6
Chemistry of Engineering Materials
6.1 Semiconducting and super conducting materials
According to conductivity elements (or) materials are broadly classified into three categories. They are
1. Conductors
2. Semiconductors
3. Insulators
Conductors
They allow the maximum portion of the applied thermal or electric field to flow through them.
Example: Metals are good conductors.
Insulators
They do not practically allow the heat (or) electricity to flow through them.
Example: Most of the organic and Inorganic solids (except graphite).
Semiconductors
The thermal and electrical conductivity of a semiconductor at normal temperature lies between that of a conductor and Insulator.
Actually semiconductors are those solids which are perfect insulators at absolute zero, but conduct electric current at room temperature.
Example: Silicon and Germanium are two very important elements used as semiconductors.
6.1.1 Semiconductor
At room temperature, semiconductors allow a portion of electric current to flow through them. The electrical conductivity of a semiconductor at normal temperature lies between that of a good conductor and an insulator; it is in range of 10–9 to 102 ohm–1cm–1.
Actually, semiconductors are those solids which are perfect insulators at absolute zero, but conduct electric current at room temperature. Silicon and germanium are two very important elements used as semiconductors. Pure samples (≥ 99.999 % pure) of these elements are obtained by zone refining and to these some impurity is added deliberately by a process called doping. These are of two types intrinsic and extrinsic.
- Intrinsic semiconductors (semiconductors due to thermal defects):
Pure silicon or germanium acts as insulators because electrons fixed in covalent bonds and are not available for conduction. At high temperatures, electrons are released by breaking of some of the covalent bonds. These electrons move freely in the crystal and can conduct electricity. Here, without introducing an external substance, these materials show conduction. Hence, these are known as intrinsic semiconductors.
- Extrinsic semiconductors (semiconductors due to impurity defects):
Silicon and germanium (group 14 elements) in pure state, have very low electrical conductivity. However, the low electrical conductivity of these elements is greatly enhanced by the addition of even traces of an element belonging to groups 13 (iii) or group 15 (v) to the crystals of group 14 (iv) elements, i.e. silicon or germanium. Induction of group 15 and group 13 elements to the crystal lattice of group 14 elements (Si or Ge) produces n-type semiconductors and p-type semiconductors respectively.
- n-type semiconductors: (n stands for negative):
This type of semiconductor is produced by
- Due to metal excess defect (explained earlier) or
- By addition of trace amount of group 15 element (P, As) to extremely pure germanium or silicon by a process called doping.
When an element of group 15(As) is added to germanium (group 14 element) crystal, some atoms of germanium are replaced by arsenic. In such cases, four electrons of the impurity element (As) are used in forming bonds to Ge, while the Fifth electron remains unused. The extra electrons can move freely and conduct electricity in the metals. Hence, arsenic-doped germanium exhibits fairly high electrical conductivity.
- p-type semiconductor: (p stands for positive):
This type of semiconductors are formed by
- Due to metal deficiency defects, or
- By addition of impurity atoms containing less electrons (i.e., atoms of group 13) then the parent insulator to the insulator lattice, behaves like a conductor – electron.
When a group 13 element (like B, Ga, In) substitutes for a germanium atom (group 14 element), i.e., when a group 13 element (say B, having only 3 electrons in the outer shell) is added in small traces to group 14 elements (say germanium), the atoms of B are not able to complete tetrahedral covalent structures because they have one electron short of the requirement. Hence some of the sites normally occupied by electrons will be left empty. This gives rise to electron-vacancies, commonly known as positive holes because the net charge at these sites is positive. With an electric field, adjacent electrons move into the positive holes and form other positive holes. The current is passed in the crystal due to migration of positive holes. Electrical conductivity of germanium (Group 14 element) crystal increases by the doping trace amount of B (Group 13 element). Here, current is carried by positive holes, hence this type of conduction is known as p-type semiconduction.
Unlike metals, the conductivity of semiconductors increases with increases in temperature. This is due to the fact that extra electron or positive hole (as the case may be) is bound weakly with the crystal and when energy supplied in the form of heat, they become force from the crystal lattice for the conduction of electricity.
- n-type semiconductors: (n stands for negative):
6.1.2 Applications of Semiconductors
A large variety of semiconductors has been prepared by the following types of combination.
- Elements of group 14 (Se, Ge) and group 15 (P, As, Sb)
- Elements of group 13 (B, Ga) and group 14 (Si, Ge)
- Elements of group 13 and group 15 (In Sb, Al, P)
- Elements of group 12 and group 16 (ZnS, CdS, CdSe, Hg, Te)
Properties of a semiconductor are considerably changed depending upon the nature of the impurity. Semiconductors are used in transistors and in exposure metals as photoelectric devices. Combination of p– and n-type of semiconductors (known as p-n junction) allow electric current from outside to flow through it in one direction. This type of p-n junction is known as a rectifier and is used for converting alternating current to direct current.
6.1.3 Super-conductors
The electrical resistance of metals depends upon temperature. Electrical resistance decreases with decreases in temperature and becomes almost zero near the absolute temperature. Materials in this state are said to possess super-conductivity. Thus super-conductivity may be defined as a phenomenon in which metals, alloys and chemical compounds become perfect conductors with zero resistivity at temperatures approaching absolute zero. Super-conductors are diamagnetic. The phenomenon was first discovered by Kammerlingh Onner in 1913 when he found that mercury becomes super–conducting at 4K. The temperature at which a substance states behaving as super-conductor is called transition temperature. Which lies between 2 and 5K is most of the metals exhibiting this phenomenon.
Efforts are going on to find materials that behave as a super-conductor at room temperature because attaining low temperature with liquid helium is highly expensive. The highest temperature at which super conductivity has been observed is 23K for alloys of niobium (Nb3Ge) since 1987, many complex metal oxides have been found to possess super-conductivity at fairly high temperatures. Some examples are given below.
YBa2Cu3O7 90K
Bi2Ca2Sr2Cu3O10 105K
Tl2Ca2Ba2Cu3O10 125K
Super-conductors have many application in electronics, building magnets, aviation transportation (trains which move in air without rails) and power transmission.
6.2 Magnetic materials
Magnetism is the ability of matter in which there is a force of attraction or repulsion between unlike or like poles. More than 2000 years ago, the ancient Greeks first discovered a mineral that attracts things made of iron. This mineral was found in Magnesia, a part of Turkey, hence it was named as magnetite. Magnets are very common things in workplace and house hold.
6.2.1 General Properties of Magnetic Materials
- Earth acts as a big bar magnet through its core. North pole of magnets and compass needles point to earth‚s magnetic south pole, which is near earth‚s geographic north pole.
- All the magnets have two poles. If a magnet is allowed to rotate freely the north pole will always point to the north and the other is called south pole.
- Opposite magnetic poles attract each other and like magnetic poles repel.
- Every magnet is surrounded by a magnetic field. Magnetic field lines can explain the shape of the field.
- According to Becquerel and Faraday, all matter including liquids and gases were affected by magnetism, but few respond to a noticeable extent and others do not.
6.2.2 Classification
According to Faraday‚s law of magnetic induction, the magnetic forces of the material electrons will be affected when a material is placed within a magnetic field. But in the magnetic field material, electrons can react quite differently (attract/repel). This mainly depends on atomic or molecular structure of the material and net magnetic field associated with the atom. Depending on attraction and repulsion in the magnetic field, materials can be classified into five categories. They are:
- Diamagnetic materials – weak repulsion to field
- Paramagnetic materials – weak attraction to field
- Ferromagnetic materials – strong attraction to field
- Ferrimagnetic materials – strong attraction to field
- Antiferromagnetic materials – no magnetic moment
(i) Diamagnetic Materials
Diamagnetic materials have a weak magnetic susceptibility, hence, they repel slightly in a magnetic field and the material does not retain the magnetic properties when the external field is removed. Such materials have no permanent net magnetic moment due to paired electrons. In the external magnetic field electron paths are realigned, hence, material shows weak repulsion.
E.g. Copper, silver, gold, etc.
(ii) Paramagnetic Materials
Paramagnetic materials have a small positive susceptibility, hence, they attract slightly in a magnetic field and the material does not retain the magnetic properties when the external field is removed. Due to presence of some unpaired electrons and the realignment of the electron path with the external magnetic field materials show paramagnetism.
E.g. Magnesium, molybdenum, lithium, tantalum, etc.
(iii) Ferromagnetic Materials
Ferromagnetic materials have a large positive susceptibility, hence, they exhibit strong attraction to magnetic field and the materials retain their magnetic properties after the external field has been removed due to presence of magnetic domains. Due to presence of unpaired electrons, ferromagnetic materials have net magnetic moment. Here, all the magnetic dipoles are aligned parallel and oriented in the same direction.
E.g. Iron, nickel, cobalt, etc.
Curie Temperature
At a particular temperature electronic exchange forces in ferramagnets are very large, thermal energy eventually overcomes the exchange and produces a randomizing effect, and that temperature is known as ‘Curie temperature.‚ Below the Curie temperature, the ferromagnet is ordered and above disordered.
Hysteresis
Retaining of magnetic properties after the removal of external magnetic field is known as hysteresis.
Magnetic Domain
In ferro and ferrimagnetic materials, below Curie temperature, large number of atom moments is aligned parallel as a small volume regions and it is known as ‘domain.‚ Adjacent domains are separated by boundaries and are shown in the figure given hereunder.

(iv) Ferrimagnetic Materials
Ferrimagnetism is similar to ferromagnetism, but it is observed in complex crystals not in atoms in which the magnetic moments of neighbouring ions are antiparallel and unequal in magnitude.
E.g. Magnatite
Magnetite was considered as a ferromagnet until 1940. In 1940, Neel provided the theoretical framework about ferrimagnetism.
(v) Antiferromagnetic Materials
Antiferromagnetism is a phenomenon exhibited by materials in which complete magnetic moment is cancelled with antiparallel coupling of adjacent atoms or ions. That is, the successive magnetic dipoles are aligned in opposite directions with same magnitude, hence, it has no net magnetic moment.
E.g. Manganous oxide, chromium, etc.
Magnetic material and their spin alignment are shown in the figure provided hereunder.

6.2.3 Applications of Magnetic Materials
Magnets are used in a vast array of products from loud speakers to space research.
- Power conversion (electrical to mechanical): As a motors (starter motor, power steering motors, washer pumps), generators and electromagnets.
- Power adaptation and signal transfer: Transformers.
- Permanent magnets: Loud speaker, sensors, navigation and information systems.
- Data storage analogue: Video and audio tapes.
- Data storage digital: Hard disk, floppy disk.
- Quantum devices: MRAM, GMR reading head.
- Instrumentation: Dashboard instruments, nanoscience and technology, medicine, research, etc.
6.3 CEMENT
Concrete is the widely used non-metallic material in construction. Cement is the important bonding material and can bond sand and rock with water in concrete. It has adhesive and cohesive nature and also having capability to bond with bricks, stones, etc.
6.3.1 Classification of Cements
Cement is broadly classified into Natural, Puzzolana, Slag and Portland cement. These are briefly discussed below.
(a) Natural Cement
Natural cement is prepared with calcination and pulverization of naturally occurring argillaceous lime stone at high temperature. During calcination, calcium silicates and aluminates are formed.
Natural cement is quite setting cement and it possesses hydraulic qualities and relatively low strength.
Combination of sand with natural cement is known as mortar and is used in laying bricks and setting stones. Mortar is also used in construction of dams and foundations as bulk masses of concrete.
(b) Puzzolana Cement
Puzzolana cement is the oldest cement. It is invented by Romans and used by them for the construction of walls and domes.
Mixing and grinding of natural puzzolana and slaked lime gives puzzolana cement. Volcanic ash produced by rapid cooling of lava, is known as natural puzzolana. It is a molten mixture of silicates of calcium, aluminium and iron, and has hydraulic properties too.
(c) Slag Cement
Slag cement is a mixture of lime and blast furnace slag. A mixture of calcium and aluminium silicates (blast furnace slag) is granulated by pouring into a stream of cold water, dried mixed with hydrated lime and pulverized to fine powder.
Setting of slag cement is too slow, poor in abrasion resistance and lower in strength. Hence, slag cement has limited applications and is used for making concrete in bulk construction.
(d) Portland Cement
In 1824, William Aspdin prepared Portland cement by heating limestone and clay by crushing the resulting product to a fine powder. Hence, he is known as father of Portland cement. The cement upon mixing with water set to give a hard stone-like mass and resembles stone of Portland, England. Hence, it is known as magic powder and Portland cement.
6.3.2 Raw Materials Used in the Manufacture of Portland Cement
It primarily consists of lime, silica, alumina and iron.
The following materials are used for manufacture of cement.
- Calcareous materials, CaO (such as limestone, chalk, marl, etc.)
- Argillaceous materials, Al2O3 (such as clay, shale, aluminium ore refuse, fly ash, etc.)
- Siliceous material, SiO2 (Such as clay, shale, sand, etc.)
- Powdered coal or fuel oil
- Gypsum (CaSO4 · 2H2O) and
- Iron components, Fe2O3 (Ferriferous materials such as clay, iron ore, etc.,)
Importance and their Effects of the Ingredients on the Cement
All the ingredients‚ proportion should be maintained properly; otherwise, the following effects may be observed on the characteristics of cement.
- Strength: Lime, silica, iron oxide and alumina plays a vital role on strength of the cement. But excess/loss amount of lime and alumina reduces the strength due to expansion and disintegration.
- Colour and hardness: Iron oxide provides colour and hardness to the cement.
- Soundness: Small amount of sulphur trioxide imparts soundness to cement; however, in excess reduces the soundness.
- Setting: Alumina and lime help in quick setting. Gypsum helps to retard the setting action of cement and enhances the initial setting time.
- Efflorescent: Excess alkali causes the efflorescent.
6.3.3 Manufacture of Portland Cement
Manufacture of portland cement involves following steps:
- Crushing
- Mixing
- Burning
- Grinding
- Packing
(1) Crushing
Crushing of raw materials is done with two crushers. The primary crushers reduces the size of raw material to an approximately 5 – inches and the secondary crusher further reduces the size to ¾ inches. Then these are ground to fine powder (in ball mills or tube mills). Each separate powdered ingredient is stored in separate hoopers (Figure 6.1).

Figure 6.1 Crushing: stone is first reduced to 5–in, size, then ¾–in, and stored
(2) Mixing
Mixing of raw materials can be done either by (a) dry process or (b) wet process.
(a) Dry Process
The following proportions of the powdered materials, i.e., lime 60–69 per cent, silica 17–25 per cent, alumina 3–8 per cent, iron oxide 2–4 per cent, magnesium oxide 1–5 per cent, alkali oxides like Na2O + K2O 0.3–1.5 per cent and sulphur trioxide 1–3 per cent are mixed and thus we get a raw mix. It is stored in silos (storage bins) and send to a rotary kiln for burning, and this process is shown in Figure 6.2.

.png)
Figure 6.2 (a) Dry process: Mixing of raw material; (b) Wet Process: Mixing of raw materials with water
(b) Wet Process
The calcareous raw material is crushed, powdered stored and the argilaceous material (clay) is thoroughly mixed with water for removing organic material in wash mills stored in basins. These two are led to grinding mills (tube mill/ball mill) through channels in the right proportions and they are mixed to form a paste called slurry. The chemical composition of slurry may be adjusted with correcting basins, and it contains about 38–40 per cent of water and is stored in tanks for feeding to a rotary kiln.
Differences between Dry and Wet Process

(3) Burning
Burning process is done in a rotary kiln containing a steel tube, lined inside with refractory bricks, having 2.5–3.0 m in diameter and 90–120 m in length. The kiln is in a slightly inclined position and is capable of rotating at 1 rpm along its longitudinal axis. Fuel and air are injected at the lower end for burning, which produce long hot flames that heat the interior of the kiln up to 1750°C temperature.
From the upper end of the kiln raw mix or corrected slurry is injected. From the lower end of the kiln, hot flames are forced with slow rotation and through slope of the kiln, fed material move towards bottom of the kiln and the material descends gradually with temperature.
Depending on the temperature, kiln is divided into three zones. They are drying zone, calcination zone and clinkering zone.
- Drying zone: It is the upper part of the kiln having temperature around 400°C, here water in the slurry gets evaporated.
- Calcination zone: It is the central part of the kiln having temperature around 1000°C. Here, limestone of dry mix or slurry is decomposed to give quick lime as small lumps, also called modules and carbon dioxide, escapes out.

- Clinkering zone: It is the lower part of the kiln having the temperature between 1500 and 1700°C. Here, chemical interaction of fusion occurs between lime and clay to form calcium aluminates and silicates.




The silicates and aluminates of calcium fuse to form about 0.5–1 cm diameter hard, greyish stones, known as clinkers, and these are hot at about 1000°C. These are cooled with cool air in another small rotary kiln at base of the main kiln and collected in small trolleys (Figure 6.3).

Figure 6.3 Burning: Burning changes raw mix chemically into cement clinker
(4) Grinding
In ball mills or tube mills the cooled clinkers are ground to fine powder and 2–3 per cent of gypsum is added to avoid quick setting and also acts as a retarding agent for early setting of cement. This is shown in Figure 6.4. Gypsum reacts with tricalcium aluminate to form insoluble tricalcium sulphoaluminate, and it prevents too quick reaction of setting and hardening (Figure 6.4).
![]()

Figure 6.4 Grinding: Clinker with gypsum added into portland cement and shipped
After the initial set, the cement water paste becomes stiff, but gypsum retards the dissolution of C3A by forming tricalciumsulphoaluminate (3CaO · Al2O3 · x · CaSO4 · + H2O), which is insoluble (Figure 6.5).
Thus:
![]()
Formation of insoluble C3A prevents too early further reactions of setting and hardening.
(5) Packing
The ground cement is stored in silos, from which it is fed to automatic packing machines. Each bag, usually contains 50 kg of cement.
Flow diagram for the manufacture of portland cement is shown in Figure 6.5.
6.3.4 Chemical Composition of Portland Cement and their Importance
Portland cement contains mainly dicalcium silicate (C2S), tricalcium silicate (C3S), tricalcium aluminate (C3A) and tetracalcium alumina ferrite (C4AFe). Each component exhibits particular special behaviour, hence the behaviour of cement can be altered by changing the relative percentages of the above compounds.
(1) Dicalcium Silicate (C2S)
Due to slow reaction with water, it gets hardened slowly and strengthen after 1 week with the formation of tobermonitegel with high surface area. Moist curing continuing up to 6 months.
![]()

Figure 6.5 Flow diagram for the manufacture of portland cement
(2) Tricalcium Silicate (C3S)
Due to rapid reaction with water, this material is responsible for initial setting and early strength with the formation of hydrolgel, it is having binding action between the aggregates.
![]()
(3) Tricalcium Aluminate (C3A)
Due to fast hydration, this compound forms hydrated tricalcium aluminate and is responsible for first few days of strength. This reaction is highly exothermic, hence cement is made with less C3A, and at that time it generates less heat, develop higher strengths and show greater resistance to sulphate attacks.
![]()
Due to high heat generation and reactiveness with soil, C3A is the least preferable component in the cement.
(4) Tetracalcium Alumina Ferrite (C4AF)
It hydrates very rapidly, reducing the clinkering temperature and gives little strength to concrete.
![]()
Importance of Gypsum in Cement
Tricalcium aluminate (C3A) combines with water very rapidly with the evolution of large amount of heat (exothermic reaction).
C3A + 6H2O → C3A · 6H2O + Heat
After the initial set, the paste becomes somewhat stiff. But in the presence of gypsum, it reacts with tricalcium aluminate to form insoluble tricalcium sulphoaluminate which helps to retard the speed of the initial set and does not show any tendency to rapid hydration.
![]()
![]()
The above reaction prevents high concentrations of alumina in the cement solution and retarding the early initial set of the cement.
6.3.5 Setting and Hardening of Cement
When water is added and mixed to cement to form cement paste, start hydration, convert into gel and crystalline material. Solidification, interlocking and binding of the aggregates into a rock-like matter is two-step process, they are setting and hardening.
Setting: It is the stiffening of the cement paste with the formation of gel setting that is divided into initial setting and final setting.
Initial setting: It is hydration and gel formation from the different constituents of cement.


During the hydration of dicalcium, silicate gives tobermonite gel, which possesses very high surface area and high adhesive property.
![]()
Final setting: It is the complete formation of tobermonite gel.
Hardening: Hardening is the development of strength with the crystallization of calcium hydroxide and hydrated tricalcium aluminate.


Two theories are proposed for explaining the hardening of the cement.
(a) Colloidal Theory by Michaels
According to this theory, silicate gels are formed with hydration and are responsible for hardening.
(b) Crystalline Theory by Le Chatelier
According to this theory, crystalline products are formed with hydration, and undergo interlocking are responsible for hardening.
Hence, setting and hardening of cement is due to the interlocking crystallization of gels, which are formed by hydrolysis of constitutional ingredients (Figure 6.6).

Figure 6.6 Schematic diagram of setting and hardening of cement
Ingredients Reaction Sequence During Setting and Hardening of Cement
When water is added to cement, various ingredients undergo hydration and crystallization with different rates (Figure 6.7).

Figure 6.7 Sequence of changes during setting and hardening of cement
6.3.6 Specifications of Cement
According to Indian Standard 269–1975, composition of ordinary portland cement shall satisfy the following conditions:
Chemical Requirement of Cement
- Ratio of the percentage of lime (CaO) to that of silica (SiO2), alumina (Al2O3) and iron oxide (Fe2O3) when calculated by the formula

- Ratio of percentage of alumina (Al2O3) to that of iron oxide (Fe2O3) shall not be less than 0.66.
- Weight of insoluble residue shall not exceed 2%.
- Weight of magnesia shall not be more than 6%.
- Total sulphur contents, calculated as sulphuric anhydride (SO3) shall not be more than 2.75%.
- Total loss on ignition shall not exceed 4%.
Physical Requirements of Cement
- Setting time:
Initial: Not less than 30 minutes, Final: Not more than 600 minutes.
- Compressive Strength (of 1:3 cement mortar cube of cement ampers; Ennore sand):
3 days: Not less than 1.6 kg/mm2 (or 16 N/mm2)
7 days: Not less than 2.2 kg/mm2 (or 22 N/mm2)
- Soundness:
By Le-Chatelier‚s method: It expresses the expansivity of the cement set in 24 hours between 25°C to 100°C.
Unaerated Cement: Maximum 10 mm.
Aerated Cement: Maximum 5 mm.
- Fineness:
Not less than 215 m2/kg. Finer the grinding the greater is the rate of reactions thereby hastening the early development of strength. However finer cement generates heat quickly, thereby the cement mortar/concrete is likely to develop cracks.
6.3.7 Analysis of Cement
The quality of cement is maintained by conducting various tests from raw material stage right upto the cement in packing stage, at every half an hour to one hour intervals. In fact various physical and chemical characteristics are tested. The quality of a sample of cement is determined from a number of measurements. For Example.
- Soundness: The analysis of a cement for soundness is done by Le-chatelier technique. It expresses expansivity of cement set for one day, between 80°F and the boiling point of water. The analysis for soundness can also be done by autoclave method. According to I.S.I specifications.
Soundness by auto clave method: Expansion not more than 0.8% and
Soundness by Le-chatelier method:
Aerated cement: maximum 5 mm
Unaerated cement: maximum 100 mm
- Fitness: By Turbidmetic method: 1600 cm2/gm. By plain permeability method (As specific surface) ≥ 215 m2/Kg.
- Compressivestrength: As per ISI specifications.
- TensileStrength: As per ISI specifications.
- SpecificGravity: Specific gravity should be 3.1–3.2.
6.3.8 Plaster of Paris/Gypsum Plaster
The mineral gypsum (hydrated calcium sulphate CaSO4 · 2H2O) is extensively used as raw material for the manufacture of plates, which are almost universally used for coating the inner walls of dwellings.
It is hemihydrate of calcium sulphate 2CaSO4 · H2O(or CaSO4 · ![]() H2O).
H2O).
Preparation: It is produced by heating pure Gypsum to a temperature of about 120°C–160°C. If the Gypsum heated above 200°C, the anhydrous sulphate is produced, which loses the power of readily combining with water.

