Chapter 9. Application of micromixers
Chapter Outline
9.1. Chemical Industry321
9.2. Applications in Chemical and Biochemical Analysis329
9.3. Outlook337
References339
Micromixers play an important role in the chemical industry because of their unique features as highlighted in Chapter 1. Generally, the fast mixing process is the key advantage of micromixers. Micromixers allow the formation of new products that are not possible in large-scale reactors.
9.1. Chemical Industry
9.1.1. Micromixers as microreactors
Micromixers play an important role in the chemical industry because of their unique features as highlighted in Chapter 1. Generally, the fast mixing process is the key advantage of micromixers. Micromixers allow the formation of new products that are not possible in large-scale reactors. Considering a simple reaction between two species A and B to form a product C, the reaction rate r is defined as the generation rate of the product under an isothermal condition:
(9.1)
(9.2)
For reactions with very fast reaction kinetics, the reaction rate is determined by diffusion k∝D. Because diffusion is the final stage in all micromixer types, the efficiency of a micromixer determines the reaction rate. Thus, the reaction rate can be controlled by a careful micromixer design.
9.1.2. Liquid–liquid reactions
Improved mixing in micromixers makes them suitable for synthesis applications based on liquid-phase reactions. Common liquid-phase reactions in chemical industry are [1]:
• Nucleophilic substitution reactions,
• Electrophilic aromatic substitution reactions,
• Addition and elimination reactions,
• Coupling reactions, and
• Oxidation and reduction reactions.
Nucleophilic substitution is a basic class of chemical reactions. In a nucleophilic substitution process, an electron nucleophile selectively bonds with or attacks the positive or partially positive charged atom or a group of atoms. This atom group is called the leaving group (LG); the positive or partially positive atom is called electrophile. Electrophiles are the positive or partially positive charged atoms [2]. A nucleophilic substitution reaction has the general form of
Aliphatic nucleophilic substitution carried out in micromixer with a cross-section of 200 by 100 microns results in a 75% yield of the desired product compared to 26% of a stirred batch reactor at the same residence time of 2 minutes [3]. A yield of 96% was achieved with a residence time of 10 minutes. The increase in yield is caused by the larger interfacial area between the reactants. The microfluidic platforms of micromixer allow the integration of reaction, separation, and detection in a single device. Belder et al. [4] carried out all three processes for the biocatalytic hydrolysis of glycidyl phenyl ether on a microfluidic platform. Aliphatic nucleophilic substitution in microreactor was used to synthesize aliphatic amines [5]. Yields up to 100% can be achieved at a throughput on the order of 10–100 g/h. Another key application of nucleophilic substitution is synthetic radiochemistry. Radiolabeled ester was synthesized in a glass micromixer at a residence time of 12 seconds and a radiochemical yield of 88%[6].
Electrophilic aromatic substitution is another important class of organic reaction where an atom (hydrogen) appended to an aromatic system is replaced by an electrophile. Examples of electrophilic aromatic substitution include aromatic nitration, aromatic halogenation, aromatic sulfonation, and acylation and alkylating Friedel–Crafts reactions. The improved mass and heat transfer in micromixers allow a safe and fast screening of process parameters for this reaction. Micromixers bring clear advantages to electrophilic aromatic substitution reactions. For alkylating Friedel–Crafts reactions as an example, aromatic hydrogen is substituted by an alkyl group. The first challenge of Friedel–Crafts reactions is its exothermic nature. The second challenge of Friedel–Crafts reactions is the increase in reactivity after the first alkylation. Further unwanted alkylation steps are caused by the improved electron-donating properties of the alkyl group. To avoid the subsequent alkylation processes, conventional Friedel–Crafts reactions are carried out with an excessive amount of aromatic material. The fast mixing process in micromixer would prevent polyalkylation and allows monoalkylation at stoichiometric condition, thus saving reactants. Suga et al. used a parallel lamination micromixer to run alkylation of 1,3,5-trimethoxybenzene with N-acyliminium ions at a stoichiometric condition and at a low temperature of –78 °C [7]. A total yield of 96% for the monoalkylation product was achieved. The high selectivity is the result of faster mixing in the micromixer. The temperature is an important parameter for Friedel–Craft reaction. A higher temperature leads to a lower yield of monoalkylation and higher yield of dialkylation.
Aromatic nitration is a process for the introduction of a nitro group into a chemical compound. Nitration is used for the production of precursors for polymers, agricultural chemicals, and explosives. The two main challenges of nitration are its highly exothermic nature and the corrosivity of nitrating agent. The increased temperature leads to secondary reactions and unwanted by-products lowering the yield of the target product. Aromatic nitration is catalyzed by acids, usually a mixture of sulfuric acid and nitric acid. Thus, micromixers used for these reactions should be made of material that can stand the extreme condition of high temperature and high corrosivity. The better heat transfer in micromixer allows carrying out nitration reactions under conditions not possible in conventional batch processes such as the extremely exothermic autocatalytic nitration of phenol by HNO3[8] or adiabatic nitration [9].
Halogenation of aromatic compounds such as bromination and iodination has been carried out in micromixers. For instance, aromatic compounds are brominated using dilute solution of bromine. The safe condition in a micromixer allows reaction at more intensified conditions such as high temperature, high pressure, short residence time, and undiluted bromine. Loeb et al. carry out bromination of toluene in a micromixer at 80–100 °C and pressure as high as 15 bar [10].
Addition and elimination reactions are powerful tools for the synthesis of a wide range of organic compounds. In an addition reaction, two or more molecules combine and form a larger one. The two main types of polar addition reactions are electrophilic and nucleophilic additions. The nonpolar addition reaction is called free radical addition. In an elimination reaction, two substituents are removed from a molecule in one or two steps. In most organic elimination reactions, the unsaturation of the molecule increases. In the case of reductive elimination, the valence of an atom in the molecule decreases by two.
Wiles et al. [11] used a micromixer to carry out addition of silyl enol ether of cyclohexanone to 4-bromobenzaldehyde. Conversion time was reduced from 24 hours in a batch reactor to only 20 minutes in the micromixer. Since micromixers can be fabricated in optically transparent materials such as glass, photochemical [2 + 2] cycloaddition reaction that requires ultraviolet (UV) light exposure can be carried out in microscale [12]. Compared to conventional batch reactors, the residence time was significantly reduced to 2 hours. Production scale (few grams per hour) of a key intermediate for furofuran lignans was achieved in a micromixer [13]. In this work, tributyltin hydride-mediated radical reactions of organic halides were carried out with a residence time of one minute. Production-scale elimination reaction was carried out in micromixers for the synthesis of pristine, an adjuvant for monoclonal antibody production [14]. Increasing conversion rate of 85–95% for dehydration of 1-hexanol to hexane was achieved in a micromixer [15]. A conventional reactor can only provide 30% conversion for the same reaction.
