Chapter 1. Introduction
Chapter Outline
1.1. Micromixers and Mixing in Microscale1
1.2. Micromixers as Microreactors5
1.3. Organization of the Book7
References8
This book discusses the design, fabrication, and characterization of micromixers, which are defined as miniaturized mixing devices for at least two different phases, which could be liquids, solids, or gases. The structures of a micromixer are fabricated partially or in whole, using microtechnology or precision engineering. The characteristic channel size of micromixers is in the sub-millimeter range. Common channel widths are on the order of 100-500μm, while channel length could be a few millimeters or more. The channel height is on the order of the channel width or is smaller. The overall volume defined by a micromixer ranges from microliters to milliliters. Compared to the molecular size scale, the length scale and volume scale of micromixers are very large. This fact leads to two key characteristics of micromixers. First, designing micromixers relies on manipulating the flow using channel geometry or external disturbances. Second, while micromixers bring advantages and new features into chemical engineering, molecular-level processes such as reaction kinetics remain almost unchanged.
1.1. Micromixers and Mixing in Microscale
This book discusses the design, fabrication, and characterization of micromixers, which are defined as miniaturized mixing devices for at least two different phases, which could be liquids, solids, or gases. The structures of a micromixer are fabricated partially or in whole, using microtechnology or precision engineering. The characteristic channel size of micromixers is in the sub-millimeter range. Common channel widths are on the order of 100–500μm, while channel length could be a few millimeters or more. The channel height is on the order of the channel width or is smaller. The overall volume defined by a micromixer is from microliters to milliliters. Compared to molecular size scale, the length scale and volume scale of micromixers are very large. This fact leads to two key characteristics of micromixers. First, designing the micromixers relies on manipulating the flow using channel geometry or external disturbances. Second, while micromixers bring advantages and new features into chemical engineering, molecular-level processes such as reaction kinetics remain almost unchanged.
Mixing is a transport process for species, temperature, and phases to reduce inhomogeneity. Mixing leads to secondary effects such as reaction and change in properties. In conventional macroscale mixing techniques, there are three established terminologies for mixing: macromixing, mesomixing, and micromixing [1]. Macromixing refers to mixing governed by the largest scale of fluid motion. For instance, the scale of macromixing corresponds to the diameter of the mixing tank. Micromixing is mixing at the smallest scale of fluid motion and molecular motion. In conventional macroscale mixing, the smallest scale of fluid motion is the size of turbulent eddies, also called the Kolmogorov scale. Mesomixing is in the scale between macromixing and microscale. Although micromixers may have dimensions on the order of micrometers, transport process in micromixers may still be classified as mesomixing. Since structures in micromixers may have a size approaching the Kolmogorov scale, this book avoids the use of micromixing for describing mixing processes.
There are many different ways to provide mixing in macroscale such as molecular diffusion, eddy diffusion, advection, and Taylor dispersion. Eddy diffusion is the transport of large groups of species and requires a turbulent flow. Because of the dominant viscous effect in microscale, turbulence is not possible in micromixers. Mixing based on eddy diffusion is therefore not relevant for micromixers. Thus, the main transport phenomena in micromixers include molecular diffusion, advection, and Taylor dispersion. Molecular diffusion is caused by the random motion of molecules. This transport mechanism is characterized by the molecular diffusion coefficient. Advection is the transport phenomena caused by fluid motion. A simple Eulerian velocity can lead to a chaotic distribution of the mixed species. A stable and laminar flow can also lead to chaotic advection. Thus, chaotic advection would be ideal for the laminar flow condition in micromixers. Taylor dispersion is advection caused by a velocity gradient. Axial dispersion occurs due to advection and inter-diffusion of fluid layers with different velocities. Due to this effect, mixing based on Taylor dispersion can be two or three orders faster than that based on pure molecular diffusion.
Designing micromixers is a completely new engineering discipline, because existing designs in macroscale cannot simply scale down for microscale applications. One of the main challenges related to miniaturization is the dominance of surface effects over volume effects. Actuation concepts based on volume forces working well in macroscale may have problems in microscale. A magnetic stirrer is a typical example of the ratio between surface forces and volume forces. It consists of a magnet bar and a rotating magnet or stationary electromagnets creating a rotating magnetic field. The driving magnetic force is proportional to the volume of the magnet bar, while the friction force is proportional to its surface. Scaling down the stirrer follows the so-called cube-square law. This means shrinking down the stir bar 10 times would roughly decrease its volume by 1000 times and its surface only by 100 times. With its original size, the external magnetic field can generate a force of the same order of the friction force and cause the stir bar to move. Scaling down the size 10 times in the same magnetic field would create a small driving force, which is only 1/10th of the friction force. As a consequence, the stir bar cannot move. A surface force-based actuation concept would allow scaling down because the ratio between driving force and friction force would remain unchanged.
