition polymer.
- Chain termination:
- Chain termination occurs with disproportionation reaction.

- Chain termination occurs with disproportionation reaction.
Anionic Addition Polymerization
The following are the important points:
- Carbanion acts as chain initiator.
- Alkali metal amide, grignard reagents, etc., act as catalysts and initiate the chain.
- Monomers containing electron-withdrawing groups participate in anionic polymerization.
- In this polymerization, there is no chain termination; hence anionic polymers are known as activated polymers.
Acrylonitrile, styrene, and methyl methocrylate participate in anionic polymerization.
E.g. Anionic polymerization of styrene in the presence of alkali metal amide:

Reaction Mechanism
- Chain initiation:
- Carbanion is formed in chain initiation.


- Carbanion is formed in chain initiation.
- Chain propagation:
- Carbanion reacts with styrene monomers and propagates the reaction and forms chain propagate polymer ion.
- In this type of polymerization, chain termination does not occur. Hence anionic polymer is active till the monomer units are present in reaction mixture, so anionic polymers are also known as active polymers.
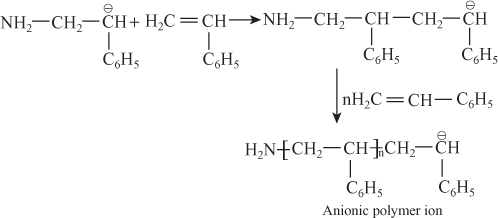
- When impurities are added to reaction mixture, chain termination occurs. So chain termination reagent is added and gets dead polymers.

4.4.3 Coordination Polymerization
Ziegler-Natta Catalyst
The mixture of titanium halides and trialkyl aluminium is known as Ziegler-Natta catalyst.
(TiCl3 + R3Al)
Reaction between titanium chloride and trialkyl aluminium forms Ziegler-Natta catalyst.
In this process, trialkyl aluminium adsorbs on the surface of titanium chloride, and forms electron-deficient bridge structure.

Ziegler-Natta structure
In this structure, titanium chloride acts as catalyst and trialkyl aluminium acts as co-catalyst.
Importance
In the presence of ziegler-natta catalyst, coordination polymerization occurs and gives isotactic polymer of olefin.
E.g.: Propylene undergoes coordination polymerization in the presence of Ziegler-Natta catalyst at 50°C and gives isotactic polymer of polypropelene

4.5 Molecular mass of a polymer
Polymers are mixture of different monomers with different molecular weights/masses. Hence, mainly three kinds of molecular masses have been identified. They are listed here under.
- Number average molecular mass

- Weight average molecular mass

- Viscosity average molecular mass

(i) Number Average Molecular Mass ![]()
This is the total mass (w) of all the molecules in a polymer sample divided by the total number of molecules present. This can be determined by measuring colligative properties like freezing point depression, boiling point elevation, osmotic pressure, lowering of vapour pressure , etc.
![]()
where Ni = the number of molecules of mass Mi
The number average molecular mass is a good index for tensile strength, but not for flow.
(ii) Weight Average Molecular Mass ![]()
This can be determined from light scattering and ultra centrifugation techniques and can be measure molecular size:
![]()
where wi = weight fraction of molecules of Mi
![]()
where Ci = weight concentration of Mi molecules
C = total weight concentration of all polymer molecules
Polydisperity index or molecular mass distribution
This is a measure of the distribution of molecular mass of a polymer. This can be calculated using the weight average molecular weight divided by the number average molecular weight.
Polydispersity index ![]()
In a monodisperse system, ![]() . But PDI value is always greater than one, i.e., the weight average molecular mass is always greater than the number average molecular mass.
. But PDI value is always greater than one, i.e., the weight average molecular mass is always greater than the number average molecular mass.
(iii) Viscosity Average Molecular Mass ( Mv)
Viscosity average molecular mass can be determined by the measuring of viscosity of that particular polymer. This can be explained by the following formula:

where a = constant.
When a = unity, the viscosity and weight average molecular masses are equal. ![]() is almost less than
is almost less than ![]() , hence a polydispersive polymer is represented as
, hence a polydispersive polymer is represented as
![]()
4.6 Plastics
Plastics are mainly of two types:
- Thermoplastics: Thermoplastics are formed with straight chains, hence when heated they soften, subsequently melt and when cooled they become hard. Due to the van der walls forces, two different chains slid over each other and plasticity of such polymers is reversible. Hence, the materials can be moulded and remoulded without damage.
E.g.: PVC, nylon, polystyrene, polyethylene.
- Thermosetting plastics: Thermosetting resins do not become soft on heating, and they never melt once they set. They are normally made from semi-fluid polymers with low molecular masses. In this case, the polymer chains are entangled with one another and hence cannot slide over each other. Deformation does not occur on heating, because only primary covalent bonds are present the entire structure, forming a three dimensional network. They have no scrap value.
E.g.: Bakelite

4.7 Important polymers – Composition, Preparation, Properties, and Engineering Uses
4.7.1 Thermo Plastics
(i) Polythene or Vinyl Resin
Polythene is the most widely used plastic. Polythene is obtained by high-pressure polymerization of ethylene, making use of oxygen as initiator. The reaction takes place at 1500 atmospheres pressure and 180°C–250°C temperature range. Ethylene polymerized into polyethylene, a waxy solid.

By using force radical initiator, low density polythene (LDPE) is obtained, while by using ionic catalysts, high density polythene is obtained.
Properties
- Polythene is a rigid, waxy, white, translucent, non-polar material, exhibiting considerable chemical resistance to strong acids, alkalis, and salt solutions at room temperature.
- It is a good insulator of electricity. However, it is swollen and permeable to most oils and organic solvents, particularly kerosene.
- Due to its highly symmetrical chain structure, polythene crystallizes very easily. The degree of crystallinity may vary from 40 to 95% depending on the degree of branching in the polythene chain.
- Polyethylene produced by high-pressure process has a branched structure and therefore, is flexible and tough. On the other hand, low-pressure process results in a completely linear polyethylene, having higher density and better chemical resistance.
- Commercial polythene can be subdivided into three groups:
- Low-density polythene (LDPE): It is polymerized under very high pressure of 1000–5000 atmospheres and temperature range of 80–250°C in the presence of O2 as initiator. It is having density 0.91–0.925 g/cm3 and M.P. 110–125°C.
- Medium-density polyethylene (MDPE): It is polymerized under medium pressure, having density 0.925–0.940 g/cm3 and M.P. 130–140°C.
- High-density polyethylene (HDPE): It is polymerized under atmospheric pressure (6–7 atmospheres) and temperature at 60°C in the presence of ziegler-natta catalyst [TiCl3 + Al(C2H5)3]. The HDPE, which is completely linear, has better chemical resistance and higher softening point but relatively brittle.

Uses
- LDPE is used in making film and sheeting. Pipes made of LDPE are used for both agricultural irrigation and domestic water line connections. It is also used for making tubes, coated wires, and cable wires.
- HDPE is used in the manufacture of toys, insulator parts, bottle caps, flexible bottles, kitchen, and domestic articles.
(ii) Polyvinyl Chloride (PVC)
It is obtained by heating a water emulsion of vinyl chloride in the presence of a small amount of benzyl peroxide or hydrogen peroxide in an autoclave under pressure.