Coupling reactions are processes where two hydrocarbon fragments are coupled with the aid of a metal catalyst. Coupling reactions are powerful tools for carbon–carbon bond formation. The two types of coupling reactions are cross-couplings and homocouplings. Cross-couplings are reactions between two different partners, while homocouplings link two identical partners.
Cross-coupling reactions were carried out in micromixers using heterogeneous Pd catalysts where the Pd/SiO2 catalyst bed was immobilized in a microchannel for the Suzuki–Miyaura reaction that couples organic halides with organoboron compounds [16]. Ionic liquid with Pd catalyst was used in a micromixer for the Mizoroki–Heck reaction, where an unsaturated halide (or triflate) reacts with an alkene and a base and palladium catalyst to form a substituted alkene [17]. The microfluidic system allows recycling of the ionic liquid and the Pd catalyst in a continuous manner. Microfabrication allows making micromixers in optically transparent materials for photochemical coupling reactions. Lu et al. carried out photochemical synthesis of benzopinacol in a micromixer made of silicon and quartz [18]. The transparent quartz allows UV light to reach the mixing channel. Both optical transparency and short residence time in a micromixer allow photochemical [2 + 3] cycloaddition in microscale [19]. In contrast to a conventional batch reactor, the reverse reaction is prevented by the shorter residence time in a micromixer.
Oxidation and reduction are basic reactions for the synthesis of both organic and inorganic compounds. Many oxidation reactions are exothermic. Thus, micromixers offer a safe reaction platform with controlled conditions. Furthermore, the danger of explosions is minimized due to the small amount of reactants involved. Hydrogen peroxide can be used as an effective liquid-phase oxidizing agent. Yube and Mae carried out the oxidation of 2-methylnaphthalene with hydrogen peroxide to 2-methyl-1,4-naphthoquinonone, an antihemorrhagic vitamin [20]. Because of the shorter residence time of 30 seconds, side reactions are minimized and a selectivity of 50% can be achieved for the intended product.
Using microtechnology, electrodes can be easily integrated in micromixers. Therefore, electrochemical oxidation and reduction of organic compound can be achieved. The large surface-to-volume ratio in microscale brings advantages of the higher mass transfer rate from and to the electrodes. Mengeaud et al. [21] carried out oxidation of furans in an electrochemical microreactor made of ceramic using H2SO4 as the support.
9.1.3. Gas–liquid reactions
Gas–liquid reactions can be carried out in micromixers in the same way as liquid–liquid micromixers. The immiscible phases will form emulsion or slug flow in the mixing channel. The small scale in micromixers allows the formation of liquid films (Fig. 9.1) or small gas bubbles (Fig. 9.2), thus increasing the contact surface between the two phases. Common gas–liquid reactions are [1]:
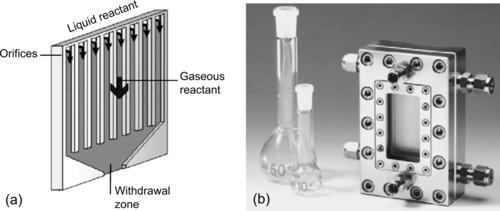 |
| FIGURE 9.1 (Reprinted with permission from [10].) |
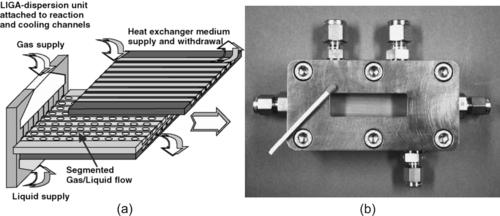 |
| FIGURE 9.2 (Reprinted with permission from [10].) |
• Substitution reactions (fluorination, chlorination, and nitration),
• Addition reactions, and
• Dehydration reactions.
Radicals for chlorination are induced thermally [24] or by irradiation [25]. Photochemical chlorination of toluene-2,4-diisocyanate was carried out in a microreactor with 32 parallel microchannels. Gas-phase chlorine was irradiated through a quartz window to form chlorine radicals. The space–time yield of this process is two orders of magnitude higher than that of a conventional reactor. The selectivity and yield of monochlorination of acetic acid are much better in microreactors than in the conventional counterpart [24].
Addition reaction such as hydrogenation involved gas-phase hydrogen. Cyclohexane hydrogenation was carried out in micromixer made of silicon [26]. The reaction occurred with platinum on alumina as catalyst under pressure and temperature close to standard room condition. A higher production of cyclohexane can be carried out with palladium as catalyst and under higher pressure and temperature [27]. The small amounts of reactants involved allow the potential use of micromixers for addition reactions with toxic reagents such as HCN for hydrocyanation and CO for carbonylation.
Dehydration reactions can also benefit from the advantages offered by micromixers. The dehydration of 1-hexanol to 1-hexene was carried out in a micromixer [15]. Compared to conventional dehydration processes, the conversion efficiency was significantly increased from 30% to over 85%. The same concept was applied to dehydration of ethanol to form ethylene, ethane, and methane.
9.1.4. Polymerization reactions
Polymerization reactions link up small monomers into a long polymer. Common polymerization reactions that can be realized in micromixers are [1]:
• Free radical polymerization,
• Living radical polymerization, and
• Cationic polymerization.
Micromixers were used for the production of acrylates [28]. The distribution of molecular weight of the polymers formed in micromixers show a significant improvement as compared to the macroscale counterpart. Large-scale production of polymers was achieved by numbering up the reactors [29]. The problem associated with numbering up is the uniformity of the flow due to the high viscosity of the reactive medium and possible clogging of some mixing channels. Iwasaki et al. [29] demonstrated that an amount of a few kilograms of PMMA can be produced continuously over several days at a constant temperature, pressure, and product quality.
Living radical polymerization (LRP) is a controlled free radical polymerization. This reaction allows control of the polymeric structure at molecular level leading to the synthesis of customized polymeric materials. The term “living” means that the polymer chain never ends by a terminal reaction. The polymer continues to grow if monomers are available. Thus, the polymer can be designed to have any molecular weight suitable for an application. A recently demonstrated LRP process is called atom transfer radical polymerization (ATRP). Homopolymerization of 2-hydroxypropyl methacrylate by ATRP was carried out in a micromixer [30]. The performance in a microreactor is similar to that in a macroscale batch process. The microreactor can be used for screening the compositions for ATRP. Furthermore, micromixers can be used for mixing viscous living polymer melts with less viscous monomer.
Cationic polymerization is a polymerization process where a cationic initiator transfers charge to a monomer, making it reactive. This reactive monomer continues to react in the same manner with other monomers to form a polymer. Monomers with electron-donating substituents and heterocycles such as olefins are needed for cationic polymerization. Cationic polymerization reactions are sensitive to the type of solvent used. Controlled living cationic polymerization based on cation stabilization was realized in micromixers [31]. Isobutyl vinyl ether (IBVE) was polymerized with a narrow molecular weight distribution in a parallel lamination micromixer. Fast mixing of initiator and monomer in micromixers allows a fast initiation. The ratio between initiator and monomer can be well controlled, leading to controlled molecular weight and its distribution.