The preparation of plaster of paris from gypsum consists the following operations:
- Crushing and grinding of gypsum
- Calcination of ground gypsum in kilns by heating about 150°C, and
- Pulverizing the calcined product.
Setting and Hardening
Plaster of paris forms a plastic mass when it is mixed with water. This plastic mass quickly sets or hardness, expanding in the process and regains the closely packed crystalline structure of gypsum. Setting of plaster of paris can be accelerated by admixing it with alkali sulphates like K2SO4, Na2SO4or alums, which initiate as well as hasten the crystallization process.
Applications
- Its slight expansion on setting renders plaster of paris suitable for making mould, since some details are there by accurately reproduced.
- It is used in making surgical bandages, structural tiles and castings.
- It is used as plaster for walls and in plaster boards, which is made up of alternate layers of a fibrous material such as felt or paper.
6.4 REFRACTORIES
Refractories are ceramic materials that can withstand high temperatures as well as abrasive and corrosive actions of molten metals, slags, and gases, without suffering a deformation in shape. The main objective of a refractory is to confine heat.
6.4.1 Characteristics of Good Refractory Materials
- A good refractory material should have excellent heat, corrosion, and abrasion resistance.
- It should possess low thermal coefficient of expansion and should expand and contract uniformly with increase and decrease of temperature, respectively.
- It should possess high fusion temperature. It should be infusible at operating temperatures.
- It should be able to withstand the overlying load of structure, at operating temperatures.
- They should be chemically inert towards corrosive action of molten metal, gases, and slags produced in its immediate contact with furnaces.
- They should not crack at the operating temperatures.
6.4.2 Failures of Refractory Materials
If a given refractory material does not have the above-mentioned good characteristics, it will fail in service. Thus we can easily summarize conditions, which lead to failures of a refractory materials as follows:
- Using a refractory material which does not have required heat, corrosion, and abrasion resistance
- Using refractory material of higher thermal expansion
- Using a refractory of refractoriness less than that of the operating temperature
- Using of lower quality refractory bricks than the actual load of raw materials in products
- Using basic refractory in a furnace in which acidic reactants and/or products are being processed and vice versa
- Using refractories that undergo considerable volume changes during their use at high temperatures.
6.4.3 Classification of Refractories
On the basis of chemical properties, refractories are broadly classified into three main categories.
- Acidic Refractories: Refractories which consist of acidic materials are known as acidic refractories. These are easily attacked by basic materials and not by acidic materials.
Eg.: Alumina, silica and fireclay refractories.
- Basic Refractories: Refractories which consist of basic materials are known as basic refractories. There are easily attacked by acidic materials and not by basic materials.
Eg.: Magnesite and dolomite.
- Neutral Refractories: Refractories which consist of weakly acidic/basic materials are known as neutral refractories.
Eg.: Zirconia, graphite, chromite and carborundum.
6.4.4 Properties of Refractories
Important properties of refractories are:
(i) Refractoriness
It is the ability of a material to withstand the heat, without appreciable deformation. It is commonly measured as the softening or melting temperature of the material. The softening temperatures of refractory materials are determined by using “pyrometric cones (seger cones) test” (Figure 6.8). Refractory should have a softening temperature much higher than the operating temperature of the furnace in which it is to be used.
Refractoriness is generally determined by comparing the behaviour of heat on cone of material to be tested with that of a series of seger cones of standard dimensions. The Refractoriness is expressed in terms of pyrometric cone equilent. Cones are 38 mm height, 19 mm long sides with triangular base pyramids, and at definite temperatures they can melt or fuse. The temperature at the apex touching the base is indication of fusion/softening of the test cone. The number of the standard cones fuses along with test cone is the pyrometric cone equivalent (PCE) of that particular refractory. If the test cone fuses later than one standard cone and earlier than next cone, the PCE is the average value of the two.

Figure 6.8 Seger cone-test
(ii) Porosity
Porosity is the property of a sold which contains opening or spaces or minute channels. It can be expressed as
![]()
where W = weight of saturated specimen
D = weight of dry specimen
α = weight of saturated specimen submerged in water
If the refractory is having pores, then entry of gases, slags, etc. is easy and can react up to a greater depth. This can reduce the life of the refractory material. Consequently, it can affect many important properties of the refractory such as decreasing the strength, resistance to corrosion, resistance to abrasion but increased resistance to thermal spalling. Hence, a good refractory should have a low porosity.
(iii) Strength or Refractorines–under Load
The refractory material must possess high mechanical strengths, even at operating temperatures to bear the maximum possible load, without breaking.
(iv) Dimension Stability
It is the resistance of a material to any volume changes, which may occur on its exposure to high temperature, over a prolonged time. It may reversible or irreversible.
(v) Chemical Inertness
A refractory does not easily form fusible products with gases, ash, slags, etc. and hence should be chemically inert.
(vi) Thermal Expansion
A refractory material should have least possible thermal expansion because
- Expansion of a refractory decreases the capacity of the furnace.
- Repeated expansion and contraction contribute much towards rapid breakdown, and wear and tear of the material structure.
(vii) Thermal Conductivity
Depending upon the type of furnace refractory material of both high thermal conductivity and low thermal conductivity are required. In most cases, furnace is lined with refractories of low heat conductivities to reduce the heat losses to the outside by radiation; otherwise maintenance of high temperatures inside the furnace will become difficult. In muffle furnace walls, coke-oven batteries a good heat conductivity of refractory is desirable for effective heat transmission.
(viii) Thermal Spalling
Under high temperature breaking, cracking, peeling off or fracturing of a refractory brick or block are known as thermal spalling.
Thermal spalling may be due to;
- Rapid change in temperature and
- Slag penetration into the refractory brick
Thermal spalling can be decreased by taking following precausions:
- Using high porosity, low coefficient of expansion and good thermal conductivity refractory bricks,
- Avoiding sudden temperature changes,
- By overfiring the refractories,
- By modifying the furnace design.
(ix) Heat Capacity
It depends on
- Thermal conductivity
- Specific heat and
- Specific gravity of refractory
(x) Resistance to Abrasion or Corrosion
Refractory is desirable that least abraded by descending hard charge, flue gases escaping at high speeds, particles of carbon or grit etc.
(xi) Electrical Conductivity
Refractories specially used for lining electric furnaces should have low electrical conductivity. Except graphite, all refractories are poor conductors.
(xii) Permeability
Rate of diffusion of gases, liquids and molten solids through a refractory is known as permeability. It mainly depends on the size and number of connected pores. Permeability increases with temperature.
(xiii) Texture
Due to large porosity, coarse- or light-textured bricks are less in weight; hence, they are more resistance to sudden temperature changes.
6.4.5 Manufacture of High-Alumina Bricks, Magnesite Bricks, and Zirconia Bricks
(i) High-Alumina Bricks
High-alumina bricks contain 50% or more of Al2O3 and are, generally, made by mixing calcined bauxite (Al2O3) with clay bind.
Properties
They possess very low coefficient of expansion, high porosity, great resistance to slags, very little tendency to spall high temperature, load-bearing capacity, excellent wear-resistance, and stability, both in oxidizing and reducing conditions, and are particularly inert to the action of gasses like CO2, H2, and natural gas. They are thus very good refractories, but they are very expensive and hence their use is limited.
Uses
“Medium-duty bricks” (i.e., containing 50–60% Al2O3) are used for zones of vertical shaft kilns for burning limes, linings of Portland Cement rotary kilns, soaking pits, reheating furnaces, hearths and walls, etc., which are subjected to high abrasion. On the other hand, “high-duty bricks” (i.e., containing 75% Al2O3) find applications specially in the sintering or hottest zones of cement rotary kilns, lower parts of soaking pits, brass melting reverberatories, lead-dressing reverberatory furnaces, aluminium melting furnaces, combustion zones of oil-fired furnaces, etc.
(ii) Magnesite Bricks
Most widely used basic refractories are magnesite bricks. Calcined magnesite is powdered to a proper size, then mixed with caustic magnesia or iron oxide as a binding material. This mixture is grounded with water and moulded into bricks, then slowly heated to 1500°C for about 8 h and then cooled slowly.
Properties
Magnesite bricks can be used without load up to 3000°C and with load of 3.5 kg/cm2 up to 1500°C. They possess good resistance to basic slags, good crushing strength and less shrinkage. They have a lot of spalling with sudden temperature changes and their resistance to abrasion is poor.
They show lot of tendency to combine with water and CO2.
Uses
These are mainly used in open-hearth furnaces where high temperature required. Also used in hot mixer linings, copper converters, reverberatory furnaces for smelting antimony, copper, lead etc., ores, refining furnaces and hot zones of cement rotary kilns.
(iii) Zirconia Bricks
These are prepared by heating Zirconite mineral (ZrO2) and colloidal Zirconite or alumina as binding material at 1700°C. This is stabilized by adding of MgO or CaO without undergoing any volume changes on heating and cooling.
Properties
Zirconia bricks are usually known as neutral refractories, but they have no quite resistance to acids, slags etc., hence they are between neutral and basic refractories. Without load they can withstand up to 2000°C, but specially prepared bricks can be used up to 2600°C and with load of 3.5 kg/cm2 up to 1900°C. These have quite resistance to thermal shocks.
Uses
These are very costly, hence used only in high frequency electric furnaces.
6.5 Lubricants
In all types of machines lot of wear and tear is observed due to friction. Due to this, large amount of energy is also lost in the form of heat and moving parts get heated and damaged. The ill effects of frictional resistance can be minimized by using a suitable substance called lubricant, which can form a thin layer in between the moving parts and keep the sliding or moving surfaces apart. Hence, frictional resistance and consequent destruction of material is minimized.
‘The process of minimizing frictional resistance between moving or sliding surfaces by the introduction of lubricants in between them is called lubrication.‚
6.5.1 Important Functions of Lubricants
- It avoids direct contact between the rubbing surfaces and reduces surface deformation, wear, tear and seizure.
- It acts as a coolant by reducing loss of energy in the form of heat.
- It enhances efficiency of a machine by reducing wastage of energy and expansion of metal by local frictional heat.
- It avoids seizure and relative motion of moving surfaces, such that running cost of the machine will be reduced.
- Lubricant used between piston and the cylinder wall of an internal combustion engine acts as a seal and can prevent the leakage of gases from the cylinder under high pressure.
6.5.2 Mechanism of Lubrication
There are mainly three types of mechanism by which lubrication is done.
- Thick film (or) fluid film or hydrodynamic lubrication: In this mechanism, moving or sliding surfaces are separated by thick film of lubricant fluid, hence it is known as thick film or fluid film lubrication. This thick film of lubricant covers entire moving surfaces and fills irregularities.
Therefore, there is no direct contact between the surfaces of machine and consequently it reduces the wear. This is shown in Figure 6.9. Here only internal resistance is observed between the particles of lubricant; hence, chosen lubricant should have minimum viscosity under the working conditions.
Hydrodynamic friction occurs in the case of shaft running places like journal bearings, which is shown in Figure 6.10. Thick film lubrication hydrocarbon oils are considered as satisfactory lubricants. Hydrocarbon lubricants are blended with selected long-chain polymers to maintain viscosity of the oil throughout the year.

Figure 6.9 Fluid-film lubrication
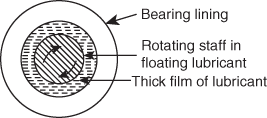
Figure 6.10 Hydrodynamic lubrications
- Boundary or thin film lubrication: In this kind of lubrication moving surfaces are separated by thin layer of lubricant, which is absorbed by physical or chemical forces on the metallic surfaces as shown in Figure 6.11. Here continuous film of lubricant cannot persist due to the following reasons:

Figure 6.11 Boundary lubrication
- a shaft starts moving from rest or
- the speed is very low (or)
- the load is very high and
- viscosity of the oil is too low.
Vegetable oils, animal oils and their soaps possess the property of adsorption either physically or chemically to the metal surfaces and form a thin film of metallic soap, which acts as a good lubricant. Fatty oils possess a greater adhesion property than mineral oil, and to improve the oiliness of mineral oils small amount of fatty oils are added. Graphite and molybdenum disulphide are also used for boundary lubrication.
- Extreme Pressure lubrication: In this mechanism the moving or sliding surfaces are under very high pressure and speed, hence this is known as extreme pressure lubrication. Under such conditions, a high local temperature is attained, and due to this liquid lubricants fail to stick and may decompose or vaporize.
Special additives are added to mineral oils to meet the extreme pressure conditions and are called extreme pressure additives. Organic compounds which are having active radicals or groups such as chlorine, sulphur or phosphorous act as good additives. These compounds react with metallic surfaces to form metallic chlorides, sulphides or phosphides as more durable films, capable of withstanding very high loads and temperatures.
6.5.3 Classification of Lubricants
On the basis of physical state, lubricants can be classified into three categories as listed hereunder.
- Liquid lubricants or lubricating oils: Lubricating oil also acts as a cooling medium sealing agent, corrosion preventer, etc. along with reducing friction and wear. According to origin, lubricating oils are classified into (a) animal and vegetable oils, (b) mineral or petroleum oils and (c) blended oils.
- Animal and vegetable oils: Vegetable and animal oils possess good oiliness but they are costly, they undergo oxidation easily, forming gummy and acidic products, get thickened on coming in contact with air, etc. Hence, they are rarely used as lubricant, but they are used as blending agent.
- Mineral or petroleum oils: Mainly mineral oils are obtained by distillation of petroleum. These are widely used lubricants because they are cheap, abundantly available and quiet stable under service conditions. The hydrocarbon oil chain length varies between 12 to 50 carbon atoms. The shorter chain hydrocarbons have lower viscosity than longer chain hydrocarbons. When compared to animal and vegetable oils mineral oils possess poor oiliness; therefore, to increase oiliness high molecular weight compounds like oleic, steric acids are added.
- Blended oils: In many modern machinery, no single oil serves as the most satisfactory lubricant. Improving of important properties by incorporating specific additives is known as blending of oils and blended oils give desired lubricating properties.
Properties of Good Lubricating Oil
A good lubricating oil must possess
- Low pressure
- High boiling point
- Adequate viscosity to particular service conditions
- Low freezing point
- High oxidation resistance
- Heat stability
- Non-corrosive property and
- Stability to decomposition at the operating temperatures.
- Greases or semi-solid lubricants: Lubricating grease is semi-solid, consisting of a soap dispersed throughout a liquid lubricating oil. The liquid lubricant may be a petroleum oil or even a synthetic oil, and it may contain any of the additives for specific requirements.
Greases are prepared by saponification of fat with alkali, followed by adding hot lubricating oil while under agitation. The total amount of mineral oil added determines the consistency of the finished grease.
The structure of lubricating greases is like that of a gel. Soaps are gelling agents, which give an interconnected structure (held together by intermolecular forces) containing the added oil. The soap dissolves in the oil at high temperature; hence, inorganic solid, thickening agents are added to improve the heat resistance of grease. Greases have higher shear or frictional resistance than oils and can support much heavier loads at lower speeds.
Greases are used:
- In situations where oil cannot remain in place, due to high load, low speed, intermittent operation, sudden jerks, etc., for example, rail axle boxes
- In bearing and gears that work at high temperatures
- In situations where bearing needs to be sealed against entry of dust, dirt, grit, or moisture, because greases are less liable to contamination by these
- In situations where dripping or spurting of oil is undesirable, because unlike oils, greases if used do not splash or drip over articles being prepared by the machine, for example, in machines preparing paper, textiles, edible articles, etc.
The main function of soap is that it acts as a thickening agent so that grease sticks firmly to the metal surfaces. However, the nature of the soap decides the temperature up to which the grease can be used, its consistency, and its water and oxidation resistance. So, greases are classified after soap is used in their manufacture.
Important greases are:
- “Calcium-based greases” or cup-greases are emulsions of petroleum oils with calcium soaps. They are, generally, prepared by adding requisite amount of calcium hydroxide to a hot oil while under agitation. These greases are the cheapest and the most commonly used. They are insoluble in water, so they are water resistant. However, they are satisfactory for use at low temperatures, because above 80°C, oil and soap begins to separate out.
- “Soda-base greases” are petroleum oils, thickened by mixing sodium soaps. They are not water resistant, because the sodium soap content is soluble in water. However, they can be used up to 175°C. They are suitable for use in ball bearings, where the lubricant gets heated due to friction.
- “Lithium-based greases” are emulsions of petroleum oils with lithium soaps. These are having high water resistance and suitable only below 15°C.
- Axle greases are cheap resin greases and are prepared by adding lime or heavy metal hydroxide to resin and fatty oils. The resulting mixture is thoroughly mixed, allowed to stand and tack or mica like fillers are added finally. These are water resistant and also suitable for less delicate equipment working under heavy loads at low speeds.
Besides the above, there are greases prepared by dispersing solids (like graphite, soapstone, etc.) in mineral oil.
- Solid Lubricants: Graphite and molybdenum disulphide are the important solid lubricants. These are used in the following conditions.
- The operating temperature or load is too high.
- Blended lubricating oil or mixed grease is unacceptable.
- To avoid combustible lubricants.
Layered structure of graphite and sandwich-like structure of molybdenum disulphide are shown in Figures 6.12 (a) and (b).

Figure 6.12 (a) Layered structure of graphite (b) Sandwitch-like structure of molybdenum disulphide
Hence, the force to shear the crystals parallel to the layers is low and consequently the parallel layers slide over one another easily. Usually some organic substances are mixed with solid lubricants so that they may stick firmly to the metal surface.
Solid lubricants are used either in the dry powder form or mixed with water or oil. Graphite is the most widely used lubricant because it is very soapy to touch, non-inflammable and not oxidized in air below 375°C. Graphite is used in the form of powder or suspension in oil or water with the help of emulsifying agent tannin. Graphite is dispersed in oil is called ‘oildag‚ and when dispersed in water it is called ‘aquadag.‚
In the absence of air, it can be used up to very higher temperature. Graphite is used either in powdered form or as suspension. Graphite greases are used at higher temperature.
6.5.4 Properties of Lubricants
- Neutralization number: The acidity or alkalinity of lubricating oil is determined in terms of neutralization number. Determination of acidity is more common and is expressed as acid value or acid number. It is defined as the “number of milligrams of potassium hydroxide required to neutralize all the free acid present in 1 gram of the lubricating oil”.
Even the most carefully refined oil may have a slight acidity. This is due to the presence of minute amount of organic constituents that are not completely neutralized during the refining treatment or due to traces of residues from the refining process. This small intrinsic acidity may not be harmful in itself, but the degree to which it increases in already used oil is usually taken as a measure of the deterioration of the oil due to oxidation or contamination. In fact, acid number greater than 0.1 is usually taken as an indication of oxidation of the oil.
- Saponification number: The saponification value of an oil is defined as the “number of milligrams of potassium hydroxide required to saponify one gram of the oil”. This is usually determined by refluxing a known quantity of the oil with a known excess of standard KOH solution and determining the alkali consumed by titrating the unreacted alkali.
Animal and vegetable oils undergo saponification but mineral oils do not. Further most of the animal and vegetable oils process their own characteristic saponification values. Hence, the determination of saponification value helps to ascertain the presence of animal and vegetable oils in a lubricant. Conversely, since each of the fined oil has got its own specific saponification number, any deviation from this value in a given sample indicates the probability and extent of adulteration.
- Aniline point: The aniline point of an oil is defined as ‘The minimum equilibrium solution temperature for equal volumes of aniline and oil sample.‚ Aromatic hydrocarbons have lot of tendency to dissolve natural and synthetic rubbers, this tendency can be determined on the basis of aniline point of an oil. A higher aniline point means lower percentage of hydrocarbons; therefore, having higher aniline point is desirable.
Aniline point is determined by thoroughly mixing equal volumes of aniline and the oil sample in a tube and heating the mixture until a homogeneous solution is obtained. Then, it is allowed to cool at a specified rate until the two phases just separate out. The temperature corresponding to this particular observation is reported as the aniline point.
- Cloud point and pour point: Petroleum oils are complex mixtures of chemical compounds and they do not show a fixed freezing point. When they are sufficiently cooled, they become plastic solids due to the formation of solid crystals or due to congealing of the hydrocarbons present. “The cloud point is the temperature at which this crystallization of solids in the form of a cloud or haze first becomes noticeable”, when the oil is cooled in a standard apparatus at a standard rate. The pour point is “the temperature at which the oil just ceases to flow when cooled at a standard rate in a standard apparatus”.
The pour point has a greater significance for lubricating oil because it determines the suitability of an hydraulic oil for low temperature installations. Refrigerator plants, air-craft engines, etc. are some important examples, which may be required to start and operate at subzero temperatures.
- Flash point and fire point: The flash point of an oil is defined as ‘the minimum temperature at which the oil gives off sufficient vapour to ignite momentarily when a flame of standard dimensions‚ is brought near the surface of the oil. The fire point of an oil is defined as ‘the lowest temperature at which the vapours of the oil burn continuously for at least five seconds‚ when the standard flame is brought near the surface of the oil.
Lubricating oil should have flash point that is reasonably above its working temperature. This ensures safety against fire hazards during usage, storage and transport. The flash point of lubricating oil can be determined by Pensky Marten‚s apparatus.
- Viscosity and viscosity index: Viscosity is one of the most important properties of lubricating oil. The formation of a fluid film of a lubricant between the friction surfaces and the generation of frictional heat under particular conditions of load, bearing spread and lubricant supply mostly depend upon the viscosity of the lubricant and, to some extent, on its oiliness. When large working clearances exist between the friction surfaces, a high viscosity oil is generally recommended to “cushion” the intermediate application of load. However, it is often necessary to sacrifice some of the cushioning effect of viscous oil by the partial substitution of a thinner oil to provide good oil circulation to dissipate the frictional heat.
The fluid lubricant film cannot be maintained between the moving surfaces, if the viscosity of the oil is too low, since with this excessive wear may take place. On the other hand, if the viscosity of lubricating oil is too high, excessive friction due to shearing of oil itself would result. Hence in hydrodynamic lubrication, the lubricant selected must possess a sufficiently high viscosity due to adherence to the bearing and prevent its being squeezed out due to high pressure and yet fluid enough so that the resistance to the shear is not too high. Hence it is essential to have knowledge of the viscosity of lubricating oil.
Viscosity is a measure of the internal resistance to the motion of a fluid and is mainly due to forces of cohesion between the fluid molecules. Absolute viscosity can be defined as the tangential force per unit area required to maintain a unit velocity gradient between two parallel planes in the fluid unit distance apart. The units of absolute viscosity η (eta) in CGS system are poise and centipoise (1/100th of a poise). Poise is equal to one dyne per second per square centimeter. The viscosity of water at 20°C is about centipoise.
The ratio of absolute viscosity to density for any fluid is known as the absolute kinematic viscosity. It is denoted by η, and in CGS system, its units are stokes and centistokes (1/100th of a stoke).

