Fibromyalgia Syndrome
Role of Obesity and Nutrients
Manisha J. Oza1,2, Mayuresh S. Garud1, Anil Bhanudas Gaikwad3 and Yogesh A. Kulkarni1, 1Shobhaben Pratapbhai Patel School of Pharmacy & Technology Management, SVKM’s NMIMS, Mumbai, Maharashtra, India, 2SVKM’s Dr. Bhanuben Nanavati College of Pharmacy, Mumbai, Maharashtra, India, 3Birla Institute of Technology and Science, Pilani, Rajasthan, India
Abstract
Fibromyalgia syndrome (FMS) is a devastating musculoskeletal disorder with characteristic symptoms of fatigue, chronic pain, headache, anxiety, morning stiffness, and memory loss that ultimately affects the quality of life. Although the clear pathogenesis of FMS is unknown, multiple lines of evidence propose a link between obesity, diet, nutritional status, and FMS. Several studies have reported that overweight and obese people have higher incidence of FMS, and weight reduction in these patients seems to be effective in improving symptoms. Vegetarian and vegan diets were reported to have beneficial effects on FMS due to the high amounts of antioxidants consumed. Nutritional deficiencies of magnesium, iron, iodine, selenium, thiamine, and manganese have been described, but there are no detailed studies available that show a direct relationship between FMS and deficiencies of these micronutrients. Extremely limited data is available about the nutritional supplements in FMS which indicates that more studies are needed to determine the types of nutritional supplements that could help reduce the symptoms of FMS. In conclusion, physical exercise, weight control, and dietary supplements, including antioxidants and micronutrients, are important in improving FMS patients’ quality of life.
Keywords
Fibromyalgia; antioxidants; obesity; nutrition
Introduction
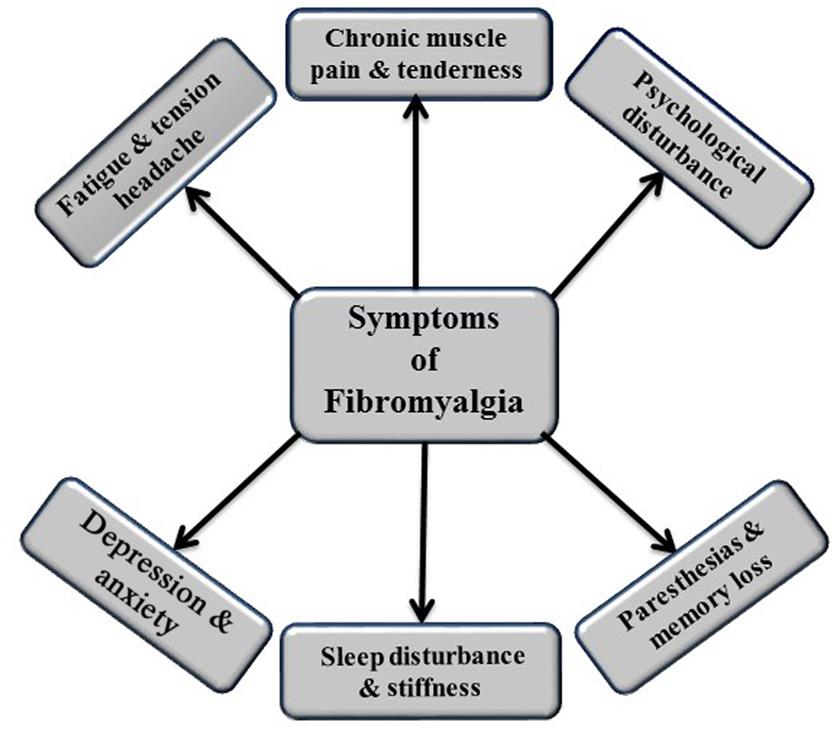
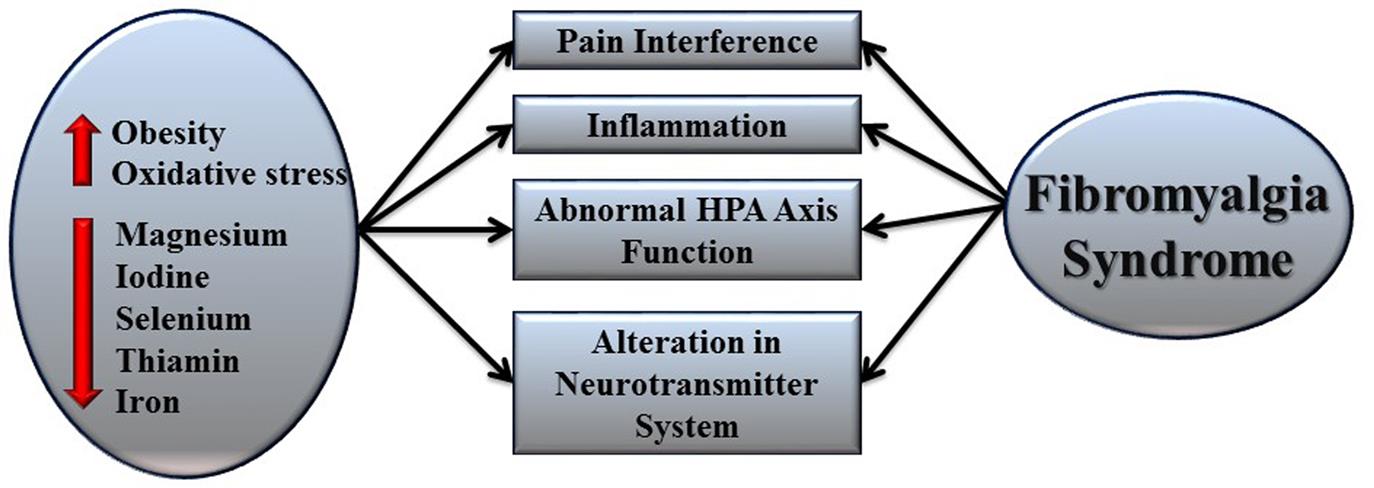
Fibromyalgia syndrome (FMS) is an idiopathic form of rheumatism characterized by diffuse nonarticular musculoskeletal pain along with generalized tender body areas such as muscles, tendons, and joints. The chronic pain in fibromyalgia is widespread and mainly affects the neck, shoulders, upper back, arms, and chest. According to American College of