9.1.5. Particles and emulsions
Improved mass transport in micromixers allows the implementation of controlled formation of solid particles. Solid-forming reaction can be realized in micromixers. Attention is to be paid to prevent contact between the formed solid particles and the channel wall, leading to possible fouling and blockage of the microchannels. One possible solution for the blockage problem is realizing the reaction with droplet-based microfluidics. The particles are formed and contained in droplets, which are transported by an immiscible phase.
In the production of pigments and colorants, fast mixing, particle size, and size distribution determine the quality of the product. Fast mixing in micromixers allows the production of smaller particles and narrower size distribution [32]. The high quality obtained with micromixers can be maintained for large-scale production by numbering up the microreactors [33].
The precisely controlled reaction conditions in micromixers make them suitable for the production of quantum dots. A quantum dot is a semiconductor nanocrystal whose excitons are confined in all three spatial dimensions. A quantum dot contains 100–100,000 atoms corresponding to a diameter of 10–50 atoms or 2–10 nanometers. Quantum dots have electronic properties between those of bulk semiconductors and those of discrete molecules. The properties of a quantum dot depend on its size and shape. If quantum dots are used as fluorescent dye, tuning the size from large to small results in a color shift of the emitted light from red to blue. Colloidal quantum dots are synthesized from three components: precursors, organic surfactants, and solvents. At a sufficiently high temperature, the precursors decompose into monomers. Once the monomers reach the supersaturation level, the growth of the nanoparticles starts with a nucleation process. Key parameters for the successful growth of nanocrystals are the precise control of temperature and the concentration of monomers. The temperature should be high enough for the rearrangement of the atoms and low enough to promote crystal growth. The concentration of the monomers affects the size of the particle and its distribution. Both temperature and concentration can be well controlled in a micromixer. Typical quantum dots are made of binary alloys such as cadmium selenide (CdSe), cadmium sulfide (CdS), indium arsenide (InAs), and indium phosphide (InP). Nakamura et al. [34] controlled the size of CdSe nanoparticles by adjusting the flow rate of the reactants in a micromixer. A shift in particle size from 2.8 nm to 4.2 nm was achieved. Chan et al. [35] tuned the size of CdS nanoparticles between 2.44 nm and 2.69 nm by controlling the temperature.
Metal nanoparticles are the other type of solid particles that can be synthesized in micromixers. Colloidal metal nanoparticles are synthesized by the reduction of metal salt or metal complex solutions. For instance, gold nanoparticles can be synthesized by reduction of tetrachloroaurate with citric acid at high temperature. Micromixers allow the fast mixing of metal salt and reducing agent as well as the rapid change of temperature between the steps of nucleation and particle growth. Wagner et al. [36] synthesized gold nanoparticles of 16–18 nm in a micromixer at room temperature using ascorbic acid as the reducing agent. The size of the nanoparticles can be tuned by adjusting the flow rate of the reactants. Higher flow rate and faster mixing result in smaller particles. Micromixers are suitable for the synthesis of metal nanoparticles with special shapes such as nanorods. Gold nanorods were formed in a micromixer by mixing tetrachloroauric, ascorbic acid, and CTAB [37]. The shape of the nanorods can be tuned by the flow rate ratio and the temperature.
9.1.6. Fuel processing
The need for clean energy sources and clean fuels leads to the recent rapid development of fuel cell technology. Hydrogen is the main fuel for fuel cells. Micromixers and microreactors are ideal platforms for the conversion of hydrocarbon fuels into hydrogen for miniature fuel cells for use in portable applications. Hydrocarbon fuels can be converted into hydrogen by catalytic partial oxidation (CPO) or oxidative steam reforming (OSR). The improved heat-transfer capability in micromixers makes them suitable for these fuel-reforming processes. The challenge in designing micromixers for this application is the integration of the reforming catalyst. The catalyst can be coated on the channel wall by sputtering or other coating techniques. However, coating the channel wall brings relatively small catalytic surface area. A packed bed or reaction chambers with microstructures such as pillars could increase the surface area and the catalytic activity. Furthermore, steam-reforming reaction is endothermic. The reformer requires external heat supply. The integration of a microcombustor and a heat exchanger into the system can utilize leftover hydrogen from the fuel cell for this purpose. Another alternative is the integration of resistive microheater in the system.
Pattekar and Kothare [38] implemented a methanol-reforming system in silicon with a conversion ratio of 90%. Holladay et al. [39] developed an integrated methanol-reforming system made of stainless steel. The system consists of two vaporizers, a heat exchanger, a catalytic combustor, and a catalytic methanol reformer. Kwon et al. [40] developed a fuel cell system made of silicon and glass. The system consists of a fuel reformer, a preferential oxidation reactor, and a fuel cell. The system was able to produce a power density of 230 mW/cm2 at 0.6 V, which is comparable to an operation with pure hydrogen.
9.2. Applications in Chemical and Biochemical Analysis
9.2.1. Concentration measurement
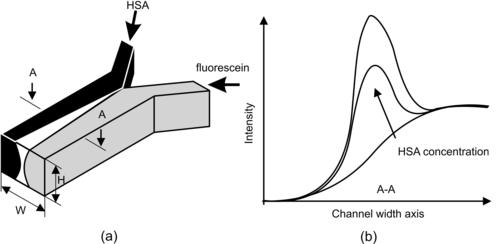
Micromixers are widely used in chemical, biological, and medical analysis fields. Almost every chemical assay requires mixing of reagents with a sample. The basic T-mixer was used in the work of Kamholz et al. [41] for the measurement of analyte concentration in a continuous flow. The concentration of a target analyte is measured with the fluorescence intensity of the region where the analyte and a fluorescent indicator have interdiffused [41]. Using this concept, the measurement of an analyte concentration can be carried out based on a continuous basis. This device is referred to as a T-sensor. The concept of a T-sensor is depicted in Fig. 9.3. Kamholz et al. [41] used the T-sensor for measurement of the concentration human serum albumin (HSA) in a sample. The fluorescein is Albumin blue 580 (AB580), which has high affinity for HSA but low affinity for other types of proteins. AB580 has an excitation wavelength of 580 nm and emits photons with a wavelength of 606 nm. The stream containing AB580 has low native fluorescence. However, the fluorescent intensity increases a few orders of magnitude when AB580 binds to HSA. In a T-sensor, the solution of interest, such as HSA sample, and the binding fluorescein, such as AB580, are introduced at the inlet. If the two streams have the same flow rate and the same viscosity, the interface of the two streams will be in the middle of the mixing channel (Fig. 9.3 (a)). The native fluorescein has its initial low level of intensity, while the intensity at the interface increases due to diffusion and subsequent binding reaction between the fluorescein and the protein. The higher the concentration of the protein, the higher the intensity peak at the interface (Fig. 9.3 (b)).
