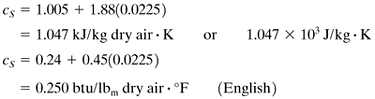9.3. VAPOR PRESSURE OF WATER AND HUMIDITY
9.3A. Vapor Pressure of Water
1. Introduction
In a number of the separation processes and transport processes, it is necessary to make calculations involving the properties of mixtures of water vapor and air. These calculations involve knowledge of the concentration of water vapor in air under various conditions of temperature and pressure, the thermal properties of these mixtures, and the changes occurring when these mixtures are brought into contact with water or with wet solids in drying.
Humidification involves the transfer of water from the liquid phase into a gaseous mixture of air and water vapor. Dehumidification involves the reverse transfer, whereby water vapor is transferred from the vapor state to the liquid state. Humidification and dehumidification can also refer to vapor mixtures of materials such as benzene, but most practical applications occur with water. To better understand humidity, it is first necessary to discuss the vapor pressure of water.
2. Vapor pressure of water and physical states
Pure water can exist in three different physical states: solid ice, liquid, and vapor. The physical state in which it exists depends on the pressure and temperature.
Figure 9.3-1 illustrates the various physical states of water and the pressure–temperature relationships at equilibrium. In Fig. 9.3-1 the regions of the solid, liquid, and vapor states are shown. Along the line AB, the phases liquid and vapor coexist. Along line AC, the phases ice and liquid coexist. Along line AD, ice and vapor coexist. If ice at point (1) is heated at constant pressure, the temperature rises and the physical condition is represented as moving horizontally. As the horizontal line crosses AC, the solid melts, and on crossing AB the liquid vaporizes. Moving from point (3) to (4), ice sublimes (vaporizes) to a vapor without becoming a liquid.
Figure 9.3-1. Phase diagram for water.

Liquid and vapor coexist in equilibrium along the line AB, which is the vapor-pressure line for water. Boiling occurs when the vapor pressure of the water is equal to the total pressure above the water surface. For example, at 100°C (212°F) the vapor pressure of water is 101.3 kPa (1.0 atm), and therefore it will boil at 1 atm pressure. At 65.6°C (150°F), from the steam tables in Appendix A.2, the vapor pressure of water is 25.7 kPa (3.72 psia). Hence, at 25.7 kPa and 65.6°C, water will boil.
If a pan of water is held at 65.6°C in a room at 101.3 kPa abs pressure, the vapor pressure of water will again be 25.7 kPa. This illustrates an important property of the vapor pressure of water, which is not influenced by the presence of an inert gas such as air; that is, the vapor pressure of water is essentially independent of the total pressure of the system.
9.3B. Humidity and Humidity Chart
1. Definition of humidity
The humidity H of an air–water vapor mixture is defined as the kg of water vapor contained in 1 kg of dry air. The humidity so defined depends only on the partial pressure pA of water vapor in the air and on the total pressure P (assumed throughout this chapter to be 101.325 kPa, 1.0 atm abs, or 760 mm Hg). Using the molecular weight of water (A) as 18.02 and of air as 28.97, the humidity H in kg H2O/kg dry air, or in English units as lb H2O/lb dry air, is as follows:
Equation 9.3-1