Rheumatology criteria, the duration of widespread pain is at least 3 months, and pain on pressure should be 11–18 specific tender points at minimum (White and Harth, 2001; Neumann and Buskila, 2003; Marcus and Deodhar, 2011; Wolfe et al., 1990). FMS is always associated with other complications, especially sleep disturbances, morning stiffness, fatigue after mild physical exertion, paresthesias, cognitive disturbances (especially memory loss), anxiety, tension headaches, irritable bowel syndrome, mitral valve prolapse, primary dysmenorrhea, depression, and psychological disturbance (Branco et al., 2010; Arnold et al., 2006). It is frequently connected with severe functional damage and work inability, and its effects are comparable to those reported for osteoarthritis and other rheumatic disorders (Walker et al., 1997; Hawley and Wolfe, 1991). Approximately 2–2.7% of the world’s population suffers from FMS, and the rate of incidence is higher in younger and middle-aged women, obese people, and aged patients (Queiroz, 2013; Fitzcharles et al., 2013). The prevalence rate of FMS in the United States is 6–15%, with a five times higher incidence among women than men (Jahan et al., 2012). From 40% to 70% of the fibromyalgia patients are suffering from obesity, and increased body mass index (BMI) is correlated with higher levels of fatigue and pain in fibromyalgia (Bennett et al., 2007; Neumann et al., 2008). The level of oxidative stress, type of diet, and nutritional status are also associated factors that increase the risk of FMS (Li and Micheletti, 2011; Arranz et al., 2010; Percival et al., 1997) (Fig. 7.1).
Pathophysiology of Fibromyalgia
The pathophysiology of FMS includes multiple abnormalities and altered mechanisms. The aberrations of the autonomic and central nervous systems, genetic factors, environmental factors, psychological factors, the hypothalamic–pituitary–adrenal (HPA) axis hormones, and oxidative stress are all involved in the pathogenesis of FMS. Abnormalities in several neuroendocrine transmitters—in particular, nerve growth hormone (GH), 5-hydroxytryptamine, cortisol, norepinephrine, and substance P—are reported in FMS (Bradley, 2009; Ozgocmen et al., 2006).