Veenstra et al. used a micromixer for the detection of ammonia in aqueous solutions [42]. The Berthelot reaction was used for the detection. Ammonia in an aqueous solution was converted into indophenol blue using a two-step reaction. The first step is the chlorination of ammonia to produce monochloramine NH2Cl. In the second step, two phenol molecules bind to form monochloramine, resulting in indophenol blue. Indophenol blue can be detected with an absorption measurement because it has a peak at 625 nm in the absorption spectrum. Because the kinetics of formation of indophenol blue is relatively slow, the micromixer should allow a residence time on the order of 1 min for a complete conversion of all ammonia molecules in the solution into indophenol blue.
9.2.2. Improving chemical and biochemical analysis
Protein folding is controlled by the solvent composition of a protein solution. The changes in protein conformation as a response to changes in solvent composition can be measured using time-resolved nuclear magnetic resonance (NMR) spectroscopy. Time-resolved measurement of reaction kinetics using nuclear magnetic resonance (NMR) can benefit from the fast mixing time in a micromixer [43]. The solvent/protein interaction time depends on the mixing length and the flow rates of the mixed streams. Adjusting these two parameters allows the measurement of NMR spectra at a precise time instance. Microcoils for NMR can be integrated with the micromixer to facilitate on-chip measurement (Fig. 9.4 (a)). The integration of an array of microcoils would allow simultaneous measurement of multiple detection points. Such micromixers with integrated microcoils for NMR spectroscopy can be used for investigations of reaction intermediates and molecular interactions. Kakuta et al. used ubiquitin as the test protein. Ubiquitin changes its conformation from native to A-state at low pH and in 40% or higher methanol/water solvents. The micromixer was used for mixing the ubiquitin solution with the methanol solution. The concentration of A-state increases with better mixing. Because the micromixer in use was a Y-mixer with parallel lamination (Chapter 2), good mixing was achieved at low Peclet number or low flow rates. The reported NMR measurement can resolve the changes in a time scale of seconds.
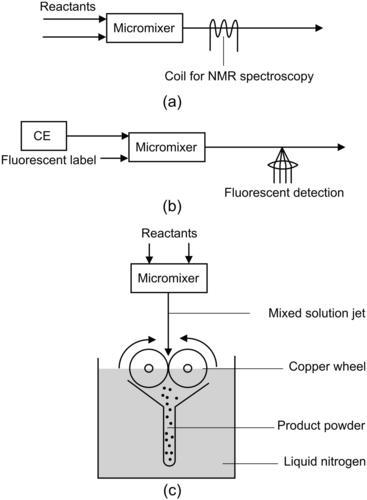 |
| FIGURE 9.4 |
Another application of micromixers is the labeling reaction of molecules after their separation using techniques such as capillary electrophoresis (CE). Because fluorescence measurement is commonly used for detecting these molecules, they must be derivatized with a fluorescent label. Micromixers can be integrated in a CE chip as postcolumn reactors. Because the reactants and the products continue to be separated in the mixing channel, micromixers for this purpose should be efficient enough to allow rapid reaction. Slow reaction may lead to band broadening caused by the different mobilities of reactants and products. Fluri et al. [44] combine capillary electrophoresis (CE) separation with a T-shaped intersection for the reaction of amino acids with the labeling reagent o-phthaldialdehyde (OPA; Fig. 9.4 (b)). Fluid flows in this system were electrokinetically driven.
Fast mixing with a micromixer was used for freeze-quenching technique, which is useful for trapping meta-stable intermediates during fast chemical or biochemical reactions [45]. The determination of the molecular properties of these intermediates leads to further understanding of chemical and biochemical reactions. The freeze-quenching technique ejects the mixed solution from a continuous-flow mixer through a small nozzle into an isopentane bath at a low temperature (–130 °C). The frozen samples contain trapped reaction intermediates that can be conveniently investigated without the time constraints (Fig. 9.4 (c)). On a macroscale, the application of freeze-quenching technique is limited because of the long mixing time and the slow freezing time of cryogenic fluids. The delay time is generally on the order of milliseconds. Before the spectroscopic investigation, the reaction should be initiated in a way such that all of the molecules are in phase. A mixer should work as a microreactor for this purpose. The earliest time allowed by conventional mixers for detection of the intermediates is on the order of milliseconds. Although the time limit can be solved by lowering the reaction temperature and fast spectroscopic tools, it is easier to reduce the mixing dead time using micromixers. The micromixer reported by Lin et al. [46] allows freeze quenching within 20 μs resulting in ultrafine frozen powder with excellent spectral quality and high packing factor.
Enzyme assays are the basic techniques in clinical and bioanalytical chemistry. Micromixers can promote the reactions between the enzyme and the substrate. These devices often use electrokinetic transport to deliver analyte and enzyme into the chip and to the detector. An electrokinetically driven T-mixer was used in [47] for performing enzyme assays. Substrate, buffer, enzyme, and inhibitor were mixed in two stages. The amount of each reagent was controlled by the applied voltages. Hadd et al. [47] used resorufin β-D-galactopyranoside (RBG), β-galactosidase (β-Gal), and phenylethyl β 4-D-thiogalactoside (PETG) as the substrate, the enzyme, and the inhibitor, respectively. The reaction forms resorufin, a fluorescent product with an emission wavelength of 585 nm. The system allows the measurement of reaction kinetics by varying the concentration of substrate and monitoring the amount of resorufin using fluorescent detection. The assay performed with the micromixers consumed about four orders of magnitude less reagents compared to a conventional assay. Micromixers can therefore help to reduce the cost of enzyme assays, especially of those with expensive reagents. Burke and Regnier [46] reported a microfabricated enzyme assay system with a micromixer to perform stopped-flow reactions. The device was tested with –galactosidase (–Gal) as the enzyme and fluorescein mono–d-galactopyranoside (FMG) as the substrate.
Micromixers can work as a reaction platform of drug production using recombinant protein production. The production process consists of several steps. In the first step, the DNA sequence of the protein to be produced is inserted into the DNA of viruses. The viruses in turn are mixed with a cell culture to allow the infection cycle. After a certain amount of time, all cells are infected with the virus and the recombinant protein can be collected. The infection process can be optimized by the right concentration of the virus. Thus, determining the right concentration of virus is crucial for the protein production. In conventional reactors, virus is diluted to various concentrations and used for infecting separated batches of cells. The optimum virus concentration is then determined by evaluating the amount of proteins harvested in each cell batch. Diffusion transport in a lamination micromixer could allow the formation of a concentration gradient where cell infection at different concentrations can be realized concurrently. Protein expression based on fluorescent measurement can be carried out on a chip. The short mixing length of a cross-mixer with hydrodynamic focusing makes fast infection of a cell with virus possible. Walker et al. [48] reported the infection of cells by virus at different concentrations in a cross-mixer. The cells are attached on the bottom of the mixing channels. A concentration gradient of virus particles was created by diffusive mixing in the flow-focusing configuration where the middle stream contains virus particles. The cells were expressed and monitored with a green fluorescent protein.
