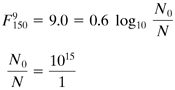9.12. UNSTEADY-STATE THERMAL PROCESSING AND STERILIZATION OF BIOLOGICAL MATERIALS
9.12A. Introduction
Materials of biological origin are usually not as stable as most inorganic and some organic materials. Hence, it is necessary to use certain processing methods to preserve biological materials, especially foods. Physical and chemical processing methods of preservation, such as drying, smoking, salting, chilling, freezing, and heating, are commonly used. Freezing and chilling of foods were discussed in Section 5.5 as methods of slowing the spoilage of biological materials. Also, in Section 9.11, freeze-drying of biological materials was discussed.
An important method is heat or thermal processing, whereby contaminating microorganisms that occur primarily on the outer surface of foods and cause spoilage and health problems are destroyed. This leads to longer storage times for food and other biological materials. A common method of preservation is to heat-seal cans of food. Likewise, thermal processing is used to sterilize aqueous fermentation media to be used in fermentation processes so that organisms that do not survive are unable to compete with the organism that is to be cultured.
The sterilization of food materials by heating destroys bacteria, yeast, molds, and so on, which cause spoilage, and also destroys pathogenic (disease-producing) organisms that may produce deadly toxins if not destroyed. The rate of destruction of microorganisms varies with the amount of heating and the type of organism. Some bacteria can exist in a vegetative growing form and in a dormant or spore form. The spore forms are much more resistant to heat. This mechanism of heat resistance is not clear.
For foods, it is necessary to kill essentially all the spores of Clostridium botulinum, which produces a toxin that is a deadly poison. Complete sterility with respect to this spore is the purpose of thermal processing. Since Cl. botulinum is so dangerous and often difficult to use, other spores, such as Bacillus stearothermophilus, which is a nonpathogenic organism of similar heat resistance, are often used for testing the heat-treating processes (A2, C1).
Temperature has a great effect on the growth rate of microorganisms, which have no temperature-regulating mechanism. Each organism has a certain optimal temperature range in which it grows best. If any microorganism is heated to a sufficiently high temperature for a sufficient time, it will be rendered sterile or killed.
The exact mechanism of thermal death of vegetative bacteria and spores is still somewhat uncertain. It is thought, however, to be due to the breakdown of enzymes, which are essential to the functioning of the living cell (B1).
9.12B. Thermal Death-Rate Kinetics of Microorganisms
The destruction of microorganisms by heating means loss of viability and not destruction in the physical sense. If it is assumed that inactivation of a single enzyme in a cell will inactivate the cell, then in a suspension of organisms of a single species at a constant temperature, the death rate can be expressed as a first-order kinetic equation (A2). The rate of destruction (number dying per unit time) is proportional to the number of organisms:
Equation 9.12-1
![]()
where N is the number of viable organisms at a given time, t is time in min, and k is a reaction velocity constant in min–1. The reaction velocity constant is a function of temperature and the type of microorganism.
After rearranging, Eq. (9.12-1) can be integrated as follows:
Equation 9.12-2
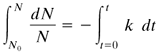
Equation 9.12-3
![]()
where N0 is the original number of organisms at t = 0 and N is the number at time t. Often N0 is called the contamination level (original number of contaminating microbes before sterilization) and N the sterility level. Equation (9.12-3) can also be written as
Equation 9.12-4
![]()
Sometimes microbiologists use the term decimal reduction time D, which is the time in min during which the original number of viable microbes is reduced by ![]() . Substituting into Eq. (9.12-4),
. Substituting into Eq. (9.12-4),
Equation 9.12-5
![]()
Taking the log10 of both sides and solving for D,
Equation 9.12-6
![]()
Combining Eqs. (9.12-3) and (9.12-6),
Equation 9.12-7
![]()
If the log10(N/N0) is plotted versus t, a straight line should result from Eq. (9.12-3). Experimental data bear this out for vegetative cells and approximately for spores. Data for the vegetative cell E. coli (A1) at constant temperature follow this logarithmic death-rate curve. Bacterial-spore plots sometimes deviate somewhat from the logarithmic rate of death, particularly during a short period immediately following exposure to heat. However, for thermal-processing purposes for use with spores such as Cl. botulinum, a logarithmic-type curve is used.
To experimentally measure the microbial death rate, the spore or cell suspension in a solution is usually sealed in a capillary or test tube. A number of these tubes are then suddenly dipped into a hot bath for a given time. Then they are removed and immediately chilled. The number of viable organisms before and after exposure to the high temperature is then usually determined biologically by means of a plate count.
The effect of temperature on the reaction-rate constant k may be expressed by an Arrhenius-type equation:
Equation 9.12-8
![]()
where a = an empirical constant, R is the gas constant in kJ/g mol · K (cal/gmol · K), T is absolute temperature in K, and E is the activation energy in kJ/g mol (cal/g mol). The value of E is in the range 210 to about 418 kJ/g mol (50–100 kcal/g mol) for vegetative cells and spores (A2) and much lower for enzymes and vitamins.