Chronic pain in FMS is a result of central sensitization, abnormal descending inhibitory pain pathways, and altered level of neurotransmitters. Central sensitization is considered the major mechanism involved in chronic pain. It is a result of spontaneous nerve action, enlarged receptive fields, and augmented response to the stimuli conveyed by the primary afferent nerve (Bellato et al., 2012; Ozgocmen et al., 2006). In FMS, central sensitization causes abnormal windup processes, which results in neuronal hyperexcitability in the spinal cord. It is mediated by N-methyl-D-aspartate receptors via nitric oxide (NO) and peroxynitrite pathways and plays a vital role in pain processing (Woolf, 2011; Staud et al., 2001; Staud et al., 2004; Latremoliere and Woolf, 2009). Glial cell activation through various stimuli also plays a significant role in pain processes. The activation of glial cell increases the release of NO, reactive oxygen species, and proinflammatory cytokines and ultimately prolongs neuronal hyperexcitability (Watkins et al., 2001). Several neurotransmitters and modulators are involved in the central sensitization and chronic pain. For example, serotonin and noradrenalin levels in the central nervous system appear to be reduced in FMS and lead to dysfunctional descending pathways, while substance P and glutamate levels are increased in cerebrospinal fluid, which increases pain sensitivity. Alteration of these neurotransmitters also affects sleep and mood in FMS patients (Becker et al., 2011; McCarley, 2007; Raison, 2009). The HPA axis functions abnormally in FMS because of chronic pain-induced stress (Crofford, 2002; Demitrack and Crofford, 1998). Furthermore, hyposecretion of corticotropin-releasing hormone in FMS patients elevates the level of adrenocorticotropic hormone and cortisol in response to stress (Neeck and Crofford, 2000; Riedel et al., 1998; Neeck and Crofford, 2002). The secretion of GH and insulin-like growth factor (IGF-1) is also decreased. Inadequate secretion of GH and IGF-1 are significant factors of sleep disturbance in FMS patients (Prinz et al., 1995; Van Cauter et al., 1998). Genetic modifications such as nucleotide polymorphism in various genes such as the serotonin transporter (5-HTT) gene, the catechol-O-methyltransferase gene, and dopamine D4 receptor gene are also reported in FMS. Environmental factors such as infections caused by various viruses such as the human immunodeficiency virus, parvoviruses, the hepatitis C virus, and some bacteria also play important roles in pathogenesis by activating glial cells and releasing cytokines (Rivera et al., 1997; Leventhal et al., 1991; Buskila et al., 1990; Nicolson et al., 1999; Furr and Marriott, 2012).
Fibromyalgia and Obesity
Obesity is defined as a complex disease with immoderate deposition of fat in adipose tissue (Ravussin and Swinburn, 1992; Ursini et al., 2011). It can be considered a major risk factor for the development of several medical problems such as hypertension, respiratory diseases, type 2 diabetes, gout, strokes, osteoarthritis, and musculoskeletal disorders, including fibromyalgia (Must and Strauss, 1999). BMI is considered worldwide as an important measure of obesity and is divided into three classes: a BMI <25 kg/m2 is considered as normal weight status, a BMI of 25–30 kg/m2 is categorized as overweight, and a BMI >30 is considered obese (Gremese et al., 2014). Physical dysfunction and musculoskeletal pain have been more commonly observed in obese individuals. Several clinical reports also revealed relationships between fibromyalgia and obesity (Peltonen et al., 2003; Hooper et al., 2007; De Sá Pinto et al., 2006). Yunus and coworkers examined a connection between fibromyalgia and obesity in 211 female patients and demonstrated significant correlation between BMI and fibromyalgia (Yunus et al., 2002). Neumann and coworkers evaluated the link between fibromyalgia symptoms (physical activity, muscle tenderness, and quality of life) and obesity in 100 FMS female patients. The result showed negative correlation between quality of life, tenderness threshold, and BMI and positive correlation between obesity and physical dysfunction. In the same study, pain sensitivity was found to be high in obese FMS patients (Neumann et al., 2008). Similar outcomes were obtained by Kim et al. and Timmerman et al. in their study conducted on 888 patients and 179 women suffering from FMS, respectively (Kim et al., 2012; Timmerman et al., 2013). Findings from a few more studies showed that low levels of physical exercise in overweight and obese patients increases the risk of FMS development (Mork et al., 2010; Vincent et al., 2014). Furthermore, FMS was found to be more common in obese and overweight twins than nonobese twins (Wright et al., 2010). All of these reported studies indicate a strong correlation between fibromyalgia and obesity.