Vinyl chloride so needed is generally prepared by treating acetylene at 1–1.5 atmospheres with hydrogen chloride at 60–80°C, in the presence of metal chloride as catalyst.
Properties
PVC is colourless, odourless, non-inflammable, and chemically inert powder, resistant to light, atmospheric oxygen, inorganic acids, and alkalis but soluble in hot chlorinated hydrocarbons such as ethyl chloride. Pure resin possesses high softening point and a greater stiffness and rigidity, but is brittle.
Uses
- Rigid PVC or unplasticized PVC has superior chemical resistance and high rigidity but is brittle. It is used for making sheets, which are employed for tank linings, light fittings, safety helmets, refrigerator components, tyres, cycles, and motor-cycle mudguards. It is also extruded in strip and tube form for use in place of nonferrous metals.
- Plasticized PVC is used for making continuous sheets, rain coats, table clothes and curtains, electric cables, toys, radio components, conveyor belts, etc.
(iii) Polystyrene (PS)
It is prepared by polymerization of styrene in the presence of benzoyl peroxide catalyst.

Properties
Polystyrene is a transparent, light-stable, and moisture-resistant material. It is highly electric insulating and highly resistant to acids, and it is a good chemical resistant. But it has less softening and is brittle. However, it has the unique property of transmitting light through curved sections.
Uses
It is used in moulding of articles like toys, combs, buttons, buckles, radio and television parts, refrigerator parts, battery cases, high-frequency electric insulators, lenses, indoor lighting panels, food containers, food packaging, umbrella handles, etc.
(iv) Teflon (Polytetrafluoroethylene (PTFE or FLUON))
In the presence of benzoyl peroxide catalyst and high pressure polymerization of tetrafluoroethylene gives Teflon.

Properties
Teflon has twisted, zigzag structure with fluorine atoms, packing tightly in a spiral around the carbon-carbon skeleton. Due to the presence of highly electronegative fluorine atoms, there are very strong attractive forces between different chains. These strong attractive forces give the material extreme toughness, high softening point, exceptionally high chemical resistance towards all chemicals, high density, waxy touch, very low coefficient of friction and extremely good electrical and mechanical properties. It can be machined, punched, and drilled. The material cannot be dissolved and cannot exist in a true molten state. Around 350°C, it sinters to form a very viscous, opaque mass, which can be moulded by applying high pressure.
Uses
- It is used as insulating material (motors, transformers, cables, wires, etc.) and for making gaskets, packings, pump parts, tank lining, chemical-carrying pipes, tubings and tanks, etc.
- Used for coating and impregnating glass fibres, asbestos fibres, and cloths, and in non-lubricating bearings and non-sticking stop-clocks, coating of frying pans, etc.
(v) Nylon
These are polyamides. The word nylon is new accepted as a generic term for the synthetic polyamides, which are characterized by a repeating acids linkage (–NHCO–). Nylon is formed with dicarboxylic acids and diamide under condensation process. It has been named on the basis of number of carbon atoms present in that two monomer units.
E.g.: Nylon 6,6, Nylon 6,10, Nylon 6,11, etc.
Nylon 6,6 is formed with the condensation reaction of hexamethylene diamine and adipic acid.

Properties
They are translucent, whitish, horny, and high melting polymers. They possess stability upto high temperature and good abrasion resistance. They are insoluble in common organic solvents and soluble in phenol and formic acid.
Properties of Nylon Fibers
- They are light, horny, and high melting.
- They are insoluble in common solvents.
- They have good strength.
- They absorb little moisture and are thus “drip-dry” in nature.
- They are very flexible and retain original shape easily.
- They have resistances to abrasion.
- On blending with wool, the strength and abrasion resistance of the latter increase.
Uses
- Nylon 6,6 is primarily used for fibers and tyre cord, which find use in making rocks, ladies hoses, undergarments, dresses, carpets, ropes, etc.
- Nylon 6 and nylon 11 are mainly used for moulding purposes for gears, bearings, electrical mountings, etc. Nylon bearings and gears work quietly without any lubrication.
- They are also used for making filaments for ropes, bristles for tooth brushes, films, etc.
4.7.2 Thermosetting Plastics
Important thermosetting plastics:
Phenol-formaldehyde Resins or Phenoplasts
These are condensation polymerization products of phenolic derivatives with aldehydes, prepared by condensing phenol with formaldehyde in presence of acidic or alkaline catalyst. Depending upon catalyst and reactants mainly three kinds of resins are formed, they are
- Novalac resin
- Resol resin
- Bakelite
Among those Bakelite is important resin.
Novalac Resin
In the presence of acid, phenol and formaldehyde condense to form novalac resin.
Here first formaldehyde takes proton from acid and form carbonium ion
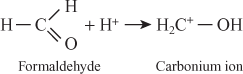
Phenol react with carbonium ion to form ortho and para methylol phenol

Ortho-methylol phenol condense to form novalac resin.

Resol Resin
Phenol and formaldehyde is refluxed with ammonia at 100°C gives resol resin. In presence of ammonia methylol phenol has greater reactivity with formaldehyde than phenol, hence it gives di and tri methylal products.

Above formed polymethylol phenol condensed and form resol resin

Bakelite
Bakelite is first prepared in Bakeland. In the presence of hexamethylene tetramine phenol react with formaldehyde and forms cross linked resin i.e. bakelite. It is hard and insoluble solid.

Properties
Novalac resin is soluble and fusible solid. Resol resin is hard and brittle solid. Bakelite set to rigid, hard, scratch-resistant, infusible, water resistant, insoluble solid. It resist to non oxidizing acids, salts and organic solvents, but are attacked by alkali due to presence of free hydroxyl groups. All phenol formaldehyde resins possess excellent electrical insulating character.
Uses
- Making electric insulator parts like switches, plugs, switch-boards, heater-handles, etc.
- Preparing moulded articles like telephone parts, cabinets for radio and television.
- For impregnating fabrics, wood and paper.
- As adhesives for grinding wheels.
- Making bearings used in propeller shafts for paper industry and rolling mills.
- As hydrogen-exchanger resins in water softening.
Polyurethanes
Diisocyanate and diol gives polyurethanes.
E.g.: Reaction between 1,4–butane diol and 1,6–hexane diisocyanate gives “Perlon – U” a crystalline polymer.
Properties
- Poly urethanes are less stable than nylons.
- These have excellent resistance to abrasion and solvents.
Uses
These are used as coatings, films, foams, adhesives and elastomers.

4.8 Rubber (Elastomers)
Rubbers are high polymers, which have elastic properties. Thus the rubber band can be stretched to 4 to 10 times its original length, and as soon as the stretching force is released, it returns to its original length. The elastic deformation in an elastomer arises from the fact that in the unstressed condition, an elastomer molecule is not straight chained, but in the form of a coil, it can be stretched like a spring consequently. The unstretched rubber is amorphous.
4.8.1 Processing of Natural Rubber
Isoprene is the basic molecule present in natural rubber. Dispersive form of isoprene units are known as latex. In the processing of natural rubber isoprene molecules polymerize and form long, coiled chains of cis-polyisoprene.