Substituting Eq. (9.12-8) into (9.12-2) and integrating,
Equation 9.12-9
![]()
At constant temperature T, Eq. (9.12-9) becomes (9.12-3). Since k is a function of temperature, the decimal reduction time D, which is related to k by Eq. (9.12-6), is also a function of temperature. Hence, D is often written as DT to show that it is temperature-dependent.
9.12C. Determination of Thermal Process Time for Sterilization
For canned foods, Cl. botulinum is the primary organism to be reduced in number (S2). It has been established that the minimum adequate heating process should reduce the number of spores by a factor of 10−12. This means that, since D is the time required to reduce the original number by 10−1, substituting N/N0 = 10−12 into Eq. (9.12-4) and solving for t,
Equation 9.12-10
![]()
Thus the time t is equal to 12D (often called the 12D concept). This time in Eq. (9.12-10) to reduce the number by 10−12 is called the thermal death time. Usually, the sterility level N is a number much less than one organism. These times do not represent complete sterilization but a mathematical concept which has been found empirically to give effective sterilization.
Experimental data for thermal death rates of Cl. botulinum, when plotted as the decimal reduction time DT at a given T versus the temperature T in °F on a semilog plot, give essentially straight lines over the range of temperatures used in food sterilization (S2). A typical thermal destruction curve is shown in Fig. 9.12-1. Actually, by combining Eqs. (9.12-6) and (9.12-8), it can be shown that the plot of log10DT versus 1/T (T in degrees absolute) is a straight line, but over small ranges of temperature a straight line is obtained when log10 DT is plotted versus T°F or °C.
Figure 9.12-1. Thermal destruction curve: plot of decimal reduction time versus temperature.

In Fig. 9.12-1 the term z represents the temperature range in °F for a 10:1 change in DT. Since the plot is a straight line, the equation can be represented as
Equation 9.12-11
![]()
Letting T1 = 250°F (121.1°C), which is the standard temperature against which thermal processes are compared, and calling T2 = T, Eq. (9.12-11) becomes
Equation 9.12-12
![]()
For the organism Cl. botulinum the experimental value of z = 18°F. This means that each increase in temperature of 18°F (10°C) will increase the death rate by a factor of 10. This compares with the factor of 2 for many chemical reactions for an 18°F increase in temperature.
Using Eq. (9.12-7),
Equation 9.12-7
![]()
Substituting T = 250°F (121.1°C) as the standard temperature into this equation and substituting F0 for t,
Equation 9.12-13
![]()
where the F0 value of a process is the time t in min at 250°F that will produce the same degree of sterilization as the given process at its temperature T. Combining Eqs. (9.12-7), (9.12-12), and (9.12-13), the F0 of the given process at temperature T is
Equation 9.12-14

This is the F0 value in min for the given thermal process at a given constant temperature T°F and a given time t in min. Values for F0 and z for adequate sterilization of Cl. botulinum vary somewhat with the type of food. Data are tabulated by Stumbo (S2) and Charm (C2) for various foods and microorganisms.
The effects of different but successive sterilization processes in a given material are additive. Hence, for several different temperature stages T1, T2, and so on, each having different times t1, t2, . . . , the F0 values for each stage are added to give the total F0:
Equation 9.12-15
![]()
EXAMPLE 9.12-1. Sterilization of Cans of FoodCans of a given food were heated in a retort for sterilization. The F0 for Cl. botulinum in this type of food is 2.50 min and z = 18°F. The temperatures in the center of a can (the slowest-heating region) were measured and were approximately as follows, where the average temperature during each time period is listed: t1 (0–20 min), T1 = 160°F; t2 (20–40 min), T2 = 210°F; t3 (40–73 min), T3 = 230°F. Determine if this sterilization process is adequate. Use English and SI units. Solution: For the three time periods, the data are as follows:
Substituting into Eq. (9.12-15) and solving, using English and SI units, Equation 9.12-15
Hence, this thermal processing is adequate, since only 2.50 min is needed for complete sterilization. Note that the time period at 160°F (71.1°C) contributes an insignificant amount to the final F0. The major contribution is at 230°F (110°C), which is the highest temperature. |
In the general case when cans of food are being sterilized in a retort, the temperature is not constant for a given time period but varies continuously with time. Hence, Eq. (9.12-15) can be modified and written for a continuously varying temperature T by taking small time increments of dt min for each value of T and summing. The final equation is
Equation 9.12-16

This equation can be used as follows. Suppose that the temperature of a process is varying continuously and that a graph or table of values of T versus t is known or can be calculated by means of the unsteady-state methods given in Chapter 5. Equation (9.12-16) can be graphically integrated by plotting values of 10(T−250)/z versus t and taking the area under the curve. In most cases a numerical integration is used to determine F0. (See Section 1.8 for methods of numerical integration.)