Saturated air is air in which the water vapor is in equilibrium with liquid water at the given conditions of pressure and temperature. In this mixture the partial pressure of the water vapor in the air–water mixture is equal to the vapor pressure pAS of pure water at the given temperature. Hence, the saturation humidity HS is
Equation 9.3-2
![]()
2. Percentage humidity
The percentage humidity HP is defined as 100 times the actual humidity H of the air divided by the humidity HS if the air were saturated at the same temperature and pressure:
Equation 9.3-3
![]()
3. Percentage relative humidity
The amount of saturation of an air–water vapor mixture is also given as percentage relative humidity HR using partial pressures:
Equation 9.3-4
![]()
Note that HR ≠ HP, since HP expressed in partial pressures by combining Eqs. (9.3-1), (9.3-2), and (9.3-3) is
Equation 9.3-5
![]()
This, of course, is not the same as Eq. (9.3-4).
EXAMPLE 9.3-1. Humidity from Vapor-Pressure DataThe air in a room is at 26.7°C (80°F) and a pressure of 101.325 kPa and contains water vapor with a partial pressure pA = 2.76 kPa. Calculate the following:
Solution: From the steam tables at 26.7°C, the vapor pressure of water is pAS = 3.50 kPa (0.507 psia). Also, pA = 2.76 kPa and P = 101.3 kPa (14.7 psia). For part (a), using Eq. (9.3-1),
For part (b), using Eq. (9.3-2), the saturation humidity is
The percentage humidity, from Eq. (9.3-3), is
For part (c), from Eq. (9.3-4), the percentage relative humidity is
|
4. Dew point of an air–water vapor mixture
The temperature at which a given mixture of air and water vapor would be saturated is called the dew-point temperature or simply the dew point. For example, at 26.7°C (80°F), the saturation vapor pressure of water is pAS = 3.50 kPa (0.507 psia). Hence, the dew point of a mixture containing water vapor having a partial pressure of 3.50 kPa is 26.7°C. If an air–water vapor mixture is at 37.8°C (often called the dry bulb temperature, since this is the actual temperature a dry thermometer bulb would indicate in this mixture) and contains water vapor of pA = 3.50 kPa, the mixture would not be saturated. On cooling to 26.7°C, the air would be saturated, that is, at the dew point. On further cooling, some water vapor would condense, since the partial pressure cannot be greater than the saturation vapor pressure.
5. Humid heat of an air–water vapor mixture
The humid heat cs is the amount of heat in J (or kJ) required to raise the temperature of 1 kg of dry air plus the water vapor present by 1 K or 1°C. The heat capacity of air and water vapor can be assumed constant over the temperature ranges usually encountered at 1.005 kJ/kg dry air · K and 1.88 kJ/kg water vapor · K, respectively. Hence, for SI and English units,
Equation 9.3-6
![]()
[In some cases cS will be given as (1.005 + 1.88H)103 J/kg · K.]
6. Humid volume of an air–water vapor mixture
The humid volume νH is the total volume in m3 of 1 kg of dry air plus the vapor it contains at 101.325 kPa (1.0 atm) abs pressure and the given gas temperature. Using the ideal gas law,
Equation 9.3-7
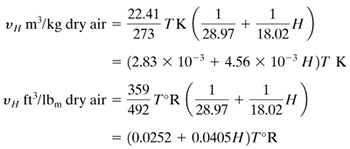
For a saturated air–water vapor mixture, H = HS, and νH is the saturated volume.
7. Total enthalpy of an air–water vapor mixture
The total enthalpy of 1 kg of air plus its water vapor is Hy J/kg or kJ/kg dry air. If T0 is the datum temperature chosen for both components, the total enthalpy is the sensible heat of the air–water vapor mixture plus the latent heat λ0 in J/kg or kJ/kg water vapor of the water vapor at T0. Note that (T − T0)°C = (T − T0) K and that this enthalpy is referred to liquid water.
Equation 9.3-8

If the total enthalpy is referred to a base temperature T0 of 0°C (32°F), the equation for Hy becomes
Equation 9.3-9

8. Humidity chart of air–water vapor mixtures
A convenient chart of the properties of air–water vapor mixtures at 1.0 atm abs pressure is the humidity chart in Fig. 9.3-2. In this figure the humidity H is plotted versus the actual temperature of the air–water vapor mixture (dry bulb temperature).
Figure 9.3-2. Humidity chart for mixtures of air and water vapor at a total pressure of 101.325 kPa (760 mm Hg). (From R. E. Treybal, Mass-Transfer Operations, 3rd ed. New York: McGraw-Hill Book Company, 1980. With permission.)