The dominant surface phenomena in microscale also affect mixing processes with immiscible interfaces. For a solid–liquid system, mixing starts with suspension of the solid particles. The dissolving process follows suspension. The large surface-to-volume ratio in microscale is an advantage for the dissolving process, making it easily achievable. Thus, the main challenge is the suspension process. Because of their relatively large sizes and the correspondingly small diffusion coefficient, the particle can only be suspended in microscale with the help of chaotic advection. Therefore, the quality of solid–liquid mixing in microscale is determined by the suspension process.
In a system of immiscible liquids, additional energy is needed to overcome interfacial tension. On the one hand, dispersing the immiscible phases is a difficult task. On the other hand, surface tension breaks the stretched fluid into segments and forms microdroplets. The advantage of microscale is that the formation process can be controlled down to each individual droplet. Therefore, an emulsion with homogenous droplet size can be achieved in micromixers.
Gas–liquid systems are other systems affected by the dominant surface phenomena. Some applications such as hydrogenation, oxidation, carbonation, and chlorination require gas–liquid dispersion. Unlike liquid–liquid emulsion, gas molecules can be absorbed into the liquid phase. The gas–liquid mixing process consists of two processes: dispersion of the gas bubble and absorption of gas molecules. While absorption is promoted due to the larger available interfacial area, dispersion of tiny gas bubbles is the main challenge in designing micromixers for a gas–liquid system.
Besides surface phenomena, the laminar flow condition is another challenge for designing micromixers. The problems in micromixers are similar to those in macroscale laminar mixers. Laminar mixers exist in many processes of food, biotechnological, and pharmaceutical industries because of the high viscosity and slow flow velocity involved. For many applications, the flow velocity in micromixers cannot be too high. The small size of micromixers leads to an extremely large shear stress in mixing devices, even at relatively slow flow velocities. This shear stress may damage cells and other sensitive bioparticles. In complex fluids with large molecules and cells, the fluid properties become non-Newtonian at high shear stress. On the one hand, the high shear compromises both the metabolic and physical integrity of cells. On the other hand, viscoelastic effects under this condition may lead to flow instability, which can be well utilized for improving mixing.
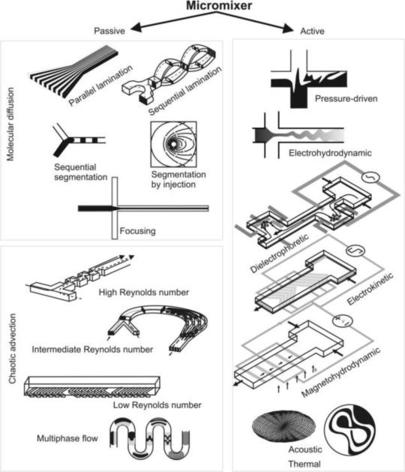
In this book, micromixers are categorized as passive micromixers and active micromixers [2] (Fig. 1.1). Except the kinetic energy of the flow itself, passive micromixers do not require external energy for disturbance. The mixing process relies entirely on diffusion or chaotic advection. Passive mixers are further categorized according to the way in which the interface between the mixed phases is arranged: parallel lamination, serial lamination, segmentation, chaotic advection, and multiphase flow. In active micromixers, disturbances are induced by an external field. Thus, active mixers can be categorized according to the physical phenomenon of the disturbance as a pressure-driven flow, electrohydrodynamics, dielectrophoretics, electrokinetics, magnetohydrodynamics, acoustics, and heat. The designs of active micromixers are often complex because of additional components. External power sources are needed for the operation of active micromixers. Thus, the integration of active mixers in a microfluidic system is both challenging and expensive. In contrast, passive micromixers do not require external actuators except those for fluid delivery. Passive micromixers are robust, stable in operation, and easily integrated in a more complex system. Figure 1.1 depicts an overview on the different types of micromixers discussed in this book.
The time scale of mixing processes changes with miniaturization. Most micromixers are used as a reaction platform for analysis or synthesis. Mixing and chemical reactions are interrelated [3]. While reaction kinetics and reaction time do not change with miniaturization, mixing time can be significantly affected by the mixer design as well as by the mixer type. This fact leads to two important issues related to chemical reaction: measurement of real reaction kinetics and control over reaction products.