Structure of natural rubber
By making small incisions on the barks of rubber trees, like having a brasiliensis and gauyule, the rubber latex can be collected into small vessels, as it oozes out. It contains 25–45% of rubber in the form of milky colloidal emulsion, the remainder of which is made mainly of water and small amounts of protein and resinous material with time, the flow of latex from the incision made start decreases. Thus at regular intervals, tapping is necessary throughout the life of the tree.
Latex is diluted to make 15–20% of rubber and is filtered to eliminate any dirt present in it. It is then coagulated in a tank, fitted with irregular partitions by adding about 1 kg of acetic acid or formic acid per 200 kg of rubber, to a soft white mars. After washing and drying, the coagulated is treated as follows:
- Crepe rubber: It is prepared by adding little sodium bisulphite to bleach the rubber and then it is passed through a creping machine so that coagulum is rolled out into sheets of about 1 mm thickness. The sheet possesses an even rough surface resembling crepe paper. The sheet is then dried at 50°C in air.
- Smoked rubber: It is made by eliminating the bleaching with sodium sulphite and rolling the coagulum into thicker sheets, having ribbed pattern on its surface. Ribbed surface pattern on the sheet prevents them from adhering together on stacking. It also facilitates consequent drying as it exposes greater surface area of the sheet. The sheets are then dried in the smoke obtained from burning wood or coconut shells at about 50°C. The rubber sheet thus obtained is translucent and amber in colour.
4.8.2 Gutta Percha
It is a trans-form of natural rubber. (In natural rubber isoprene units are linked with cis-form). It is obtained from the matured leaves of dichopsis gutta and palagum gutta trees, grown mostly in Malaya and Sumatra. Gutta percha can be recovered by solvent extraction process, when insoluble resins and gums are separated. Alternatively, the matured leaves are grounded carefully and is treated with water at about 70°C for half an hour and then poured into cold water, when gutta percha floats on water surface it is removed.

Properties
- At room temperature, gutta percha is horny and tough, but it softens and becomes tacky at about 100°C.
- It is soluble in aliphatic hydrocarbons, but insoluble in aromatic and chlorinated hydrocarbons.
Uses
In the manufacturing of golf ball covers, submarine cables, adhesives, and tissues for surgical purposes.
4.8.3 Vulcanization of Rubber
Drawbacks of Raw (Natural) Rubber
The drawbacks of raw (natural) rubber are as follows:
- It is plastic in nature. A crude rubber becomes soft and sticky in hot summer, while in cold weather it becomes hard and brittle. Its usage is limited to a particular temperature range of about 10–60°C.
- It is weak, due to low tensile strength (200 kg/cm2).
- It has large water absorption capacity.
- It is non-resistant to non-polar solvents like vegetable and mineral oils, gasoline, benzene, and carbon tetra chloride.
- It is attacked by oxidizing agents (HNO3, H2SO4 etc). It perishes due to the oxidization in air.
- In organic solvents it undergoes, swelling and gradual disintegration.
- It possesses tackiness, which means that under pressure two fresh raw rubber surfaces coalesce to form a single piece.
- It posses very less durability.
- When stretched to a great extent, some molecular chains undergo sliding or slipping over each other, hence it suffers from permanent deformation.
To improve the properties of rubber, it is compounded with some chemicals like sulphur, hydrogen sulphide, benzoyl chloride and the rubber mix is prepared for vulcanization. The addition of compounding agents is facilitated by the process of mastication. Mastication of rubber means it is subjected to severe mechanical working. Oxidative degradation accompanied by a marked decrease in the molecular weight of the rubber occurs. This converts rubber into soft and gummy mass.
Vulcanization
Heating of raw rubber with sulphur around 100–140°C is known as vulcanization. Sulphur combines chemically at the double bonds of rubber chains and forms cross links. With these crosslinks rubber becomes stiff and the percentage of sulphur determines the stiffness of rubber.
E.g.: A tyre rubber contains 3–5% of sulphur.

Advantages of Vulcanization
- The tensile strength of vulcanized rubber is very good and has extensibility about 10 times the tensile strength of raw rubber; when a tensile force is applied, it can bear a load of 2000 kg/cm2 before it breaks.
- After the removal of deforming force, the articles made from vulcanized rubber regain their original shape, so it has excellent resilience.
- It has broader useful temperature range (–40 to 100°C) compared to raw rubber‚s temperature range (10 to 60°C).
- It has much high resistance to moisture, oxidation, and abrasion.
- It has much higher resistance to wear and tear as compared to raw rubber.
- It is a better electrical insulator, although it tends to absorb small amounts of water.
E.g.: Ebonite is a better insulator.
- It has resistance to organic solvents like benzene, carbontetra chloride, petrol, etc., but it swells in them.
- It is very easy to manipulate the vulcanized rubber to produce the desired shapes.
The superior properties of vulcanized rubber compared to raw rubber are summarized below.

4.8.4 Compounding of Rubber
Compounding is “mixing of the raw rubber with other chemicals so as to impart the product-specific properties suitable for particular job.” The following substances are generally mixed with raw rubber:
- Antioxidant: Natural rubber has a tendency to “perish” due to oxidation, retarding the deteriorating of rubber by preventing its oxidation by light and air; about 1% of the antioxidants are used. These are phenolic substances, phosphites, and complex organic amines like phenyl naphthyl amine.
- Colouring agents: Colouring agents impart the desired colour to the rubber product.
E.g.: For white products, titanium dioxide (TiO2) is the usual pigment. For colour products, the following pigments are used:

- Vulcanizing agents: The main vulcanizing agent is sulphur. Among these sulphur monochloride, hydrogen sulphide, and benzoyl chloride are also used as vulcanizers. Depending on the nature of the product required, the percentage of S added varies between 0.15 and 32.0 percent. The process of vulcanization brings about excellent changes in the properties of crude rubber.
- Accelerators: They are positive catalysts for vulcanization process as they drastically shorten the time required for vulcanization. Generally, benzothiozole, 2–mercaptol, zinc alkyl xanthate, and thiocarbamates are used 0.5–1% as most usual accelerators.
- Reinforcing agents: These agents give rigidity, strength, and toughness to the rubber and are present up to 35% of the rubber compound. Commonly used reinforcers are carbon black, zinc oxide, CaCO3, MgCO3, etc.
E.g.: Addition of carbon black in the elastomer is used in the manufacture of automobile tyres.
- Plasticizers and softness: They impart greater tenacity and adhesion to the raw rubber. The important plasticizers are stearic acid, waxes, vegetable oils, rosin, etc.
- Inert fillers: They lower the cost of the product and alter the physical properties of the mix for simplifying the subsequent manufacturing operations.
4.8.5 Synthetic Rubbers or Artificial Rubber
The landmark discovery of rubber is the greatest achievement in polymer industry and with the efforts of scientists and technologists the first useful synthetic rubber Buna-S was prepared.
Advantages of Synthetic Rubbers
Because of the better performance properties of synthetic elastomers, natural rubber failed to give stiff competition.
- They are produced from petrochemical raw materials in abundant amounts.
- They are economically beneficial.
- They are not only replacements but are superior to natural rubber in certain cases.
- They are tailor-made elastomers with diverse applications.
- They have high abrasion resistance and high tensile strength.
- Certain elastomers like silicones have low temperature (–80°C), flexibility, and high temperature stability.
- Silicones are valuable in surgical prosthetic devices.
4.8.6 Important Artificial Rubbers
(i) Styrene Butadiene Rubber (GR-S or Buna-S)
It is a copolymer of styrene (25% by weight) and butadiene (75% of weight). The monomers are emulsified in water using soap or detergent. The reaction is initiated by peroxide initiators. Polymerization is carried out at 5°C, and therefore, the product is known as cold SBR.