Symptoms of FMS and Obesity
Obesity deleteriously affects the musculoskeletal system. Mechanical loading, inflammation, and psychological status are three major mechanisms involved in obesity-related musculoskeletal pain (Vincent et al., 2013; Messier et al., 2005; Kaur, 2014; Marcus, 2004). Mechanical loading is responsible for anabolic stimulus to the bone and improves bone strength, size, and shape by improving tissue density (Turner and Robling, 2004). Obesity induces joint pain by increasing mechanical loading on the musculoskeletal system. It subsequently reduces strength to control loading on cartilaginous areas of the joint and affects alignment of the joints. Furthermore, loading may increase toward a small cross-sectional area of the joint and cause tissue damage (Andriacchi et al., 2004). Axial joints are also victim of central obesity and cause back pain (Menegoni et al., 2009). Tissues such as tendons, cartilages, and fascia are also affected by obesity (Wearing et al., 2006). Recent evidence suggests that in obese person adipocytes enlarge and cause alteration in systemic metabolism (Greenberg and Obin, 2006). Furthermore increases in BMI and fat volume also cause adiposopathy and increased tissue pain because more macrophages are able to enter adipose tissue (Seaman, 2013). The release of several inflammatory markers such as C-reactive proteins, cytokines, and interleukins (specifically IL-6) is higher in obese individuals suffering from chronic musculoskeletal pain compared to lean individuals (Deere et al., 2012; Briggs et al., 2013). Cytokines are known to play a role in diverse clinical processes and phenomena such as fatigue, fever, sleep, pain, stress, and aching (Wallace, 2006). Increases in the release of these inflammatory markers also increases pain severity (Bas et al., 2014). Obesity also alters functions of the HPA axis and increases cortisol levels (Okifuji et al., 2009). Psychological factors also play a major role in severity of pain in obese patients, who have more fear of movement and thus are more inactive and lethargic, which ultimately increases multisite pain (Vincent et al., 2010; Seaman, 2013). Obesity is also associated with insomnia, sleep disturbances, and excessive daytime sleepiness, creating a major risk for sleep apnea. It has also been reported that sleep duration is inversely propositional to BMI while daytime sleepiness in directly proportional to BMI. Obesity reduces sleep duration and quality of sleep in fibromyalgia patients (Hargens et al., 2013; Dixon et al., 2001; Watenpaugh, 2009; St-Onge et al., 2009; Algul et al., 2009; De Araújo et al., 2015). Okifuji and coworkers observed similar outcomes in their study conducted on 215 patients suffering from fibromyalgia. The pain sensitivity was high in obese patients along with poor sleep quality and reduced physical strength (Okifuji et al., 2010). Aparicio and coworkers found that weight status affected fibromyalgia symptoms in 175 and 177 women suffering from fibromyalgia, which showed increased levels of anxiety, fatigue, stiffness, morning tiredness, and depression in overweight and obese patients compared to nonobese patients. Furthermore, the quality of life, motor agility, cardiorespiratory fitness, and upper-body flexibility was damaged more in obese patients (Aparicio et al., 2011; Aparicio et al., 2013; Aparicio et al., 2014).
Based on the fact that obesity is playing an important role in pathophysiology of fibromyalgia, many studies have been carried out by various groups of researchers that showed positive improvement in the symptoms of fibromyalgia after weight reduction. In 2005, Shapiro and coworkers investigated the relationship between BMI and FMSs in 42 obese women to determine the effect of behavioral weight-loss treatment in these patients. The outcome of the 20-week treatment showed significant reduction in FMS and pain interference and improved the patients’ quality of life (Shapiro et al., 2005). Evidence showed that weight loss through bariatric surgery in obese subjects reduced FMS up to 92% and also improved musculoskeletal health in obese people (Hooper et al., 2007; Saber et al., 2008).
