Appetite Regulation in Healthy Aging
Stijn Soenen and Ian Chapman, Royal Adelaide Hospital, Adelaide, SA, Australia
Abstract
Compared to healthy young adults, older people on average are less hungry, consume smaller meals more slowly, and have fewer snacks between meals; as a result, their energy intake is about 20–30% lower than younger people. In particular, control of energy intake is less responsive (to stimulation or inhibition) in older than younger subjects. The gastrointestinal tract has important actions in regulating appetite and food intake, which are modified by healthy aging. These include the effects of gut hormones and the rate of gastrointestinal motility. The duration of long-lasting satiety and hunger suppression after a meal are proportional to these gut hormone concentrations.
Keywords
Aging; food intake; hunger; malnutrition; gastrointestinal mechanisms; body weight
Introduction
Aging is associated with various physiological changes in appetite and body weight, and many older people are obese or undernourished. Compared with younger adults, older adults have reduced hunger and energy intake; in some cases, suppression of energy intake comes with the ingestion of nutrient preloads (Wurtman et al., 1988; Rolls et al., 1995; Clarkston et al., 1997; MacIntosh et al., 2001a,b; Sturm et al., 2003, 2004; Soenen and Chapman, 2013; Soenen et al., 2014, 2015, 2016; Giezenaar et al., 2016). Malnutrition is a common condition in elderly residents in long-term care (85%) as well as in hospitalized (23–62%) and community dwelling elderly (15%) (Wysokinski et al., 2015). Both low body weight and weight loss are strong predictors of poor outcomes (Newman et al., 2001; Somes et al., 2002), including the development of pathological undernutrition and sarcopenia and reduced functional capacity and frailty (Rolland et al., 2011). The increased prevalence of obesity among older people results largely from the increasing proportion of people entering old age already obese; body weight increases on average to peak at about age 55–60 years before stabilizing and then slowly decreasing thereafter (Ng et al., 2014). The effects of obesity are modified by age. The body weight and body mass index (BMI) associated with maximum life expectancy increase with age; the BMI associated with greatest life expectancy in people older than 65 is in the range of 27–30 kg/m2 compared to 20–25 kg/m2 in younger adults (Thinggaard et al., 2010). Both over- and undernutrition in the elderly—a BMI of less than 22 or more than 30 kg/m2—are associated with substantial reductions in functional independence and quality of life, as well as increases in morbidity, mortality, and health-care utilization (Chapman, 2006; De Hollander et al., 2012; Soenen and Chapman, 2013). More recently, dynamic weight change (i.e., weight change per year)—both increases and decreases in body weight—is increasingly recognized as a critical factor that directly affects health and both all-cause as well as cause-specific mortality risk (i.e., cardiovascular disease and cancer) in older people (French et al., 1997; Somes et al., 2002; Korkeila et al., 2009).
Reduced Appetite and Energy Intake During Aging
On average, healthy older people are less hungry and more full and consume less food and energy compared to healthy younger adults. This physiological process has been termed the physiological anorexia of aging (Morley and Silver, 1988; Soenen and Chapman, 2013). Compared with younger adults, some older adults also have diminished energy intake from ingesting nutrient preloads (Fig. 5.1) (Rolls et al., 1995; Soenen and Chapman, 2013; Soenen et al., 2014, 2015, 2016; Giezenaar et al., 2016).