In biochemical sensors, the analyte often needs to be transported to immobilized receptors to make binding and subsequent detection possible. Receptors are surface-immobilized biomolecules that are complementary to the biomolecules to be detected. Vijayendran et al. used micromixers based on chaotic advection to promote analyte transport to receptors immobilized on a surface [49]. Soluble rabbit IgG antibodies were passed through the micromixer allowing them to bind to protein A immobilized on one microchannel wall. The binding reactions were detected using surface plasmon resonance (SPR) concept. Since the binding kinetics is two or three orders higher than the diffusion of analytes, the quality of detection depends on the extent of chaotic advection. Experiments showed that compared to a simple T-mixer, a chaotic mixer can double the rate of analyte detection.
Kim et al. [50] used the F-shaped chaotic micromixer depicted in Fig. 6.15 for blood typing on a disposable chip. Blood typing is an important blood test because transfusion of incompatible blood groups (A, B, AB, and O) of recipients or donors may lead to intravascular hemolysis in the recipient. The blood group is determined by agglutination results of red blood cells reacting with the corresponding blood serum. The reaction shows the presence of antigens (agglutinogens) on the red blood cells corresponding to antibodies (agglutinins) in the serum. The sera of anti-A, anti-B, and anti-AB are obtained from the sera of blood group B persons, A persons, and O persons, respectively. The micromixers work as reactors for the agglutination process. The small size of the device allows blood typing with a very small sample blood volume on the order of 1 μL. Besides the chaotic micromixers, the reported lab-on-a-chip device also contains flow-splitting microchannels, reaction chambers, and detection microfilters (Fig. 9.5). The blood sample was divided into multiple equal volumes through the flow-splitting microchannel so that multiple tests can be performed in parallel. The reaction chambers were used to keep the mixture of the blood and serum for a few minutes before filtering. The gradually decreasing multistep detection microfilters were designed for separation of the reacted agglutinated red blood cells. The separation results allow visual detection of blood groups A, B, and AB.
9.2.3. Purification and preconcentration
Sensitive detection is crucial for biochemical analysis. The sample condition may affect the quality of processes such as polymerase chain reactions (PCR). To improve the accuracy of pathogen detection, preconcentration and purification of a DNA sample are necessary before PCR. Furthermore, higher sample concentration also leads to better detection sensitivity. Depending on the filtering or trapping concept, micromixers can be used for controlling buffer concentration or generating chaotic advection as described in the following two examples.
Lee et al. [51] used a serpentine chaotic micromixer for DNA purification. Because DNA has a negative charge, it is strongly adsorbed by the glass surface under high-salt buffer conditions. The binding forces to glass of other contaminants, such as proteins or sugars, are relatively weak. Thus, packed beads can be used for DNA purification. The adsorbed DNA can subsequently be released and collected if a low-salt buffer is introduced into the packed chamber. A micromixer can realize the stepwise change of salt concentration in a buffer solution before flushing it through the packed chamber. Lee et al. used the micromixer to change the concentration of MgCl2 from 500 mmol/L to 15 mmol/L. For this purpose, the mixing ratios were controlled at 1:1 and 1:66.
Dielectrophoresis (DEP) can be utilized for trapping, manipulating, and separating bioparticles, such as virus, DNA molecules, bacteria, and cells. Planar interdigitated electrodes (IDEs) can be used to generate the nonuniform electric field required for dielectrophoresis. However, the electrophoretic force is only effective if the sample particles are brought close to the surface with the IDEs, similar to the case of surface-immobilized receptors. Lee and Voldman [52] used the micromixers depicted in Figure 6.19 and Figure 6.21 to bring more sample particles closer to the IDEs. Chaotic advection in the flow increases the amount of particles trapped onto the surface. Using the mixer, the amount of trapped particles increased by 50% compared to the case of a straight and smooth channel.
9.2.4. Biological assays
Micromixers in the form of gradient generators have a number of biological applications. The major biological assays based on a concentration gradient are cancer chemotaxis, immune response, stem cell differentiation, axon guidance, and angiogenesis [53] and [54].
Cancer metastasis is the late stage of the disease where cancer cells spread to other organs. Metastasis consists of two main steps: intravasation and extravasation. In the first step of intravasation, cancer cells are transported in the circulatory system. In the second step of extravasation, cancer cells migrate and spread from blood vessel to the organ tissues. These steps are regulated by a number of chemoattractants such as growth factors and chemokines, which are chemotactic cytokines. For instance, chemokine receptor type 4/chemokine (C-X-C motif) ligand 12 (CXCR4/CXCL12), epidermal growth factor (EGF), and insulin-like growth factor 1 (IGF1) are potent mediators of breast cancer. The gradient of the above-mentioned chemoattractants significantly affect the migration of cancer cells. Gradient generators can mimic the environment in tissues under controlled conditions. Understanding cancer metastasis in gradient generators can lead to the development of new therapeutics against cancers.
Wang et al. studied metastasis of breast cancer cells in a concentration gradient of EGF generated by a parallel lamination generator [55]. The results show that the cancer cells show a more directional movement in a nonlinear gradient than in a linear one (Fig. 9.6). Abhyankar et al. [56] used a free-diffusion generator forming an EGF gradient in a 3-D gel matrix to study metastasis of rat mammary adenocarcinoma cells. The extracellular matrix (ECM) represents an additional controllable factor for the study. The platform allows detailed investigation of the interaction of the cells with the ECM substrate in an EGF gradient.
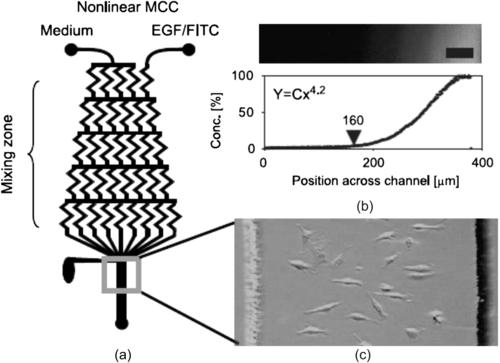 |
| FIGURE 9.6 Parallel lamination gradient generator: (a) device concept; (b) generated concentration distribution; and (c) cell spreading after subjecting to a nonlinear EGF gradient [55]. |
Immune response is affected by chemokines and their receptor. Chemokines recruit leukocytes to the infection site. Therefore, detailed studies on the ability of immune cells to sense the gradient are important for the understanding of immune response. Neutrophils or polymorphonuclear neutrophils (PMNs) are the most abundant type of white blood cells in mammals. During the early phase of inflammation, neutrophils are one of the first responders to migrate toward the infection site. Neutrophils migrate through the blood vessels, then through interstitial tissue, following the gradient of chemoattractants such as interleukin-8 (IL-8), C5a, and leukotriene B4 (LTB4). Jeon et al. [57] used a parallel lamination generator to investigate chemotaxis of human neutrophils in a concentration gradient of IL-8. The results show that the behavior of neutrophils depends not only on the gradient but also on the shape of the concentration distribution. Further investigations on the same platform [58] reveal that the mean concentration of linear gradients strongly affects the directed motility of neutrophils.