The curve marked 100% running upward to the right gives the saturation humidity HS as a function of temperature. In Example 9.3-1, for 26.7°C, HS was calculated as 0.02226 kg H2O/kg air. Plotting this point for 26.7°C (80°F) and HS = 0.02226 on Fig. 9.3-2, it falls on the 100% saturated line.
Any point below the saturation line represents unsaturated air–water vapor mixtures. The curved lines below the 100% saturation line and running upward to the right represent unsaturated mixtures of definite percentage humidity HP. Going downward vertically from the saturation line at a given temperature, the line between 100% saturation and zero humidity H (the bottom horizontal line) is divided evenly into 10 increments of 10% each.
All the percentage humidity lines HP mentioned and the saturation humidity line HS can be calculated from the data for vapor pressure of water.
EXAMPLE 9.3-2. Use of Humidity ChartAir entering a dryer has a temperature (dry bulb temperature) of 60°C (140°F) and a dew point of 26.7°C (80°F). Using the humidity chart, determine the actual humidity H, percentage humidity HP, humid heat cS, and humid volume νH in SI and English units. Solution: The dew point of 26.7°C is the temperature when the given mixture is at 100% saturation. Starting at 26.7°C (Fig. 9.3-2), and drawing a vertical line until it intersects the line for 100% humidity, a humidity of H = 0.0225 kg H2O/kg dry air is read off the plot. This is the actual humidity of the air at 60°C. Stated in another way, if air at 60°C and having a humidity H = 0.0225 is cooled, its dew point will be 26.7°C. In English units, H = 0.0225 lb H2O/lb dry air. Locating this point where H = 0.0225 and t = 60°C on the chart, the percentage humidity HP is found to be 14%, by linear interpolation vertically between the 10 and 20% lines. The humid heat for H = 0.0225 is, from Eq. (9.3-6),
The humid volume at 60°C (140°F), from Eq. (9.3-7), is
In English units,
|
9.3C. Adiabatic Saturation Temperatures
Consider the process shown in Fig. 9.3-3, where the entering gas of air–water vapor mixture is contacted with a spray of liquid water. The gas leaves having a different humidity and temperature and the process is adiabatic. The water is recirculated, with some makeup water added.
Figure 9.3-3. Adiabatic air–water vapor saturator.
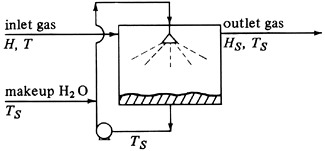
The temperature of the water being recirculated reaches a steady-state temperature called the adiabatic saturation temperature, TS. If the entering gas at temperature T having a humidity of H is not saturated, TS will be lower than T. If the contact between the entering gas and the spray of droplets is enough to bring the gas and liquid to equilibrium, the leaving air is saturated at TS, having a humidity HS.
Writing an enthalpy balance (heat balance) over the process, a datum of TS is used. The enthalpy of the makeup H2O is then zero. This means that the total enthalpy of the entering gas mixture = enthalpy of the leaving gas mixture, or, using Eq. (9.3-8),
Equation 9.3-10
![]()
Or, rearranging, and using Eq. (9.3-6) for cS,
Equation 9.3-11