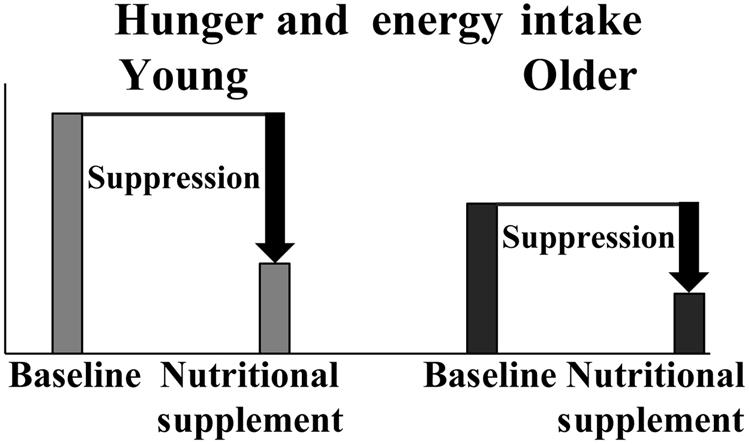
A recent meta-analysis indicated that energy intake decreases by approximately 0.5% per year of increasing age, and this progressive reduction is likely to contribute to weight loss in older people and the development of pathological undernutrition (Giezenaar et al., 2016). This meta-analysis examined the effect of healthy aging on appetite and energy intake in adults, including data from >7500 subjects on energy intake and >500 subjects on appetite derived from 59 studies. Energy intake was less in healthy older (~70 years) than younger (~26 years) adults. The calculated reduction fell into quite a narrow range at 16–20%, despite studies of the fasting and fed states and energy intake being calculated by a variety of methods, including intake at an acute study meal, during prolonged periods at home or using weighed food records, 24-h food intake recalls, and food frequency questionnaires—i.e., a robust finding regardless of the method of intake evaluation. Earlier studies indicated a reduced energy intake of ~30% between ages 20 and 80 (Wurtman et al., 1988; Briefel et al., 1995). A 7-year New Mexico longitudinal study of 156 persons aged 64–91 years reported a decrease of 19 kcal/day/year in women and 25 kcal/day/year in men (Koehler, 1994), while a Swedish longitudinal study of 98 people found an even greater decline of energy intake of 610 kcal/day in men and 440 kcal/day in women between ages 70 and 76 (Sjogren et al., 1994). A population-based study indicated that older people ages 60–74 years consume ~500–700 kcal/day less than their younger counterparts ages 20–39 (Briefel et al., 1995).
Perceptions of hunger are predictive of energy intake in both healthy younger and older subjects (Parker et al., 2004). The results of the meta-analysis by Giezenaar et al. show that older people (~73 years) feel less hungry than younger adults (~26 years), both fasting (25%) and after they have consumed food (39%), and they also feel more full in the fasting state (37%)—i.e., changes of about 0.5% per year for hunger and about 0.7% per year for fullness, respectively (Giezenaar et al., 2016).
Less Suppression of Appetite and Energy Intake in Older People
The regulation of appetite and energy intake may be impaired in the elderly. For example, the acute suppression of energy intake by dietary protein, whether ingested orally or infused directly into the small intestine (i.e., bypassing orosensory and intragastric factors), is less in healthy older than younger adults, and in the elderly it may even increase overall energy intake (Soenen et al., 2014; Giezenaar et al., 2015). In the meta-analysis by Giezenaar et al., energy intake was measured in 203 subjects during a single ad libitum buffet-style meal at the research facility both after overnight fasting and in the postprandial state, energy intake decreases on average 11% less in the older than younger adults (Giezenaar et al., 2016). Older people do not show the same ability to regulate food intake after prolonged over- or underfeeding as young individuals (Roberts et al., 1994; Rolls et al., 1995; Moriguti et al., 2000; Parker and Chapman, 2004). For example, younger and older men were underfed for 21 days, during which the younger and older groups lost comparable amounts of weight. After the underfeeding period, the men were allowed to again eat ad libitum. The younger men were shown to eat more than at baseline (before underfeeding) and promptly returned to normal weight, whereas the older men failed to compensate and returned only to their baseline intake and not above it, so they did not regain the weight they had lost (Roberts et al., 1994). This strongly supports the concept that after an anorectic insult (such as major surgery), older adults usually take longer than younger adults to regain the weight, particularly muscle lost, and are at increased risk of vitamin and other dietary deficiencies as well as being more susceptible to superimposed illnesses, often infections.
Gastrointestinal Regulation of Appetite and Energy Intake
Appetite and energy intake are dependent on the precise coordination of interrelated intragastric and small intestinal mechanisms triggered by the interaction with the nutrients ingested. Gastric emptying reflects the coordinated motor activity of the proximal stomach, distal stomach (antrum and pylorus), and duodenum, which is controlled primarily by feedback from neural and humoral signals generated by the interaction of nutrients with the small intestine. Ghrelin is secreted by the stomach and stimulates appetite and energy intake, whereas cholecystokinin (CCK), peptide tyrosine tyrosine (PYY), and glucagon-like polypeptide-1 (GLP-1), among others, are secreted by the small intestine in response to food intake and suppress food intake. The gastric and small intestinal motor and humoral mechanisms underlying normal gastric emptying in humans are complex and highly variable: ingested food must be stored, mixed with digestive enzymes, ground into small particles, and delivered in a liquefied form into the duodenum at a rate that allows efficient digestion and absorption.