Axon guidance is important for the regeneration of nerve cells. Chemotaxis of neuronal precursors and their differentiation are the key processes of axonal regeneration. The concentration gradient of both diffusible and surface-bound molecules can modulate axon guidance. Dertinger et al. used a parallel lamination generator to investigate the effect of laminin gradient on the axon specification of rat hippocampal neurons [59]. Axons were oriented toward the higher concentration of laminin. Another important factor affecting axon guide is the mechanical stiffness of the substrate. Generating a concentration gradient in gel matrices with controllable stiffness could open up new tools for investigating axon guidance.
Stem cells are special types of cells that can grow into any specialized cell type in tissues and organs. Several biochemical and biophysical factors control the differentiation of stem cells. A gradient generator can be used to control the spatial and temporal distribution of cytokines and growth factors. Chung et al. used a parallel lamination gradient generator to form a stable gradient of a growth factor mixture of epidermal growth factor, fibroblast growth factor 2 (FGF2), and platelet-derived growth factor (PDGF) [60]. Human neural stem cells (hNSCs) are grown into astrocytes in this environment. The differentiation of hNSCs shows a clear dependence on the concentration gradient of the growth factor mixture (Fig. 9.7). Amadi et al. used a free-diffusion gradient generator to investigate the differentiation of embryoid [61]. The cells were grown in a 3-D collagen matrix under a concentration gradient of morphogen.
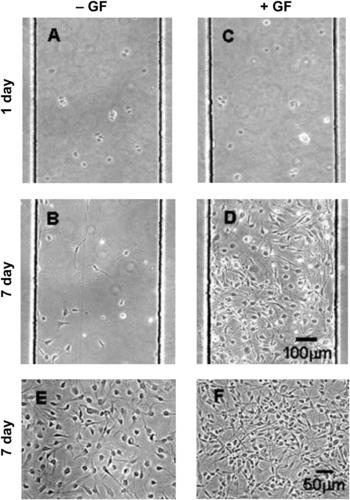 |
| FIGURE 9.7 Proliferation of hNSCs with (+GF) and without (2GF) growth factors [60]. |
Angiogenesis is the growth process of new blood vessels from existing vessels. It is a vital process in growth, development, and wound healing. However, angiogenesis is also a key step in the transition of tumors from a dormant state to a malignant one. Controlled concentration gradient of signaling molecules such as vascular endothelial growth factor A (VEGF-A) can guide the sprouting of new vessels. Barkefors used a simple parallel lamination micromixer in flow-focusing configuration with three streams to investigate angiogenesis of human umbilical vein (HUVEC) and artery endothelial cells (HUAEC) [62]. A concentration gradient of VEGF-A was generated. HUVECs migrated toward the maximum concentration and switched to nonmigratory phenotypes at the peak of the concentration distribution. Shamloo et al. investigated the response of HUVECs in a gradient of VEGF [63]. The linear gradient was formed in a free-diffusion generator (Fig. 9.8). The results show that both mean concentration and gradient affect the directional migration of HUVECs.
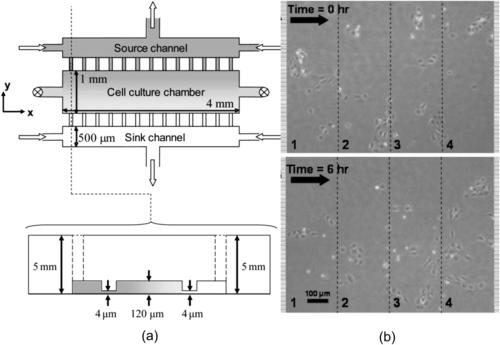 |
| FIGURE 9.8 Free-diffusion gradient generator for the investigation of angiogenesis: (a) device concept and (b) HUVEC chemotaxis in the cell culture chamber [63]. |
9.3. Outlook
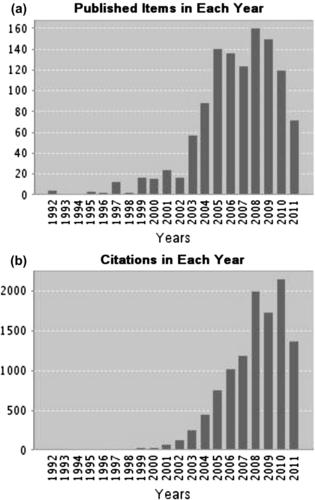
Micromixers continue to receive attention of the research community, because of the enormous potential and impact in chemical analysis and chemical industry. Figure 9.9 shows the statistics on the number of published research works on micromixers and their citations until June 2011. After the initial surge of interest, the research output on micromixers has been stabilizing at 100–150 papers per year. This solid number proves the importance of micromixers in the quest for miniaturization in chemical and biochemical analysis and chemical industry.
 |
| FIGURE 9.9 |
The recent trend points to the development of micromixers without moving parts. Passive and active micromixers with chaotic advection are preferred due to their effectiveness in microscale. Mixing based on chaotic advection caused by flow-guiding structure could be the focus of future research work on passive micromixers. With technologies such as hot embossing or molding, the flow-guiding structures and the mixing channel can be fabricated in the same process. Mixing based on electrokinetic disturbance could be the favorite one among the active mixing concepts because of the simplicity in the fabrication. Only electrodes are needed for actuation. As described in Section 7.5, mixing based on electrokinetic instability would require a relatively strong DC electric field and, consequently, a high voltage. Electrokinetic mixing based on an AC field could have the potential for creating chaotic advection at a much lower electric field and supply voltage.
In industrial applications, solid and reliable designs for high throughput are required. Pressure-driven passive mixers made in materials such as ceramics and stainless steel are suitable for this purpose. For extremely aggressive conditions such as high temperature and high pressure, materials such as silicon carbide may be needed for the fabrication of the micromixers. However, finding a suitable micromachining technology for these materials could be a challenge. Furthermore, the micromixers for chemical production may need to fulfill other requirements such as energy consumption and fouling resistance. Besides mixing function, microreactors should be able to provide a controlled environment for the industrial production of chemicals. Additional functions such as heating and temperature sensing would be needed for controlling the temperature of the reaction. For a wide adoption of micromixers as platforms for microreactor technology, issues related to intellectual properties and the cost of the first user should be considered. A high cost for setting up a production process based on microreactor technology may hinder or delay the transfer of this technology from academic research to industrial production [64].
References
[1] V. Hessel, A. Renken, J.C. Schoten, J. Yoshida (Eds.), Micro process engineering, vol 2: Devices, reactions and applications, Wiley-VCH, Weinheim.
[2] March, J., Advanced organic chemistry. fourth ed (1992) Wiley, New York.
[3] Ueno, M.; Hisamoto, H.; Kitamori, T.; Kobayashi, S., Phase-transfer alkylation reactions using micro reactors, Chem. Commun. (2003) 936–937.
[4] Belder, D.; Ludwig, M.; Wang, L.; Reetz, M.T., Enantioselective catalysis and analysis on a chip, Angew. Chem. Int. Ed. 45 (2006) 2463–2466.