where n = absolute kinematic viscosity
η = absolute dynamic viscosity
r = density of the fluid.
The dimensions of dynamic viscosity are HL–1T–1, and the dimensions of kinematic viscosity are L2T–1.
For academic purposes, viscosity is usually expressed in centistoke, but a more common practical measure of the viscosity of an oil is the time in seconds for a given quantity of the oil to flow through a standard orifice under the specified set of conditions. Thus viscosities are usually determined with Redwood viscometer in commonwealth countries with Engler‚s viscometer in the Europe and with Saybolt‚s viscometer in USA. In these commercial viscometers, a fixed volume of the liquid is allowed to flow through the standard orifice of particular standard apparatus used, for example, viscosity of the oil is 156. Redwood (No. 1) seconds at 25°C. The viscosity of the oil so determined in the time units is sometimes called relative viscosity. Since the instruments used are of standard dimensions, kinematic viscosity of the oil in centistokes can be calculated from the time taken by the oil to flow through the standard orifice of the instrument, with the help of the following equations:
m = Ct (for fluids whose kinematic viscosity is more than centistokes) and
m = Ct – b/t (for fluids having kinematic viscosities lesser than or equal to 10 centistokes)
m = Kinematic viscosity in centistokes
t = Time of flow in seconds
C = Viscometer constant
B = Coeffiecient of kinetic energy, which may be determined experimentally or eliminated by choosing long flow times
For routine purposes, the test viscometer may be calibrated and the constant C determined by using solutions of known viscosity. The primary standard used is freshly distilled water whose kinematic viscosity is 1.0008 centistokes. Other standards usually employed are:
40% sucrose solution:
n = 4.390 cs at 25°C, r = 1.17395
60% sucrose solution:
n = 33.66 cs at 25°C, r = 1.28335
For Redwood No. 1 viscometer, the values for the constants are as below:

These constants are based on the results of the work carried out at the National Physical Laboratory at a temperature of 70°F (21.11°C) and with the ranges of viscosity; at that temperature, the results are accurate to ±1%.
Redwood No. 2 viscometer is used for every viscous liquids and gives 1/10th the value of Redwood No. 1 viscometer.
- Viscosity index: The viscosity of an oil decreases with increase of temperature as a result of decrease in intermolecular attraction due to expansion. Hence, it is always necessary to state the temperature at which the viscosity is determined.
In many applications, lubricating oil will have to function in a machinery over considerably wide range of operating temperatures. If this is due to seasonal variations in atmospheric temperature adjustments can be affected by selecting different oils of appropriate viscosity for different seasons. However, in case of internal combustion engines, aeroplanes, etc, the lubricant used must function both at low starting temperatures as well as at very high operating temperatures. Since the viscosity of lubricating oils decreases with temperature, it is impossible to select an oil having same viscosity over such a wide range of operating temperatures. However, one can select an oil whose variation in viscosity with temperature is minimum. This variation can be indicated either by viscosity temperature curves or by means of the viscosity index. Viscosity index is the numerical expression of the average slope of the viscosity temperature curve of lubricating oil between 100°F to 210°F. The oil under examination is compared with two standard oils having the same viscosity at 210°F as the oil under test. Oils of the Pennsylvanian type crudes thin down the least with increase of temperature, whereas oils of Gulf coast origin thin down the most as the temperature is increased.
Hence the viscosity index of Pennsylvanian oil is taken as 100 and that of the Gulf oil as zero. Then the viscosity of the oil under investigation is deducted as follows:
Viscosity index of the oil under test =

where VL = Viscosity at 100°F of Gulf oil standard, which has the same viscosity at 210°F as that of oil under test
VX = Viscosity of the oil under test
VH = Viscosity at 100°F of Pennsylvanian standard oil, which has the same viscosity at 210°F as that of oil under test
Thus the higher the viscosity index, the lower the rate at which its viscosity decreases with increase of temperature. Hence, oils of high viscosity index, that is, those having that viscosity temperature curves are demanded for air-cooled internal combustion engines and aircrafts engines. In general, oils of high specific gravity have steeped viscosity temperature curves. However, all oils tend to attain the same viscosity above 300°C.
By and large, light oils of low viscosity are used in plain bearings for high-speed equipment such as turbines, spindles, and centrifuges, whereas high viscosity oils are used with plain bearings of low-speed equipment.
- Mechanical stability: Four-balls extreme-pressure test is one of the important mechanical tests to judge the suitability of a lubricant under conditions of very high pressure, and the same is shown in Figure 6.13. In this test lubricant under test is powered in a machine containing four balls. Here, upper ball is rotated and lower three balls are stationary. Load is gradually increased, the ball is withdrawn and examined at specific intervals for scale formation, etc. and under a given load, the ball bearings after the test comes out clean if the lubricant desirable. When the load is progressively increased the liberated heat welds the ball together, here lubricant is said to be failed completely. Hence this test enables us to determine the maximum load that can be carried safely by a lubricant. Four – balls extreme – pressure lubricant tester is shown in Figure 6.13.

Figure 6.13 Four-balls extreme-pressure lubricant tester
6.5.5 The Redwood Viscometer
The Redwood viscometer is made in two sizes. The Redwood No. 1 viscometer is commonly used for determination of viscosities of lubricating oils and has on efflux time of 2,000 seconds or less. The Redwood No. 2 viscometer is similar to Redwood No. 1 type but the jet for the out flow of the oils is of a larger diameter and hence gives an efflux time of approximately 1/10th of that obtained with Redwood 1 instrument under otherwise identical experimental conditions. Redwood No. 2 instrument is therefore used for the oils having higher viscosities such as fuel oils.
The Redwood viscometer does not give a direct measure of viscosity in absolute units but it enables the viscosities of oils to be compared by measuring the time of efflux of 50 ml of oil through the standard orifice of the instrument under standard conditions. The results given by these two viscometers are reported as Redwood No. 1 viscosity or Redwood No. 2 viscosity followed by the efflux time in seconds of the experimental temperature.
Description
The Redwood No. 1 viscometer shown in Figure 6.14 essentially consists of a standard cylindrical oil cup made up of brass and silvered from inside and has 90 mm height and 46.5 mm in diameter. The cup is open at the upper end. It is fixed with an agate jet in the base. The diameter of the orifice is 1.62 mm and the internal length is 10 mm. The upper surface of the agate is ground to concave depression into which a small silver-plated brass ball is attached to a stout wire can be placed in such a way that the channel is totally closed and no leakage of the oil from the cup through the orifice can take place. The cup is provided with a pointer, which indicates the level up to which the oil should be filled in a cup. The lid of the cup is provided with an arrangement to fix a thermometer to indicate the oil temperature. The oil cup is surrounded by a cylindrical, copper vessel containing water, which serves as a water bath used for maintaining the desired oil temperature with the help of electrical heating oils or by means of a gas burner as the case may be. A thermometer is provided to measure the temperature of water. A stirrer with four blades is provided in a water bath to maintain uniform temperature in the bath and hence enabling uniform heating of the oil. The stirrer contains a broad, curved flange at the top to act as a shield for preventing any water splashing into the oil cylinder. The entire apparatus rests on a sort of tripod stand provided with levelling screws at the three legs. The water bath is provided with an outlet for removing water as and when needed. A sprit level is used for levelling the apparatus, and a 50 ml flask for receiving the oil from the jet outlet is also provided.

Figure 6.14 Redwood viscometer No. 1
Working
The instrument is levelled with the help of the levelling screws on the tripod. The water bath is filled with water to the height corresponding to the tip of the indicator up to which the oil is to be filled in the cylindrical cup. The orifice is sealed by keeping the brass ball in position. Then the oil under test is carefully poured into the oil cup up to the tip of the indicator. The 50 ml flask is placed in position below the jet. The oil and water are kept well stirred; respective temperatures are noted. The ball is raised and suspended from the thermometer bracket. Simultaneously, a stop-watch is started. When the level of the oil dropping into the flask just reaches the 50 ml mark, the stop-watch is stopped, and time is noted in seconds. The ball value is replaced in the original position to prevent the overflow of the oil. The experiment is repeated, and the mean value of time of flow for 50 ml of the oil is reported as t seconds, Redwood 1 at T°C. The usual test temperatures stipulated are 21.11°C (70°F), 60°C (140°F), and 93.33°C (200°F).
During the test, the measuring flask should be shielded from draughts with the help of metal shields usually supplied with instrument.
6.5.6 Englers Viscometer
This instrument is diagrammatically presented in Figure 6.15. The water bath is heated by a gas ring, and its temperature is kept uniform with the help of the stirrer. The oil cylinder is fitted with three gauge points, which indicate the amount of oil required and also serve as a means of levelling the instrument. The loosely fitting cover carrying thermometer can be gently rotated to agitate the oil. The jet is slightly tapered and is made of platinum for standard work and nickel for general work. The valve pin, which seats itself in the jet, is lifted at the commencement of a test and supported in the cover by a cross pin. As the valve pin is lifted, stop-watch is started, and the time of outflow of 200 ml of the oil is determined.

Figure 6.15 Englers viscometer
The viscosity is expressed in Engler degrees or degree E by using water as standard. The time of outflow of 200 ml of water at 20°C is taken as 52 seconds. The viscosity in degrees E is calculated by dividing the time m seconds for the outflow of 200 ml of oil by time of outflow of 200 ml of water at 20°C.
6.5.7 Saybolt Viscometer
A single unit Saybolt universal viscometer is shown in Figure 6.16. In the multiple-unit viscometer, a number of oil cups can be accommodated in the same bath thus enabling tests on a number of oils to proceed at the same time. Instruments can be fitted with an electric immersion heater, a U-tube for steam heating or water cooling, and a gas ring, which is placed inside the air jacket surrounding the water bath. The bath liquid is stirred by rotating the cover by means of the two handles as a turn-table arrangement. Its temperature can be regulated by running cold or warm water through the U-tube whatever may be the heating arrangement used. The jet is made of a hard non-corrodible metal such as monel or stainless steel. The lower end of the jet opens into a larger tube. This tube when stoppered by a cork becomes a closed air chamber preventing the oil flowing out (Figure 6.16).

Figure 6.16 Saybolt viscometer
To start with the test, the bath is brought to the test temperature and the oil is heated to the same temperature in a separate vessel. The oil is then poured into the oil cylinder and stirred with the oil thermometer, any excess oil flowing over into the surrounding gallery. When the oil and the bath are at the same temperature, the oil thermometer is removed, the excess oil drawn off from the gallery with a pipette, the cork withdrawn, and the stop-watch started. The collecting flask is arranged such that the oil stream will strike its neck, thus avoiding the formation of foam. The time of outflow of 60 ml of the oil is the viscosity in seconds Saybolt universal at the test temperature.
For very viscous fuels, a viscometer with a larger jet known as the Saybolt furol viscometer is used. The Saybolt universal viscometer can be used for oils having flow times of more than 32 seconds. There is no maximum unit, but in general, for liquids having flow times over 1000 seconds, Saybolt furol viscometer is better.
6.5.8 U-Tube Viscometer
Standard U-tube viscometer (Figures 6.17(a) and (b)) is an improved form of the Ostwald viscometers, which is used for the determination of the absolute viscosity of lubricating oils. The determination of absolute viscosity of lubricating oils by the U-tube viscometer based on Poiseuille‚s law.

Figure 6.17 U-tube viscometers (a) Standard U-tube viscometer (b) Ubbelohde suspende level viscometer
![]()
where V = volume of the liquid flowing through a capillary tube of length l (cm) of uniform radius ‘r‚ (cm) in a times ‘t‚ (seconds) and η (poise) is the coefficient of viscosity of the liquid at the particular temperature.
The determination of absolute viscosity by the U-tube viscometer essentially consists of measurement of the time of passage through the capillary of a fixed volume of liquid under a fixed mean hydrostatic head ρ of the liquid. If the density of the liquid is ‘d‚, then P ∝ d, and since, for a given viscometer, η ∝ td.
η = ktd
where k is the constant of proportionality and has to be determined for each viscometer from its known dimensions or by caliberation with a liquid (such as water) whose viscosity coefficient is known
![]()
6.5.9 Conversion of Redwood, Engler and Saybolt Viscosities into Absolute Units
Redwood, Engler and Saybolt instruments are not the ideal methods of determining the absolute viscosities, the conversion values are only considered as good approximations and that too only when taken at the same temperature.
So the conversion of the above relative viscosities to absolute viscosities is done with the help of the following equations
n = ct – b/t
where n = Kinematic viscosity in centistokes,
t = time flow in seconds,
c and β are constants.
Values of c and β are tabulated below.

6.6 Explosives and Propellants
Explosives
An explosive is a “substance (or) compound (or) mixture, which when subjected to thermal and mechanical shock, gets very rapidly oxidized exothermically with a sudden release of potential energy”.
The explosive reaction is exothermic, so the products get heated to a high temperature and a high pressure is exerted on the surrounding. The amount of power available from a given weight (or) volume of explosive, is called “power to weight ratio”.
6.6.1 Some Important Terms about Explosives
- Explosive strength: It is the energy liberated per unit mass of the explosive (cal/g).
- Velocity of detonation: It is the velocity with which the given explosive detonates.
- Sensitivity: It is to impact (or) friction (or) heat (or) electric spark (or) detonator wave. Some explosives may detonate by a feather touch, some may not detonate even with hammer blow. Sensitivity plays a key role in the selection of explosives for a particular purpose.
- Brisance: It indicates to the shattering power of explosive.
6.6.2 Classification of Explosives
Explosives are broadly classified into three groups.
- Detonators (or) Primary explosives (or) Initiating explosives:
There are highly sensitive, which explode on receiving a slight-shock or by fire. So they should be handled with the atmost care. Some of examples are,
- Lead azide (PbN6 ): It is very popular for military uses due to low cost, excellent initiating action and stability in storage.
- Mercury fulminate [Hg(CNO2 )]: It is more sensitive as well as more expensive them lead azide. It is slightly toxic.
- Tetracene (C2η7N7O): It is low initiating ignites easily, has high heat of explosion and produces a large volume of gases.
- Diazodinitro Phenol (DDNP): It is quite sensitive and has high brisance and consequently can initiate explosion even in less sensitive high explosives.
- Propellents (or) Low explosives:
These are simply burns and do not explode suddenly. The chemical reactions taking place in such explosives are slow and their burning proceeds from the surface inwards in layers at an approximate rate. The gases evolved disperse readily without building high pressures and consequently they can be controlled easily. Some of examples are,
- Gun-Powder (or) Black Powder :
It is a mixture of 75% potassium nitrate, 15% charcoal and 10% sulphur. The explosive reaction is
10KNO3 + 3S + 8C (3K2SO4) + 2K2CO3 + 6CO2 ↑ + 5N2↑
If excess of carbon and sulphur take part in slower processes leads to evolution of more gases.
It is an excellent and cheap explosive for blasting down of coal, as its low velocity gives it a slow heaving action that does not shatter the coal unduly. It is time in delay-fuses, for blasting and in shells, igniters and primer assemblies for propellants, practice bombs and saluting charges.
- Nitrocellulose:
It is prepared by treating cellulose with nitric and sulphuric acids. Formed nitrocellulose is dissolved in a mixture of ether and alcohol and then solvent is evaporated, when a jelly like solid is left behind. It is stead by adding stabilizer like diphenylamine and pressed into cylindrical rods. It is called smokeless powder due to producing of smokeless gases like CO2, CO, N2 and water vapour.
- Gun-Powder (or) Black Powder :
- High explosives:
Explosives which have high energy content than the primary explosives are high explosives. These are quite stable and insensitive to fire, mechanical shocks, etc. Hence, to start a rapid chemical reaction some amount of primary explosives are placed with high explosives.
These are broadly divided into four groups:
- Single compounds explosives:
These contain only one chemical compound. Some of examples are,
- Ammonium nitrate: It is very stable, nontoxic, cheap and has low brisance-value. It is about half as powerful as TNT and mostly employed in making binary explosives. It is dangerous to store near any inflamable material.
- TNT (Trinitrotoluene): It is high explosive. It is most widely used in shell-firing and under-water explosions and is well-suited for loading in containers, due to low melting point. It is safe explosive in manufactures, transportation, storage, non–hydroscopic, violent disruptive explosive, does not react with metals so it is used as military use.
- PETN (Pentaerythritol tetra nitrate): It is an extremely powerful, sensitive and standard military explosive.
- RDX (or) Cyclonite: It is a powerful most sensitive, less toxic high explosive. RDX came into prominence as military as well as an industrial explosive.
- Binary explosives:
Binary explosives are a mixture of TNT and other explosives, and these are more convenient and superior than single explosives. Due to its low melting point, TNT is one of the ingredients in all binary explosives.
Ex:
- TNT + Ammonium nitrate is amatol.
- TNT + PETN is pentolite.
- TNT + Tetryl is tetrytol.
- TNT + RDX + Al powder is tropex.
- TNT + Al flakes is titronal.
- Plastic explosive:
These are combination of explosives which are in plastic state, they can be hand moulded and made into various shapes without any serious risk. They are mainly used for industrial applications and military uses. A simple combination to get plastic explosive is a high explosive. Due to their engineering applications they are available as flexible-sheets.
- Dynamites:
These are explosives containing nitroglycerin as the main ingredient, by pressure or shock detonates.

The explosion is so sudden it would shatter the breech of rifile, before the bullet had time to move. It also pulverizes rock, instead of breaking it into fragments of unable size. It is danger in handling and transporting. So it is usually mixed with an insert absorbent likes would pulp, starch,meals etc. Some of examples are,
- Straight-dynamites: It contains 15 to 60% nitroglycerine in wood meal with sodium nitrate. Important uses are blasting of hard rocks, coal and other mineral.
- Blasting gelatin-dynamites: It is the combination of NG (Nitro glycerene) (91.5%) with partly gelatinized by nitro-cotton (8%) (or) colladion cotton and CaCO3 (0.5%). These are very powerful, water proof, stick well in holes into which they have been loaded. They can be used under wet conditions, where high loading density is desired. Important uses are submarine blasting tunnel driving, deep-well shooting, and at places where maximum shattering effects are desired.
- Gun cotton (or cellulose nitrate): Cotton is steeped for half-an hour in a cooled mixture of conc.nitric and conc.sulphuric acids it is formed.
C6H7O2(OH)3 + 3HNO3( C6H7O2(NO3)3 + 3H2O
- Cordite: It is (a form of smokeless powder) is made by dissolving gun-cotton (65 parts), nitroglycerine (30 parts), and petroleum jelly or “Vaseline” (5 parts) in acetone. The resulting paste is rolled and cut into pieces of different dimensions, according to the rate of explosion desired. When the Acetone evaporates, the horny cordite remains. The Vaseline acts as stabiliser and cooling agent on the powder, as it has a tendency to lower the temperature of explosion. The gun-cotton slows down the explosive reaction of NG and makes cordite an excellent propellent for large caliber naval guns.
- Gelignite: It consists of 65% blasting gelatine and 35% of absorbing powder. It is a powerful explosive, which can be used under water.
- Single compounds explosives:
6.6.3 Precautions During Storage of Explosive
Following precautions should be taken while storing the explosives:
- Explosives should be stored separately, i.e., without mixing with others.
- Detonators and explosives should be stored totally separate.
- Flame lantern should be not use in any case. When power failure takes place, use of only torches should be made for lighting purposes.
- All the electric fittings and wirings should be properly insulated and frequently checked.
- Only authorised persons should be allowed to the explosive store. The authorised person must wear magazine shoes having not be allowed.
- One should take care regarding jerks or drops while handling explosives.
- Smoking/fire should be strictly prohibited around the explosive store.
- The magazine should not be constructed with in 500 m from any working kiln or furnace.
- Explosive store boundary should be protected by high barbed wire fencing and should be with proper ‘caution boards.‚
- Magazine (explosive store) should be provided with heightening conductors as a safeguard.
- During thunders, magazine should not be opened and also no person should be allowed.
6.6.4 Blasting Fuses
A fuse is “a thin water-proof canvas length of tube containing gun-powder (or TNT) arranged to burn at a given speed for setting off charges of explosives. Fuses are of two types:
- Safety fuse: It is employed in initiating caps, where electrical firing is not used. A safety fuse consists of a small diameter core of black powder, enclosed in a covering of wrapper of water proofed fabrics. It is made to have an approximate burning speed of 30 to 40 seconds per foot (or about 1 cm s–1). When a fuse is used to fire a shot in blasting, a sufficient length is used so that ample time is allowed for the shot firer to reach a point of safety.
- Detonating Fuse: It has a velocity of over 6,000 m/s and consists of a charge of high velocity explosive, such as TNT, contained in a small-diameter bent tube. The line of fuse (called Cordean) is in contact with the charge through out its length and this causes practically instantaneous detonation of the whole charge, regardless of its velocity. Such fuses are used principally for exploding charges of explosive in deep-holes.
6.6.5 Important Explosives and their Preparation
- Lead AzidePb(N3)2 is prepared by reacting sodamide (formed by reacting ammonia with sodium) with nitrous oxide (N2O), followed by treating the sodium azide (NaN3) so-formed with lead acetate.
Thus;
6NH3 + 2Na (2NaNH2 + 3H2)
NaNH2 + N2O → (NaN3 + H2O)

- Mercury Fulminate Hg(ONC)2 is prepared by dissolving mercury in excess of HNO3 and then pouring the resulting solution of ethyl alcohol, when the solution starts boiling and mercury fulminate is precipitated.
Decomposition Reaction: Hg(ONC)2 Hg(g) + N2 + CO + 117 k Cal
- Diazodinitrophenol (DDNP) is prepared by diazotizing picranicacid.

- Trinitrotoluene (TNT) is prepared by the nitration of toluene (C6H5CH3) using a nitrating mixture of conc. HNO3 and conc. H2SO4 in 1:1 ratio in a tank reactor, in which contents are continuously stirred.

Then liquid product (TNT) so-formed is taken out, washed with ammonical solution of Na2SO3 and then with cold water, when TNT crystallises out. Crystals of TNT are filtered and purified by melting. The melt is dried (by passing warm air) and poured in containers.
Decomposition Reaction: TNT (3.5CO + 3.5C + 2.5H2O + 1.5N2 + 190 K cal
- Nitroglycerine (NG) or glycerol trinitrate (GTN): is prepared by adding glyceral to a cooled mixture of conc. H2SO4 (60%) and conc. HNO3 (40%) at 10 °C with constant stirring.

