9.11. FREEZE-DRYING OF BIOLOGICAL MATERIALS
9.11A. Introduction
Certain foodstuffs, pharmaceuticals, and biological materials, which may not be heated even to moderate temperatures in ordinary drying, may be freeze-dried. The substance to be dried is usually frozen by exposure to very cold air. In freeze-drying, the water is removed as a vapor by sublimation from the frozen material in a vacuum chamber. After the moisture sublimes to a vapor, it is removed by mechanical vacuum pumps or steam jet ejectors.
As a rule, freeze-drying produces the highest-quality food product obtainable by any drying method. A prominent factor is the structural rigidity afforded by the frozen substance when sublimation occurs. This prevents collapse of the remaining porous structure after drying. When water is added later, the rehydrated product retains much of its original structural form. Freeze-drying of biological and food materials also has the advantage of little loss of flavor and aroma. The low temperatures involved minimize the degrading reactions which normally occur in ordinary drying processes. However, freeze-drying is an expensive form of dehydration for foods because of the slow drying rate and the use of vacuum.
Since the vapor pressure of ice is very small, freeze-drying requires very low pressures or high vacuum. If the water were in a pure state, freeze-drying at or near 0°C (273 K) at a pressure of 4580 μm (4.58 mm Hg abs) could be performed. (See Appendix A.2 for the properties of ice.) However, since the water usually exists in a solution or a combined state, the material must be cooled below 0°C to keep the water in the solid phase. Most freeze-drying is done at −10°C (263 K) or lower at pressures of about 2000 μm or less.
9.11B. Derivation of Equations for Freeze-Drying
In the freeze-drying process the original material is composed of a frozen core of material. As the ice sublimes, the plane of sublimation, which started at the outside surface, recedes, and a porous shell of material already dried remains. The heat for the latent heat of sublimation of 2838 kJ/kg (1220 btu/lbm) ice is usually conducted inward through the layer of dried material. In some cases it is also conducted through the frozen layer from the rear. The vaporized water is transferred through the layer of dried material. Hence, heat and mass transfer are occurring simultaneously.
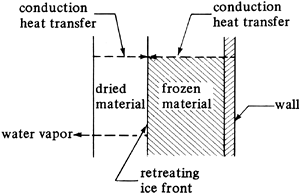
In Fig. 9.11-1 a material being freeze-dried is pictured. Heat by conduction, convection, and/or radiation from the gas phase reaches the dried surface and is then transferred by conduction to the ice layer. In some cases heat may also be conducted through the frozen material to reach the sublimation front or plane. The total drying time must be long enough that the final moisture content is below about 5 wt % to prevent degradation of the final material on storage. The maximum temperatures reached in the dried food and the frozen food must be low enough to keep degradation to a minimum.
Figure 9.11-1. Heat and mass transfer in freeze drying.
The most widely used freeze-drying process is based upon the heat of sublimation being supplied from the surrounding gases to the sample surface. Then the heat is transferred by conduction through the dried material to the ice surface. A simplified model by Sandall et al. (S1) is shown in Fig. 9.11-2.
Figure 9.11-2. Model for uniformly retreating ice front in freeze drying.

The heat flux to the surface of the material in Fig. 9.11-2 occurs by convection and in the dry solid by conduction to the sublimation surface. The heat flux to the surface is equal to that conducted through the dry solid, assuming pseudo-steady state:
Equation 9.11-1
![]()
where q is heat flux in W (J/s), h is external heat-transfer coefficient in W/m2 · K, Te is external temperature of the gas in °C, Ts is surface temperature of the dry solid in °C, Tf is temperature of the sublimation front or ice layer in °C, k is thermal conductivity of the dry solid in W/m · K, and ΔL is the thickness of the dry layer in m. Note that (Ts − Tf)°C = (Ts − Tf) K.