Properties
Styrene rubber is slightly inferior to natural rubber in its physical properties. It possesses high abrasion resistance, high load-bearing capacity, and resilience. However, it gets readily oxidized, especially in the presence of traces of ozone present in the atmosphere. It swells in oils and solvents. It can be vulcanized in the same way as natural rubber, but it requires less sulphur and more accelerators for vulcanization.
Uses
- It is mainly used for the manufacture of motor tyres, (passenger car tyres, motor cycle tyres, and scooter and cycle tyres.) but not used for truck tyres.
- It is also used for floor tiles, shoe soles, gaskets, footwares, conveyor belts, wire and cable insulations, carpet backing, adhesives, tank linings, etc.
(ii) Buna-N or Nitrile Rubber or GR-A
It is a copolymer of a 1,3–butadiene and acrylonitrile. They are also prepared in emulsion systems. They are noted for their oil resistance but not suitable for tyres.

Properties
It possesses excellent resistance to heat, sunlight, oils, acids, and salts, but it is less resistant to alkalis than natural rubber because of the presence of cyano groups. As the proportion of acrylonitrile is increased, the resilience to acids, salts, oils, solvents, etc., increases, but the low temperature resilience suffers. Vulcanized rubber is more resistant to heat and ageing than natural rubbers and may be exposed to high temperature.
Uses
- They are extensively used for fuel tanks, gasoline hoses, creamery equipment, conveyor belts, and high-altitude aircraft components.
- They are also used as adhesives, latex, gaskets, printing rollers, leather, and textiles.
(iii) Poly Sulphide Rubber or Thiokol or GR-P
Thiokols are those elastomers in which sulfur forms a part of the polymer chain. It is a copolymer of sodium poly sulphide (Na2S4) and ethylene dichloride.

Properties
Thiokols have outstanding resistance to swelling and disintegration by organic solvents, mineral oils, fuels, solvents, oxygen, ozone, gasoline, and sunlight. Thiokol films have low permeability to gases.
It has the following limitations:
- It tends to flow or lose shaped under continued pressure. As it cannot be vulcanized, it does not form hard rubber.
- Its tensile strength is lesser than that of natural rubber.
- It cannot be vulcanized.
Uses
- Thiokols are used for barrage balloons, life rafts, and jackets, which are inflated by CO2.
- It is also used for lining hoses for conveying gasoline and oil, in paints, for gaskets, diaphragm and seats in contact with solvents, and for printing rolls.
(iv) Polyurethanes (Isocyanate Rubber)
These polymers are formed by the reaction between diisocyanates and polyalcohols.

Properties
Polyurethane elastomers have outstanding abrasion resistance and hardness combined with good elasticity and resistance to oils, greases, chemical, weathering, and solvents.
Uses
They are used in applications where extreme abrasion resistance is required such as in heel lifts, surface coatings, manufacture of foams, spandex fibers, and small industrial wheels.
(v) Silicone Rubbers
Silicones are organic silicone polymers. They are having alternate Si-O–bonds.
Preparation: For preparation of silicones, dialkyl-substituted silanes are used as raw materials. They undergo hydrolysis and condensation polymerization to form silicone polymers.
Silicones are of mainly two types:
- Straight-chain polymers
- Cross-linked polymers
- Straight-chain polymers: Dialkyl-substituted silanes are subjected to hydrolysis and undergo condensation polymerization to form straight-chain thermo plastic silicones.

- Cross-linked polymers: Mono alkyl-substituted silanes are subjected to hydrolysis and proceeds condensation reaction gives cross-linked polymers.

Alkyl chloro silanes are prepared by this process.

Properties
- Silicones are having resistance to heat, cold, oxidation, etc.
- They obtain as liquids, resins, and elastomers.
- Silicon liquids are having repulsion with water. They have also resistance with chemicals.
- Silicone rubber may form reaction between silicone resins, silica, and alumina.
Uses
- Silicones are used as anti-forms in the preparation of food stuff.
- They are used as elastics, insulators, gascatoles, and ceiling joints, and also used in medicine.
- They are used as surface-coating agents.
- Silicones are used for lamination.
(vi) Reclaimed Rubber
Reclaimed rubber is rubber obtained from waste rubber articles like worn out tyres, tables, gaskets, hoses, foot wears, etc. The process of reclaimation of rubber is carried out as follows:
The waste is cut to small pieces and powdered by using a craker which exerts powerful grinding and tearing action. Then ferrous impurities, if any are removed by the electromagnetic separator. The purified waste powdered rubber is then digested with caustic soda solution at about 200°C under pressure for 8–15 hours in “steam-jacketed autoclave”. By this process, the fibres are hydrolysed. After the removed of fibres reclaimed agents (like petroleum and coal tar based oils) and softeners are added sulphur gets removed as sodium sulphide and rubber becomes devulcanised. The rubber is then thoroughly washed with water sprays and dried in hot air driers. Finally, the reclaimed rubber is mixed with small proportion of reinforcing agents (like clay, carbon black, etc).
Properties
The reclaimed rubber is of less tensile strength lower in elasticity and possesses lesser wear-resistance than natural rubber. However, it is much cheaper, uniform in composition and has better ageing properties. Moreover it is quite easy for fabrication.
Uses
For manufacturing tyres, tubes, automobile floor mats, belts, hoses, battery containers, mountings, shoes and heals, etc.
4.9 Reinforced or Filled Plastics
The polymers, generally of low strengths and modulii of elasticity are needed for structural purposes. For these reasons, the polymers are combine with fillers (which are primary silicates) to get better products. The fillers are solid additives, which modify the physical properties, particularly, the mechanical properties of basic polymeric materials. For example, they improve: (i) thermal stability (ii) mechanical strength (iii) insulating characteristics (iv) water resistance (v) external appearance (vi) rigidity (vii) finish (viii) hardness (ix) opacity and (x) workability beside reducing (xi) cost (xii) Shrinkage on setting and brittleness.
Usually, specific fillers are added to a polymeric compound to impart special charecters to the final products. For example:
- To improve hardness, fillers like carborundum, quartz, and mica are added.
- To make the plastic impervious to x-ray, barium salts are fillers.
- To provide heat and corrosion resistances, asbestos is added.
The combination of polymeric substance with solid fillers, is called filled or reinforced plastics. The filler acts as a reinforcing material while the polymer acts as binder, which links the filler particles. The polymer serves as stress transforming agent from filler to filler particles.
Most commonly used fillers are:
(i) wood-flour (ii) saw-dust (iii) ground cork (iv) asbestos (v) marble flour (vi) china clay (vii) paper pulp (viii) coru husk (ix) mica (x) pumice powder (xi) carbon (xii) cotton fibres (xiii) boron fibres (xiv) silicon carbide (xv) silicon nitrade (xvi) graphite (xvii) alumina (xviii) glass fibres (xix) kelvar fibres (xx) cotton fibres (xxi) metallic oxides like ZnO, PbO etc and (xxii) metallic powder like Al, Cu, Pb, etc.
Composition
Fillers are usually employed in sizable weight percentage. The percentage of filler can be upto 50% of the total moulding mix.
Nature of Polymers Used
Polymers used are thermoplastics, thermosetting polymers as well as rubber (elastomers) such as polyethylene, polypropylene, Nylon–6, PET, polystyrene, melamine, silicone, natural and synthetic rubbers, epoxy etc.
Examples of filled plastics
- Carbon black on addition to natural rubber brings about 40% increase in tensile strength and such filled plastic is used in automobile tyres.
- Addition of china clay to PVC enhances electrical insulation characteristics of PVC. So this filled plastic is used for electrical insulation purposes.
- PVC and calcium carbonate combination is used for general purposes like tubings, seat covers, wire and cable insulations.
- Fibrous fillers like wood flour, short length of synthetic fibres, cotton floc, macerated paper and cloth etc are used in the thermosetting polymers, phenol-formaldehyde, melamine, formaldehyde, melamine urea etc, to get filled plastics of higher impact strength.
- Asbestos filled plastics are employed in electrical appliances.
- Fibre-reinforced plastics posses good thermal and shock resistance, good dimensional stability, good processability and moulded articles can be repaired easily.
Application of Filled Plastics (Reinforced Plastics)
Filled or reinforced plastics find numerous applications. For example:
- In automobiles for making door handles battery cases, engine cooling fans, etc (using base polymers polyethylene, polypropylene, and nylon–66).
- In defence for making nose cones, pistol grips and riffle bullets (using base polymers polystyrene and nylon–66).
- In textile for making shuttle (nylon–66 as base polymer).
- In electrical/electronic industry for making exhaust fan, computer tape, insulators, wire and cable insulation, switch gear parts, spools etc, (using polypropylene, PET, nylon and SAN as base polymers.
- In consumer goods like door handles, and window, hinge, chair shells, camera housing etc., (using polypropylene and ABS as base polymers).
- Miscelleneous like water meter housings, chemical pump housings, tubings, seat coverings etc., (using nylon–66, PVC and polypropylene as polymers).
4.10 Conducting Polymers
Due to nonavailability of free electrons most of the normal polymers are insulators. Scientists have taken this property as an advantage, and with their curiosity and challenging nature they prepared conducting polymers as promising materials.
‘Conducting polymer is an organic polymer having highly delocalized π–electron system and electrical conductance.‚
Conducting polymers are broadly classified into two categories such as intrinsically conducting polymers and extrinsically conducting polymers.
Intrinsically Conducting Polymer (ICP) or Conjugated p–Electrons Conducting Polymer
The polymers which contain conjugated π–electron backbone or delocalized electron pairs act as intrinsic conducting polymers.
E.g.:
- Polyacetylene polymers like Poly-p–phenylene, polyquinolone, etc.
- Condensed aromatic polymers like polyaniline, polyphenanthrylene, etc.
- Heteroaromatic and conjugated aliphatic polymers like polypyrole, polyazomethine, etc.
If conductivity of intrinsically conducting polymers is less, they are doped with positive or negative charges and this process is known as doping.
It is mainly oxidative (or) p-doping, reductive (or) n-doping and protonic acid doping.
- Oxidative (or) P-doping: Treating of intrinsically conducting polymers with Lewis acid like I2, Br2, FeCl3, CCl4, HBF4, etc. is known as p-doping.