A randomized controlled trial was also carried out using 86 obese fibromyalgia patients to study the effect of weight loss in improving the FMS. After a 6-month dietary weight loss program, significant improvement was observed in terms of quality of life, improved sleep quality, reduced depression, and reduced tender-point count. The C-reactive protein and interleukin-6 level were also reduced after weight-loss treatment (Senna et al., 2012). There is evidence for the beneficial role of exercise in obese fibromyalgia patients to reduce fatigue because exercise leads to increased resistin and IGF-1 levels in serum that are inversely proportional to fatigue (Bjersing et al., 2013) (Fig. 7.2).
Role of Diet and Micronutrients in Fibromyalgia
The eating habits, type of food, and nutritional level of the diet play significant role in the improvement of fibromyalgia symptoms along with pharmacological therapies (Batista et al., 2015; Arranz et al., 2012). It has been reported that nutritional factors are associated with immune and inflammatory processes and can modify the symptoms of FMS (Henderson and Panush, 1999). According to the Brazilian Rheumatology Society, nutrients play a potential role in FMS. Increased consumption of salt, sugar, fat, and alcohol worsens the symptoms of FMS while increased intake of fruits, vegetables, fluids, and fibers have beneficial effects for FMS patients. In addition, nutritional supplementation of micronutrients such as magnesium, iodine, calcium, manganese, iron, thiamine, vitamin D, melatonin, malic acid, thiamine, and sources of tryptophan are important in improving fibromyalgia symptoms (Batista et al. 2015; Arranz et al., 2010).
Diet
Vegetarian diets rich in antioxidants, beta carotene, minerals, and fibers have been reported to improve some of the fibromyalgia symptoms. These diets mainly reduce inflammation in the body by regulating the level of antioxidants, essential fatty acids and arachidonic acid (Smedslund et al., 2010). According to the Donaldson’s observational study, fibromyalgia symptoms such as chronic pain in the shoulder and neck, quality of life, and psychosocial behavior in the patients can be improved by dietary intervention of pure vegetarian diet (Donaldson et al., 2001). A study by Kaartinen showed that vegan diets rich in lactobateria improve joint stiffness, quality of sleep, and visual analog scale. It also improved BMI, serum cholesterol and peroxide levels, apolipoproteins, and plasma fibrinogen levels in fibromyalgia patients (Kaartinen et al., 2000; Hostmark et al., 1993). Another study found that a vegan diet rich in antioxidants that mainly contains carotenoids, vitamin C, and vitamin E reduces pain and self-reported morning joint stiffness in rheumatic disorders (Hänninen et al., 2000). Brain tryptophan level is important in the synthesis of serotonin, which is an important neurotransmitter in pain pathway. Increased intake of large neutral amino acids present in animal proteins decreases brain tryptophan levels in fibromyalgia patients and affects the pain pathway (Juhl, 1998). Azad and coworkers conducted an open, controlled, and randomized trial on 78 fibromyalgia patients to investigate the effects of a vegetarian diet (free of animal proteins) in reducing pain and morbidity. The study outcome showed a significant reduction in pain score (Azad et al., 2000). The reports showed that food additives containing aspartate and glutamate, monosodium glutamate, aspartame, autolyzed yeast extract, and branched-chain amino acids present in food act as excitatory neurotransmitters and play a potential role in pain via central sensitization. In addition, food coloring, cow’s milk, chocolate, caffeine, and shellfish also trigger fibromyalgia symptoms (Holton et al., 2009; Li and Micheletti, 2011). According to a community-based study, immune reactants such as monosodium glutamate, food colors, chocolate, caffeine, dairy products, and aspartame alter the neuroimmune hormonal feedback control system in fibromyalgia patients. In the same study, replacement of foods containing these substances with dietary supplements that included antioxidants and minerals consumed by patients for 6 months brought improved fibromyalgia symptoms, including reductions in pain, depression, morning stiffness, and fatigue (Deuster and Jaffe, 1998).
