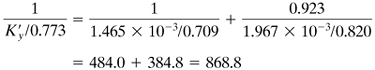10.4. MASS TRANSFER BETWEEN PHASES
10.4A. Introduction and Equilibrium Relations
1. Introduction to interphase mass transfer
In Chapter 7 we considered mass transfer from a fluid phase to another phase, which was most often a solid phase. The solute A was usually transferred from the fluid phase by convective mass transfer and through the solid by diffusion. In the present section we shall be concerned with the mass transfer of solute A from one fluid phase by convection and then through a second fluid phase by convection. For example, the solute may diffuse through a gas phase and then diffuse through and be absorbed in an adjacent and immiscible liquid phase. This occurs in the case of absorption of ammonia from air by water.
The two phases are in direct contact with each other, such as in a packed, tray, or spray-type tower, and the interfacial area between the phases is usually not well defined. In two-phase mass transfer, a concentration gradient will exist in each phase, causing mass transfer to occur. At the interface between the two fluid phases, equilibrium exists in most cases.
2. Equilibrium relations
Even when mass transfer is occurring, equilibrium relations are important to determine concentration profiles for predicting rates of mass transfer. In Section 10.2 the equilibrium relation in a gas–liquid system and Henry's law were discussed. In Section 7.1C a discussion covered equilibrium distribution coefficients between two phases. These equilibrium relations will be used in discussion of mass transfer between phases in this section.
10.4B. Concentration Profiles in Interphase Mass Transfer
In the majority of mass-transfer systems, two phases, which are essentially immiscible in each other, are present together with an interface between these two phases. Assuming solute A is diffusing from the bulk gas phase G to the liquid phase L, it must pass through phase G, through the interface, and then into phase L in series. A concentration gradient must exist to cause this mass transfer through the resistances in each phase, as shown in Fig. 10.4-1. The average or bulk concentration of A in the gas phase in mole fraction units is yAG, where yAG = pA/P, and in the bulk liquid phase in mole fraction units it is xAL.
Figure 10.4-1. Concentration profile of solute A diffusing through two phases.

The concentration in the bulk gas phase yAG decreases to yAi at the interface. The liquid concentration starts at xAi at the interface and falls to xAL. At the interface, since there would be no resistance to transfer across this interface, yAi and xAi are in equilibrium and are related by the equilibrium distribution relation
Equation 10.4-1
![]()
where yAi is a function of xAi. They are related by an equilibrium plot such as Fig. 10.1-1. If the system follows Henry's law, yAP or pA and xA are related by Eq. (10.2-2) at the interface.
Experimentally, the resistance at the interface has been shown to be negligible for most cases of mass transfer where chemical reactions do not occur, such as absorption of common gases from air to water and extraction of organic solutes from one phase to another. However, there are some exceptions. Certain surface-active compounds may concentrate at the interface and cause an “interfacial resistance” that slows down the diffusion of solute molecules. Theories for predicting when interfacial resistance may occur are often obscure and unreliable.
10.4C. Mass Transfer Using Film Mass-Transfer Coefficients and Interface Concentrations
1. Equimolar counterdiffusion
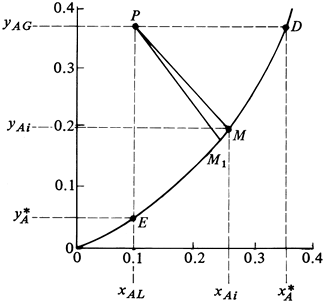
For equimolar counterdiffusion the concentrations of Fig. 10.4-1 can be plotted on an x-y diagram as in Fig. 10.4-2. Point P represents the bulk phase compositions xAG and xAL of the two phases and point M the interface concentrations yAi and xAi. For A diffusing from the gas to liquid and B in equimolar counterdiffusion from liquid to gas,
Equation 10.4-2
![]()
Figure 10.4-2. Concentration driving forces and interface concentrations in interphase mass transfer (equimolar counterdiffusion).