After nitration, the mixure is run into a tank, when NG rises to top, while excess acids form lower layer. The NG layer is separated, washed first with water, then with dilute sodium carbonate solution (2%) to remove traces of acids completely. It is then converted into different desired types of dynamites by absorbing in specific inert materials.
Decomposition Reaction: 4C3H5(NO3)2(12CO2 + 6N2 + 10H2O + O2)
- Pentaertythrital Tetranitrate (PETN): is prepared by using cannizaro reaction between formaldehyde and acetaldehyde in molar ratio 4:1 Thus;

Decomposition Reaction: C(CH2ONO2)4(3CO2 + 2CO + 4H2O + 2N2 + 180 K Cal
- RDX or Cyclonite (Cyclomethylene trinitro amine) is prepared by treating hexamethylene tetra amine with nitric acid.
Thus
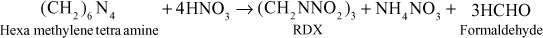
6.6.6 Rocket Propellants
Rocket propellant is a high oxygen-containing fuel plus oxidant, whose combustion takes place, in a definite and controlled manner with the evolution of a huge volume of a gas. A propellant reacts quickly, producing a very large volume of hot gases (usually at a temperature of 3,000 °C and a pressure of 300 Kg/cm2), which exit through a small opening (called “jet” or nozzle) at supersonic velocity. This act of pushing the gas backwards produces an equal and opposite reaction. i.e. Newton‚s 3rd law of motion, which moves the rocket forward. With the increase of exhaust velocity the rocket performance increases. Rockets are used for pyrotechnic effect signalling, carrying a life, hurling explosives at an enemy, putting space capsule into orbit, etc (Figure 6.18).

Figure 6.18 Use of bipropellant in a rocket. Combustion reaction between kerosene and liquid oxygen produces a huge volume of gaseous CO2 and H2O, the thrust of which forces the rocket upwards with a high speed
6.6.7 Characteristics of a Good Propellant
- It should have high specific impulse (specific impulse is the thrust delivered divided by the rate of propellant (fuel plus oxidant) burnt.
- It should produce low molecular weight products (like H2, CO, CO2 and N2) during combustion.
- It should burn at a slow and study rate.
- It should possess low ignition delay (ignition delay is the time taken by propellant to catch fire in the presence of an oxidizing agent. It is expressed in milli seconds)
- It should possess high density.
- It should be stable over a wider ranges of temperature.
- It should be safe to handle and store under ordinary condition. i.e., it does not detonate under shock, heat or impact.
- It should be non-corrosive & non-hydroscopic.
- It should be readily ignitable at predictable burning rate.
- It should leave no solid residue after ignition.
- It shouldn‚t produce toxic products.
- It should produce high temp on combustion.
Note: Specific impulse is the thrust in Kg – per second per Kg on the fuel burnt. The value of thrust (or propulsive force) due to momentum of the exiting gases and is given by
![]()
where F = Thrust (kg/m/kg)
m = Mass flow (kg)
g = Aceleration due to gravity (9.81 m2/s)
v = exhaust velocity (m/s)
Pe = exit pressure (kg/m2)
Pa = ambient gas pressure (kg/m2)
and αe = nozzle exit area (m2)
6.6.8 Classifications of Propellants
The chemical propellants are classified into solid and liquid categories.
- Solid Propellants may be “homogeneous” or “composite”. When solid propellant or a mixture of propellants is thoroughly mixed in a colloidal state, it is called homogenous solid propellant. When a single propellant is employed, it is called a single-base propellant. For example, nitro cellulose, also known as gun-cotton or smokeless powder. On the other hand, a solid propellant which contains two materials is called double-base propellant. For example, ballistite, containing nitro cellulose and nitroglycerine mixture, is a powerful double-base solid propellant. Cordite, composed of 65% nitrocellulose 30% of nitro cellulose and (40–45)% nitro glycerine. Diethlyphthalate, upto 5% is also added and this acts as solvent cum plasticiser, thereby giving a homogenous plastic mass, which can be worked smoothly. In addition to it, upto 1% of diphenylamine is added, which acts as a stabilizer. Such propellant gives a flame temperature of about 2,700 °C and the volume of gases is about 1,500 times the original volume.
When an oxidising agent is dispersed in a fuel mass, the solid propellant is called heterogeneous or composite. Gun powder is the oldest composite propellant. It gives a flame temperature of 800–1500 °C and volume of gases is about 400 times the volume of the charge. Other composite solid propellants are 75% potassium per chlorate plus 25% asphalt oil; 80% ammonium perchlorate plus 20% resin (rubber) binder; 46% ammonium picrate, 48% sodium nitrate, and 8% plastic resin binder. In selecting the oxidiser, it should be seen that it is non-hygroscopic, stable in contact with fuel and doesnot form any corrosive products. Oxidant potassium perchlorate leaves behind a white residue of potassium chloride particles; while ammonium perchlorate leaves no solid residue, but its combustion products contain hydrogen chloride and water, which form a toxic and corrosive fog.
- Liquid Propellants possess many advantages over solid propellants. Thus, liquid propellants are more versatile and the engine using them can be checked and caliberated more easily. However, unlike solid propellants, the engine using liquid propellant is quite delicate and cannot with stand any rough handling. Liquid propellants may be mono propellants (or) bipropellants.
Mono Propellant: It has fuel as well as oxidiser in the same molecule or in a solution containing both these. For example, hydrogen peroxide, nitro methane, ethylene oxide hydrazine propyl nitrate and a mixture of 21.4% methanol and 78.6% hydrogen peroxide are some of the common propellants (mono). A mono propellant must be safe to state and at the same time, it should burn smoothly. Hydrogen peroxide is not easy to store and handle, due to its reactivity. Moreover, metal oxides catalyse its decomposition. So, storage tanks for it are made of special materials.
Bipropellants: These are more widely used. In these, liquid fuel plus oxidiser, kept separately, are injected in the combustion chamber separately. Commonly used fuels are liquid hydrogen, hydrazine, ethyl alcohol, aniline, and kerosene oil. Ethyl alcohol admixed with 25% water is a good fuel. Addition of water, although reduces flame temperature, yet it reduces the molecular mass of combustion gases, which compensates for reduction in performance. The common oxidisers employed are liquid oxygen, ozone, hydrogen peroxide, fuming nitric acid and liquid flourine. Liquid oxygen is non-toxic, safe and good oxidizing agent, but it has to be stored under pressure in insulated containers. Ozone is very powerful oxidising agent, but it is quite toxic and can explode at high concentrations. Liquid flourine is volatile, toxic, corrosive, very reactive, but very good oxidizing agent. Also, it is difficult to store and handle.
6.7 Nanomaterials
Nanoscience and technology is considered to be one of the most promising fields having huge potential to bring countless opportunities in many areas of research and development. It is the study of tiny structures at nanometer scale, which forms a basis for number of core technologies.
“Nanotechnology plays a key role in many areas”.
Definition
“One nanometer is the one billionth of a meter ”.
1 nm = 1/1000000000 of a meter which is close to 1/1000000000 of a yard.
For getting easy sense of the nanoscale, it is suffice to know that a human hair has around 50,000 nm and a commonly used microchip has around 150 nm. The normal human eye can see the things which have the size above 10,000 nm only.
A Note on Measures
Almost all nanosciences are discussed using SI measurements.

6.7.1 Synthesis of Nanomaterials
Nanotechnology has sprung into prominence nowadays, due to the recent development of various synthesis techniques/methodologies and the discovery of modern tools for the characterization and manipulation of nanomaterials. Synthesis techniques are broadly categorized as top-up approach and bottom-up approach. Some of the methods for synthesis of nanomaterials are as follows:
- The vapour–liquid–solid growth
- Solution–liquid–solid growth
- Template–mediated growth
- Electron beam lithography
- Reverse micellar route, etc.
Preparation of copper and nickel nanooxalates by reverse micelle route: The reverse micellar route is the best method for synthesis of variety of nanoparticles due to ability to mix the reactants efficiently and control the size of nanoparticles effectively.
The synthesis of these nanorods have been achieved using two micro-emulsions as described below:
- Microemulsion I is the mixture of cetyl trimethyl ammonium bromide (CTAB) as a surfactant and n-butanol as the co-surfactant. Here, isooctane or n-octane is used as a hydrocarbon phase and 0.1 M copper nitrate/nickel nitrate solution is used as the aqueous phase.
- Microemulsion II comprised of the same constituents as microemulsion I, except for having 0.1 m ammonium oxalate instead of copper nitrate or nickel nitrate as the aqueous phase. The weight fractions of various constituents in these microemulsions are 16.76% of CTAB, 13.90% of n-butanol, 59.29% of isooctane, and 10.05% of the aqueous phase. These two microemulsions were mixed slowly and stirred overnight on a magnetic stirrer, and the resulting precipitate was separated from the apolar solvents and surfactants by centrifuging and washing it with 1:1 mixture of methanol and chloroform. The precipitate was then dried in air.
6.7.2 Characterization
The nano materials are characterized by X-ray diffraction, transmission electron microscopy, scanning electron microscopy, (TEM, SEM, and AFM), dynamic light scattering studies (DLS), thermal analysis (TGA/DTA), fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), field dependent magnetization studies, etc.
6.7.3 Importance
- Synthesis of nanowires and nanorods has generated a lot of interest in the recent years due to their importance as objects for understanding microscopic systems.
- Nano-structured wires and rods are expected to have interesting optical, electrical, magnetic, and mechanical properties as compared to micron-sized whiskers and fibers.
- There is also find potential use as nanowires in the area of biosensors, where a specific molecule is attached to the tip of the nanowire, which then identifies a particular molecule in the living system.
6.7.4 Broad Classification of Nanomaterials
According to atoms (or) molecules arrangements nanomaterials are broadly classified into three types as follows:
- Materials which have one dimension in the nanoscale.
E.g.: Surface coatings and thin films
- Materials which have two dimensions in nanoscale.
E.g.: Nanowires and nanotubes
- Materials which have three dimensions in nanoscale or quantum dots.
E.g.: Fullerenes, cadmium-selenium quantum dots, gold quantum dots, ZnO quantum dots etc.,
Some Other Nanomaterials
Super-paramagnetic iron oxide nanoparticle, ZnO nanoparticle, Titanium oxide nanoparticle, carbon-coated silver nanoparticles, gold nanoparticle, NayF4 nanophosphors, CdS nanowires, ZnS nanorods, Fe3O4 nanoparticles etc.,
6.7.5 Properties
The advent of nanotechnology has resulted in increased use of nanomaterial-based products in daily life. A significant increase in surface-area-to-volume ratio at the nanoscale, giving rise to novel and enhanced magnetic, mechanical, electronic, catalytic, conducting, and optical properties of nanomaterials, has made nanotechnology the most promising tool of this century.
- Size: Nanostructures are the smallest of human-made things but the largest molecules of natural things and nanometer is magical point on the scale. Nanoscience and technology provides advanced materials and systems which are intermediate between isolated atoms and bulk materials, with controlling of transitional properties. Due to drastically increased surface-to-volume ratio, physical and chemical properties are quite different from the bulk but tend to dominate at nanoscale.
- Optical properties: Due to large surface area, nanomaterials exhibit better optical properties. Luminescent nanocrystals, termed as nanophosphors, with unique optical properties make them ideal for a wide spectrum of applications ranging from flexible displays, lasers to biological imaging, and therapeutic agents. For example, NaYF4 is a highly multifunctional material with promising potential application in IR to visible up conversion process. Silicon nanowires show strong photoluminescence.
- Mechanical properties: Mechanical behaviour of nanomaterial are found to be high strength, good ductility, superior superplasticity, a low-friction coefficient, good thermal stability, high wear resistance, enhanced high-cycle fatigue life, and good corrosion resistance.
E.g.: Tensile strength of carbon nanotube is approximately 20 times greater than the steel.
- Magnetic property: Magnetic property of a nanomaterial is most useful in biological systems. Due to unique properties and biocompatibility of nanoparticles, they easily combine with biological materials like proteins, antibodies, enzymes, nucleus acids, etc.
- Electrical properties: Nanomaterials show electrical conductivity from semiconductors to superconductors, depending upon the diameter and chirality of the molecules. Nano-structured conducting polymers have generated much interest for their potential use in nanoelectronics.
E.g.: The fullerenes are a class of allotropes of carbon, which are basically graphene sheets rolled into tubes or spheres. They include the carbon nanotubes because of their mechanical strength and electrical properties. Carbon nanotubes have been identified basically to have three structures namely, armchair, zigzag, and chiral structure (Figure 6.19a, b, c).

Figure 6.19 (a) Armchair (n, n), (b) Zigzag (n, 0), (c) Chiral (n, m)
Carbon nanotubes have higher electrical conductivity than copper wires. The high electrical conductivity of nanotubes is mainly negligible amount of defects, so they possess low resistance.
- Semiconductors: Most of the nanomaterials show semiconductivity.
E.g.: Carbon nanotubes, nanowires, MoS2, etc. They are used mainly as field-effect transistors, p-n diodes, etc.
- Superconductors: Some of the nanomaterials at low temperature show superconductivity.
E.g.: At normal temperature, NbS2 is metallic in nature, but at low temperature, it becomes a superconductor.
- Catalytic activity: Due to the increasing surface area, nanomaterials act as a good catalyst in different homogeneous and heterogeneous phases.
E.g.:
- In2O3–Ga2O3/Al2O3 nanocomposite used in selective catalytic reduction of nitric oxide.
- Platinum, a precious transition metal, which has outstanding catalytic and electrical properties and superior resistant characteristics to corrosion, has been widely applied in chemical, petrochemical, pharmaceutical, electronic, and automotive industries. Both platinum metal and its alloys possess distinctive ability in catalyzing partial oxidation, hydrogenation, and dehydrogenation of a variety of important molecules that are essential in many industrial processes.
6.7.6 Applications of Nanomaterials
Due to the advent of nanotechnology, applications of nanomaterials are increasing very fastly day-by-day.
- Conducting polymers are widely employed as coatings conferring the electrode systems, antifouling properties, and possibly activating electrocatalytic redox processes, conducting polymers for commercial applications due to their high conductivity; easy preparation and environmental stability have generated much interest for their potential use in nanoelectronic and organic conductors.
- Aurum nanoparticles have been used for the enhancement of radiation effects on bovine aortic endothelial cells of superficial X-ray radiation therapy and megavoltage electron radiation therapy beans.
- Nanoparticles are used as important catalyst in different chemical reactions.
- Quantum dots and quantum wires have been used in the design of new super computers.
- Magnetic nanoparticles have wide application in medicine as drug transport and biosensor.
- Carbon nanotubes having fascinating physical and chemical properties such as electrical conductance, high mechanical stiffness, light weight, electron spin resonance field emission, electrochemical actuation, transistor behaviour, piezoresistance, contact resistance, coulomb drag power generation, thermal conductivity, luminescence, electrochemical bond expression, opto-mechanical actuation, and the possibilities of function-aligned CNTs to change their intrinsic properties are the reasons for their use in different ways as given below:
- It is used as a novel biosensor as enzyme electrodes, immunosensors, DNA, RNA, electrodes, etc.
- Due to well-defined geometry, exceptional mechanical properties and extraordinary electric characteristics, among other outstanding physical properties of CNTs, qualify them for potential applications in the preparation of nanoelectronic circuits, nanoelectromechanics systems, nanorobotics systems, structural elements, probes, grippers, tweezers, scissors, actuators, bearings, syringes, switches, memories, diodes, transistors, logic gates, wires, cables, position sensors, displacement sensors, circuits, thermal actuators, etc.
- It is used in field emission light devices for fluorescent displays.
- It acts as a storage device in lithium batteries.
- From household uses to industries, space, army, medicine, and agriculture, and in every field nanotechnology applications are there.
6.7.7 Carbon Nanotubes
Due to the well-defined geometry, exceptional mechanical properties and extraordinary electrical characteristics of carbon nanotubes (CNTs) are used as nanoelectric circuits, nanoelectrochemical systems, nonorobots, etc.
Synthesis of Carbon Nanotubes
Carbon nanotubes including powder and array types are generally produced by three main techniques.
- Electric arc discharge method
- Laser ablation method and
- Thermal or plasma-enhanced chemical vapour deposition method
Laser ablation and arc discharge methods are modified physical vapour deposition techniques and involve the condensation of hot gaseous carbon atoms generated from the evaporation of solid carbon. In arc discharge, a vapour is created by an arc discharge between two carbon electrodes with or without a catalyst. Nanotube self-assemble from the resulting carbon vapour laser ablation produces a small amount of clean nanotubes, whereas arc discharge methods generally produce large quantities of impure material. Both the laser ablation and arc discharge techniques produce powder-type nanotubes with impurities in the form of amorphous carbon and catalyst particles because of the high temperature of the heat source. The chemical vapour deposition method is a better technique for high yield and low impurity production of carbon nanotube arrays at moderate temperatures. Plasma-enhanced chemical vapour deposition can grow individual, free-standing nanofibers with special controlling ability. On the other hand, high-density aligned nanotubes can be mass-produced using thermal chemical vapour deposition. The CVD method also provides good control over the size, shape, and alignment of the nanotubes.
Recently, Kang et al. have synthesized dense, perfectly aligned arrays of single-walled nanotubes, and Vijayaraghavan et al. have been able to horizontally align individual nanotubes on a large scale using dielectrophoretic force. Nanotube materials for analytical applications should be clean with low impurities from metal catalysts and amorphous carbon. Synthesis of nanotubes with a uniform size and special density is critical before post-processing and functionalization.
Applications of Carbon Nanotubes
- Carbon nanotubes are used in the preparation of nanoelectric circuits, nanoelectromechanical systems and nanorobotic systems.
- Multi-walled carbon nanotubes can serve as bearings, switches, gigahertz oscillators, memories, shuttles, syringes, etc.
- The hallow carbon nanotubes can be used as containers, conduits, pipettes, coaxial cables, etc.
Carbon Nanotubes as Nanobiosensors
Carbon nanotubes have become the forces of intensive research by analytical chemists, as electrodes to transmit electrical signals or as sensors to detect concentrations of chemicals, biological materials. Fascinating physical and chemical properties such as electrical conductance, high mechanical stiffness, light weight, electron-spin resonance, field emission, electrochemical actuation, transistor behaviour, piezoresistance, contact resistance, coulomb drag power generation, thermal conductivity, luminescence, electrochemical bond expansion, opto-mechanical actuation, and the possibilities of functionalizing carbon nanotubes to change their intrinsic properties are the reasons for their use as novel biosensors.
The structure of nanotubes can be described as a rolled-up tabular shell of graphite sheet with the carbon atoms covalently bound to their neighbours. Helicity of the shell categorizes nanotubes into metallic or semiconducting types. Based on the capture and promotion of electron transfer reaction from analytics, ballistic conductivity of metallic nanotubes is extremely attractive. Semiconducting nanotubes can be used as biosensors directly because these are optimized by changing the gate voltage. Hence, CNT electrodes have high sensitivity with low detection limit. CNT can be described as single wall nanotubes (SWNT), double wall nanotubes (DWNT), or multi-wall nanotubes (MWNT). CNT diameters range from about 1.4 nm to 60 nm and their length varies from microns to above one centimeter.
To use nanotube electrodes for electro analytic purposes, proper conjugation strategies between biological molecules such as enzymes, single stand DNA/RNA/PNA, antibodies, receptors, and aptamers need to be developed. Appropriate functionalization methods and immobilization of biomaterials on nanotubes are critical since functional groups create defects in the nanotubes that will eventually alter or degrade the intrinsic electrical properties of the nanotube. Nanotubes also have contact resistance that should be minimized to provide the highest sensitivity when used as a sensor.
Carbon Nanotubes as Nanorobotics
Robots can do all kind of physical and mechanical work more in various situations instead of manpower. Now, large-size of robots are used, and shrinking the robot size to nanoscale with carbon nanotube is fascinating advantage. Nanorobots can measure mass in femtogram ranges, sensing forces at piconewton scales, etc. and with a provision for advanced features nanorobots can introduced in army, intelligence, security, mechanochemical synthesis, etc.
6.8 Liquid Crystals
The study of liquid crystals began in 1888 with the observation of two distinct melting points to cholesteryl benzoate by Friedrich Reinitzer.
The liquid crystal constitute a fascinating state of aggregation that is intermediate between the crystalline solid and the amorphous liquid. They exhibit rheological behaviour similar to those of liquids and anisotropic physical properties similar to crystaline solids. Their dual nature and easy response to electric, magnetic and surface forces have generated innumerable applications, which are extended over diverse fields. The explosive growth of application of liquid crystals has generated immense interest amongst the scientists and technologists to synthesize, characterize and understand the substances.
“Liquid crystal is formed in between the temperature of transition point and melting point, the cloudy liquid shows double refraction. Hence the cloudy liquid is known as liquid crystal (or) mesomorphic state”.
(or)
“The liquid crystals are highly anisotrophic fluids that exists between the boundaries of the solid and conventional, isotropic liquid phase”.
The liquid crystal is made up of liquid and crystals. The term liquid is used because the tendency to take the shape of the container and the word crystals is used because they still contain 1 or 2 dimensional arrays.
6.8.1 Characteristics of Liquid Crystal Phase
The liquid crystalline state is the tendency of the mesogens (molecules) to point along the director (common axis), which have no intrinsic order. The average alignment of the molecules in solid, liquid crystal and liquid are shown in figure.
Crystalline material possesses long range periodic order in three dimentions, but liquid crystals does not possess order as a solid, since they have some degree of alignment order only. They show positional, orientational, bond orientational order, etc.
These are characterized by partial ordering, that is, one or more degrees of freedom, but not all, will have long range order.

6.8.2 Classification of Liquid Crystals
Liquid crystals may be divided into two broad categories, according to the principal means of breaking down the complete order of the solid state.
- Thermotropic liquid crystals.
- Lyotropic liquid crystals.
6.8.3 Thermotropic Liquid Crystals
There are established solely by the adjustment of temperature. There are divided into three types namely nematic, cholesteric and smectic. These three classes are distinguished by the different kinds of molecular order they exhibit.
(i) Nematic Structure
The molecules in the nematic structure maintain a parallel or nearly parallel arrangement to each other along the long molecular axes. They are mobile in three directions and can rotate about one axis. Schematic representation of nematic liquid crystals are shown in Figure 6.20.

Figure 6.20 Schematic representation of molecular order in nematic liquid crystals
Properties
Nematic structure is the highest-temperature mesophase in thermotropic liquid crystals. In this structure molecules have no particular positional order, but tend to point vertically in same direction as shown in figure. Like liquids molecules are free to flow, their centre of mass positions are randomly distributed, but they maintain long range directional or orientational order. The molecular order of nematics is shown schematically in figure. As in any liquid, the molecules possess no translational order. There exists, however, a significant degree of long-range orientational order.
(ii) Cholesteric Structure
The cholesteric-mesophase is a nematic type of liquid crystal except that it is made up of optically active molecules.
(or)
The cholesteric liquid crystal phase is typically composed of nematic-mesogenic molecules containing a chiral centre, which produces intermolecular forces that favour alignment between molecules at a slight angle to one another. Helical structure (6.21.a) and Pitch of helix of the cholesteric liquid crystal (6.21.b) are shown in figures.