In macroscale, mixing time is usually much larger than reaction time. The reaction rate is therefore mostly determined by the mixing time. In microscale, mixing time can be reduced to the same order or even less than the reaction time. Measurement of real reaction kinetics is therefore possible in microscale.
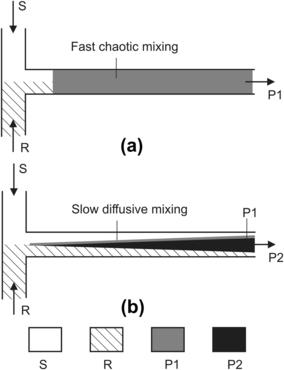
Mixing time and, consequently, the reaction products can be possibly controlled in microscale. If the reaction results in only one product, mixing time can only affect the reaction rate. If there are more than one product, mixing time determines the product composition and distribution. The following example shows the impact of mixing type on reaction results. Assuming a reaction between the substrate S and reagent R:
(1.1)
(1.2)
1.2. Micromixers as Microreactors
Since 2000, we have witnessed increasing activities in the use of microfluidic technology in analytical chemistry and chemical production. Mixing is the central process of most microfluidic devices for medical diagnostics, genetic sequencing, chemistry production, drug discovery, and proteomics. The impact of micromixers on microfluidic systems for chemical analysis and synthesis is similar to that of transistors in integrated circuits. Although micromixers for analysis and synthesis are different, some applications require both classes. For instance, in combinatorial chemistry and screening microdevices, micromixers are analytical tools for information gathering and synthetic tools for providing minute quantities of products.
In micromixers for analysis, information gained from this product is the purpose of the mixing process and the reaction. The amount of the reaction product only needs to fulfill the delectability requirements. In contrast, reaction products in synthesis applications are used to make materials with improved properties at favorable conditions given by micromixers. A large amount of the product may be needed. Thus, the design of micromixers for synthesis should be ready for numbering up in the case of a large-scale production [4].
Micromixers as microreactors will potentially have a large impact on chemical technology. Because of their small size, micromixers allow control over a number of parameters of production processes in chemistry and the pharmaceutical industry. Reaction conditions that are unusual in macroscale are technically possible in micromixers. The advantages of reaction in micromixers are small thermal inertia, uniform temperature, high gradient of the different physical fields, short residence time, and a high surface-to-volume ratio. The small thermal inertia allows fast and precise temperature control in micromixers. Miniaturization leads to higher rates of heat and mass transfer. Compared to their macroscale counterparts, micromixers can offer more aggressive reaction conditions. The large surface-to-volume ratio allows for an effective suppression of homogenous side reactions in heterogeneously catalyzed gas-phase reactions. The small size makes reaction in micromixers safe because of the suppression of flames and explosions. Explosions can be suppressed by using mixing channels with hydraulic diameter less than the quenching distance [5]. For instance, the fluorination of toulene with fluorine can be carried out at –10°C in micromixers. Conventional reactors would require a temperature of –70°C due to the explosive nature of the reaction [5]. In the case of accidents, the small amounts of hazardous reaction products are easy to contain.
Micromixers as microreactors enable a faster transfer of research results into production. Since scaling up the mixer design is not possible, lab setup can immediately be transferred into large-scale production by numbering up. Since numbering up is the only option for micromixers, scaling law leads to high ratio between device material and reaction volume. This means, fixed production costs will increase with miniaturization because of the higher costs of materials and infrastructure. If microreactors deliver a similar performance to their conventional macroscale counterparts, the higher production costs will make micromixers unprofitable for chemical production. However, for some particular products, the smaller production capacity may save costs through other factors such as replacing a batch process by a continuous process. For instance, due to slow mass and heat transfer in macroscale reactors, reaction time for fine chemicals is determined by mixing and is much longer than needed for reaction kinetics. Replacing a batch-based macroscale reactor by a continuous-flow microreactor can significantly reduce the reaction time. The reactor volume is smaller, but the total throughput per unit time is higher. As a result, for the same amount of products the reaction process would be carried out faster in microreactors.
In addition, as illustrated in Fig. 1.2, selectivity of the reaction may increase with micromixers. Production yields of microreactors could exceed those of batch-based macroscale reactors. The next cost-saving factor of micromixers for chemical production is the intensification process. The larger surface-to-volume ratio provides more surface for catalyst incorporation. Compared to its macroscale counter part, the amount of catalyst needed in a microreactor can be decreased by a factor of 1000. If the cost of the catalysts is significant in the overall production, saving catalysts can compensate for the large amount of construction materials needed for numbering-up microreactors.