[5] Schwalbe, T.; Autze, V.; Hohmann, M.; Stirner, W., Novel innovation systems for a cellular approach to continuous process chemistry from discovery to market, Org. Proc. Res. Dev. 8 (2004) 440–454.
[6] Lu, S.; Watts, P.; Chin, F.T.; Hong, J.; Musachio, J.L.; Briad, E.; Pike, V., Synthesis of 11C- and 18F-labeled carboxylic esters within a hydrodynamically-driven micro reactor, Lab Chip 6 (2004) 523–525.
[7] Suga, S.; Nagaki, A.; Yoshida, J., Highly selective Friedel-Crafts monoalkylation using micromixing, Chem. Commun. 3 (2003) 354–355.
[8] Ducry, L.; Roberge, R.M., Controlled autocatalytic nitration of phenol in a microreactor, Angew. Chem. Int. Ed. 44 (2005) 7972–7975.
[9] Henke, L.; Winterbauer, H., A modular microreactor for mixed acid nitration, Chem. Eng. Technol. 28 (2005) 749–752.
[10] Loeb, P.; Loewe, H.; Hessel, V., Fluorinations, chlorinations and brominations of organic compounds in micro reactors, J. Fluor. Chem. 125 (2004) 1677–1694.
[11] Wiles, C.; Watts, P.; Haswell, S.J.; Pombo-Villar, E., The aldol reaction of silyl enol ethers within a micro reactor, Lab Chip 1 (2002) 100–101.
[12] Fukuyama, T.; Hino, Y.; Kamata, N.; Ryu, I., Quick execution of [2+2] type photochemical cycloaddition reaction by continuous reaction by continuous flow system using a glass-made microreactor, Chem. Lett. 33 (2004) 1430–1431.
[13] Fukuyama, T.; Kobayashi, M.; Rahman, M.T.; Kamata, N.; Ryu, I., Spurring radical reactions of organic halides within hydride and TTMSS using microreactors, Org. Lett. 10 (2008) 533–536.
[14] Tanaka, K.; Motomatsu, S.; Koyama, K.; Tanaka, S.I.; Fukase, K., Large-scale synthesis of immunoactivating natural product, pristine, by continuous microfluidic dehydration as the key step, Org. Lett. 10 (2007) 299–302.
[15] Wilson, N.G.; McCreedy, T., On-chip catalysis using a lithographically fabricated glass microreactor – the dehydration of alcohols using sulfated zircon, Chem. Commun. 9 (2000) 733–734.
[16] Greenway, G.M.; Haswell, S.J.; Morgan, D.O.; Skelton, V.; Styring, P., The use of a novel microreactor for high throughput continuous flow organic synthesis, Sensor. Actuator. B Chem. 63 (2000) 153–158.
[17] Shi, G.; Hong, F.; Liang, Q.; Fang, H.; Nelson, S.; Weber, S.G., Capillary-based serial-loading, parallel microreactor for catalyst screening, Anal. Chem. 78 (2006) 1972–1979.
[18] Lu, H.; Schmidt, M.A.; Jensen, K.F., Photochemical reactions and on-line UV detection in microfabricated reactors, Lab Chip 1 (2001) 22–28.
[19] Mukae, H.; Maeda, H.; Nashihara, S.; Mizuno, K., One-step synthesis of benzotetra- and benzopentacyclic compounds through intramolecular [2+3] photocycloaddition of alkenes to naphthalene, Angew. Chem. Int. Ed. 45 (2006) 6558–6560.
[20] Yube, K.; Mae, K., Efficient oxidation of aromatics with peroxides under severe conditions using a microreaction system, Chem. Eng. Technol. 28 (2005) 331–336.
[21] Mengeaud, V.; Bagel, O.; Ferrigno, R.; Girault, H.H.; Haider, A., A ceramic electrochemical microreactor for the methoxylation of methyl-2-furoate with direct mass spectrometry coupling, Lab Chip 2 (2002) 39–44.
[22] Chambers, R.D.; Holling, D.; Rees, A.J.; Sandford, G., Microreactors for oxidations using fluorine, J. Fluor. Chem. 119 (2003) 81–82.
[23] Chambers, R.D.; Fox, M.A.; Holling, D.; Nakano, T.; Okazoe, T.; Sandford, G., Elemental fluorine. Part 16. Versatile thinfilm gas-liquid multi-channel microreactors for effective scale-out, Lab Chip 5 (2005) 191–198.
[24] Chambers, R.D.; Holling, D.; Spink, R.C.H.; Sandford, G., Elemental fluorine. Part 13. Gas-liquid thin film microreactors for selective direct fluorination, Lab Chip 1 (2001) 132–137.
[25] Ehrlich, H.; Linke, D.; Morgenschweis, K.; Baerns, M.; Jahnisch, K., Application of microstructured reactor technology for the photochemical chlorination of alkylaromatics, Chimia 56 (2002) 647–653.
[26] Losey, M.W.; Schmidt, M.A.; Jensen, K.F., Microfabricated multiphase packed bed reactors: characterization of mass transfer and reaction, Ind. Eng. Chem. Res. 40 (2001) 2555–2562.
[27] Trachsel, F.; Hutter, C.; von Rohr, Ph. R., Transparent silicon/glass microreactor for high-pressure and high-temperature reactions, Chem. Eng. J. 135 (2008) 309–316.
[28] Iwasaki, T.; Yoshida, J.I., Free radical polymerization in microreactors, significant improvement in molecular weight distribution control, Macromolecules 38 (2005) 1159–1163.
[29] Iwasaki, T.; Kawano, N.; Yoshida, J.I., Radical polymerization using microflow system: numbering-up of microreactors and continuous operation, Org. Proc. Res. Dev. 10 (2006) 1126–1131.
[30] Wu, T.; Mei, Y.; Cabral, J.T.; Xu, C.; Beers, K.L., A new synthetic method for controlled polymerization using a microfluidic system, J. Am. Chem. Soc. 126 (2004) 9880–9881.
[31] Inagaki, N.; Ando, T.; Sawamoto, M.; Kamigaito, M., Living cationic polymerization with micromixer: syntheses of end-functionalized polymers and multiblock copolymer, Polym. prepr. Jpn. 53 (2004) 2416–2417.
[32] Pennemann, H.; Forster, S.; Kinkel, J.; Hessel, V.; Lowe, H.; Wu, L., Improvement of dye properties of the azo pigment yellow 12 using a micromixer-based process, Org. Proc. Res. Dev. 9 (2005) 188–192.
[33] Wille, C.; Gabski, H.P.; Haller, T.; Kim, H.; Unverdorben, L.; Wehle, D., Synthesis of pigments in a three-stage microreactor pilot plant-an experimental technical report, Chem. Eng. J. 101 (2004) 179–185.