Mechanism of conduction: The removal of an electron from the polymer π–back bone using a suitable oxidizing agent leads to the formation of delocalized radical ion called polarion.
A second oxidation of a chain containing polarion, followed by radical recommendation, yields two charge carriers on each chain. The positive charges sites on the polymer chains are compensate by anions formed by the oxidizing agent.
The delocalized positive charges on the polymer chain are mobile, not the dopant anions. Thus, these delocalized positive charges are current carriers for conduction. These charges must move from chain to chain as well as along the chain for bulk conduction.
- Reductive (or) n-doping: Treating of intrinsically conducting polymers with Lewis bases like Li, Na, tetrabutyl ammonia, napthylamine, etc. is known as n-doping

Mechanism of conduction: The addition of an electron to the polymer π–back bone by using a reducing agent generates a polarion. A second reduction of chain containing polarian, followed by the recombination of radicals, yields two negative (–ve) carriers on each chain. These charge sites on the polymer chains are compensated by cations formed by the reducing agent.
- Protonic acid doping: The synthesis of conducting polyaniline is a typical example of this type of doping technique. In this technique, current-carrying charged species (–ve/+ve) are created by the protonation of imine nitrogen.
Polyaniline is partially oxidized first, with a suitable oxidizing agent, into a base form of aniline, which contains alternating reduced and oxidized forms of aniline polymer backbone. This base form of aniline when treated with aqueous HCl (IM) undergoes protonation of imine nitrogen atom, creating current due to +ve sites in the polymer backbone. These charges are compensated by the anions (Cl–) of the doping agent, giving the corresponding salt. This doping results increase conductivity up to 9–10 orders of magnitude.
Applications: Conducting polymers are the most important materials to be used in electric and electronic applications:
- As electrode material for commercial rechargeable batteries, for higher power-to-weight ratio.
- As conductive tracks on printed circuit boards.
- As sensors: Gas sensor, radiation sensor, humidity sensor, bio sensor for glucose and galactose, etc.
- As film membranes for gas separations.
- As light-emitting diode.
- In electrochemical display windows.
- In fuel cells, as the electro catalytic materials.
- In information storage devices.
Conducting Polyaniline
Alan MacDiarmid investigated polyaniline as an electrically conducting polymer in 1985.

Properties
- Except specific conductivity, all other properties of polyaniline are considered as that of an organic metal.
- It is transparent in thin layers.
- When heated, it is stable in air.
- It is highly reactive, redox active material.
- In conducting state, it is green and changes its colour in different media.
E.g.: Under reducing condition – yellow
Oxidizing or basic condition – blue
Advantages
- It is the foremost air stable conducting polymer.
- It has a wide and controllable range of conductivity.
- It shows a number of interesting properties such as multicolour, electrochromism, chemical sensitivity, etc.
- It is considered to be unique among all the conducting polymers as it can be synthesized chemically or electrochemically as a bulk powder or film.
Disadvantages
- It is insoluble in common solvents except strong acids and n-methyl pyrolodine.
- It has poor mechanical properties.
- It decomposes prior to melting, and hence it is difficult to process.
Uses
- Due to its reversible electrochemical response during anodic oxidation and cathodic reduction, it is useful as a secondary electrode in rechargeable batteries and electrochromic display devices.
- It is used for coating of films and semi-finished articles.
- It is also used for corrosion protection, sensors, smart windows, printed circuit boards, conductive pipes for explosives, and conductive fabrics.
Extrinsically Conducting Polymers
Polymers whose conductivity is due to externally added ingredient are known as extrinsically conducting polymers. They are conductive element filled polymers and blended conducting polymers.
- Conductive element-filled polymers: In this kind of polymers, conducting materials like carbon black, metal oxides, etc. are added to the polymers.
- Blended conducting polymers: In this kind of polymer, conducting polymers are mixed with conventional polymer.
Application of Conducting Polymers
Conducting polymers have many uses because they are light weight, easy to process, and have good mechanical properties. They are used in
- In chargeable light-weight batteries based on perchlorate doped polyacetylene lithium systems. These are approximately ten times lighter than conventional, lead storage batteries. They have flexibility to fit into different designs.
- In optical display devices, based on polythiophene and polyaniline. When the structure is electrically biased, the optical density of the film changes and its colour changes. Such electrochromic systems produce colour displays with faster switching time and better viewing than conventional liquid display devices. Polyaniline, due to redox activity in conducting state, is green; under reducing condition, it is in yellow colour, and oxidizing condition, it appears blue.
- In telecommunication systems
- In electromagnetic screening materials
- In antistatic coatings for clothing
- In electronic devices such as transistors and diodes
- In wiring in aircrafts and aerospace components
- In solar cells and as biosensors in metabolic reactions and drug delivery system for human body
- In photo voltaic devices
- In non-linear optical material
- In molecular wires and molecular switches.
4.11 Polyphosphazenes/phosphonitritic polymers
Polyphosphazenes are hybrid inorganic organic polymers with a number of different skeletal structures that contain a backbone of alternating phosphorous and nitrogen atoms, and are interesting, commercially promising materials. A variety of substituents can substitute the basic backbone and hence we can get a variety of products. Basic backbone of polyphosphazene is