Micromixers have an indirect impact on national security due to the possibility of on-side portable detection systems for chemical weapons and explosive. However, due to its portability, micromixers could be misused by criminals and terrorists [5]. A miniature chemical plant fitted into a suitcase could be misused for the production of drugs and hazardous gases. Raw chemicals may not be detectable prior to reactions in these miniature plants. Lethal nerve gases could be formed by two primary less-toxic compounds in micromixers. Detection facilities should be extended to these pre-compounds to counter this potential misuse.
1.3. Organization of the Book
This book offers a wide spectrum for the study of the mixing processes in microscale, from fundamental transport effects to a variety of designs to specific applications in chemistry and life sciences. After the introduction in Chapter 1, Chapter 2 and Chapter 3 discuss the basic terminology and fundamental physics of transport effects that will be used for designing micromixers. Chapter 2 discusses in detail the three key mass transport effects often used in micromixers: molecular diffusion, Taylor dispersion, and chaotic advection. The challenges and advantages of miniaturization in mixing are highlighted in this chapter with the help of scaling laws. The scaling laws are discussed based on nondimensional numbers which represent relationships between different transport effects. Chapter 3 discusses the fundamentals of the different numerical schemes for modeling the transport phenomena in micromixers.
Chapter 4 gives an overview on available microtechnologies for making micromixers. Basic techniques of conventional silicon-based microtechnologies are covered. Since polymers are chemically and biologically compatible, polymeric micromachining is the focus of this chapter. Technologies for bonding and sealing are necessary for making a micromixer. This chapter also discusses the design and fabrication of fluidic interconnects that are needed for interfacing micromixer to larger-scale devices and equipments.
Different concepts and designs for micromixers are discussed in Chapter 5, Chapter 6 and Chapter 7. Although all mixing concepts involve molecular diffusion, Chapter 5 only discusses concepts where molecular diffusion is the primary mass transfer process. Based on the arrangement of the mixed phases, the four mixer types discussed in this chapter are parallel mixer, serial mixer, sequential mixer, and injection mixer.
Chapter 6 is dedicated to micromixers based on chaotic advection. In contrast to the micromixers discussed in Chapter 5, this class of micromixers relies on bulk mass transport for mixing. The general concepts for generating chaotic advection are stretching and folding of fluid streams. These stretching and folding actions can be implemented in a planar design or in a complex three-dimensional channel structure. A special case of chaotic advection is mixing in microdroplets. Manipulation of the flow field inside a droplet can lead to the same stretching and folding effects as achieved in a continuous-flow platform.
Chapter 7 discusses active mixers, where mixing is achieved with energy induced by an external source. Active mixers are similar to conventional macroscale mixers where fluid motion is driven by an impeller. However, as discussed in Section 1.1, miniaturization of the impeller concept would not work because of the dominant viscous force in microscale. This chapter discusses different concepts for inducing a disturbance into the flow field. The use of electrohydrodynamic, dielectrophoretic, electrokinetic, magnetohydrodynamic, acoustic, and thermal effects in micromixers is discussed here.
Chapter 8 summarizes key diagnostics techniques for characterization of micromixers. Since both velocity field and concentration field are important for good mixing, diagnostics techniques for these fields are the focus of this chapter. The quantification of the extent of mixing is important for the evaluation of performance as well as the design optimization of micromixers.
Chapter 9 discusses the current applications of micromixers. Different applications need different design requirements. The chapter discusses applications from the two major areas: lab-on-a-chip for chemical and biochemical analysis and chemical production. This chapter also recommends materials and mixer types for each application area.
References
[1] Paul, E.L.; Atiemo-Oberg, V.A.; Kresta, S.M., Handbook of Industrial Mixing. (2004) Wiley, New York.
[2] Nguyen, N.T.; Wu, Z.G., “Micromixers – a review”, Journal of Micromechanics and Microengineering vol. 15 (2005) R1–R16.
[3] Bourne, J.R., “Mixing and Selectivity of Chemical Reactions”, Organic Process Research & Development vol. 7 (2003) 471–508.
[4] K.F. Jensen, The Impact of MEMS on the chemical and pharmaceutical industries, in: Technical Digest of the IEEE Solid State Sensor and Actuator Workshop, Hilton Head Island, SC, 4–8 June, 2000, pp. 105–110.
[5] Löwe, H.; Hessel, V.; Muller, A., “Microreactors. Prospects already achieved and possible misuse”, Pure Applied Chemistry vol. 74 (2002); 2271–2276.
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.