Intragastric mechanisms that reduce energy intake include a slowed rate of gastric emptying—i.e., nutrients empty from the stomach at an overall rate of 1–4 kcal/min irrespective of volume in young adults (Meyer et al., 1981; Brener et al., 1983; Horowitz et al., 1994; Gentilcore et al., 2006), increased antral distension (Sturm et al., 2004; Gentilcore et al., 2006), and inhibition of plasma ghrelin concentrations (Sturm et al., 2003; Bowen et al., 2006; Pilichiewicz et al., 2007a). Energy intake is inversely related to antral area and directly to plasma ghrelin concentrations (Jones et al., 1997; Sturm et al., 2004; Bowen et al., 2006).
Small intestinal mechanisms are highly sensitive to the nutrients ingested, and small amounts of nutrients delivered directly into the small intestine have the capacity to reduce appetite and energy intake associated with the suppression of antral motility and increased pyloric motility (Brennan et al., 2008), which results in slowing of gastric emptying and stimulation of gut hormone secretion (i.e., CCK, GLP-1, PYY, and gastric inhibitory peptide, or GIP) (Jones et al., 1997; Pilichiewicz et al., 2007a,b) and the suppression of ghrelin (Pilichiewicz et al., 2007a,b). Appetite and energy intake have been shown to be related inversely to plasma CCK (Bowen et al., 2006) and GLP-1 (Lejeune et al., 2006) as well as the number of isolated pyloric pressure waves (Brennan et al., 2008).
Appetite Regulation in Healthy Older Subjects
The senses of smell and taste deteriorate with age (Doty et al., 1984), leading to a reduced capacity to enjoy food and develop sensory-specific satiety (Rolls and McDermott, 1991). This normal decline in the pleasantness of a particular food’s taste after it has been consumed leads to a decrease in its consumption and a tendency to shift consumption to other food choices during a meal. Age-related reduction in sensory-specific satiety favors a less varied, more monotonous diet and the development of micronutrient deficiencies.
The gastrointestinal mechanisms underlying appetite and energy intake are affected by healthy aging as well; motor function is generally well preserved, whereas deficits in sensory function are more apparent. Healthy aging is associated with the modest slowing of gastric emptying of both solids and liquids (Evans et al., 1981; Moore et al., 1983; Horowitz et al., 1984; Wegener et al., 1988; Clarkston et al., 1997; O’Donovan et al., 2005; Giezenaar et al., 2015, 2016; Soenen et al., 2016), but the rate of emptying generally remains within the range for healthy young subjects (i.e., 1–4 kcal/min) (Soenen et al., 2015). We have recently shown that aging appears especially to affect the initial phase of gastric emptying of protein drinks (Giezenaar et al., 2015), although the dose-dependent slowing of gastric emptying with ingestion of increasing loads of protein was of comparable magnitude in both healthy young and older men. The slightly slower gastric emptying in older subjects is indicative of changes in intragastric mechanisms.
Healthy aging is accompanied by loss of enteric neurons and interstitial cells of Cajal throughout the gut; motor function is generally well preserved, whereas deficits in sensory function are more apparent. Perception of gastric distension is diminished in the healthy elderly (Rayner et al., 2000), indicating a reduction in visceral sensitivity. As a group, older adults have greater antral area (Sturm et al., 2004) and lower plasma ghrelin concentrations (Rigamonti et al., 2002; Sturm et al., 2003) than younger adults. There is evidence that the higher prevalence of Helicobacter pylori infection and atrophic gastritis in the elderly compared with the young is associated with a decline in levels of the orexigenic peptide ghrelin (Sturm et al., 2003; Salles, 2007).
In addition to mechanical stimuli, perception of chemical stimuli such as acid and humoral responses to duodenal nutrient exposure decrease with age. There is evidence for altered responses to the presence of nutrients in the small intestine in older people when compared to younger people, including greater stimulation of phasic pyloric pressure waves by intraduodenal lipid infusion (Cook et al., 1997), a greater satiating effect of intraduodenal glucose infusion (MacIntosh et al., 2001a,b), and higher fasting and postprandial CCK and GLP-1 concentrations, which may contribute to slowing of gastric emptying (Berthelemy et al., 1992; Gutzwiller et al., 1999; MacIntosh et al., 2001a,b; Sturm et al., 2003, 2004). It is uncertain whether these changes are due to aging per se or reflect changes in nutrient intake. Healthy older people seem to retain their sensitivity to the satiating effects of exogenous GLP-1 (Gutzwiller et al., 1999), and they may have increased sensitivity to the satiating effects of CCK (MacIntosh et al., 2001a,b). Aging is associated with increased postprandial circulating insulin concentrations (Fraze et al., 1987), mainly due to insulin resistance and associated impaired glucose tolerance (Scheen, 2005), which may reflect increased adiposity and changes in the secretion of incretins GLP-1 and GIP (Trahair et al., 2012). Plasma concentrations of the anorectic hormone leptin may increase with aging. In women, this may be largely attributable to the increased fat mass that also accompanies aging (Baumgartner et al., 1999) and in men, the age-related decrease in circulating testosterone concentrations, which is potentiated by obesity (Hislop et al., 1999).