Preparation: The most popularly used method for preparing polyphosphazenes is ring opening and substitution method. Allcock and co-workers discovered that cyclic trimer (hexachlorocyclo triphosphazene) can be thermally ring opened and can give high molecular weight soluble poly (dichlorophosphazene). After the replacement of the chlorine atoms in poly (dichlorophosphazene) by reaction with organic/organometallic nucleophiles, they give a variety of polyphosphazenes.

Substitution Reaction
Reaction with Sodium Alkoxide
Poly (dichlorophosphazene) react with sodium alkoxide and give poly (dialkoxyphosphazene).

Reaction with Amine
Poly (dichlorophosphazene) reacts with amine and gives poly (dialkylaminephasphazene).

Reaction with Metal Alkyde
Poly (dichlorophosphazene) reacts with metal alkyde and gives poly (dialkylphasphazene).
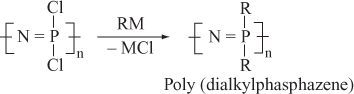
Important Characteristics of Polyphasphazenes
Polyphasphazenes have so many important properties, and among those biocompatibility, high dipole moment, flexibility, chemical inertness, broad range of glass transition temperature (Tg), elastomeric property and impermeability are the most important.
Applications
Based on their wide range of unique properties, polyphosphazenes have countless and advanced applications. They have potential for formation of new compounds. The applications include in challenging areas of biomedical research such as tissue generation, macromolecules, etc. These are also used as ion conductive membranes for rechargeable lithium batteries and fuel cell membranes. These are advanced material of elastomers for aerospace engineering. Polyphosphazenes are good photonic materials and fire-resistant polymers.
4.12 Composites
Composites are the multiphase material that exhibits a significant proportion of the properties of both the constituent materials.
(or)
Materials composed of at least two distinctly dissimilar materials acting in harmony. A Judicians combination of two or more distinct materials can provide better combination of properties.
(or)
An artificially prepared multiphase material in which the chemically dissimilar phases are separated by a distinct interface.
E.g.: Wood is the composite of cellulose and lignin, bone is the composite of a soft, strong protein collagen, and brittle, hard apatite material.
Packing paper impregnated with bitumen or wax, rain-proof cloth (cloth impregnated with water-proof material), insulating tape, reinforced concrete, etc.
4.12.1 Constituents of Composites
Composite material comprises mainly of
- Matrix phase and
- Dispersed phase
Matrix Phase
Matrix phase is the continuous body constituent enclosing the composite and given in its bulk form. Depending upon the matrix phase, composites are known as ceramic matrix composites, metal matrix composites, polymer matrix composites, etc.
Functions of Matrix Phase
- Matrix phase binds the dispersion phase, act as a medium, applied stress is transmitted and distributed uniformly.
- Protects the surface from damage due to chemical reactions, mechanical abrasion, etc.
- It prevents the propagation of brittleness, cracking, etc.
Hence, a good matrix phase should be ductile, having corrosion resistant and possess high binding strength.
Dispersed Phase
Dispersed phase is the structural constituent of composite. Fibres, flakes, whiskers, etc. are some important dispersed phases.
4.12.2 Classification of Composites
Composites are broadly classified into three categories:
- Particle-reinforced composites: In these composites, the dispersed phase is equi-axed, i.e., the dimensions of the particles are nearly the same in all directions. They are subdivided into the following:
- Large-particle composites
- Dispersion-strengthened composites
- Fiber-reinforced composites: In these composites, the dispersed phase is in the form of fibers. These are subdivided into (a) continuous aligned (b) discontinuous.
Discontinuous composites are further divided into (a) aligned (b) randomly oriented.
- Structural composites: In these composites, the properties depend not only on the constituent material but also on this geometrical design. These are subdivided into (a) laminates (b) sandwich panels.
Among these, fibre-reinforced polymer composites are widely used.
Fiber-reinforced Polymer Composites
These are prepared by reinforcing a plastic matrix with a high-strength fiber material.
Fiber-reinforced composites involve three components, namely filament, a polymer matrix, and encapsulating agent (which ties fiber filaments to polymer). Glass fibers and metallic fibers are commonly employed for this purpose. The fibers can be employed either in the form of continuous lengths, staples, or whiskers. Such composites possess high specific strength (tensile strength/specific gravity) and high specific modulus (elastic modulus/specific gravity), stiffness, and lower overall density.
Characteristics
The fiber-reinforced composites posses superior properties like higher yield strength, facture strength, and fatigue life. The fibers prevent slip and crack propagation and inhibit it, thereby increasing mechanical properties. When a load is applied, there is a localized plastic flow in matrix, which transfers the load to the fibers embedded in it. When a soft phase is present in hard matrix, the shock resistance of the composite is increased. On the other hand, if hard reinforcing fibers are present in a soft matrix, the strength and modulus of composite are increased. To obtain composites having the maximum strength and modulus, it is essential that there should be maximum number of fibers per unit volume, so that each fiber takes its full share of the load. The fiber-reinforced composites are, generally, anisotropic (i.e., having different directions), and the maximum strength is in the direction of alignment of fibers. For getting isotropic properties, the fibers are oriented randomly within the matrix, E.g.: ordinary fiber glass. It may be pointed here that the cost of laying fibers aligned in a particular direction is much higher than that for random orientation. For preparing fiber-reinforced composites, it is essential that
- The coefficient of expansion of the fiber matches closely that of the matrix.
- The fiber and matrix should be chemically compatible with each other and no undesirable reaction takes place between them.
- The fiber should be stable at room temperature and should retain a good percentage of strength at elevated temperatures.
Some important reinforced composites are described hereunder.
- Glass fibre-reinforced polymer composites: For improving the characteristics of nylon, polyester, etc. containing polymer matrices, glass fibres are employed. These have lower densities, higher tensile strengths and resistance to corrosion and chemicals.
Limitations:
- Since the most polymeric matrices start deteriorating or flow or melt at high temperatures, they find application with limited temperature service conditions.
- They cannot be employed as structural components, since these materials do not possess the desired stiffness and rigidity.
Applications: They are used in automobile parts, storage tanks, floorings (industrial), transportation industries, plastic, pipes, etc.