Figure 6.21a Helical structure of the cholesteric liquid crystal

Figure 6.21b Pitch of a helix of cholesteric liquid crystal
Properties
The cholesteric structure acquires a spontaneous twist about an axis normal to the preferred molecular directions. The twist may be right-handed or left-handed depending on the molecular conformation.
In the structure of cholesteric phase, the local molecular ordering is identical to that of the nematic phase. The chiral nematic phase exhibits chirality, hence it is called cholesteric phase. Chiral molecules those have no internal planes of symmetry can give cholesteric phase. This phase shows a spontaneous twisting of the molecular axis parallel to the director. This twist may be right or left-handed and depends on the molecular conformation.
In the structure of cholesteric phase, the local molecular ordering is identical to that of the nematic phase. Due to asymmetric packing the finite twist angle between adjacent molecules is a long range chiral order.
A cholesteric liquid crystal rotates the direction of linearly polarized light, and it is roughly 1000 times stronger than the activity of an ordinary optically active substance such as quartz, hence it is optically active. The chiral pitch refers to the distance over which the liquid crystal molecule undergoes a full 360° twist.
(iii) Smectic Structure
The word “smectic” is derived from the Greek word for soap. This seemingly ambiguous origin is explained by the fact that the thick, slippery substance often found at the bottom of a soap dish is actually a type of smectic liquid crystal. Smectic liquid crystal is shown in Figure 6.22.
Properties
The smectic state is another distinct mesophase of liquid crystal substances. Molecules in this phase slow a degree of translational order not prevent in the nematic. In the smectic state, the molecules maintain the general orientational order of nematics, but also tend to align themselves in layers or planes. Motion is restricted to within these planes, and separate planes are observed to flow part each other. The increased order means that the smectic state is more “solid-like” than the nematic.

Figure 6.22 Smectic liquid crystal
Up to now nine thermotropic smectic phases have been identified on the basis of appearance under a polarizing microscope, miscibility with known phases and X-ray scattering. These phases are labelled by the chronological order of their discovery and designated as smectic A, B … I.
Some thermotropic liquid crystalline compounds are tabulated below:


6.8.4 Lyotropic Liquid Crystals
Mixtures of two or more components that change phase with changes of concentration are called lyotropic. These mesophases occur in concentrated solutions of rodlike molecules in an isotropic solvent. The stability of these mesophases is as readily influenced by concentration of solute as by temperature. Lyotropic mesophases are important in soaps, gels and colloids and are of great interest in biology.
Lyotropic crystal having one of the components is an amphiphile (containing polargroup attached to long hydrocarbon chains) and another is water. When a crystalline amphiphile is added to water, several mesophases can be observed, ranging from a true solution to the crystal state.
Example: Sodium Stearate-CH3(CH2)16COO–Na+
α–Lecithin–
6.8.5 Chemical Properties of Liquid Crystals
According to chemical properties, liquid crystals can be broadly classified into two categories; they are thermotropic liquid crystals and lyotropic liquid crystals. These two are similar in many ways and distinguished by the mechanisms that drive their self-organization.
Thermotropic liquid crystals are formed by the raising of temperature of a solid or decreasing the temperature of a liquid. Usually, thermotropic liquid crystal occurs with anisotropic dispersion forces of the molecules and their packing interactions. Generally thermotropic liquid crystals occur because of anisotropic dispersion forces between the molecules and because of packing interactions.
According to their properties thermotropic liquid crystal classified into two types.
- Enantiotropic liquid crystals: Those crystals can be changed into the liquid crystal state from either lowering the temperature of a liquid or raising of the temperature of a solid are enantiotropic crystals.
- Monotropic liquid crystals: Those crystals can only be changed into the liquid crystal state from any one of these but not both, either an increase in the temperature of a solid or decrease in the temperature of a liquid.
Lyotropic liquid crystals are formed with the influence of solvents, not by changing of temperature. It occurs with the results of solvent-induced aggregation of the constituent mesogens into micellar structures. This kind of crystals shows amphiphilic properties, i.e., they have both lyophilic and lyophobic ends. With this property, in presence of a solvent form as micellar structures, due to which lyophobic ends will stay together and the lyophilic ends stay outward of the solution and with increasing the concentration of solution, cooled and micellar structures increase in size and eventually coalesce and separate the newly formed liquid crystal from the solvent.
6.8.6 Applications of Liquid Crystals
Liquid crystal science had a major effect in different fields of Science and Technology. They are widely used in research work, medicine, displays, Radiation-sensors, Thermometers, nondestructive testers etc.
- Displays: These are more desirable for displays than other materials are lower power consumption and the clarity of display in the presence of bright light. The power requirements are so low for digital display and the mechanism which runs the watch. The two mades most widely used in liquid crystal displays are dynamic-scattering and field-effect.
- Radiation and sensors: Cholesteric liquid crystals have been used in versatile and inexpensive radiation sensors, where an impinging invisible radiation is registered as a colour change by local heating and the change of structure. These devices can be used with UV, infrared, microwave, ultrasonic, ionizing radiation transducers etc. where a practical absorber in contact with the liquid crystals exists. Cholesteric liquid-crystal films have been used as recording media in holograms.
- Thermometers: The temperature-dependent variation in the colour of cholesteric liquid crystals has led to the use of these substances in the measurement of temperature and gradients of temperature. A cholesteric substance or a mixture of cholesteric substances always exhibits the same colour at the same temperature, the colour is very sensitive to change in the ambient temperature.
Cholesteric liquid-crystal substances, when applied to the surface of the skin, have been used to locate veins, arteries, infections, tumors and the fetal placenta which are warmer than the surrounding tissue.
- Research field: Nematic liquid crystals are most useful research tools in the applications magnetic resonance. Molecules that are dissolved in nematic liquid-crystals due to anisotropic environment they give a very highly reduced NMR spectrum. Analysis of the spectra of molecules in liquid crystal solvents yields information regarding the anisotropy of chemical shifts, chemical structure, bond angles, bond lengths, direct magnetic dipole-dipole interactions, indirect spin-spin couplings, molecular order, and relaxation processes. Some liquid crystals have been used in chromatographic separations, as solvents to direct the course of chemical reactions and to study molecular rearrangements and kinetics and as anisotropic host fluids for visible, UV&IR spectrosper of molecules.
- Nondestructive testing: Cholesteric-nematic liquid crystals are colour sensitive with temperature this property can be used for nondestructive testing.
- Biological systems and medicine: The liquid crystals are used in medicine, used as an optical discs, full colour “electronic slides” light modulators etc. Most of the biological systems exhibit the properties of liquid crystals. Considerable concentrations of mesomorphic compounds have been found in many parts of the body, often as sterd or lipid derivatives. A liquid crystal phase has been implicated in degenerative diseases Ex: atherosclerosis, sickle-cell anemia etc. Living tissues like muscle, tender, ovary, adrenal cortex, nerve etc. Show the optical birefriurgenee properties that are characteristic of liquid crystals.
6.9 non conventional energy sources and storage devices
Nuclear energy has gained importance and has become essential as an alternative source of energy with the realisation that the supply of fossil fuels is depleting fast and very limited. Due to rapid increase in industry and urbanization, together with booming increase in population, it is estimated that the present fossil fuels (coal and petroleum) will not last more than a few decades to supply the demand of electric power. So attention has been focused mainly on nuclear energy, which seems to offer an infinite source of energy.
This field began with the discovery of radioactivity. The release of a tremendous amount of energy through nuclear reaction became possible with the development of artificial radioactivity.
6.9.1 Nuclear Energy
Nuclear energy is the energy released when nuclei of certain atoms undergo induced reactions such as fission and fusion. The materials that make such energy available are called nuclear fuel. Nuclear fuels release energy by entirely different mechanism as compared to chemical reactions. In the nuclear fuel, different elements are produced, and some of the binding energy of the nucleus is released, during bombardment of neutron on radioactive element such as Uranium–238, etc. Therefore, a large amount of energy is released in less than a millionth of a second. For example, complete fission of 1 kg of uranium provides the energy equivalent of about 2 × 107 kWh; such amount of energy can be obtained by burning of about 3,000 tonnes of high grade coal.
6.9.2 Mass Defects and Nuclear Binding Energy
The mass of an atomic nucleus has been found to be always less than the sum of the masses of the constituent nucleons (i.e., protons, neutrons, and electrons). This difference is called mass defect. The mass thus lost appears in the form of energy in accordance with Einstein‚s mass-energy relationship; the energy emitted is called the binding energy of the nucleus.
Consider an isotope having atomic number ‘Z‚ and mass number ‘A‚. Evidently, its atom contains protons = Z, electrons = Z, Neutrons = (A – Z).
Let mp, me and mn are, respectively, masses of proton, electron, and neutron.
Thus, calculated mass of this isotope,

![]()
Thus the mass defect (ΔM) is the loss of mass in the formation of nucleus from its constituents. This loss of mass represents the amount of energy, which would be released if an atom of mass number A were synthesized from its constituents. This form of energy is according to Einstein‚s mass-energy relationship.
Thus the binding energy of the nucleus may thus be defined as the energy released during the formation of a nucleus from its constituents nucleons.
Thus, if ΔM is the mass defect,
then binding energy B.E. = ΔMC2, ![]()
![]()
Binding energy per nucleon is calculated by dividing the binding energy of a nucleus by the number of nucleons.
The binding energy corresponding to one atomic mass defect is 931.5 MeV (Million electron Volts)
![]()
The greater is B.E./nucleon, the greater is the stability of the nucleus.
The energy equivalent to one atomic mass unit (1 amu) is given by,

Example to Calculate the Binding Energy per Nucleon
The formation of a helium atom can be written as,
![]()
Change in mass is given by,

Since the energy equivalent to 1 amu of mass is 931.5 MeV, the energy change during the formation ![]() of atom is,
of atom is,

Further, since there are four nucleons in ![]() nucleus, the binding energy per nucleon (EB/A) is obtained by dividing the total binding energy with the number of nucleons, that is,
nucleus, the binding energy per nucleon (EB/A) is obtained by dividing the total binding energy with the number of nucleons, that is,
![]()
This binding energy per nucleon is a direct measure of the stability of the nucleus.
Binding Energies and Stability of Nuclei
Mean binding energies, that is, the binding energies per nucleon, drastically increase with increasing atomic mass in lighter elements, reach a maximum at mass numbers 55–58, and gradually decrease in the case of heavier elements. The mass number 55–58 correspond to iron and nickel, the most stable elements, and this stability is also reflected in the abundant presence of these elements in the inner core of the earth.
From Figure 6.23, it is seen that the maximum occurs at about mass number 56 (iron). Thus nucleus of iron is thermodynamically most stable. It is also seen that the points for helium (mass number = 4), carbon (mass number = 12), and oxygen (mass number = 16) lie quite high in the graph. This shows that the nuclei of these elements are exceptionally stable. The maximum binding energy is seen to be about 8.7 MeV. This is the energy required to remove a proton or a neutron from the most stable nucleus.

Figure 6.23 Mean binding energies of various nuclei
6.9.3 Nuclear Reactions
Nuclear reactions involve the changes in the number of nucleons present in the nucleus. Hence there is a formation of new atomic species. Thus, nuclear reactions lead to atomic transformations. In addition to that, a huge amount of energy is released in nuclear reaction, involving a small but measurable loss in mass. This mass is transformed into energy in accordance with Einstein‚s equation (E = mc2).
Rutherford carried out the first nuclear reaction by bombarding nitrogen (target nucleus) with α–particles (projectile) to produce an oxygen isotope.

Types of Nuclear Reactions
- Nuclear fission:
E.g.:

- Nuclear fusion:
E.g.:

Nuclear Fission
Very high nuclei have a lower binding energy per nucleon than the nuclei with intermediate mass. Thus, the former are less stable than the latter. Otto Hahn and F. Strassmann observed during 1934–1938 that when 235U is bombarded with slow-moving thermal neutrons (energy = 0.025 eV), it undergoes fission giving barium (z = 56) as one of the products of fission. In order to explain this observation, L. Meither and O.R. Frisch suggested that after the capture of a neutron, the uranium nucleus gets excited and then splits into two fragments of approximately equal mass, which is known as nuclear fission.
There is invariably mass defect during fission, that is, the total mass of products of fission is less than the total mass of neutron and the 235U atom. The loss of mass appears in the form of energy according to Einstein‚s mass-energy relation, E = mc2.
The fission reaction is represented as follows:
![]()
The splitting of a heavier atom like that of Uranium-235 into a number of fragments of much smaller mass by suitable bombardment with sub-atomic particles with liberation of huge amount of energy is called “nuclear fission”.
In this reaction, in addition to the release of enormous amount of energy, the most significant feature of nuclear fission is that more neutrons are produced than those consumed in the reaction. This process is schematically represented in Figure 6.24.

Figure 6.24 A schematic diagram representing the uranium fission process
Mechanism of Nuclear Fission – The Liquid Drop Model
In the fission process, a heavy nucleus splits apart into nearly two equal fragments of more stable nuclei of intermediate mass. This fission reaction that occurs due to absorption of neutron may be treated as analogous to the behaviour of a liquid drop as it contracts, elongates, and eventually splits apart into two droplets as shown in Figure 6.25. According to this liquid-drop model, the absorption of neutron by a nucleus causes the nucleus to oscillate like a liquid drop and then split‚s it into two small, stable nuclei.

Figure 6.25 Schematic diagram representing the fission process by the Bohr-Wheeler liquid drop model for heavy nuclei
There are 14 isotopes of uranium, and their mass number ranges from A = 227 to A = 240. The most important isotopes are 235U and 238U whose natural relative abundance is 0.72% and 99.28%, respectively. Both 235U and 238U undergo fission upon absorbing a neutron. 238U undergoes fission with ‘fast‚ neutrons while 235U with both ‘fast‚ and ‘slow‚ neutrons.
Among naturally occurring nuclides, only 235U undergoes fission, but 238U and 232Th are converted into 239Pu and 233U by neutron capture and two successive β–decay. These two converted nuclides then undergo fission. Both 235U and 233U can be excited to fissionable state by slow neutrons more easily than 238U as both are less stable.
The division of 235U occurs in many different ways, and nearly 34 elements were identified in the fission products. Enormous amount of energy is also liberated. In any single reaction, two particular nuclides are produced along with two or three secondary neutrons. These neutrons have about 200 MeV of kinetic energy. The daughter nuclei produced in fission reactions have different Z and A values.
Some of many pathways through which 235U undergoes fission with fast neutrons are mentioned below:

Since emission of more than one neutron occurs in the fission process, there is a possibility of a chain reaction in which the releases of energy increases in geometric fashion. The neutrons released in one fission reaction cause a second fission reaction and so on, and a chain reaction continues, which, if goes unchecked, would quickly lead to the release of enormous amount of energy. A chain reaction is possible only when the amount of fissionable material exceeds its critical mass (Figure 6.26). The critical mass is defined as the amount of fissionable material, which is just large enough to recapture one neutron, on an average, for every fission reaction. If the amount of fissionable material is less than the critical mass, less than one neutron is recaptured; the rate of fission events does not grow, and the rate of energy release is low. In addition, if the amount of fissionable matter is more than the critical mass, the number of fission events increases, and a chain reaction is set up, which quickly grows into explosive proportions.

Figure 6.26 Self-propagating nuclear chain reactions
Release of Fission Energy
On this basis, nuclear fission reactions may be categorized into two types:
- Uncontrolled nuclear fission used for construction of nuclear weapons, the atomic bombs.
- Controlled nuclear fission, which is exploited for the controlled generation of energy in nuclear reactors.
- Uncontrolled nuclear fission: The atom bomb: In a fission reaction, two or three neutrons are produced. These neutrons can collide with other fissionable atoms to sustain and multiply the fission reactions. If the amount of fissionable material exceeds the critical mass, an uncontrolled explosive chain reaction may result. An enormous amount of energy is produced in the chain reaction. A nuclear bomb is a frightening example of the enormous amount of energy released by nuclear fission, which is not controlled.
The central feature of a fission explosion is a growing chain of fission reactions. For this, there are three requirements as given below:
- The fissionable nuclide must be concentrated enough so that it becomes critical.
- Sub-critical portions of this fissionable nuclide must be combined into critical mass.
- The critical mass must be held together for a long period so that the chain multiplies to immense size.
The potential of uncontrolled nuclear fission was first realized in the atomic bomb (Figure 6.27). It contains two sub-critical portions of fissionable materials.

Figure 6.27 A simple design of atomic bomb
One portion is propelled into another to form supercritical mass by carefully designed detonation of an ordinary chemical explosive such as trinitrotoluene (TNT). The fission chain multiplies, and then a nuclear fission explosion occurs. Tremendous amount of heat energy and many other radio nuclides are also released whose effects are disastrous to life and environment. The radioactive dust and debris are called fall-out.
In 1945, the United States started the nuclear age by dropping two nuclear bombs on Hiroshima and Nagasaki, Japan. Both these bombs were fission weapons of tremendous power. The bomb, which was dropped on Hiroshima, contained 235U (Uranium–235) while the Nagasaki bomb has 239Pu (Plutonium–239) as fissionable materials.
- Controlled nuclear fission: Nuclear reactor: For controlled release of fission energy, the chain reaction is carried out in a device called a nuclear reactor. The fission is controlled in such a manner that on an average, only one neutron is left from each fission; to excite further fission, the large amount of energy released in nuclear fission can be used to generate electrical power. This requires a delicate balance between neutron generation and neutron loss, and this is achieved by proper use of moderator and control rods in the nuclear reactors.
Two types of nuclear reactors are in common use. They are:
- Thermal reactors: Most thermal reactors in the United States are ‘light water reactors‚ in which ordinary water is used as moderator to slow the neutrons. However, heavy water reactors had been developed in Canada in which heavy water, D2O is used as moderator in place of ordinary water.x
Light water reactor (LWR), which is a type of thermal reactor, used normal water as its coolant as well as neutron moderator.
There are three varieties of light water reactors:
- Pressurized water reactor (PWR)
- Boiling water reactor (BWR)
- Supercritical water reactor (SCWR)
- Reactor design: The light water reactor produces heat by controlled nuclear fission. The nuclear reactor core is the major portion of a nuclear reactor where the nuclear reactions take place (Figure 6.28).

Figure 6.28 Light water nuclear reactor
It consists essentially of the following parts:
- Fuel: The fissionable material used in the reactor is called fuel. The fuel used is enriched Uranium–235 (in the form of U3O8). This is obtained from the naturally occurring U–235. The solid fuel is made into the form of rods or pellets, shielding by placing them in stainless steel tubes.
- Moderator: The most efficient fission reactions occur with slow neutrons. Thus, moderators, which are atoms of comparable mass, slow down the fast neutrons produced in fission and do not absorb them. Light water reactor uses ordinary water, also called light water, as its neutron moderator (Figure 6.28).
- Control rods: To control the fission process, rods made of cadmium or boron are suspended between the fuel rods. These rods can be raised or lowered and control the fission process by absorbing neutrons. That is why they are called control rods.
Cadmium and boron are good neutron absorbers.

- Cooling system: The light water reactor uses ordinary water to keep the reactor cooled. The light water is circulated in the reactor core to absorb the heat, which is generated due to fission reaction. This transfers the heat to a steam generator, which converts water into steam, and this steam is then taken to turbines, which drive generators to produce electricity.
- Shielding: To prevent the losses of heat and protect the persons operating the reactor from radiation and heat, the entire reactor core is enclosed in a steel containment vessel. This vessel is housed in a thick-walled concrete building. The operating people are protected by a thick layer of organic material made of compressed wood fibres, which absorb the neutrons, β particles, and γ–rays.
- Breeder reactors: It may be mentioned that at the rate at which U–235 is being used to produce power, the stocks are likely to exhaust very soon. Therefore, scientists have been actively engaged in investigating other fissionable materials. They have found plutonium–239 (Pu–239) and Uranium–233 (U–233) to be quite suitable. These are produced by bombardment of more abundantly available U-238 and Th-232 with neutrons.
A breeder reactor is a nuclear reactor that ‘breeds‚ fuel. It consumes fissile and fertile material at the same time as it creates new fissile material. In this reactor, neutrons produced from fission of U–235 are partly used up for carrying on fission of U–235 and partly to produce Pu–239 or U–233. Such reactors produce more fissionable materials (as Pu-239 or U-233) than they consume (as U-235).
The sequence of reactions that take place when U–238 and Th–232 are bombarded with fast neutron producing the fissionable nuclei of Pu–239 and U–233 are represented as follows:
- Fissionable Pu–239 by neutron bombardment followed by two successive β–decays.

- Fissionable U–233 by neutron bombardment on Th–232 by two successive β–decays.

The neutrons produced by fission reactions are absorbed in a blanket of uranium or thorium. Since higher temperature is required to operate, water cannot be used as a coolant. Liquid sodium is used as a coolant. This type of reactor uses fast neutrons; therefore, no moderator is required.
The nuclides such as U–238 and Th–232, which can be converted into fissionable nuclides, are called fertile nuclides, whereas nuclides such as U–235 and Pu–239, which are fissionable and are called fissile nuclides.
- Fissionable Pu–239 by neutron bombardment followed by two successive β–decays.
- Thermal reactors: Most thermal reactors in the United States are ‘light water reactors‚ in which ordinary water is used as moderator to slow the neutrons. However, heavy water reactors had been developed in Canada in which heavy water, D2O is used as moderator in place of ordinary water.x
Nuclear Fusion
Nuclear fusion may be defined as a process of combination of two lighter nuclides to form a heavier nuclide with the release of energy.
The following reactions have been successfully investigated as fusion reactions:

These processes are generally known as thermonuclear reactions, because they require that the colliding nuclei must possess very high kinetic energies before they are initiated. The high kinetic energies of the reacting nuclei overcome the coulombic repulsion between positive particles of reactants. This is only possible of extremely high temperature of millions of degrees (≈ 4 × 106 °C), so these processes generally occur in the sun and other stars because of tremendous high temperatures that are nearly impossible to achieve and contain on earth. It is, therefore, believed that in the sun, the following process takes place:
![]()
There is evolution of 26 MeV of energy, and this energy is available to us from the sun and keeps the sun at extremely high temperature.
The fission reactions take place only with a few rare and extremely heavy nuclides. On the other hand, fusion reactions are possible for light nuclides such as 1H, which are abundant. Furthermore, fusion reactions release more energy per unit mass than fission reactions. For example, fission of U–235 yields 7.9 × 107 KJ/g energy while fusion of two isotopes such as hydrogen, deuterium, and tritium releases 3.4 × 108 KJ/g energy. Another advantage of fusion reaction is that the fusion reaction produces radio nuclides of very short half lives, so there would be no long-term waste disposal problem.
There are several ways to study fusion reactions. There are:
- By using particle accelerators.
- Through stellar nuclear reactions, the process occurs in the sun and other stars.
- By fusion bombs, which use a fission bomb to produce a temperature high enough for fusion.
Fusion Bombs (Uncontrolled Nuclear Fusion Reactions)
A hydrogen bomb uses nuclear fusion. A conventional explosive first triggers a fission bomb, which then induces the fusion reaction. These bombs are more powerful than fission bombs because they can incorporate large masses of nuclear fuel to produce unlimited energy.
A hydrogen bomb has an arrangement of nuclear fission in the centre, which is surrounded by a mixture of deuterium (![]() ) and lithium 6 isotope (
) and lithium 6 isotope (![]() ).
).
The nuclear fission provides heat and neutrons. The neutrons are used up for converting lithium isotope into tritium (![]() ), and the heat liberated is required for the fusion between
), and the heat liberated is required for the fusion between ![]() to start. The fusion reactions are then accompanied by the liberation of a large amount of energy.
to start. The fusion reactions are then accompanied by the liberation of a large amount of energy.
Thus the reactions taking place in a hydrogen bomb may be represented as follows:
Fission (in the centre) → Heat + Neutrons

All these processes occur in an extremely short time to release an immense amount of energy, and the bomb blasts.
Controlled Nuclear Fusion
The major problem in a nuclear fusion reaction is the attainment of high temperature required for the purpose. So far, attempts to maintain such a high temperature to generate energy on a large scale have been successful for only a very small fraction of a second, because all known containers would vapourize at such a high temperature. At this temperature, the atoms become fully ionized, and the ions (i.e., nuclei and electrons) form the plasma state in which the particles move about independently but in the form of inter penetrating gases. The plasma is electrically neutral.
Lastly, it can be mentioned that the attempts to make fusion reactors have not met with any success so far because of many technical difficulties involved, for example, that of the container material, which can withstand very high temperatures as required for the fusion to start. However, if a solution is found, it will place at our disposal a tremendous source of energy at a very cheap rate because deuterium is present in huge amount in sea water (in the form of D2O).
Differences between nuclear fission and nuclear fusion are listed in Table 6.1.