Micronutrients
Micronutrients are vital constituents of biological structures. A diet’s nutritional status is an important aspect to be considered in fibromyalgia patients because nutritional deficiencies are possible with FMS. Arranz and coworkers have reported the role of nutritional deficiency of magnesium, iodine, iron, selenium, and other micronutrients in the pathogenesis of fibromyalgia (Arranz et al., 2010). The following discusses important nutrients that have a role in FMS.
Magnesium
Magnesium is an important micronutrient required for the production of energy through adenosine triphosphate (ATP) synthesis in the presence of oxygen, substrates, adenosine diphosphate, and phosphate. High levels of cytosolic calcium and aluminum reduces ATP production by inhibiting glycolysis and oxidative phosphorylation. An adequate amount of magnesium is required to maintain low levels of cytosolic calcium and reduce aluminum toxicity (Siesjö et al., 1988; Allen, 1987). Intracellular magnesium and calcium concentrations play a vital role in muscle contraction. The results obtained from a study conducted by Magaldi et al. also showed the potential role of magnesium and calcium in FMS patients for muscular hypertonus (Magaldi et al., 1999). It has been reported that the level of magnesium is below normal range in fibromyalgia patients. A deficiency of magnesium affects the Krebs cycle and reduces lung capacity which ultimately leads to the development of such symptoms as fatigue, headache, muscle pain, irritable bowel syndrome, and depression in FMS patients (Abraham and Flechas, 1992; Romano and John, 1994). A sufficient concentration of magnesium is required to reduce relative hypoxia condition in FMS patients by reducing blood lactate levels and oxygen consumption (Schmidt et al., 1989). A magnesium deficiency also causes mitochondrial swelling and reduces the number of mitochondria per cell (Heaton and Rayssiguier, 1987). Yunus and coworkers also observed similar mitochondrial changes in tender-point muscle biopsies of FM patients (Yunus et al., 1988) and has been associated with muscle pain. Based on this association, Eisinger and coworkers examined magnesium levels in 22 patients suffering from muscle pain in fibromyalgia. The outcome of the study showed increased leukocyte level of magnesium while erythrocyte levels of magnesium decreased in the patients (Eisinger et al., 1994a,b). In one study, the correlation between clinical symptoms of fibromyalgia, especially fatigue and serum magnesium levels, were evaluated in 32 fibromyalgia patients. The results of the study showed significant reduction in serum magnesium levels, and significant correlation has been found between fatigue and serum magnesium levels in patients (Sendur et al., 2008).
Since magnesium concentration in serum plays an important role in fibromyalgia symptoms, magnesium supplementation may improve the symptomatic condition in FMS. In one study conducted in an open clinical setting, the effect of magnesium supplementation at a dosage of 300–600 mg along with malic acid (1200–2400 mg) was evaluated for 4–8 weeks in 15 FMS patients. The outcome of the study showed improvements in the pain condition and significant reductions in the tender-point index after magnesium supplementation (Abraham and Flechas, 1992). Similar finding were obtained in another randomized, double-blind, crossover study carried out by Russell et al. in 24 fibromyalgia patients. In this study, dose escalation of magnesium for 6 months showed significant reduction in pain and tenderness (Russell et al., 1995). A group of researcher evaluated the effect of magnesium citrate and magnesium citrate in combination with amitriptyline in the clinical symptoms of FMS such as pain threshold, number of tender points, pain intensity, tender-point index, Beck Anxiety Scale score and depression in 60 premenopausal women. The outcome of the study showed reduction in tender points, the tender-point index, and the Beck Depression Scale after magnesium citrate treatment and magnesium citrate in combination of amitriptyline showed significant improvement in all fibromyalgia symptoms (Bagis et al., 2013).