- Carbon fiber-reinforced polymer composites: They are also known as advance polymer matrix composites or high performance composites and are employed in situations requiring (i) excellent resistance to corrosion (ii) lighter density, and (iii) retention of desired properties, even at elevated temperatures. However, the general use is limited due to their higher costs.
Applications: They are used as structural components (like wing, body, and stabilizer) of aircrafts (military and commercial) and helicopter‚s recreational equipments (fishing rod), sport materials (golf clubs), etc.
4.12.3 Advantages of Composites Over Conventional Materials
Composites have the following advantages over conventional materials like metals, polymers, ceramics, etc.
- Have higher specific strength and stiffness. They can maintain strength up to higher temperatures.
- Have lower specific gravity, electrical conductivity and thermal expansion.
- They have better toughness, impact, thermal shock resistance, fatigue strength, corrosion and oxidation resistance.
4.12.4 Applications of Composites
- In automobile industries, transportation industries, turbine engines, wire drawing dies, valves, pump parts, spray nozzles, storage tanks, fabrication of roof and floors, furniture, sport goods (lawn, tennis rackets), high-speed machinery, etc.
- Marine applications like propellers, shafts, hulls, spars (for racing boats), and other ship parts.
- Aeronautical applications like components of rockets, aircrafts (business and military), helicopters, missiles, etc.
- Communication antennae and electronic circuit boards
- Safety equipments like ballistic protection
4.13 Review questions
4.13.1 Fill in the Blanks
- Repeating units present in polymer is _______.
[Ans.: monomer]
- Two or more different monomer units are participates in polymerization _______ polymers are formed.
[Ans.: oligo]
- _______ plastics cannot be remould.
[Ans.: Thermosetting]
- Monomer units participated for formation of fluon is _______.
[Ans.: Tetrafluroethylene]
- Bakelite is _______ plastic.
[Ans.: Thermosetting]
- _______ units are present in natural Rubbers.
[Ans.: Isoprene]
- Monomers are involved in formation of nylon 66.
[Ans.: Hexamethylene diamine and Adipic acid]
- Monomers posses the double bonds undergo _______ polymerization mechanism.
[Ans.: addition]
- An organic polymer with highly delocalized pi-electron system, having electrical conductance is called a _______.
[Ans.: conducting polymer]
- _______ is considered as an organic metal.
[Ans.: polyaniline]
4.13.2 Multiple-choice Questions
- High polymers are:
- Liquids
- Gases
- Solids
- Colloidal
[Ans.: d]
- Polymerization in which two or more chemically different monomers takes part, is called
- Addition polymerization
- Co polymerization
- Chain polymerization
- None of these
[Ans.: b]
- Structural units of high polymers, are called;
- Fibers
- Thermo units
- Monomers fabrics
- Fabrics
[Ans.: c]
- GR-S rubber is an example of:
- Condensation polymerization
- Co polymerization
- Addition polymerization
- Cross-linked polymer
[Ans.: b]
- A thermo plastic is formed by the phenomenon of:
- Chlorination
- Condensation polymerization
- Nitration
- Chain polymerization
[Ans.: d]
- A copolymer is formed by the union of two or more:
- A molecules of some monomer by the elimination of small molecules like H2O
- Monomer molecules in a chain without the elimination of H2O etc.
- Monomer molecules with the elimination of small molecules like H2O
- Thermosetting and thermo plastic resins
[Ans.: b]
- Phenol-formaldehyde resin is commercially known as:
- pvc
- Elastomer
- Bakelite
- Nylon
[Ans.: c]
- Polymer commonly used for making fibre/cloth is:
- Rubber
- pvc
- nylon
- bakelite
[Ans.: c]
- Bakelite is prepared by the condensation of:
- Benzene and formaldehyde
- Phenol and formaldehyde
- Phenol and acetaldehyde
- Glycerol and phthalic desired
[Ans.: b]
- A high molecular weight material that can easily be moulded in any desired shape is:
- Graphite
- Resin
- Jelly
- Grease
[Ans.: b]
- A plastic which can be softened on heating and hardened on cooling is called:
- Thermo elastic
- Thermo plastic
- Thermosetting
- Thermite
[Ans.: b]
- Phenol-formaldehyde (Bakelite) is an example of:
- Thermo elastic
- Thermoplastic
- Thermosetting
- Thermite
[Ans.: c]
- Natural rubber is basically a polymer of:
- Propylene
- Isoprene
- Ethylene
- Propane
[Ans.: b]
- Which one of the following is an elastomer?
- pvc
- bakelite
- natural rubber
- nylon
[Ans.: c]
- Raw rubber on vulcanization becomes
- plastic
- racky
- soft
- less elastic
[Ans.: d]
- Most commonly used vulcanizing agent is:
- graphic
- carbon black
- sulphur
- dryice
[Ans.: c]
- Ebonite is:
- Polyethelene
- highly vulcanized rubber
- Natural
- synthetic rubber
[Ans.: b]
- One of the important uses of Bakelite is for making
- cables
- electrical switches
- cloth
- hose pipe
[Ans.: b]
- The fibre obtained by condensation of hexamethylene diamine and adipic acid is:
- decorn
- nylon
- rayon
- terylene
[Ans.: b]
- Nylon is
- a polythene derinative
- a polyster fibre
- a polyamide fibre (c) none of these
[Ans.: c]
- Bakelite is a co polymer of:
- urea and formaldehyde
- Phenol and formaldehyde
- urea and phenol
- butadiene and ethylene
[Ans.: c]
- Bun-s is a co polymer of:
- vinyl chloride + vinyl alcohol
- butadiene and acrylonitirile
- butadiene and styrene
- butadiene and ethylene
[Ans.: c]
- Which one is not a macro molecule?
- protein
- insulin
- ice
- cellulose
[Ans.: c]
- Which one is a co polymer?
- Nylon–66
- Teflon
- pvc
- polybutadiene
[Ans.: a]
- An example of chain-growth polymer is:
- nylon–66
- bakelite
- terylene
- Teflon
[Ans.: d]
- What is the repeat unit in teflon?
- F2C = CF2
- H2C = CH2
- H2C = CHCl
- H2C = CHC6H5
[Ans.: a]
- Which one is not thermoplastic?
- bakelite
- terylene
- polystyrene
- polythene
[Ans.: a]
- Which one is a step growth polymer
- Teflon
- PVC
- polybutadiene
- bakelite
[Ans.: d]
- Which of the following is a synthetic polymer
- cellulose
- PVC
- proteins
- nucleic acids
[Ans.: b]
- Which of the following is a linear polymer?
- nylons
- bakelite
- low density polythene
- formaldehyde polymer
[Ans.: a]
- Which of the following has the largest molecular mass
- monomer
- dimer
- oligomer
- polymer
[Ans.: d]
- Isoprene is monomer of
- Starch
- synthetic rubber
- natural rubber
- PVC
[Ans.: c]
- Which of the following is thermosetting polymer
- linear
- branched chain
- cross-linked
- thermo plastic
[Ans.: c]
- Which of the following is an addition polymer?
- terylene
- nylon
- polyethylene
- all the above
[Ans.: c]
- Which of the following is thermosetting polymer?
- nylon
- polyethylene
- bakelite
- PVC
[Ans.: c]
- The monomer of PVC is:
- ethylene
- vinyl chloride
- tetrafluroethylene
- styrene
[Ans.: b]
- Chloroprene is the repeating unit in:
- polystyrene
- neoprene
- PVC
- polythene
[Ans.: b]
- The process of vulcanization makes rubber:
- soluble in water
- hard
- soft
- more elastic
[Ans.: b]
- A common catalyst used in addition polymerization is:
- nickel
- y-zeolite
- Ziegler-natta catalyst
- platinum
[Ans.: c]
- Functionality of phenol is
- one
- two
- three
- six
[Ans.: c]
4.13.3 Short Answer Questions
- What is a monomer?
Ans.: The repeating unit present in the formation of a polymer is known as a monomer.
- What is a polymer?
Ans.: Polymers are macromolecules of high molecular masses built up by the linking together of a large number of small, repeated units by a covalent bond.