6.9.4 Solar Energy
The sun is a source of enormous energy. Solar energy originates with thermo-nuclear fusion in the sun involving the fusion of hydrogen into helium either through the proton-proton chain or through the carbon-nitrogen cycle. Earth is receiving the solar energy as a radiant energy which reaches the top of the atmosphere at 1366 watts per square meter. This energy ranges from ultraviolet, visible and infrared light. About half of this energy actually makes it to earth‚s surface, 30 per cent is reflected and 20 per cent is absorbed by the atmosphere. Thus, when the sun is directly overhead full sunlight can deliver about 700 watt per square meter, at that rate sun can deliver 700 MW of power to an area of 390 square miles.
The total amount of solar energy reaching earth is very vast and almost beyond belief. For an example, one year expenditure of fossil fuel in the United States is equivalent to just 40 min of sunlight striking the particular land surface of the United States.
Solar energy technology broadly comprises thermal conversion and photoconversion. Thermal conversion takes place through direct heating, ocean waves and currents, and wind. Photo conversion includes photosynthesis, photochemistry, photo electrochemistry, photo galvanism and photovoltaics. Solar radiation is collected and converted by natural collectors such as the atmosphere, the ocean and plant life, as well as by man-made collectors of many kinds (Figure 6.29). There are a number of solar technologies by which it can be harnessed (Figure 6.30).

Figure 6.29 Natural and man-made collectors

Figure 6.30 Solar technologies
The earliest use of direct solar energy by mankind was – drying the body or warming it in the sun during winters. Indeed, drying of clothes, fodder, timber, agricultural and animal products, salt water (to get salt), and passive space heating-remained the most extensive form of use of direct solar energy in the history of mankind. All other active solar technologies/devices for harnessing direct solar energy have fairly recent origins (Figure 6.30). A variety of active solar collectors provide a broad range of applications, namely:
- solar heating of water
- solar space heating of buildings
- solar air-conditioning
- solar refrigeration
- solar drying
- solar cooking
- solar green-houses
- solar furnaces
- solar desalination
- salt production
- solar electricity – thermal
- solar electricity – photovoltaic.
Solar Heating of Water
Solar water heating is very popular in warm, sunny climates. Flat-plate collectors for heating water consists of a thin broad box with a glass or plastic top and a black bottom in which water tubes are embedded as shown in Figure 6.31. These collectors are faced towards the sun and the black bottom gets hot when it absorbs sunlight. Thus water circulating tubes are heated and are conveyed to a tank where it is stored.

Figure 6.31 The principle of a flat-plate solar collector
Solar heating system may be ‘Active‚ or ‘Passive‚. The heated water is moved by means of pumps in an active system. But in a passive system, the collector is lower than the tank. By natural convection heated water rises into the tank from collector and cool water descends into the collector from tank. This is shown in Figure 6.32. The tank will usually have a source of auxiliary heat (electric or gas) in order to get the temperature to a desired level or to provide heat when solar energy is insufficient.

Figure 6.32 Solar water heaters. In nonfreezing climates, simple water-convection systems may suffice. In freezing climates, an antifreeze fluid is circulated. Solar heat is augmented by an auxiliary heat source in the hot-water tank
Solar Space Heating of Buildings
Flat-plate collectors such as those used in water heating with same concept and less expensive can be used for space heating. Because here only air circulation through the collector box is necessary.
The efficiency of solar space heating can be enhanced by proper designing of a building to act as its own collector. Here, the basic principle is to have windows facing the sun. In cool days it depends on sun‚s angle of incidence, and sunlight can heat the interior of the building; however, at nights, insulated drapes or shades can be pulled down to trap the heat inside. Excessive heat in hot days can be avoided by using an awning or overhang to shield the window from the sun.
Solar Air-conditioning
Solar Air-conditioning includes solar-powered refrigeration system of ranking cycle system, absorption refrigerator systems and solar-regenerated desiccant cooling systems. In such type of system, ambient air is adiabatically cooled, dehumidified, cooled both sensibly and evaporatively, and then ducted to the living area. In the regenerative stage, air is evaporatively cooled, heated as it cools the supply air stream, heated again by solar collectors, and humidified.
Solar Refrigeration
Solar refrigeration is closely related to air-conditioning. It is generally required for food preservation or for storage of medical and biological materials. Although, most of the units/machines fabricated are simple in design; but are generally too complicated to operate and therefore are not usable by the people.
Solar Drying
Solar drying of agriculture and animal products is the most ancient, traditional and wide spread method of utilizing direct solar energy.
Agricultural products like grain, hay, copra, fruits, nuts and vegetables are still dried with sun drying all over the world, including highly industrialized and well-developed countries. Because it is cheapest and simplest way to dry crops and also abundant availability of sunlight is ensured.
Solar Cooking
Solar cooker is a perfectly insulated shallow rectangular metal box, and inner side of this box is blackened and fitted with a flat glass cover. When this cooker is placed in sunlight, the solar energy penetrates the glass cover and is absorbed by the inner blackened surface. Thus, temperature will increase inside the box, and the food gets cooked quickly.
The collector area of such a solar cooker can be increased by providing a plane reflector mirror of size equal to the size of the box and is hinged on one side of the glass frame. With the help of reflector mirror, a temperature rise of 15 to 20 °C can be achieved inside the solar cooker.
Solar Greenhouses
A greenhouse is a closed structure covered with transparent material (glass or plastic) which acts as a solar collector and utilizes solar radiant energy for the growth of plants.
The incoming short-wave solar radiations can pass through the green house glazing; but the long wave thermal radiations emitted by the objects within the greenhouse cannot escape through the glazed surface. As a result, the radiations get trapped within the greenhouse and result in an increase in temperature. Further, the air inside the greenhouse gets enriched with carbon dioxide and the moisture loss is reduced due to restricted transpiration. All these factors help the plant growth to sustain during night and colder months.
Solar Furnaces
Extremely high temperatures with very clean condition around 3500°C can provide solar furnaces. This can be used to melt refractory material.
In a solar furnace, high temperature is obtained by concentrating the solar radiations on to a specimen using a number of heliostats (turnable mirrors) arranged on a sloping surface. The biggest advantage of a solar furnace is that heating can be accomplished without any contamination and temperature can be easily controlled by changing the position of the material in focus. It is anticipated that in future, solar furnaces can be utilized in the production of nitric acid and fertilisers from air.
Solar Desalination
In this method, solar radiation is admitted through a transparent air tight cover of sloping sheets of glass into a shallow blackened pool containing brine. Solar radiations passes through the cover and is absorbed and converted into heat causing the water to evaporate from the brine. The vapours produced get condensed to form purified water on the cooler glass cover, flow down the sloping roof, collected in the troughs and from there into a water storage tank (as shown in Figure 6.33). The excess brine that has not evaporated is run to waste.
The per liter distilled water cost obtained by this process is cheaper than distilled water obtained by other electrical energy-based processes.

Figure 6.33 Basin type solar-still
Salt Production
It is most widely used method for salt production today in many developing countries of the world. The basic concept is that – in areas where evaporation exceeds rainfall, a shallow pool of brine is exposed which results in evaporation of water, leaving behind the salt.
Solar Electricity – Thermal
Solar energy may be used to heat a fluid, which then generates electricity through a conventional heat engine. To obtain an adequate working temperature, some form of concentration of solar energy is required, so that for most designs there is little contribution from diffuse sunlight.
Broadly the systems fall into two categories:
- Systems in which individual mirrors track the sun continuously; and
- Systems in which the mirrors are fixed
In continuous tracking systems, large number of plane or curved mirrors is used. Each mirror steered to reflect sunlight onto a single tower mounted boiler and gives a high temperature with high efficiency. But mirrors require complex, rugged and accurate mechanism.
Non-tracking system consists of assemblies of trough-shaped collector, aligned east to west. Above the each collector, absorber is fitted in the form of tube. A general plan of a solar-thermal electric power plant is shown in Figure 6.34. The hopes are that cost effective and utility scale solar-thermal power plants seem likely to be built in the near future.

Figure 6.34 General plan of a solar-thermal electric power plant
Solar Electricity – Photovoltaic
A solar cell-more properly called a photovoltaic, or PV, cell. It convert directly incident solar radiation to electrical current. PV cell looks like a simple wafer of material with one wire attached to the top and one to the bottom. As sunlight shines on the wafer, it puts out an amount of electric current roughly equivalent to that emitted by a flashlight battery. Thus, PV cells collect light and convert it to electric power in one step. However, several cells can be connected together to obtain large amount of power.
Working Principle
The simple appearance of PV cells belies a highly sophisticated level of science and technology. Each cell consists of two very thin layers of semiconductor materials separated by a junction layer. The lower layer has atoms with single electrons in their outer orbital that are easily lost. The upper layer has atoms lacking electrons in their outer orbital; these atoms readily gain electrons. The kinetic energy of light photons striking the two-layer “Sandwich” dislodges electrons from the lower layer, creating a current that can flow through a motor or some other electrical device back to the upper side.
The major material used in PV cells is silicon. PV cells consists of a single crystal of p–type silicon with a surface layer of n–type silicon. When light falls on junction, electrons and holes move in opposite directions across the p-n junction and electric current will flow if an external circuit is connected. This is shown in Figure 6.35.

Figure 6.35 Semiconductors functioning as solar cells
Cost
The cost of PV power (cents per kilowatt-hour) is the cost of the PV cells divided by the total amount of power they may be expected to produce over their lifetime currently around 25 cents per kilowatt-hour while the cost of power from other power alternative is 6–12 cents per kilowatt-hour for residential electricity. PV power had its first significant application in the 1950s, in the solar panels of space satellites. Cost comes down dramatically if more efficient cells with less expensive production techniques are evolved.
Uses
- PV cells are widely used in watches, calculators, toys, etc.
- Panels of PV cells provide power for traffic signals, radio transmitters, light houses, irrigation pumps, earth orbiting satellites, etc.
The Future of Solar Energy
Solar electricity is growing at a phenomenal rate – the solar PV business is a $ 20 billion industry, and it is growing at a rate of 40% a year. The sun provides power only during the day, but 70% of electrical demand occurs during day time hours, when industries, offices, and stores are in operation. Thus, considerable savings still can be achieved by using solar panels just for daytime needs and continuing to rely on conventional sources at night.
6.10 green house concepts
A green house is also known as a glasshouse.
In the green house, plants are grown in a building or other complex. Building of the green house is made up either glass or plastic material. In the green house, incoming UV light is absorbed inside the structure and temperature of air inside increases and retained in the building by the roof and wall, and air is warmed near the ground and flowing within the complex.
6.10.1 Types of a Green House
A green house is divided into two types as listed hereunder.
- Glass green house
- Plastic green house
In both types of green house, plastics used are polyethylene, polycarbonate or PMMA glass.
Uses of Glass House
In a green house, temperature, level of light and shade are maintained.
- In the green house, growth of plants is controlled by controlling temperature, cooling and lighting of house.
- It is also used to improve the qualities of a piece of land and also improve food production by providing good environment.
- Green house is also used for growing flowers, vegetables, fruits and transplantation of specific plants.
- In addition to all of these, the green house is also used to produce solar fields which produce steam for solar-enhanced oil.
6.10.2 Mechanism of Green House Effect
In green house effect, UV radiations from sun of short wavelength are absorbed through a transparent medium, but IR radiations of longer wavelength are unable to pass back from that medium. As a result, inside temperature increases. Such effect is called the green house effect. This effect is shown due to absorption of excess heat by carbon dioxide present in it.
Example of Green House
- Bright sun light warms a car on a cold, clear day by the green house effect.
- Global warming.
- Increasing atmospheric carbon dioxide level.
6.10.3 Requirements for Green House
(a) Green House Carbon Dioxide
The presence of carbon dioxide in green house is responsible for absorption of radiations by it. Carbon dioxide in green house is also responsible for enhanced plant growth.
(b) Green House Heating
Heating is most important in colder climates. For good heating, the heat lost by solid opaque wall is prevented by providing greenhouse design.
(c) Green House Ventilation
For a good greenhouse, and for a good plant, proper ventilation is an important factor. The main aims of ventilation are to regulate the temperature and humidity in a green house. This also ensures a fresh air supply for photosynthesis and plant respiration. Ventilation in a green house is controlled either automatically via a computer or recirculation fans.
(d) Green Synthesis
Green chemistry is also called sustainable chemistry. Synthesis of any product based on the fact that use of minimizing the hazards and maximizing the efficiency of chemical chosen for the synthesis.
Green synthesis is identified as development in the fields of:
- Use of supercritical carbon dioxide as a solvent called green solvent
- Oxidant used as an aqueous hydrogen peroxide
- Use of hydrogen in asymmetric synthesis
Green synthesis methods have a good impact on the environment.
In such type of processes, there is less exposure to pollutants, reduce waste and consume less energy.
In research, green synthesis methods include the use of microwave reactors to minimize energy needs and use of microfluidic reactors to minimize solvent waste.
Green chemistry is also called sustainable chemistry in which design of chemical products and processes that reduce the substance which are hazardous to humans, animals, plants and the environment.
6.11 Green Chemistry
In 1990 the pollution prevention act in the United States, initiated to create a modus operandi for dealing with pollution in an innovative and sustainable way and paved the way to green chemistry concept.
Green chemistry or sustainable chemistry or environmentally benign chemistry is the frontiers of science, with the utilization of set of principles that attempts to reduce or eliminates the use or generation of hazardous substances in the design and manufacture of environmentally and economically sustainable products. It can help to solve large global problems such as climate change, energy consumption of effective utilization of natural resources especially renewable resources.
6.11.1 12 Principles of Green Chemistry
Paed Anatas and John Warmer of the U.S. Environmental Protection Agency coined the two-letter word “green chemistry” and formulated 12 principles of green chemistry. They are listed hereunder.
- Waste prevention instead of remediation: Waste prevention is better than treat or clean up after it is formed.
- Atom economy (or) efficiency: Safer synthetic methods should be designed to give a good yield of final product.
- Use of less hazardous and toxic chemical synthesis: Synthetic methodologies should be designed to use and generate environmentally benign, which possess little or no toxicity.
- Designing of safer chemicals/products: Chemical products should be designed to preserve efficacy of function while reducing toxicity.
- Innocuous solvents and auxiliaries: The use of auxiliary substances like solvent separating agents should be made unnecessary wherever possible and innocuous when used.
- Design for energy efficiency: Energy requirements of chemical processes should be recognized for their environmental and economic impacts and should be minimized. Preferably, synthetic methods should be conducted at ambient temperature and pressure.
- Use of renewable feedstocks or raw material: A raw material or feedstock should be renewable rather than depleting environment whenever technically and economically possible.
- Reduce derivatives (or) shorten synthetic routes: Unnecessary derivatigation like use of blocking groups protection/deproduction, temporary modification of physical/chemical processes should be minimized or avoided. Because such steps require additional reagents and can also generate waste.
- Catalysis rather than stoichiometric reagents: Catalytic reagents are superior to stoichiometric reagents.
- Design for degradation: Chemical products should be designed to degrade, so that at the end of their function break down into innocuous degradation products and do not remain in the environment.
- Real-time analysis for pollution prevention: Analytical methodologies required to be further developed to allow for real-time, in-process monitoring and control prior to pollution prevention.
- Inherently safer processes for accident prevention: Substances derivatives and products in a chemical process should be chosen to minimize the potential for chemical accidents, including releases, explosions and fires.
6.11.2 Importance of Green Synthesis
Green chemistry addresses many challenges by opening a wide and multifaceted research scope and allowing the invention of novel reacting chemical that can maximize the desired products and minimize the waste products, as well as the design of new synthetic schemes that are inherently, environmentally and ecologically benign.
- This prevents pollution at the molecular level by atom economy.
- It gives innovative scientific solutions to real-world environmental problems.
- It provides alternative synthetic routes for feedstock and starting material.
- Biocatalysis and bioleaching are prominent applications in green chemistry.
- Utilization of carbon dioxide as a green solvent.
- Biosorption is one of the important phenomena based on 12 principles of green chemistry. In this, number of agricultural materials like wool, palm kernel hunk, apple residues, banana hunk and sawdust are using to remove toxic metals from waste water.
- Effective utilization of renewable resources as alternate energy sources such as solar energy, wind energy, hydro energy, etc.
6.11.3 Methods for Green Synthesis
Over the past decades, green chemistry has convincingly demonstrated how fundamental scientific methodologies can be devised and applied to protect human health and the environment in ecofriendly and economically beneficial manner.
- Alternative synthetic route for feedstocks and starting materials
Ex: Production of dimethyl carbonate (DMC)
Dimethyl carbonate is a versatile and environmentally friendly material for chemical industry. Due to high oxygen content and blending properties, it is used as a good component of fuel.
Traditional Method: In this method, phosgene (COCl2) and methanol (CH3OH) are used to produce DMC.

Here, phosgene (reactant) and Hydrochloride acid (by-product) are environmentally harmful.
Greener Method (Alternative Method): This method makes use of copper chloride, methanol, oxygen and carbon monoxide.

Here copper chloride further comes as a by-product and usage of CO in this method is cheap and indirectly decreases the pollution.
- Biocatalysis or bioleaching: Bioleaching is the extraction of specific metals from their ores by using microorganisms such as bacteria.
Ex: Extraction of gold
Traditional method: Heap leaching method by using cyanide is the traditional method in the extraction of gold. Here, cyanide is hazardous to health and environment.
Greener Method: In this method, Acidithiobacillus ferrooxidans and acidithiobacillus thiooxidans bacteria are used to oxidize ferrous and sulphur. The gold will be easily separated from the ore and solution. This method is much cleaner than the traditional heap leaching method.
- Catalysis: Catalytic methods are superior than stoichiometric methods.
Ex: Synthesis of adipic acid
Adipic acid is a monomer for nylon and starting material for cathode.
Traditional Method: In the past, for the production of adipic acid benzene is used as a starting material. However, it is highly carcinogenic and causes leukaemia. Afterwards, the starting material became cyclohexanone or a mixture of cyclohexanone and cyclohexanol. In oxidation with nitric acid, it produces toxic fumes of nitric oxides, which are contributors to the greenhouse effect, acid rains and the destruction of ozone layer.
Greener Method: In this method cyclohexane is oxidized by 30 per cent of hydrogen peroxide in presence of catalyst. Catalyst is a salt of the metal wolfram and dissolved in an organic solvent such as aliquot 336.