Equation (9.3-11) is the equation of an adiabatic humidification curve when plotted on Fig. 9.3-2, which passes through the point HS and TS on the 100% saturation curve and other points of H and T. These series of lines, running upward to the left, are called adiabatic humidification lines or adiabatic saturation lines. Since cS contains the term H, the adiabatic lines are not quite straight when plotted on the humidity chart.
If a given gas mixture at T1 and H1 is contacted for a sufficiently long time in an adiabatic saturator, it will leave saturated at HS1 and TS1. The values of HS1 and TS1 are determined by following the adiabatic saturation line going through point T1, H1 until it intersects the 100% saturation line. If contact is not sufficient, the leaving mixture will be at a percentage saturation less than 100% but on the same line.
9.3D. Wet Bulb Temperature
The adiabatic saturation temperature is the steady-state temperature attained when a large amount of water is contacted by the entering gas. The wet bulb temperature is the steady-state nonequilibrium temperature reached when a small amount of water is contacted under adiabatic conditions by a continuous stream of gas. Since the amount of liquid is small, the temperature and humidity of the gas are not changed, contrary to the case of adiabatic saturation, where the temperature and humidity of the gas are changed.
The method used to measure the wet bulb temperature is illustrated in Fig. 9.3-4, where a thermometer is covered by a wick or cloth. The wick is kept wet by water and is immersed in a flowing stream of air–water vapor having a temperature of T (dry bulb temperature) and humidity H. At steady state, water is evaporating to the gas stream. The wick and water are cooled to TW and stay at this constant temperature. The latent heat of evaporation is exactly balanced by the convective heat flowing from the gas stream at T to the wick at a lower temperature TW.
Figure 9.3-4. Measurement of wet bulb temperature.

A heat balance on the wick can be made. The datum temperature is taken at TW. The amount of heat lost by vaporization, neglecting the small sensible heat change of the vaporized liquid and radiation, is
Equation 9.3-12
![]()
where q is kW (kJ/s), MA is the molecular weight of water, NA is kg mol H2O evaporating/s · m2, A is surface area m2, and λW is the latent heat of vaporization at TW in kJ/kg H2O. In English units, q is btu/h, NA is lb mol/h · ft2, and λW is btu/lbm H2O. The flux NA is
Equation 9.3-13
![]()
where ![]() is the mass-transfer coefficient in kg mol/s · m2 · mol frac, xBM is the log mean inert mole fraction of the air, yW is the mole fraction of water vapor in the gas at the surface, and y is the mole fraction in the gas. For a dilute mixture xBM ≅ 1.0 and
is the mass-transfer coefficient in kg mol/s · m2 · mol frac, xBM is the log mean inert mole fraction of the air, yW is the mole fraction of water vapor in the gas at the surface, and y is the mole fraction in the gas. For a dilute mixture xBM ≅ 1.0 and ![]() . The relation between H and y is
. The relation between H and y is
Equation 9.3-14
![]()
where MB is the molecular weight of air and MA the molecular weight of H2O. Since H is small, as an approximation,
Equation 9.3-15
![]()
Substituting Eq. (9.3-15) into (9.3-13) and then substituting the resultant into Eq. (9.3-12),
Equation 9.3-16
![]()
The rate of convective heat transfer from the gas stream at T to the wick at TW is
Equation 9.3-17
![]()
where h is the heat-transfer coefficient in kW/m2 · K (btu/h · ft2 · °F).
Equating Eq. (9.3-16) to (9.3-17) and rearranging,
Equation 9.3-18
![]()
Experimental data on the value of h/MBky, called the psychrometric ratio, show that for water vapor–air mixtures, the value is approximately 0.96–1.005. Since this value is close to the value of cS in Eq. (9.3-11), approximately 1.005, Eqs. (9.3-18) and (9.3-11) are almost the same. This means that the adiabatic saturation lines can also be used for wet bulb lines with reasonable accuracy. (Note that this is only true for water vapor and not for other vapors, such as benzene.) Hence, the wet bulb determination is often used to measure the humidity of an air–water vapor mixture.
EXAMPLE 9.3-4. Wet Bulb Temperature and HumidityA water vapor–air mixture having a dry bulb temperature of T = 60°C is passed over a wet bulb, as shown in Fig. 9.3-4, and the wet bulb temperature obtained is TW = 29.5°C. What is the humidity of the mixture? Solution: The wet bulb temperature of 29.5°C can be assumed to be the same as the adiabatic saturation temperature TS, as discussed. Following the adiabatic saturation curve of 29.5°C until it reaches the dry bulb temperature of 60°C, the humidity is H = 0.0135 kg H2O/kg dry air. |