Iodine
Iodine is an essential micronutrient required for a healthy life (Prashanth et al., 2015). Approximately 70 μg/day of iodine is used by the thyroid gland to synthesis required amounts of thyroxine (T4) and triiodothyronine (T3) to regulate metabolism, normal growth, and development (Miller, 2006). It acts as an antioxidant by scavenging free hydroxyl radicals and increasing antioxidant levels in human serum (Winkler et al., 2000). An iodine deficiency causes thyroid dysfunction, and this leads to clinical symptoms like those found in fibromyalgia such as chronic aches, abnormal tenderness, sleep disturbances, fatigue, lethargy, and reduced physical and mental functions (Navia, 1970). Evidence showed a link between hypothyroidism, thyroid autoimmunity, and FMS (Friedman, 2013). In one clinical study conducted on 92 fibromyalgia patients, 52 patients were found to have either primary or central hypothyroidism (Lowe et al., 1998). The rate of occurrence of thyroid antibody mainly thyroglobulin and thyroid peroxidase in fibromyalgia patients is double compared to healthy humans (Bazzichi et al., 2007; Pamuk and Çakir, 2007). In addition, the characteristic pain, pressure pain threshold, and pain distribution observed in fibromyalgia patients may be positively associated with increased levels of thyroid antibodies in muscle proteins, low concentration of intracellular T3, and hypothyroidism (Bazzichi et al., 2007; Ruchala et al., 2007; Lowe et al., 2006). Reduced T3 levels in fibromyalgia patients cause increases in the synthesis and secretion of substance P, a neuropeptide that is responsible for pain signaling (Savard et al., 1984). Moreover, mitochondrial dysfunction, one of the factors responsible for impaired thyroid transport, has also been present in fibromyalgia patients (Friedman, 2013). This indicates that thyroid functions become disturbed in FMS. Because iodine deficiency is a major factor associated with thyroid dysfunctions, iodine supplementation may improve thyroid functions and symptoms in FMS patients.
Manganese
Manganese is an important micronutrient with its highest concentration found in grains, nuts, and cereals (Burch et al., 1975). It is an important component of metalloenzymes and plays a significant role in oxidative phosphorylation and metabolism as an enzyme activator (Rehnberg et al., 1982). It is an essential component of antioxidant defense in mitochondria in the form of manganese superoxide dismutase. Since the antioxidant defense has been altered in fibromyalgia patients, a manganese deficiency might be considered in the syndrome’s pathophysiology (Kim et al., 2011). Fatigue is one prominent symptom of FMS that can be linked with manganese-dependent neuroendocrine changes because they directly affect the metabolic rate via manganese’s participation in the hypothalamic–pituitary–thyroid axis (Ferraccioli et al., 1990).
Selenium
Selenium is a vital element of selenoprotein enzymes and acts as a redox center for these enzymatic functions (O’Dell and Sunde, 1997). Selenium-dependent glutathione peroxidase reduces hydrogen peroxide, organic hydroperoxidases, and main membrane integrity and reduces oxidative damage to biomolecules (Diplock, 1994). A selenium nutritional deficiency is one causative factor in the musculoskeletal noninflammatory disorder known as Kashin–Beck Disease (KBD). KBD symptoms such as morning stiffness in the joint and joint pain are comparable with the symptoms of FMS (Allander, 1994; Rayman, 2000). Low levels of selenium are also observed in rheumatoid arthritis and psoriatic arthritis (Tarp, 1994; Michaelsson et al., 1988). A selenium deficiency, along with an iodine deficiency, aggravates hypothyroidism (Vanderpas et al., 1990). A selenium insufficiency leads to depressed mood, anxiety, fatigue, and depression, which are also observed in fibromyalgia patients (Hawkes and Hornbostel, 1996; Benton and Cook, 1990). The above facts revealed a link between selenium deficiency and the symptoms of fibromyalgia. Based on these facts, a study was conducted by Reinhard and coworkers of 68 fibromyalgia patients to check their serum concentrations of selenium. The outcome of the study showed significant differences in serum selenium levels between FM patients and healthy blood donors, with the fibromyalgia patients showing lower serum selenium levels (Reinhard et al., 1998). The reports showed that dietary supplements of selenium significantly improved mood, anxiety, depression, and tiredness in a US study and double-blind crossover study conducted in the United Kingdom in FMS patients (Finley and Penland, 1998; Benton and Cook, 1990).