In a similar manner, the mass flux of the water vapor from the sublimation front is
Equation 9.11-2
![]()
where NA is flux of water vapor in kg mol/s · m2, kg is external mass-transfer coefficient in kg mol/s · m2 · atm, psw is partial pressure of water vapor at the surface in atm, pew is partial pressure of water vapor in the external bulk gas phase in atm, T is the average temperature in the dry layer, D' is an average effective diffusivity in the dry layer in m2/s, and pfw is the partial pressure of water vapor in equilibrium with the sublimation ice front in atm.
Equation (9.11-1) can be rearranged to give
Equation 9.11-3
![]()
Also, Eq. (9.11-2) can be rearranged to give
Equation 9.11-4
![]()
The coefficients h and kg are determined by the gas velocities and characteristics of the dryer and hence are constant. The values of Te and pew are set by the external operating conditions. The values of k and D' are determined by the nature of the dried material.
The heat flux and mass flux at pseudo-steady state are related by
Equation 9.11-5
![]()
where ΔHs is the latent heat of sublimation of ice in J/kg mol. Also, pfw is uniquely determined by Tf, since it is the equilibrium vapor pressure of ice at that temperature; or
Equation 9.11-6
![]()
Substituting Eqs. (9.11-3) and (9.11-4) into (9.11-5),
Equation 9.11-7
![]()
Also, substituting Eqs. (9.11-1) and (9.11-4) into (9.11-5),
Equation 9.11-8
![]()
As Te and, hence, Ts are raised to increase the rate of drying, two limits may possibly be reached. First, the outer-surface temperature, Ts cannot be allowed to go too high because of thermal damage. Second, the temperature Tf must be kept well below the melting point. For the situation where k/ΔL is small compared to kg and D'/RT ΔL, the outer-surface temperature limit will be encountered first as Ts is raised. To further increase the drying rate, k must be raised. Hence, the process is considered to be heat-transfer-controlled. Most commercial freeze-drying processes are heat-transfer-controlled (K1).
In order to solve the given equations, ΔL is related to x, the fraction of the original free moisture remaining:
Equation 9.11-9
![]()
The rate of freeze-drying can be related to NA by
Equation 9.11-10
![]()
where MA is molecular weight of water, VS is the volume of solid material occupied by a unit kg of water initially (VS = 1/X0ρS), X0 is initial free moisture content in kg H2O/kg dry solid, and ρS is bulk density of dry solid in kg/m3.
Combining Eqs. (9.11-3), (9.11-5), (9.11-9), and (9.11-10), we obtain, for heat transfer,
Equation 9.11-11
![]()
Similarly, for mass transfer,
Equation 9.11-12
![]()
Integrating Eq. (9.11-11) between the limits of t = 0 at x1 = 1.0 and t = t at x2 = x2, the equation for the time of drying to x2 is as follows for h being very large (negligible external resistance):
Equation 9.11-13
![]()
where ΔHs/MA is heat of sublimation in J/kg H2O. For x2 = 0, the slab is completely dry.
Assuming that the physical properties and mass- and heat-transfer coefficients are known, Eq. (9.11-8) can be used to calculate the ice-sublimation temperature Tf when the environment temperature Te and the environment partial pressure pew are set. Since h is very large, Te ≅ Ts. Then Eq. (9.11-8) can be solved for Tf, since Tf and pfw are related by the equilibrium-vapor-pressure relation, Eq. (9.11-6). In Eq. (9.11-8) the value to use for T can be approximated by (Tf + Ts)/2.
The uniformly retreating ice-front model was tested by Sandall et al. (S1) against actual freeze-drying data. The model satisfactorily predicted the drying times for removal of 65–90% of the total initial water (S1, K1). The temperature Tf of the sublimation interface did remain essentially constant as assumed in the derivation. However, during removal of the last 10–35% of the water, the drying rate slowed markedly and the actual time was considerably greater than the predicted time for this period.
The effective thermal conductivity k in the dried material has been found to vary significantly with the total pressure and with the type of gas present. Also, the type of material affects the value of k (S1, K1). The effective diffusivity D' of the dried material is a function of the structure of the material, Knudsen diffusivity, and molecular diffusivity (K1).