Appetite Regulation in Malnourished Older Subjects
In young adults, there is evidence to support the concept of BMI-related differences in responses to energy intake. Young obese individuals exhibit a less precise compensatory response to ingested energy than lean individuals (Rolls et al., 1994; Ebbeling et al., 2004). For example, suppression of energy intake at a buffet-style meal 30 min after oral mixed macronutrient yogurt ingestion of 161 kcal compared to control (no yogurt) was less in 12 obese than in 12 lean young women (p<.05) (Rolls et al., 1994).
Although only a limited number of studies have examined the effects of undernutrition on the regulation of appetite and food intake in older individuals, there is persuasive evidence of substantial differences between undernourished and well-nourished older people. These differences may be the outcome of undernutrition and contribute to the undernourished state (Sturm et al., 2003; Serra-Prat et al., 2009, 2013). Undernourished older adults had significantly reduced hunger in the fasted state and in the postprandial state and significant greater fullness in the fasted state when compared to healthy older (Serra-Prat et al., 2013) and young adults (Sturm et al., 2003; Serra-Prat et al., 2013). In undernourished older women, energy intake was not suppressed by a mixed nutrient preload unlike well-nourished older and young women. The undernourished were also characterized by higher fasting and postpreload ghrelin concentrations, irrespective of a reduction in hunger ratings (Sturm et al., 2003). Hence, it is likely that increased plasma ghrelin concentrations represent a compensatory response to undernutrition at any age, particularly as there is a rise in fasting ghrelin concentrations in normal weight individuals before meals (Cummings et al., 2001), with diet-induced weight loss in the obese (Cummings et al., 2002), and in association with anorexia nervosain young adults (Otto et al., 2001). In another study of undernourished older subjects, plasma concentrations of CCK were shown to be higher than in well-nourished older subjects (Berthelemy et al., 1992), further suggesting that increased CCK activity may be a cause of undernutrition in older people and may act to perpetuate it.
Loss of Body Weight During Aging
When compared to younger adults, older adults are more likely to lose than gain weight (Evans and Campbell, 1993). Even apparently healthy, illness-free people exhibit a tendency to lose weight as they age (Wurtman et al., 1988). In some cases, weight loss is due to an illness, which is primarily responsible for the poor outcome. Weight loss in older people occurs because there is a decrease in daily energy intake that is greater than the decrease in energy expenditure. Reduced energy expenditures in the elderly are due to reduced physical exercise, loss of energy-demanding lean tissue, and decreased metabolic cost of metabolizing the smaller amount of consumed food (Fukagawa et al., 1990; Vaughan et al., 1991; Roberts et al., 1995). Various physiological and nonphysiological factors have been identified as being associated with and probably contributing to weight loss in older people (Morley and Kraenzle, 1994; Chapman, 2011; Soenen and Chapman, 2013). These factors include dementia, depression, reduced functional status, medical conditions and medications, poor dentition, social isolation, and poverty (Kerstetter et al., 1992; Gilmore et al., 1995; Chapman, 2007).
It is well documented in population-based, cross-sectional, and longitudinal studies that weight loss is more common than weight gain in adults aged 65 years and older (Wright, 1993; Morley and Kraenzle, 1994; Blaum et al., 1995; Newman et al., 2001; Somes et al., 2002). For example, in the prospective US Cardiovascular Health Study, weight loss over 3 years of ≥5%, was more common than weight gain of ≥5% (17% compared with 13%) and associated with a 70% increase in mortality; weight stability and weight gain were not associated with increased mortality (Newman et al., 2001). Similarly, in the Systolic Hypertension in the Elderly Program, weight loss of 1.6 kg/year in people aged 60 and older was associated with an approximately five times greater death rate than those without significant weight change (i.e., −0.7 to +0.5 kg/year) (Somes et al., 2002).