Oxidation with H2O2 is very effective and environmentally benign. Scientists improved the reaction with other metal catalysts like tungsten and molybdenum. The process also promoted towards biocatalytic method by using genetically transgenic bacteria like Klebriella pneumonia, a non-toxic strain of E. coli. Dr. Karen M. Draths and Professor John W. Frost were awarded the ‘Presidential Green Chemistry challenge Award‚ in 1998 in the USA for this achievement.
6.11.4 Applications of Green Synthesis
Green synthesis has wide application is many fields. Few of them are listed hereunder.
- Preparation of antibacterial products which are alternative of traditional chlorine or tin containing antibacterial agents such as bandages, sutures, hospital gowns, acne medication, toothpastes, air filters, antiviral agents, etc.
- Used for cleaning clothes such as
- TAML catalyst activates hydrogen peroxide which inhibits dye transfer and good for washing machines that use less water.
- Total impact programme (TIP) as laundry formulation incorporates neutral pH, detergents, enzymes, surfactants, oxygen bleach and biodegradable softness.
- Dry cleaning with liquid carbon dioxide which is non-flammable, non-toxic and renewable substance.
- Use of sodium iminodisuccinate for cleaning clothes which is a biodegradable, environment friendly chelating agent.
- Used for cleaning water by using
- Chlorine disinfection which is toxic to aquatic life but important for preventing diseases.
- Polymer technology for manufacture of high molecular weight, water-soluble polymers in aqueous salt solution.
- Used for industrial cleaning by using simple green non-toxic, biodegradable surfactants, replacing traditional organic solvents.
- Use of carbon dioxide blowing agent for polystyrene foam production; polystyrene foam is used in packing and food transportation.
- The conversion of waste glycerine from biodiesel production to propylene glycol.
- Synthesis of nanoparticles of metals. The stabilization of small particles is done by using polymers, ligands, solid matrix and surfactants. The preparation of nanomaterials in green solvent such as water and other non-toxic solvents is very popular nowadays.
6.12 Review Questions
6.12.1 Fill in the Blanks
- _______ are good conductors.
[Ans.: metals]
- At absolute zero temperature solid semiconductors act as perfect _______.
[Ans.: insulators]
- _______ are important semiconductors.
[Ans.: Si and Ge]
- The materials which are used to prevent the loss of electricity through certain parts in an electrical systems are _______.
[Ans.: dielectrics]
- Semiconductors due to impurity defects is _______ semiconductors.
[Ans.: extrinsic]
- Addition of Group–15 elements to Group–14 elements gives _______ semiconductors.
[Ans.: n-Type]
- At absolute temperature _______ of metal becomes zero.
[Ans.: electrical resistance]
- The phenomena of metals become perfect conductors with zero resistivity at absolute temperature is known as _______.
[Ans.: super conductors]
- ________ is the material possessing adhesive and cohesive properties and capable of bonding material.
[Ans.: cement]
- Natural cement is made by calcination of a naturally occurring ________ line stone.
[Ans.: argillaceous]
- ________ was an English mason, invented the cement.
[Ans.: Aspdin Joseph]
- ________ cement is the oldest cement invented by Romans.
[Ans.: puzzolana]
- ________ cement resembled in color and hardness to Portland stone.
[Ans.: Portland]
- Formula of gypsum is________.
[Ans.: CaSO4 · 2H2O]
- ________ acts as a retarding agent for early setting of cement
[Ans.: Gypsum]
- The process of solidification of cement consists ________ and ________.
[Ans.: setting, hardening]
- Calcarious material is rich in ________.
[Ans.: lime]
- According to I. S. I specifications of cement the ratio
.png) shall not be less than ________.
shall not be less than ________.
[Ans.: 0.66]
- Plaster of Paris setting and hardening ________ can initiates as well as hasten the crystallization.
[Ans.: Alums or alkali sulphur]
- ___________ are ceramic materials that can withstand high temperatures.
[Ans.: Refractories]
- Al2O3 and SiO2 are ___________ refractories.
[Ans.: Acidic]
- ___________ are the examples of basic refractories.
[Ans.: CaO, MgO]
- Introducing of the lubricant to reduce frictional resistance between the moving or sliding surfaces is known as ________.
[Ans.: Lubrication]
- Fluid film lubrication mechanism is also known as ________.
[Ans.: Thick-film or Hydrodynamic]
- ________ oils are considered to be satisfactory lubricants for fluid film lubrication.
[Ans.: Hydrocarbon]
- Improving of properties of petroleum by incorporating specific additives are called ________.
[Ans.: Blended oils]
- Calcium based greases are emulsions of petroleum oils with ________.
[Ans.: Calcium soaps]
- The most usual solid lubricants are ________.
[Ans.: Graphite and molybdenum disulphide]
- When graphite is dispersed in oil is called ________.
[Ans.: Oil dag]
- ________ is the property of a liquid or fluid by virtue of which it offers resistance to its over flow.
[Ans.: Viscosity]
- Primary explosives also known as ________.
[Ans.: detonators]
- Propellants is known as ________.
[Ans.: Low explosives]
- Mixture of 75% potassium nitrate, 15% charcoal and 10% sulphur is ________.
[Ans.: Gun-powder]
- Mixture of TNT and ammonium nitrate is ________.
[Ans.: Amatol]
- Principal ingredient in dynamites are ________.
[Ans.: nitroglycerine]
- ________ is a thin water-proof causes length of the containing gunpowder or (TNT).
[Ans.: A Fuse]
- A good propellant should have ________.
[Ans.: high specific impulse]
- ________ is the energy liberated per unit mass of the explosives.
[Ans.: explosive strength]
- ________ explosives have higher energy content than the primary explosives.
[Ans.: High]
- ________ is an extremely powerful, sensitive and standard military explosive.
[Ans.: PETN]
- TEM means __________.
[Ans.: Transmission electron microscopy]
- SEM means __________.
[Ans.: Scanning electron microscopy]
- XRD means __________.
[Ans.: X-ray diffraction]
- ___________ crystals are highly anisotropic fluids that exists between the boundaries of the solid aid liquid phase.
[Ans.: Liquid]
- ___________ crystal move in three directions and can rotate about one axis.
[Ans.: Nematic]
- ___________ crystal structure is optically active.
[Ans.: Cholesteric]
- Lyotropic crystal having ___________ and water.
[Ans.: Amphiphile]
- ___________ mesophases are important in soaps, gels and colloids.
[Ans.: Lyotropic]
- Complete the reactions
(a) 7N14 + 01n → 714C + _______
(b) 13Al27 + ________ → 15P30 + 01n
[Ans.: a = 1H1; b = 24He]
- ________ is used as a coolant in nuclear reactor.
[Ans.: Light water]
- Atom bomb is based on ________.
[Ans.: Nuclear fission]
- ________ isotope of uranium is used in nuclear reactor.
[Ans.: U235]
- Nuclear reaction is produced large quantities of energy according to ________ equation.
[Ans.: Einstein energy mass E = mc2]
- A fission reactor which produces more fissionable material than its consumed in its operation is ________ reactor.
[Ans.: Breeder]
- ________ changes alter the number of protons and neutrons in the nuclei of atoms consumed while simple chemical changes involve in reorganization of ________ only.
[Ans.: Nuclear, electrons]
- Sun‚s energy is given by fusion of ________ nuclei.
[Ans.: Hydrogen]
- Photovoltaic cell convert directly incident solar radiations to ________.
[Ans.: Electric current]
- The major material used in pv cell is ________.
[Ans.: silicon]
6.12.2 Multiple-choice Questions
- The following compound act as super conductors
- YBa2Cu3O7
- Bi2Ca2Sr2Cu3O10
- Tl2Ca2Ba2Cu3O10
- All
[Ans.: d]
- Doping of Germanium with Group–13 elements gives
- n-type semiconductors
- p-type semiconductor
- Super conductor
- Conductor
[Ans.: b]
- On hydration of cement producers small volume changes it is known as
- Dehydration
- Soundness
- Calcination
- Hardening
[Ans.: b]
- Formula for plaster of paris is
- CaSO4 · H2O
- CaSO4 · H2O
- CaSO4 ·
 H2O
H2O - CaSO4
[Ans.: c]
- Formula for tricalcium alumina ferrite
- 4CaO · Al2O3 · Fe2O3
- 4CaO · Fe2O3
- CaO · Al2O3
- Al
[Ans.: a]
- Which is responsible for high ultimate strength in cement
- C3S
- C2S
- C3A
- C4AF
[Ans.: a]
- Major component of Portland cement is
- MgO
- C3A
- CaO
- C2A
[Ans.: c]
- Tobermonite gel is chemically
- Hydrated tricalcium aluminate
- Hydrated tricalcium silicate
- Hydrated dicalcium ferrite
- Hydrated dicalcium silicate
[Ans.: b]
- The argillaceous material is rich in
- Lime
- Silica
- Stone
- Ferriferrite
[Ans.: b]
- According to I.S.I specification insoluble residue should not exceed
- 5%
- 3%
- 10%
- 2%
[Ans.: d]
- Any materials that can with stand high temperatures without either breaking or suffering a deformation in shape is called
- Dielectric
- Thermal insulator
- Refractory
- Insulator
[Ans.: c]
- In acidic environment, preferably refractory should not be
- Acidic
- Basic
- Neutral
- None of these
[Ans.: b]
- Alumina is an example of:
- Acidic refractory
- Basic refractory
- Neutral refractory
- None of these
[Ans.: a]
- High resistance to spalling is shown by:
- Magnesia refractory
- Dolomite refractory
- Alumina refractory
- Lime refractory
[Ans.: c]
- Breaking, cracking or peeling off a refractory material under high temperature, is called
- Thermal expansion
- Spalling
- Fusion
- Chemical cracking
[Ans.: b]
- A refractory material, generally obtained from bauxite
- Fire clay
- Dolomite
- Chromite
- Alumina
[Ans.: d]
- Which of the following is neutral refractory?
- Dolomite
- Graphite
- Silica
- Magnesia
[Ans.: b]
- Porosity in a refractory brick, generally, increases
- Density
- Resistance to spalling
- Strength
- Melting point
[Ans.: b]
- A good refractory material must:
- Be chemically inactive in use
- Possess low softening temperature
- Undergo spalling
- Possess high thermal expansion
[Ans.: a]
- Spalling of refractory material can be reduced by
- Rapid changes in temperature
- Using porous refractory material
- Using high coefficient of expansion refractory material
- Using good thermal conductivity refractory material
[Ans.: b]
- Good thermal conductivity of a refractory material is desirable if it is to be used in the construction of walls of a
- Blast furnace
- Muffle furnace
- Reverberatory furnace
- All of these
[Ans.: b]
- Porosity of a refractory brick increases its:
- Strength
- Abrasion resistance
- Corrosion resistance
- Resistance to thermal spalling
[Ans.: d]
- Which one of the following refractories can not be used in oxidizing conditions?
- Dolomite bricks
- Magnesite bricks
- Carbon bricks
- Silica bricks
[Ans.: c]
- Which one of the following refractories is neutral in characters
- Dolomite bricks
- Silica Bricks
- Chromite bricks
- Fire clay bricks
[Ans.: c]
- Which one of the following refractories is used in nuclear engineering as modulator?
- Chromite bricks
- Carborundum
- Beryllia bricks
- Fire clay bricks
[Ans.: c]
- An example of acid refractory is:
- Chromite
- Dolomite
- Silica
- Magnesite
[Ans.: c]
- Silica bricks belong to
- Acidic refractories
- Basic refractories
- Neutral refractories
- None of these
[Ans.: a]
- High resistance to spalling is shown by
- Magnesia refractory
- Dolomite refractory
- Alumina refractory
- Lime refractory
[Ans.: c]
- The porosity of refractory brick increases, its mechanical strength
- Increases
- Decreases
- Slightly change
- No change
[Ans.: b]
- Refractoriness is measured by using the test:
- RUL test
- Seger cone test
- Conductivity test
- Spalling test
[Ans.: b]
- Refractoriness under load is determined by
- RUL test
- Seger cone test
- Conductivity test
- None of these
[Ans.: a]
- A refractory should be
- Chemically active
- Chemically inactive
- Chemically unstable
- None of these
[Ans.: b]
- Formula for porosity
[Ans.: d]
-
- From the formula
 W indicates:
W indicates:
- Wt of saturated specimen
- Wt of dry specimen
- Wt of dry specimen in water
- Wt of saturated specimen in water
[Ans.: a]
- Silica bricks are used in
- Roofs of open hearth furnaces
- Coke oven walls
- Roofs of electric furnaces
- All of these
[Ans.: d]
- Refractory used in the linings of port land cement rotary kilns
- Silica bricks
- Fire clay bricks
- High-alumina bricks
- Magnesite bricks
[Ans.: c]
- Magnesite bricks are used in
- Acidic refractories
- Basic refractories
- Neutral refractories
- None of these
[Ans.: b]
- Refractories used in the construction of electrodes
- Magnesite
- Alumina
- Graphite
- Chromite
[Ans.: c]
- Refractory used to separate acidic and basic refractory lining
- Chromite bricks
- Alumina bricks
- Graphite
- All of these
[Ans.: a]
- Refractories used in high frequency electric furnaces:
- Beryllia bricks
- Zirconia bricks
- Carborundum
- None of these
[Ans.: b]
- Higher the porosity of refractory:
- Higher the thermal conductivity
- Higher the refractoriness
- Lesser is its thermal conductivity
- None of these
[Ans.: a]
- Cause of thermal spalling
- Porosity
- Rapid change of furnace temperature
- Maintaining low temperature
- None of these
[Ans.: b]
- Most widly used refractories are:
- Fire clay bricks
- Alumina bricks
- Chromite bricks
- None of these
[Ans.: a]
- High alumina bricks contain about 50 to 80% Al2O3 and 40–45%
- MgO
- CaO
- SiO2
- Carbon
[Ans.: c]
- A refractory lining in a blast furnace should possess:
- Low thermal conductivity
- High thermal conductivity
- Medium thermal conductivity
- None of these
[Ans.: a]
- A lubricant is used with the object of
- Increasing frictional heat
- Increasing resistance
- Decreasing frictional resistance
- Providing direct contact between rubbing surfaces
[Ans.: c]
- A lubricant should possess high
- Volatility
- Acidity
- Oiliness
- None of these
[Ans.: c]
- A lubricant is used primarily to prevent
- Corrosion of metals
- Wearing out of rubbing metallic surfaces
- Oxidation of metal
- Reduction of metals
[Ans.: b]
- A suitable lubricant for watches is
- Grease
- Graphite
- Hazel-nut oil
- Palm oil
[Ans.: c]
- A good lubricant should have
- Low viscosity index
- High viscosity index
- Low fire point
- High
[Ans.: b]
- Lubricant used in a machine working at low temperature should possess
- High pour point
- Low flash point
- High cloud point
- Low pour point
[Ans.: d]
- Capacity of an oil to stick on to the surfaces of machine parts under conditions of heavy load, is called
- Volatility
- Oiliness
- Acid value
- Flash point
[Ans.: b]
- Oiliness is least in case of
- Greases
- Mineral oils
- Animal oils
- Palm oil
[Ans.: b]
- In case of liquid lubricants, generally
- Flash point is higher than the fire point
- Fire point is higher than the flash point
- Fire point is lower than the flash point
- Flash and fire points are identical
[Ans.: b]
- When the resistance to movement of sliding parts is only due to the internal resistance between the lubricant itself, then lubrication is called
- Fluid film
- Boundary
- Thin film
- Extreme pressure
[Ans.: a]
- Mineral oils are
- Very costly
- Poor in oiliness
- Unstable
- Easily oxidized
[Ans.: b]
- Animal and vegetable oils are
- Very cheap
- Not oxidesed easily
- Not thickened in use
- Good in oiliness
[Ans.: d]
- Greases are not used to lubricate
- Rail axle boxes
- Bearings working at high temperatures
- Gears
- Delicate instruments
[Ans.: d]
- Machines operating under high temperatures and loads are best lubricated by
- Mineral oils
- Solid lubricants
- Greases
- Animal oils
[Ans.: b]
- When graphite is dispersed in oil, it is called
- Grease
- Aqua dag
- Oil dag
- Blended oil
[Ans.: c]
- Pick the odd out
- Viscosity
- Carbon residue
- Pour-point
- RUL test
[Ans.: d]
- The single most important property of lubricating oil is its
- Fire-point
- Cloud point
- Oiliness
- Viscosity index
[Ans.: d]
- Type of lubrication involved in delicate machines like watches
- Fluid-film
- Thin film
- Boundary
- Extreme pressure
[Ans.: a]
- Type of oils suitable for thick film lubrication
- Animal oils
- Vegetable oils
- Blended oils
- Hydro carbon oils
[Ans.: d]
- One of the important properties of greases
- Consistency
- Oiliness
- Thermal stability
- All of these
[Ans.: a]
- Quality of grease will be measured by using:
- Refractometer
- Caliometer
- Penetrometer
- Vaporimeter
[Ans.: c]
- Precipitation number indicates:
- Ash content
- Asphalt
- Moisture
- None of these
[Ans.: b]
- Aromatic content in lubricant is determined by
- Saponification number
- Aniline point
- Precipitation number
- Neutralization number
[Ans.: b]
- Estimation of carbon residue is generally carried out by
- Grease penetrometer
- Conrodson‚s apparatus
- Vapourimeter
- None of these
[Ans.: b]
- A good lubricant should deposite:
- More amount of carbon
- Least amount of carbon
- more amount of ash
- None of these
[Ans.: b]
- Oiliness of a lubricant will be increased by the addition of
- Mineral
- Vegetable or animal oil
- grease
- Solid lubricants
[Ans.: b]
- Diameter of the jet in red wood – I viscometer
- 1.42 mm
- 1.62 mm
- 3.4 mm
- 3.6 mm
[Ans.: b]
- Jet bore length in red wood – II viscometer
- 5 mm
- 10 mm
- 15 mm
- 20 mm
[Ans.: b]
- Formula used to measure the viscosity index
[Ans.: c]
-
- Gulf oils are called
- L – Oils
- H – Oils
- L and H Oils
- None of these
[Ans.: a]
- The unit of viscosity is
- Calorie
- Eta
- Poise
- Seconds
[Ans.: c]
- Force per unit area F = ________
- η/dv
- η ∝ v/d
- η/d/v
- η ∝ d/v
[Ans.: b]
- Amount of oil collected during viscosity determination using red wood viscosity
- 50 ml
- 60 ml
- 200 ml
- 400 ml
[Ans.: a]
- Absolute viscosity will be calculated by using the formula
- Kinematic viscosity × density
- Commercial viscosity × density
- Commercial viscosity/density
- None of these
[Ans.: a]
- Abels apparatus is used to determine
- Carbon residue
- Consistency
- Viscosity
- Flash point
[Ans.: d]
- Graphite and molebdenim disulphide are
- Synthetic lubricants
- Antioscidants
- Solid lubricants
- Additives
[Ans.: c]
- The usual coefficient of friction between solid lubricants is between
- 0.001 and 0.01
- 0.005 and 0.05
- 0.005 and 0.007
- 0.005 and 0.01
[Ans.: d]
- Hexanol is
- Viscosity index improver
- Thickness
- Corrosion prevention
- Oiliness carrier
[Ans.: a]
- Lubricants also act as
- Sealing agent
- Corrosion prenenter
- Cooling medium
- All of these
[Ans.: d]
- The coefficient of friction in fluid film lubrication is
- 0.01 to 0.03
- 0.01 to 0.3
- 0.01 to 0.05
- 0.001 to 0.03
[Ans.: d]
- Tropex is a mixture of
- 40% RDX, 40% TNT and 20% Al powder
- TNT and PETN
- 70% Tetryl and 30% TNT
- None
[Ans.: a]
- Ammonium Nitrate is a
- Low single compound explosive
- High single compound explosive
- a and b
- None
[Ans.: b]
- Lead azide is a
- High explosive
- Low explosive
- Primary explosive
- Propellant
[Ans.: c]
- Gun powder is
- Low explosive
- High explosive
- Blasting fuse
- All
[Ans.: a]
- A propellant used in rocket engine is
- Fuel
- oxidant
- None of these
- a and b
[Ans.: d]
- The term nano means
- One billionth of a kilometer
- One billionth of a meter
- One billionth of an inch
- One billionth of a millimeter
[Ans.: b]
- Who is the father of nanoscience?
- Rutherford
- Richard Feynmen
- Newton
- Qurie
[Ans.: b]
- Which of the following nanomaterials act as sensors of gases like NO2 and NH3 on the basis of increase in electrical conductivity?
- Carbon nanotubes
- Thin film
- ZnO
- Palladium
[Ans.: a]
- The nanotubes of MoS2 and CoS2 are used as
- Semiconductors
- Insulators
- Storage device
- Solid lubricants
[Ans.: d]
- Which of the following nanowires shows photoluminescence?
- Zinc oxide
- Palladium
- Silicone
- MoS2
[Ans.: c]
- Which nanomaterial effectively catalyzes hydrogenation of oil?
- Rane CuO
- Rane pd
- Rane Ni
- Rane ZnO
[Ans.: c]
- Nanowires and nanotubes are __________ in nanoscale.
- One dimensional
- Two dimensional
- Three dimensional
- None
[Ans.: b]
- __________ type of nanomaterials is having three dimensional structure.
- Thin film
- Nanowires
- Quantum dots
- All
[Ans.: c]
- According to molecular arrangements, nanomaterials are broadly divided into __________ types.
- Two
- Three
- Four
- Many
[Ans.: b]
- Thermotropic liquid crystals are
- Enantiotropic
- Monotropic
- a and b
- None
[Ans.: c]
- Crystals which can change into the liquid crystal state from either lowering temperature of a liquid of raising of the temperature of solid
- Enantiotropic
- Monotropic
- Nematic
- all
[Ans.: a]
- Which mesophase is a nematic type of liquid crystal and optically active?
- Semeiotic
- Cholesteric
- a and b
- none
[Ans.: b]
- The source of energy in nuclear fuel is due to
- Chemical reaction
- Nuclear reaction
- Nuclear fission
- Nuclear fusion
[Ans.: c]
- Binding energy of a nucleus is related with
- ΔMc2
- Mc2
- ΔMc
- hn
[Ans.: a]
- Fissionable material used in nuclear reactor is
- U235
- U238
- Th232
- Pu239
[Ans.: a]
- 12H + 12H → ?
- 23He
- 24H
- 24He
- None
[Ans.: c]
- Control rods used in nuclear reactor is made of
- Na
- B
- CO2
- U235
[Ans.: b]
- Atom bomb is based on principle of
- Nuclear fusion
- Nuclear fission
- Chemical reaction
- None
[Ans.: b]
- Uncontrolled nuclear fusion reaction takes place in
- Atom bomb
- H – bomb
- Nuclear bomb
- Radium bomb
[Ans.: b]
- Solar energy originates from the reaction taking place in the sun
- Nuclear fusion
- Nuclear fission
- Chemical reaction
- Nuclear reaction
[Ans.: a]
- Photovolatic cells are commonly known as
- Primary cell
- Solar cell
- Secondary cell
- Fuel cell
[Ans.: b]
- Example of indirect solar energy is
- Nuclear energy
- Wind energy
- Chemical energy
- Surface energy
[Ans.: b]
6.12.3 Short Answer Questions
- Define semiconductors.
Ans.: Semiconductors are those solids which are perfect insulators at absolute zero, but conduct electric current at room temperature and lies between that of a good conductor and insulator.
- What is meant by doping?
Ans.: Addition of some impurities to pure semiconductors to enhance conductivity is known as doping.
- Explain intrinsic semiconductors and conduction.
Ans.: At normal temperature and pressure pure silicon and germanium are insulators, because no electron is free and all the electrons are fixed in covalent bonds. But at high temperature covalent bonds are broken and the electrons so released become free to move in the crystal and thus conduct electric current. This type of conduction is known as intrinsic conduction and the conductors are intrinsic semiconductors. It will happen in the crystal without adding any external substance.
- Classify extrinsic semiconductors with examples.
Ans.: n-type semiconductors: Upon addition of trace amount of group 15 elements like phosphorous, arsenic to pure germanium n-type semiconductors are formed. Extra electrons come from the impurity and conduct the electricity.
P-type semiconductors: Upon addition of trace amount of group 13 elements like boron to pure germanium p-type semiconductors are formed. Formed positive holes by doping are responsible for conduction.
- Define superconductors and give examples.
Ans.: Materials which show zero electrical resistance at absolute zero temperature are superconductors and that state is superconductivity.
Ex: YBe2Cu3O7, Bi2Ca2Sr2Cu3O10.
- Who invented magnet and how it is named as magnet?
Ans.: More than 2000 years ago, the ancient Greeks first discovered a mineral that attracts things made of iron. This mineral was found in Magnesia, a part of Turkey, hence it is named as magnetite.
- Give the classifications of magnetic materials.
Ans.: 1. Diamagnetic
2. Paramagnetic
3. Ferromagnetic
4. Ferrimagnetic
5. Anti-ferromagnetic materials.
- Give examples for diamagnetic materials.
Ans.: Copper, silver, gold, etc. are the examples for diamagnetic materials.
- Define hysteresis.
Ans.: Retaining of magnetic properties after the removal of external magnetic field is known as hysteresis.
- What is meant by magnetic domain?
Ans.: In ferro and ferromagnetic materials, below Curie temperature, large number of atom moments is aligned parallels as a small volume region and are known as magnetic domains.
- Give broad classifications of cement.
Ans.: Cement is broadly classified into natural, puzzolana, slag and Portland cement.
- Who invented puzzolana cement and how it can be prepared?
Ans.: Puzzolana cement is the oldest cement invented by Romans. This is prepared by mixing of natural puzzolana and slaked lime.
- What is known as magic powder and what are the main ingredients of it?
Ans.: A Portland cement is also known as magic powder. It primarily consists of lime, silica, alumina and iron.
- What is the function of iron oxide in cement?
Ans.: Iron oxide provides colour, strength and hardness to the cement.
- Give the equation for final setting and hardening of the cement.
Ans.:

- What are the theories that explain the hardening of the cement?
Ans.: Colloidal theory by Michaels and crystalline theory by Le Chatelier explain the hardening of the cement.
- What are the chemical constituents present in Portland cement?
Ans.: Tricalcium silicate (3CaO SiO2), dicalcium silicate (2CaO SiO2), tricalcium aluminate (3CaOAl2O3) and tetracalcium alumino ferrite (4CaOAl2O3Fe2O3).
- What is the main function of gypsum in cement?
Ans.: The presence of gypsum in the cement helps to retard the speed of the initial set, due to the formation of insoluble calcium sulphoaluminate. This does not show tendency to rapid hydration.
C3A + Gypsum → Tricalcium sulphoaluminate
- Define a refractory.
Ans.: Refractories are ceramic materials that can withstand high temperatures as well as abrasive and corrosive actions of molten metals, slags and gases without deformation.
- Explain any two important properties of a refractory.
Ans.: (a) Refractoriness: Refractoriness is the ability of a material to withstand heat, without appreciable deformation.
(b) Dimension stability: It is the resistance of a material to any volume changes, which may occur on its exposure to high temperature and load.
- Explain porosity with an equation.
Ans.:

where W = weight of saturated specimen
D = weight of dry specimen
α = weight of saturated specimen submerged in water
A good refractory should have low porosity.
- Give examples for all types of refractories.
Ans.: Acidic refractories: Alumina, silica
Basic refractories: Magnesite, dolamite
Neutral refractories: Graphite, zirconia
- What is meant by permeability?
Ans.: Permeability is a measure of rate of diffusion of gases, liquids and molten solids through a refractory. It depends upon the size and number of connected pores. Permeability will increase with temperature.
- What are the uses of magnesite bricks?
Ans.: Magnesite bricks are used in open hearth furnaces, reverberatory furnaces, rotary kilns and refining furnaces.
- What test is used to determine the refractoriness of the refractory?
Ans.: Seger cone test.
- Define thermal spalling.
Ans.: Breaking, cracking, peeling off or fracturing of a refractory brick under high temperature is known as thermal spalling.
- Define lubricants.
Ans.: The process of reducing frictional resistance between moving or sliding surfaces by the introduction of lubricants in between them is called lubrication.
- What are the main mechanisms of lubrication?
Ans.:
(i) Fluid film or thick film lubrication
(ii) Boundary or thin film lubrication
(iii) Extreme pressure lubrication
- What are the main types of lubricants?
Ans.: On the basis of physical state, lubricants are classified into liquid lubricants, greases or semisolid lubricants and solid lubricants.
- Give some examples for greases.
Ans.: Calcium-based greases, sodium-based greases, lithium-based greases and axle greases.
- Why graphite acts as a good lubricant?
Ans.: Due to its unique layer structure, graphite acts as a good lubricant.
- Explain oildag and aquadag.
Ans.: In the presence of tannin, dispersion of graphite in oil is oildag and dispersion of graphite in water is aquadag.
- What are the units for absolute viscosity?
Ans.: Eta (η) or poise or centipoise are units for absolute viscosity.
- Give the equation for viscosity index.
Ans.: Viscosity index

- Name the different types of viscometers.
Ans.: Redwood viscometers, Englers viscometers, saybolt viscometers, U-tube viscometers, etc.
- How can we measure the mechanical stability of a lubricant?
Ans.: Four-balls extreme pressure is the one of the important mechanical tests to judge the mechanical stability of a lubricant under load.
- Give some important properties of explosives.
Ans.: Explosive strength, velocity of detonation, sensitivity, Brisance, etc. are some important properties of explosives.
- Give some examples for detonators.
Ans.: Lead azide, mercury fulminate, tetracene, diazodinitro phenol.
- What is the main difference between detonators and propellants?
Ans.: Detonators are highly sensitive and can explode with slight shock or fire, whereas propellants burn simply but do not explode.
- How high explosives are classified?
Ans.: Depending on the components present on the explosives, they are classified into single compound explosives, binary explosives, plastic explosives, and dynamites.
- What is the main component present in the binary explosives? Give some examples.
Ans.: TNT is the main component present in the binary explosives. Examples are amatol, pentolite, tetrytol, tropex, etc.
- Define blasting fuses.
Ans.: A fuse is a thin water-proof canvas length of tube containing gun powder, which is arranged to burn at a given speed for setting of charges of explosives.
- Define rocket propellants and give their classification.
Ans.: Rocket propellant is high oxygen containing fuel and oxidant, whose combustion takes place in a definite and controlled manner with the evolution of a huge volume of a gas. Propellants are mainly classified into solid and liquid propellants.
- What are the advantages of liquid propellants?
Ans.: Liquid propellants possess many advantages over solid propellants because they are versatile and the engine using them can be checked and calibrated more easily. The engine using the liquid propellant is quite delicate and cannot withstand any rough handling.
- Define a nanometre.
Ans.: Nanometre is the one-billionth of a metre, 1 nm = 1/1000000000 metre = 1 × 10–9 m.
- Classify the nanomaterials according to molecular arrangements.
Ans.: According to atoms/molecular arrangements, nanomaterials are broadly classified into three types.
(a) Materials which have one dimension in the nanoscale. Ex: Surface coatings, thin films, etc.
(b) Materials which have two dimensions in the nanoscale. Ex: Nanowires, nanotubes, etc.
(c) Materials which have three dimensions in nanoscale or quantum dots. Ex: Fullerenes.
- What are the important properties of nanomaterials?
Ans.: A significant increase in surface-area-to-volume ratio at the nanoscale, gives rise to novel and enhanced magnetic, mechanical, electronic, catalytic, conducting and optical properties.
- Give some examples of semiconducting and superconducting nanomaterials.
Ans.: Semiconductors: Carbon nanotubes, nanowires, MoS2, etc.
Superconductors at high temperature: NbS2.
- What are the most important applications of carbon nanotubes?
Ans.: Well-defined geometry, exceptional mechanical properties and extraordinary electrical characteristics of carbon nanotubes are used in nanoelectric circuits, nanoelectromechanical systems, nanorobotics, nanobiosensors, etc.
- Give the interconversions of a nanometre.
Ans.: One nanometre is the one-billionth of a meter. 1 nm = 10–9 m = 10–9 yards approximately.
- Give the different synthetic routes for nanomaterials.
Ans.: The vapour–liquid–solid growth, solution–liquid, solid growth, template-mediated growth, electron beam lithography, reverse micellar route, etc.
- How can we characterize the nanomaterials?
Ans.: Nanomaterials are characterized by X-ray diffractions (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), thermal analysis like TGA/DTA, Fourier transform infrared spectroscopy (FITR), etc.
- Give the broad classification of nanomaterials.
Ans.: (1) Materials which have one dimension in the nanoscale. Ex: Surface coatings or thin films.
(2) Materials which have two dimension in the nanoscale. Ex: Nanowires and nanotubes.
(3) Materials which have three dimension in the nanoscale. Ex: Quantum dots.
- Which property plays a vital role in the nanomaterials?
Ans.: A significance increase in surface area-to-volume ratio at the nanoscale gives rise to novel and enhanced magnetic, mechanical, electronic, catalytic, conducting, optical properties, etc.
- Give a brief note about fullerenes and carbon nanotubes.
Ans.: The fullerenes are a class of allotropes of carbon, which are basically graphene sheets rolled into tubes or spheres. They include the carbon nanotubes because of their mechanical strength and electrical properties.
- What are the important uses of carbon nanotubes?
Ans.: Nanoelectronic circuits, nanoelectromechanics, nanorobotics, probes, grippers, nanobiosensors, etc. are important uses of carbon nanotubes.
- Give the broad classification of liquid crystals.
Ans.: Liquid crystals are broadly classified into thermotropic liquid crystals and hydropic liquid crystals.
- Name the three kinds of thermotropic liquid crystals.
Ans.: The three types are nematic, cholesteric and smectic liquid crystals.
- What is the main difference between nematic and cholesteric structure?
Ans.: Cholesteric structure is a nematic type of liquid crystal but it is optically active
- Give examples for nematic liquid crystals.
Ans.: P-methoxybenzylidene p1–N-butyl-aniline and P-n–Hexyl-P1–cyanobiphenyl.
- Why lyotropic crystals are known as amphiphilic?
Ans.: Lyotropic crystals are composed of both lyotropic and lyophobic parts.
- Define liquid crystals. Why are they named as liquid crystals?
Ans.: The liquid crystals are highly anisotropic fluids that exists between the boundaries of the solid and conventional, isotropic liquid phase.
The liquid crystals are made up of both liquid and crystal. The term liquid is used because the tendency to take the shape of the container and the word crystal is used because they still contain 1 to 2 dimensional arrays.
- Name different types of thermotropic liquid crystals.
Ans.: Nematic, cholesteric and smectic structures are main types of thermotropic liquid crystals.
- Define lyotropic liquid crystal and give one example.
Ans.: Lyotropic liquid crystals are mixtures of two or more components that change phase with changes of concentration.
Ex: Sodium stearate, α–Lecithin.
- Give some applications of liquid crystals.
Ans.: Liquid crystals are widely used in research, medicine, displays, radiation sensors, thermometers, non-destructive testers, etc.
- Give the importance of liquid crystals in medicine.
Ans.: The liquid crystals are widely used in medicine as optical disks, full colour electronic slides, light modulators, etc. because most of the biological systems exhibits the liquid crystal properties.
- What is the reason for liquid crystals being used as thermometers?
Ans.: The temperature-dependent variation in the colour of cholesteric liquid crystals has led to the use of substances in the measurement of temperature and gradients of temperature. A cholesteric substance or a mixture of cholesterics always exhibits the same colour and the same temperature. Hence, these are also used to locate veins, arteries, infections, tumours, etc. which are warmer than surrounding tissues.
- Define green chemistry.
Ans.: Green chemistry is the frontier of science, with the utilization of set of principles that attempts to reduce or eliminate the use or generation of hazardous substances in the design and manufacture of environmentally and economically sustainable products.
- Define biocatalysis or bioleaching, Explain with one example.
Ans.: Bioleaching is the extraction of specific metals from their ores by using microorganisms such as bacteria.
Ex: Extraction of gold.
Traditional method: Heap leaching method by using cyanide.
Greener method: Acidithiobacillus ferrocious and acidithiobacillus thiooxidous bacteria are used.
- Explain greener route for synthesis of adipic acid.
Ans.: In greener route, cyclohexane is oxidized by 30 per cent of hydrogen peroxide in presence of catalyst.

6.12.4 Descriptive Questions
- Define semiconductors and explain intrinsic and extrinsic semiconductors with example.
- Write a note on n-type and p-type semi conductors.
- Give brief note on importance and application of semiconductors.
- Explain super conductors with examples.
- Define magnetism and explain dia and paramagnetic materials.
- Explain in detail a classification of magnetic materials.
- Give a brief note on important properties of magnetic materials.
- Explain the applications of magnetic materials.
- What is portland cement? Explain the different ingredients of cement.
- Give an account of
a. Chemical composition of cement
b. Chemical constitution of portland cement.
c. Why Portland cement so-named.
- Explain the setting and hardnening of cement with suitable chemical reactions.
- Write a brief account on the following:
a. The raw materials and the ingredients of cement.
b. Function of gypsum in cement.
c. Discuss merits and demerits of dry process and wet process.
- Explain analysis of cement.
- Draw a labeled diagram of a rotary kiln used for the manufacture of portland cement by wet process and discuss the various reactions taking place in furnace.
- What are the microscopic constituents or Constitutional compounds present in portland cement? How do they contribute towards the properties of the cement?
- What do you mean by setting and hardening of cement? Discuss the various reactions involved with the help of equations.
- “The properties of portland cement depend upon the relative proportions of its constitutional compounds”. Justify statement.
- What are the different methods of manufacturing cement? Discuss their relative merits and demerits.
- Write informative notes on the following:
a. Reactions taking place in the rotary kiln.
b. Constitutional compounds in cement and its derivatives.
c. Additives for cement.
d. Important properties of cement.
e. How are the cement classified.
f. Define soundness of cement.
- a.What is pyro metric cone equivalent? How it is determined for a refractory? What is its significance?
b. Write a short note on:
i. Porosity
ii. Thermal conductivity
iii. Dimensional stability
iv. Strength
- a.Define refractories and what are the criteria of a good refractory?
- b. Give the classification of refractories with suitable examples.
- a. What are refractories? How important are the properties – refractoriness under load and thermal conductivity for industrial applications?
- b. Compare acidic and basic refractories with examples.
- a. How are the refractories are classified? Give one example for each class.
- b. Write a note on the conditions leading to failure of a refractory material.
- Discuss any four essential properties of a good refractory in detail.
- a. What are the causes leading to failure of a refractory?
b. Describe
i. Fire-Clay Bricks
ii. Sic bricks
- Write short note on:
a. Refractoriness
b. Refractoriness under load or strength
c. Dimensional stability
d. Thermal conductivity
e. Porosity
- State some important industrial applications of refractories.
- Discuss the important properties of refractories which have a direct bearing on their industrial use.
- Write informative notes on:
a. Fire clay refractories
b. Silica refractories
c. Magnesite refractories
- a. What are the raw materials for refractories?
b. What are the different steps in the manufacture of refractories?
c. What do you mean by super refractories?
- Discuss various physical and chemical factors which affect the industrial uses of refractories.
- a. Give the functions of lubricants.
b. Describe the mechanism of extreme pressure lubrication.
c. How a viscous lubricant is converted into grease?
- Discuss the important properties of lubricating oils, which are useful for their evaluation.
- a. Distinguish between hydrodynamic lubrication and boundary lubrication.
b. Distinguish between hydrodynamic lubrication and extreme pressure lubrication.
- Explain the following two theories for the mechanism of the lubricants:
a. Boundary lubrication
b. Exterme pressure lubrication
- Write notes on
a. Blended oils
b. Petroleum oils
c. Extreme pressure additives
d. Antioxidants
- Explain how the following acts as lubricants:
i. Graphite
ii. Molybdenum disulphide
- Write a note on lubricants with special reference to their classification, mode of action, examples, and applications.
- How to select lubricants for the following:
a. Cutting tools
b. I.C. engines
c. Steam Engines
d. Steam turbines
e. Gears
- Explain the various mechanisms of lubrication in detail.
- Define lubricant. Discuss the important properties of the lubricating oils.
- Describe the various types of lubrication.
- Define the flash and fire points.
- a. Describe thick-film lubrication.
b. Write a note on semi-solid lubricants.
- Write short notes on the following properties of lubricants:
a. Pour point
b. Aniline point
- Explain the hydrodynamic lubrication.
- Explain the following properties of lubricants and discuss their significance:
a. Viscosity and viscosity index
b. Flash point
c. Aniline point
d. Saponification value
- Distinguish between fluid film and boundary lubrication.
- Lubricating oil has the same viscosity as standard naphthenic and paraffinic type oils at 210°F. Their viscosities at 100°F are 320 S.U.S. (Saybolt Universal Second), 430 S.U.S., and 260 S.U.S., respectively. Find the viscosity index of the oil.
- a. What do you mean by viscosity index of lubricating oil?
b. Lubricating oil has a S.U.S. of 58 seconds at 210°F and of 600 seconds at 100°F. The high viscosity index standard (i.e., Pennsylvanian) oil has S.U.V. (Saybolt Universal Viscosity) of 58 seconds at 210°F and 400 seconds at 100°F. The low viscosity index standard (i.e., Gulf ) oil has a S.U.V. of 58 seconds at 210°F and 800 seconds at 100°F. Calculate the viscosity index of oil.
- Write an essay on solid lubricants with emphasis on their classification, mechanism of action, examples, and applications.
- How are semi-solid lubricants prepared? In what situations a semi-solid lubricant is preferred? Mention some important tests for evaluating semi-solid lubrications.
- How are liquid lubricants classified? Discuss the various methods available for refining mineral oils.
- What do you mean by blended or compounded oils? What are the various additives used to induce or improve the necessary properties of lubricating oil?
- Discuss the use of lubricating emulsions.
- Write informative notes on the following:
a. Cup greases
b. Fixed oils
c. Gas lubrication
d. Biodegradable lubricants
e. Neutralization number
f. Extreme pressure lubrication
g. Oiliness
g. Redwood viscometer
i. Cryogenic bearing lubricants
j. Lubricants for nuclear reactor systems
k. Lubricants for food processing
l. Environmental and health factors in the use of lubricants
- Justify the following statements:
a. Flash point determination by the closed-cup apparatus gives a lower value than that determined by an open-cup apparatus.
b. Closed-cup apparatus gives more reliable value than the open-cup apparatus for the determination of flash point.
c. The relative viscosity determined by Saybolt viscometer or Redwood viscometer can be converted into absolute kinematic viscosity by calculations.
- Write short notes on saponification and iodine values.
- Discuss the significance of viscosity in lubricating oil. How is it determined by Redwood viscometer?
- a. Write the structure of graphite. Based on this, suggest why this can be used as a solid lubricant.
b. Discuss the classification of lubricants.
- Define lubricants. Discuss the classification of lubricant with suitable examples.
- a. Explain the following properties of lubricants and their significance:
i. Carbon residue
ii. Aniline point
b. Write an informative note on synthetic lubricants.
- Define the term lubricants. Mention their important functions. Explain and discuss the significance of any two properties of lubricants.
- Discuss, in brief, lubrication, its mechanism, and significance. Explain viscosity index of lubricating oil.
- a. Define the terms lubrication and lubricants. What are the different types of lubricants? Discuss the basic principle of lubrication.
b. What are the chief functions of lubricants?
c. What are the different types of lubricants?
d. Discuss the classification of lubricants with example.
- a. How are lubricating oils produced and refined? Which oil is a better lubricant?
b. Define dewaxing. What are the characteristic features of synthetic lubricating oils?
- a. Write the names of two semi-solid lubricants.
b. Give brief note on graphite and molybdenum disulphide.
c. Write a short note on the following:
i. Semi-solid lubricants
ii. Synthetic lubricants
iii. Solid lubricant and its advantage
- a. Write a short note on extreme-pressure lubrication?
b. What are the different synthetic lubricants used? How are they superior over petroleum lubricants?
- a.What are greases and under what situations are they employed? Discuss the composition and uses of:
i. Calcium-based greases
ii. Soda-based greases
iii. Axle greases.
b. Write a note on extreme-pressure additives to mineral oil.
- a. Draw a neat and labelled diagram determination viscosity of lubricant by Redwood viscometer
b. Write a short note on aniline point.
- a. How does viscosity determine the operating characteristics of the lubricants?
b. Suggest the suitable properties of lubricating oil used for steam engines and transformers.
- a. Cotton seed oil is used as dry oil in paints. Is it true or false?
b. Write a note on additive for lubricating oils.
- a. How are greases made?
b. What is a lubricant? Discuss the classification and its basic characteristics with examples.
- a. What are the characteristic features of synthetic lubricating oils?
b. Write an explanatory note on solid lubricants.
- a. Name any four solid lubricants.
b. Describe any four desirable properties of lubricant oil.
c. Write brief note on greases.
- a. Explain clearly the importance of the following in selecting lubricating oil for a particular use:
i. Viscosity
ii. Flash point
iii. Acidity
iv. Carbon residue
b. How is the viscosity of lubricating oil determined in the laboratory?
- a. How will you select a lubricant?
b. Explain the properties of lubricants such as viscosity and viscosity index.
- a. What is meant by lubricant? Explain the mechanism of lubrication.
b. Write a note on the following:
i. Lubricating greases
ii. Lubricant emulsions
c. Describe a method to manufacture lubricating oils.
d. Write a note on the lubricating action of greases.
- a. How are lubricants classified?
b. Define flash point. Describe any one method of determining flash point.
c. With the help of a neat diagram, explain the working of Redwood viscometer.
d. What is lubrication? Explain any one type of lubrication in detail.
- a. Fatty oils are no longer used as lubricants. Why?
b. What do you mean by viscosity of a lubricating fluid? How does it change with temperature?
c. How do viscosity and viscosity index influence the selection of lubricants for particular purposes?
d. What are flash point and fire point of a liquid lubricant? Are they directly related to the quality of lubricants?
e. Write a short account on solid lubricants.
- a. Under what situation greases are used? What are the main functions of soap in grease?
b. What do you understand by consistency and drop point of grease? Explain their importance.
- a. What is meant by ‘oiliness‚ of a lubricant? How can this be improved?
b. Explain boundary and fluid lubrications and mention the conditions therein.
- a. What are propellants and explains the characteristics of a good propellants?
b. Explain the properties of solid and liquid propellants.
- a. What are explosives? What are the basic requirement of chemical explosives?
b. Write short note on Dynamite.
c. Write short note on TNT.
d. Distinguish between primary and secondary explosives.
- Write short note on
- a. Classification of explosives
- b. Plastic explosives
- c. Rocket fuels
- d. Propellants
- e. Blasting fuses.
- a. What is detonation?
b. What are the requirements of a good propellants.
c. What are the requirements of a good explosives.
- a. What two factors for the selection of a propellant.
b. Explain Mono and bi propellants with examples.
- Define nanomaterial. Explain some of the important properties.
- Give brief description about applications of nanomaterials.
- What are nanomaterials? Explain their characteristics.
- Give broad classification of nanomaterial with example.
- Explain the following properties of nanomaterial:
a. Catalytic property
b. Mechanical property
c. Electrical property
d. Optical property
- What are nanoparticles? Give brief description on their properties.
- Give brief explanation about preparation of nanomaterials and their importance.
- Give brief explanation about characterization of nanomaterials.
- Discuss about carbon nanotubes and their importance.
- Define Liquid crystal and explain characterizing of Liquid crystal phase.
- Give brief explanation about Thermotropic liquid crystals with example.
- Write short note on Lyotropic liquid crystals.
- Explain important applications of Liquid crystals.
- Give brief note on
a. Smectic structure
b. Enantiotropic crystal
c. Monotropic crystals.
- Discuss the theoretical principles involved in the generation of power by nuclear fission and nuclear fusion.
- Describe the various components of a nuclear power reactor and their functions.
- Discuss the environmental aspects of nuclear power generation.
- Write informative notes on
a. Breeder reactors
b. Energy from nuclear fusion.
- Write notes on nuclear fission and fusion.
a. Explain how fission grade U235 is obtained.
b. Write short note on nuclear binding energy.
- What are the functions of following in a nuclear reactor?
i. 235U
ii. Cadmium rods
- Define nuclear reaction.
- Calculate the binding energy in Mev of 24He, if its experimentally determined mass is 4.00390 amu. The masses of a proton, an electron and a neutron are respectively 1.007825, 0.0005852 and 1.008668 amu.
- Explain how binding energy is useful about the stability of a nuclei.
- Write the difference between nuclear fusion and nuclear fission.
- Write the principle involved in atomic bomb and its reactions.
- Write the reaction taking place in sun and stars.
- Discuss the use of solar energy for space heating, water heating and production of electricity.
- Discuss the use of indirect solar energy for generation of electrical power.
- Write short notes on the following:
a. Solar greenhouse
b. Solar production of electricity.
- Explain the solar desalination process and solar cooking process.
- Write informative note on wind power with its merits and limitations.
- Define green chemistry and explain 12 principles of green chemistry.
- Explain any two greener methods with examples.
- Give a brief note on greenhouse concept.
- What is the importance of green synthesis?