[34] Nakamura, H.; Tashiro, A.; Yamaguchi, Y.; Miyazaki, M.; Watari, T.; Shimizu, H.; Maeda, H., Application of a microfluidic reaction system for CdSe nanocrystal preparation: their growth kinetics and photoluminescence analysis, Lab Chip 4 (2004) 237–240.
[35] Chan, E.M.; Mathies, R.A.; Alivisatos, A.P., Size-controlled growth of CdSe nanocrystals in microfluidic reactors, Nano Lett. 3 (2003) 199–201.
[36] Wagner, J.; Kirner, T.; Mayer, G.; Albert, J.; Kohler, J.M., Generation of metal nanoparticles in a microchannel reactor, Chem. Eng. J. 101 (2004) 251–260.
[37] Sonnichsen, C.; Alivisatos, A.P., Gold nanorods as novel nonbleaching plasmon-based orientation sensors for polarized single-particle microscopy, Nano Lett. 5 (2005) 301–304.
[38] Pattekar, A.V.; Kothare, M.V., A microreactor for hydrogen production in micro fuel cell applications, J. Microelectromech. Syst. 13 (2004) 7–18.
[39] Holladay, J.D.; Jones, E.O.; Phleps, M.; Hu, J., High efficiency and low carbon monoxide micro-scale methanol processors, J. Power Sourc. 131 (2004) 69–72.
[40] Kwon, O.J.; Hwang, S.M.; Chae, J.H.; Kang, M.S.; Kim, J.J., Performance of a miniaturized silicon reformer-PrOx-fuel cell system, J. Power Sourc. 165 (2007) 342–346.
[41] Kamholz, A.E.; et al., Quantitative analysis of molecular interactive in microfluidic channel: the T-sensor, Anal. Chem. 71 (1999) 5340–5347.
[42] Veenstra, T.T., Characterization method for a new diffusion mixer applicable in micro flow injection analysis systems, J. Micromech. Microeng. 9 (1999) 199–202.
[43] Kakuta, M.; et al., Micromixer-based time-resolved NMR: Applications to ubiquitin protein conformation, Anal. Chem. 75 (2003) 956–960.
[44] Fluri, K.; et al., Integrated capillary electrophoresis devices with an efficient postcolumn reactor in planar quartz and glass chips, Anal. Chem. 68 (1996) 4285–4290.
[45] Lin, Y.; et al., Ultrafast microfluidic mixer and freeze-quenching device, Anal. Chem. 75 (2003) 5381–5386.
[46] Burke, B.J.; Regnier, F.E., Stopped-flow enzyme assays on a chip using a microfabricated mixer, Anal. Chem. 75 (2003) 1786–1791.
[47] Hadd, A.G.; et al., Microchip device for performing enzyme assays, Anal. Chem. 69 (1997) 3407–3412.
[48] Walker, G.M.; Ozers, M.S.; Beebe, D.J., Cell infection within a microfluidic device using virus gradients, Sensor. Actuator. B Chem. 98 (2004) 347–355.
[49] Vijiayendran; et al., Evaluation of a three-dimensional micromixer in a surface-based biosensor, Langmuir 19 (2003) 1824–1828.
[50] Kim, D.S.; Lee, S.H.; Ahn, C.H.; Leed, J.Y.; Kwon, T.H., Disposable integrated microfluidic biochip for blood typing by plastic microinjection moulding, Lab Chip 6 (2006) 794–802.
[51] Lee, N.Y.; Yamada, M.; Seki, M., Development of a passive micromixer based on repeated fluid twisting and flattening, and its application to DNA purification, Anal. Bioanal. Chem. 5 (2005) 776–782.
[52] Lee, H.Y.; Voldman, J., Optimizing micromixer design for enhancing dielectrophoretic microconcentrator performance, Anal. Chem. 79 (2007) 1833–1839.
[53] Kim, S.D.; Kim, H.J.; Jeon, N.L., Biological applications of microfluidic gradient devices, Integr. Biol. 2 (2010) 584–603.
[54] Chung, B.G.; Choo, J.B., Microfluidic gradient platforms for controlling cellular behavior, Electrophoresis 31 (2010) 3014–3027.
[55] Wang, S.J.; Saadi, W.; Lin, F.; Nguyen, C.M.C.; Jeon, N.L., Differential effects of EGF gradient profiles on MDA-MB-231 breast cancer cell chemotaxis, Exp. Cell Res. 300 (2004) 180–189.
[56] Abhyankar, V.V.; Toepke, M.W.; Cortesio, C.L.; Lokuta, M.A.; Hutenlocher, A.; Beebe, D.J., A platform for assessing chemotactic migration within a spatiotemporally defined 3D microenvironment, Lab Chip 8 (2008) 1507–1515.
[57] Jeon, N.L.; Baskaran, H.; Dertinger, S.K.W.; Whitesides, G.M., Neutrophil chemotaxis in linear and complex gradients of interleukin-8 formed in a microfabricated device, Nat. Biotechnol. 20 (2002) 826–830.
[58] Lin, F.; Nguyen, C.M.C.; Wang, S.J.; Saadi, W.; Gross, S.P.; Jeon, N.L., Effective neutrophil chemotaxis is strongly influenced by mean IL-8 concentration, Biochem. Biophys. Res. Commun. 319 (2004) 576–581.
[59] Dertinger, S.K.W.; Jiang, X.Y.; Li, Z.Y.; Murthy, V.N.; Whitesides, G.M., Gradients of substrate-bound laminin orient axonal specification of neurons, Proc. Natl. Acad. Sci. USA. 99 (2002) 12542–12547.
[60] Chung, B.G.; Flanagan, L.A.; Rhee, S.W.; Schwartz, P.H.; Lee, A.P.; Monuki, E.S.; Jeon, N.L., Human neural stem cell growth and differentiation in a gradient-generating microfluidic device, Lab Chip 5 (2005) 401–406.
[61] Amadi, O.C.; Steinhauser, M.L.; Nishi, Y.; Chung, S.; Kamm, R.D.; McMahon, A.P.; Lee, R.T., A low resistance microfluidic system for the creation of stable concentration gradients in a defined 3D microenvironment, Biomed. Microdevices 12 (2010) 1027–1041.
[62] Barkefors, I.; Le Jan, S.; Jakobsson, L.; Hejll, E.; Carlson, G.; Johansson, H.; Jarvius, J.; Park, J.W.; Jeon, N.L.; Kreuger, J., Endothelial cell migration in stable gradients of vascular endothelial growth factor a and fibroblast growth factor 2-Effects on chemotaxis and chemokinesis, J. Biol. Chem. 283 (2008) 13905–13912.
[63] Shamloo, A.; Ma, N.; Poo, M.M.; Sohn, L.L.; Heilshorn, S.C., Endothelial cell polarization and chemotaxis in a microfluidic device, Lab Chip 8 (2008) 1292–1299.
[64] Roberge, D.M.; Durcy, L.; Bieler, N.; Cretton, P.; Zimmermann, B., Microreactor technology: a revolution for the fine chemical and pharmaceutical industries?Chem. Eng. Technol. 28 (2005) 318–322.
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.