Longitudinal studies have shown that body weight decreases in older people at approximately 0.5% per year (Wallace et al., 1995; Newman et al., 2001; Arnold et al., 2010), and the rate of weight loss in nursing home residents is slightly higher than in their community-dwelling peers (Blaum et al., 1995; Pizzato et al., 2015; Wirth et al., 2015). Weight loss of >4–5%, probably irrespective of starting weight (Newman et al., 2001), is associated with increased mortality in older people, both those in the community (Wallace et al., 1995; Reynolds et al., 1999; Newman et al., 2001; Murphy et al., 2014) and those in nursing homes (Ryan et al., 1995; Sullivan et al., 2004; Wirth et al., 2015). Low body weight is also associated with adverse outcomes in older people (Murden and Ainslie,1994; Ryan et al., 1995; Wallace et al., 1995; Flacker and Kiely, 1998; Reynolds et al., 1999; Sullivan et al., 2004; Murphy et al., 2014; Wirth et al., 2015). The body weight and BMI associated with maximum life expectancy increases with increasing age (Decaria et al., 2012), as does the BMI value below which there is an increase in associated mortality. Studies in older people indicate that a BMI less than approximately 23 kg/m2 is associated with increased mortality (Soenen and Chapman, 2013).
Loss of Muscle Mass During Aging
Weight loss in older people has been associated consistently with adverse outcomes, including increased mortality. This is particularly so in people who lose weight involuntarily and who are already of low body weight (Thinggaard et al., 2010). Adverse effects of weight loss in older people are likely to result, at least in part, from decreases in already reduced skeletal muscle mass. Skeletal muscle mass decreases after ages 20–30, with a decrease of lean mass, mainly muscle, of approximately 3 kg per decade after age 50 (Evans and Campbell, 1993). The loss of muscle mass in the elderly is associated with reduced physical performance, loss of function, increased rates of falls, and increased prevalence of chronic metabolic diseases such as type 2 diabetes (Mathus-Vliegen and Obesity Management Task Force of the European Association for the Study of 2012). When muscle loss is excessive, it results in sarcopenia, which is characterized by generalized loss of muscle mass and strength, and it is associated with increased rates of functional limitation and disability and the need to move to nursing home care (Pajecki et al., 2014). Sarcopenia is usually defined as the combination of very low skeletal muscle mass (e.g., more than two standard deviations below the young adult mean as measured by dual-energy X-ray absorptiometry (Rolland et al., 2009)), and functional impairments such as reduced grip strength or decreased gait speed (Malafarina et al., 2013). The prevalence of sarcopenia in older people varies according to the population studied and diagnostic criteria used, but it is in the range of 6–15% in people 65 and older (Maleki et al., 2000) and up to four times higher in those over 85 than in those 70–75 years of age (Castillo et al., 2003).
In contrast to the loss of lean tissue with age, fat tissue, particularly visceral tissue, increases with age. As a result of these contrasting changes, the percentage of body fat is as much as twice as high in the elderly as in young adults of the same weight (Prentice and Jebb, 2001). With increasing age, fat is increasingly deposited in the skeletal muscle and liver, which is associated with increasing insulin resistance and the development of glucose intolerance (Dominguez and Barbagallo, 2007). Both aging and obesity are characterized by increased inflammation, with reduced immune function, and increased circulating concentrations of tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and C-reactive protein. TNF-α and IL-6 have catabolic effects on muscle mass and further predispose the older person to the development of sarcopenia and frailty. Perhaps it is not surprising, therefore, that obesity in older people is associated with high rates of sarcopenia, a combination referred to as sarcopenic obesity. Sarcopenic obesity is associated with two to three times higher rates of disability than either obesity or sarcopenia alone (Dominguez and Barbagallo, 2007). Increased cytokine levels, which may reflect the “stress” of aging per se or the amplified stressful effects of other pathologies, could partly account for the decline in appetite and body weight that occurs in most older people (Yeh and Schuster, 1999). Circulating levels of the cytokines IL-1 and IL-6 appear to decrease energy intake and reduce body weight via a number of central and peripheral pathways. IL-1 and IL-6 levels are elevated in older people with cachexia, while plasma IL-6 concentrations increase as a function of normal aging and correlate inversely with functional ability in older people (Yeh and Schuster, 1999).
Conclusion
Healthy older people are less hungry, more full, and consume less food and energy compared to healthy younger adults. They also have a diminished suppression of energy intake by ingestion of a nutrient preload. The gastrointestinal tract has important actions in regulating appetite and food intake that are modified by healthy aging. The reduction in energy intake equates to approximately 0.5% per year of increasing age, and this is likely to contribute to loss of weight in older people and the development of pathological undernutrition in predisposed older people.
Potential competing interests: None of the authors have any conflicts of interest to declare.
Sources of support: SS was supported by Royal Adelaide Hospital Florey Fellowship.