In many cases the temperature of a process that is varying continuously with time is determined experimentally by measuring the temperature in the slowest-heating region. In cans this is the center of the can. Methods given in Chapter 5 for unsteady-state heating of short, fat cylinders by conduction can be used to predict the center temperature of the can as a function of time. However, these predictions can be somewhat in error, since physical and thermal properties of foods are difficult to measure accurately and often can vary. Also, trapped air in the container and unknown convection effects can affect the accuracy of predictions.
EXAMPLE 9.12-2. Thermal Process Evaluation by Numerical IntegrationIn the sterilization of a canned purée, the temperature in the slowest-heating region (center) of the can was measured, giving the following time–temperature data for the heating and holding time. The cooling-time data will be neglected as a safety factor.
The F0 value of Cl. botulinum is 2.45 min and z is 18°F. Calculate the F0 value of the process above and determine if the sterilization is adequate. Solution: In order to use Eq. (9.12-16), the values of 10(T−250)/z must be calculated for each time. For t = 0 min, T = 80°F, and z = 18°F,
These calculated values are then used in Eq. (9.12-16) to perform a numerical integration to give F0 = 2.50 min. The process value of 2.50 min is greater than the required value of 2.45 min, and the sterilization is adequate. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
9.12D. Sterilization Methods Using Other Design Criteria
In types of thermal processing which are not necessarily involved with sterilization of foods, other types of design criteria are used. In foods the minimum adequate heat process should reduce the number of spores by a factor of 10−12, that is, N/N0 = 10−12. However, in other batch-sterilization processes, such as in the sterilization of fermentation media, other criteria are often used. Often the equation for k, the reaction velocity constant for the specific organism to be used, is available:
Equation 9.12-8
![]()
Then Eq. (9.12-9) is written as
Equation 9.12-17
![]()
where ∇ is the design criterion. Usually, the contamination level N0 is available and either the sterility level N or the time of sterilization at a given temperature is the unknown. In either case a numerical or graphical integration is used to solve the problem.
In sterilization of food in a container, the time required to render the material safe is calculated at the slowest-heating region of the container (usually the center). Other regions of the container are usually heated to higher temperatures and are overtreated. Hence, another method used is based on the probability of survival in the whole container. These details are given by others (C2, S2). In still another processing method, a short-time, continuous-flow process is used instead of a batch process in a container (B2).
9.12E. Pasteurization
The term pasteurization is used today to apply to a mild heat treatment of foods that is less drastic than sterilization. It is used to kill organisms that are relatively low in thermal resistance compared to those which the more drastic sterilization processes are designed to eliminate. Pasteurization usually involves killing vegetative microorganisms and not heat-resistant spores.
The most common process is the pasteurization of milk to kill Mycobacterium tuberculosis, which is a non-spore-forming bacterium. This pasteurization does not sterilize the milk but kills the M. tuberculosis and reduces the other bacterial count sufficiently so that the milk can be stored if refrigerated.
For the pasteurization of such foods as milk, fruit juices, and beer, the same mathematical and numerical procedures covered for sterilization processes in this section are used to accomplish the degree of sterilization desired in pasteurization (B1, S2). The times involved are much shorter and the temperatures used in pasteurization are much lower. Generally, the F0 value is given as 150°F (65.6°C) or a similar temperature rather than 250°F as in sterilization. Also, the concept of the z value is employed, in which a rise in temperature of z°F will increase the death rate by a factor of 10. An F0 value written as ![]() means the F value at 150°F with a z value of 9°F (S2).
means the F value at 150°F with a z value of 9°F (S2).
In pasteurizing milk, batch and continuous processes are used. U.S. health regulations specify two equivalent sets of conditions; in one, the milk is held at 145°F (62.8°C) for 30 min, and in the other, at 161°F (71.7°C) for 15 s.
The general equations used for pasteurization are similar to sterilization and can be written as follows. Rewriting Eq. (9.12-13),
Equation 9.12-18
![]()
Rewriting Eq. (9.12-14),
Equation 9.12-19
![]()
where T1 is the standard temperature being used, such as 150°F, z is the value of z in °F for a tenfold increase in death rate, and T is the temperature of the actual process.
EXAMPLE 9.12-3. Pasteurization of MilkA typical F value given for the thermal processing of milk in a tubular heat exchanger is Solution: The z value is 9°F (5°C) and the temperature of the process is 150°F (65.6°C). Substituting into Eq. (9.12-18) and solving,
This gives a reduction in the number of viable cells of 1015. |
9.12F. Effects of Thermal Processing on Food Constituents
Thermal processing is used to cause the death of various undesirable microorganisms, but it also causes undesirable effects, such as the reduction of certain nutritional values. Ascorbic acid (vitamin C) and thiamin and riboflavin (vitamins B1 and B2) are partially destroyed by thermal processing. The reduction of these desirable constituents can also be given kinetic parameters such as F0 and z values in the same way as for sterilization and pasteurization. Examples and data are given by Charm (C2).
These same kinetic methods of thermal death rates can also be applied to predict the time for detecting a flavor change in a food product. Dietrich et al. (D1) determined a curve for the number of days to detect a flavor change in frozen spinach versus temperature of storage. The data followed Eq. (9.12-8) and a first-order kinetic relation.