Thiamine
Thiamine or vitamin B1 is important to the respiratory chain. It acts as a coenzyme (magnesium-coordinated thiamin pyrophosphate) in metabolizing carbohydrates and amino acids. A thiamine deficiency leads to reduced carbohydrate and branched-chain amino acid metabolism, which subsequently affect the formation of acetylcholine, which is required for normal neuronal functions (Vorhees et al., 1977; Mann and Quastel, 1940). Moreover, a thiamine deficiency alters the brain’s turnover rate of serotonin, which plays a significant role in FMS (Plaitakis et al., 1982). The symptoms of thiamine deficiency mainly include anorexia, weakness, apathy, muscle tenderness, fatigue, burning sensations in feet and hands, confusion, sleep disturbance, low blood pressure, reduced metabolism, and depression (Prinzo, 1999). The majority of symptoms observed for a thiamine deficiency are also found in FMS patients. An important study published by Monroe revealed a metabolism abnormality of thiamine in fibromyalgia mainly due to a reduction in the activation of thiamine to thiamine pyrophosphate (Monroe et al., 1998). This abnormality causes impaired glycolysis, and decreased NO and glutathione and causes serotonin depletion in FM, which is responsible for symptoms such as muscle soreness, fatigue, and abnormal muscle relaxation (Eisinger et al., 1994a,b; Eisinger et al., 1997; Eisinger and Ayavou, 1990). In their study results, Costantini et al. commented that the classical symptoms of fibromyalgia such as depression, anxiety, fatigue, and insomnia are the result of thiamine deficiency and can be overcome by high-dose thiamine therapy in fibromyalgia patients (Costantini and Pala, 2013).
Iron
Iron is an essential element used by cytochrome oxidase enzyme to generate energy. It is required for the synthesis of neurotransmitter such as serotonin, norepinephrine, and dopamine, which are involved in the pathophysiology of fibromyalgia. An iron deficiency results in chronic tiredness, myalgia, unusual fatigue, poor endurance, and sleep disturbances (Beard et al., 1993; Gerwin, 2005). An iron deficiency also reduces the pain threshold and increases pain sensation (Dowling et al., 2009). This indicates that iron might play a significant role in the pathophysiology of fibromyalgia. Ortancil and coworkers suggested from their study carried out on 46 fibromyalgia patients that iron deficiency may have a role in etiology of some fibromyalgia symptoms because it act as a cofactor in serotonin and dopamine synthesis (Ortancil et al., 2010). The clinical study carried out on 205 patients with iron deficiency anemia (IDA) patients, 40 patients with thalassemia minor (TM), and 100 healthy volunteers detected a higher frequency of fibromyalgia in IDA patients and TM patients compare to the healthy volunteers (Pamuk et al., 2008).
Summary
FMS is a rheumatic disease that affects the quality of life of people around the world. Its etiology is unknown, but various scientific and nonscientific reports show the strong involvement of obesity and lowered nutritional levels of antioxidants and essential micronutrients in its pathophysiology. Furthermore, current therapies used to treat fibromyalgia symptoms include not only pharmacological agents but also physical activities and other alternative regimens (i.e., weight-loss programs, diet modifications, and the use of antioxidant and micronutrient supplements).
The assessed clinical data showed that obesity plays a major role in FMS. The severity of symptoms in obese people is more than in nonobese fibromyalgia patients. Various clinical studies demonstrated improvement in the FMS after the use of weight-loss programs and nutritional intervention of vegetarian and vegan diets rich in antioxidants. Nevertheless, more complete and detailed studies are required to confirm the positive effects of these diets on FMS. Some nutritional deficiencies have been suggested to be involved in FMS, although detailed clinical reports on these are lacking. Few studies have been carried out that point to nutritional deficiencies of magnesium, iodine, selenium, iron, thiamine, and manganese as linked with FMS or related conditions such as chronic pain syndrome and hypothyroidism. The importance of some micronutrients in FMS has been evaluated in some studies with positive outcome. This suggests that more detailed and specific investigations are required in FMS patients to understand the beneficial effect of the cited nutrients. In conclusion, current dietary advice is necessary for FMS patients so they can maintain normal weight and enjoy the nutritional benefits of micronutrients that will reduce the severity of their symptoms.