- What is meant by polymerization?
Ans.: The chemical process leading to the formation of a polymer is known as polymerization.
- What is meant by degree of polymerization?
Ans.: The number of monomer units in a polymer is known as the degree of polymerization.
- Differentiate between homopolymer and copolymer.
Ans.: Homopolymers are formed with same monomer units.
E.g.: PE, PS, PVC, etc.
Copolymers are formed with two or more different monomers.
E.g.: Nylon 66 (Hexamethylene diamine + adipic acid)
- Differentiate between addition and condensation polymerization.
Ans.: Addition polymerization is the process of polymerization by the addition of monomer units which have unsaturated double or triple bonds.
E.g.: Polyethylene, polyvinyl chloride, etc.
Condensation polymerization takes place where the monomer units have two or more reactive functional groups.
E.g.: Polyester, nylon, polyamide, etc.
- Define thermoplastics.
Ans.: Linear, long chain polymers which can be softened on heating and hardened on cooling are known as thermoplastics.
E.g.: Polythene, polyvinyl chloride, etc.
- What are the monomers present on nylon 66?
Ans.: Hexamethylene diamine and adipic acid.
- What is meant by vulcanization of rubber?
Ans.: Vulcanization is heating of the raw rubber at 100–140°C with sulphur.
- What is natural rubber?
Ans.: Cis-polyisoprene is a natural rubber.
- What is gutta percha?
Ans.: Trans-polyisoprene is gutta percha.
- What is crepe rubber?
Ans.: Rubber with sodium bisulphite is passed through a creping machine and the coagulum is rolled into sheets. The sheet is hence having the surface like crepe paper; hence, it is known as crepe rubber.
- What is an elastomer?
Ans.: An elastomer is vulcanizable rubber like polymer, which can be stretched to at least twice its length and returned to its original shape and dimensions as soon as stretching force is released.
- Why does raw rubber need vulcanization?
Ans.: In vulcanization sulphur combines chemically at the double bonds of different rubber spring and provides cross-linking between the chains. Hence, for stiffening the rubber needs vulcanization.
- Give examples for natural polymers.
Ans.: Cotton, silk, wool, nucleic acid, proteins, starch, cellulose, etc.
- Give examples for inorganic polymers.
Ans.: Polyphorpazins, polysilanes, polygermanes, etc.
- What are the substances mixed in the compounding of rubber?
Ans.: Antioxidants, colouring agents, vulcanizing agents, accelerators, plasticizers and inert fillers are adding in the compounding of raw rubber.
4.13.4 Descriptive Questions
- a. Write a note on the properties and uses of Teflon.
b. Differentiate between natural polymer and synthetic polymer.
c. Write a note on silicone rubbers.
- a. Distinguish between addition and condensation polymerizations.
b. Explain the differences between thermoplastics and thermosetting plastics with examples.
c. What is meant by degree of polymerization?
- Write the structures of four addition polymers and four condensation polymers with their respective monomers.
- a. Describe the preparation, properties, and engineering uses of polyethylene.
b. What is meant by fabrication of plastics? Mention the different fabrication techniques.
- a. What are elastomers? Give examples.
b. What are the ingredients used in the compounding of plastics? What are their functions?
- a. What is a plastic?
b. Write the merits and demerits of using plastics in the place of metals.
- a. Identify the thermosets and thermoplastics among the following:
i. PVC
ii. Polyethylene
iii. Silicone
iv. Polyester fiber
v.
bakelite
b. What is bakelite? How is it manufactured? Mention its uses.
- a. Explain the process of extrusion moulding with a neat diagram.
b. How are the following polymers prepared? Mention their properties and uses.
i. PVC
ii. LDPE
- a. What are elastomers? Give the preparation, properties, and uses of Buna-S.
b. Describe a method for moulding of thermoplastic resin.
- a. Explain the preparation, properties, and uses of bakelite.
b. Describe with a neat sketch the process of compression moulding.
- a. Why are silicones called inorganic polymers? Discuss the synthesis of linear chain silicones.
b. Why bakelite cannot be remoulded? Write its repeating unit.
c. Describe condensation polymerization with an example.
- a. What is a homochain polymer? Give examples.
b. What is polymerization? Explain the different types of polymerization with examples.
- a. How is HDPE prepared? Give its properties and uses.
b. Explain the injection moulding process with a neat diagram. Mention its advantages.
- Write informative notes on the following:
a. Classification of polymers
b. Mechanism of radical polymerization
c. Anionic and cationic polymerization
d. Thermodynamics of a polymerization process
- What are the common constituents of plastics and what are their functions?
- Write short notes on the following:
a. Vulcanization of rubber
b. Polyvinyl chloride
c. Compounding of rubber
d. Reclaimed rubber
- Write an essay on the preparation, properties, and uses of the following:
a. Polyvinyl acetate
b. Cellulose acetate
c. Phenol formaldehyde resins
d. Teflon
e. Polyethylene
f. Polystyrene
g. Polymethylmethacrylate
- Discuss the various polymers related to natural rubber with emphasis on their preparation, properties, and uses.
- Write an account of application of polymers in bio-medical electronic fields.
- Write an essay on the various types of synthetic rubber with brief description of the preparation, properties, and uses of any three of them.
- Write an essay on fiber-reinforced plastics.
- What type of rubber would you recommend for the following?
a. For a flexible connection to a steam line
b. As a gasket for a pipe containing a chlorinated solvent
c. In a solvent to form an adhesive
- Write short notes on the following:
a. Molecular weights of polymers
b. Conductive polymers
c. Ionic polymers
d. Liquid crystal polymers
e. Engineering polymers
f. Photo conductive polymers
g. Polymer structure and properties of polymers
- Define the following terms and give examples: monomer, polymer, polymerization, and degree of polymerization.
- Distinguish clearly between the following:
a. Thermoplastic and thermosetting plastic
b. Natural and synthetic rubber
c. Addition and condensation polymerization
- Discuss the addition polymerization reaction mechanism.
- Explain condensation polymerization with example.
- Write informative notes on the following:
a. Vulcanization of rubber
b. Compounding of rubber
c. Gutta percha
d. Silicone rubber
- Give preparation, properties, and engineering uses of the following:
a. Teflon
b. Polystyrene
c. Polyethene
d. Polyurethane
e. HDPE
f. Conductive polymers
- Discuss the various polymers related to natural rubber with emphasis on their preparation, properties, and uses.
- Write an essay on the various types of synthetic rubbers.
- What are elastomers? Write the structure for natural rubber and gutta percha.
- What is vulcanization of rubber? Mention its uses. Explain why natural rubber needs vulcanization. How is it carried out?
- How is crepe rubber obtained from latex?
- What is latex? How natural rubber isolated from it?
- Write short notes on silicones. How are they prepared?
- Give a brief talk about conducting polymers.
- Give the structural unit of gutta percha.