where ![]() is the gas-phase mass-transfer coefficient in kg mol/s · m2 · mol frac (g mol/s · cm2 · mol frac, lb mol/h · ft2 · mol frac) and
is the gas-phase mass-transfer coefficient in kg mol/s · m2 · mol frac (g mol/s · cm2 · mol frac, lb mol/h · ft2 · mol frac) and ![]() the liquid-phase mass-transfer coefficient in kg mol/s · m2 · mol frac (g mol/s · cm2 · mol frac, lb mol/h · ft2 · mol frac). Rearranging Eq. (10.4-2),
the liquid-phase mass-transfer coefficient in kg mol/s · m2 · mol frac (g mol/s · cm2 · mol frac, lb mol/h · ft2 · mol frac). Rearranging Eq. (10.4-2),
Equation 10.4-3
![]()
The driving force in the gas phase is (yAG − yAi) and in the liquid phase it is (xAi − xAL). The slope of the line PM is ![]() This means that if the two film coefficients
This means that if the two film coefficients ![]() and
and ![]() are known, the interface compositions can be determined by drawing line PM with a slope
are known, the interface compositions can be determined by drawing line PM with a slope ![]() intersecting the equilibrium line.
intersecting the equilibrium line.
The bulk-phase concentrations yAG and xAL can be determined by simply sampling the mixed bulk gas phase and sampling the mixed bulk liquid phase. The interface concentrations are determined by Eq. (10.4-3).
2. Diffusion of A through stagnant or nondiffusing B
For the common case of A diffusing through a stagnant gas phase and then through a stagnant liquid phase, the concentrations are shown in Fig. 10.4-3, where P again represents bulk-phase compositions and M interface compositions. The equations for A diffusing through a stagnant gas and then through a stagnant liquid are
Equation 10.4-4
![]()
Figure 10.4-3. Concentration driving forces and interface concentrations in interphase mass transfer (A diffusing through stagnant B).

Now,
Equation 10.4-5
![]()
Equation 10.4-6
![]()
Equation 10.4-7
![]()
Then,
Equation 10.4-8
![]()
Note that (1 − yA)iM is the same as yBM of Eq. (7.2-11) but is written for the interface, and (1 − xA)iM is the same as xBM of Eq. (7.2-11). Using Eq. (10.4-8) and rearranging,
Equation 10.4-9

The slope of the line PM in Fig. 10.4-3 to obtain the interface compositions is given by the left-hand side of Eq. (10.4-9). This differs from the slope of Eq. (10.4-3) for equimolar counterdiffusion by the terms (1 − yA)iM and (1 − xA)iM. When A is diffusing through stagnant B and the solutions are dilute, (1 − yA)iM and (1 − xA)iM are close to 1.
A trial-and-error method is needed to use Eq. (10.4-9) to get the slope, since the left-hand side contains the terms yAi and xAi that are being sought. For the first trial (1 − yA)iM and (1 − xA)iM are assumed to be 1.0, and Eq. (10.4-9) is used to get the slope and yAi and xAi values. Then for the second trial, these values of yAi and xAi are used to calculate a new slope to get new values of yAi and xAi. This is repeated until the interface compositions do not change. Three trials are usually sufficient.
EXAMPLE 10.4-1. Interface Compositions in Interphase Mass TransferThe solute A is being absorbed from a gas mixture of A and B in a wetted-wall tower with the liquid flowing as a film downward along the wall. At a certain point in the tower the bulk gas concentration yAG = 0.380 mol fraction and the bulk liquid concentration is xAL = 0.100. The tower is operating at 298 K and 1.013 × 105 Pa and the equilibrium data are as follows:
The solute A diffuses through stagnant B in the gas phase and then through a nondiffusing liquid. Using correlations for dilute solutions in wetted-wall towers, the film mass-transfer coefficient for A in the gas phase is predicted as ky = 1.465 × 10−3 kg mol A/s · m2 · mol frac (1.08 lb mol/h · ft2 · mol frac) and for the liquid phase as kx = 1.967 × 10−3 kg mol A/s · m2 · mol frac (1.45 lb mol/h · ft2 · mol frac). Calculate the interface concentrations yAi and xAi and the flux NA. Solution:
Since the correlations are for dilute solutions, (1 − yA)iM and (1 − xA)iM are approximately 1.0 and the coefficients are the same as Figure 10.4-4. Location of interface concentrations for Example 10.4-1.
A line through point P with a slope of −1.342 is plotted in Fig. 10.4-4 intersecting the equilibrium line at M1, where yAi = 0.183 and xAi = 0.247. For the second trial we use yAi and xAi from the first trial to calculate the new slope. Substituting into Eqs. (10.4-6) and (10.4-7),
Substituting into Eq. (10.4-9) to obtain the new slope,
A line through point P with a slope of −1.163 is plotted and intersects the equilibrium line at M, where yAi = 0.197 and xAi = 0.257. Using these new values for the third trial, the following values are calculated:
This slope of −1.160 is essentially the same as the slope of −1.163 for the second trial. Hence, the final values are yAi = 0.197 and xAi = 0.257 and are shown as point M. To calculate the flux, Eq. (10.4-8) is used:
Note that the flux NA through each phase is the same as in the other phase, which should be the case at steady state. |
10.4D. Overall Mass-Transfer Coefficients and Driving Forces
1. Introduction
Film or single-phase mass-transfer coefficients ![]() and
and ![]() or ky and kx are often difficult to measure experimentally, except in certain experiments designed so that the concentration difference across one phase is small and can be neglected. As a result, overall mass-transfer coefficients
or ky and kx are often difficult to measure experimentally, except in certain experiments designed so that the concentration difference across one phase is small and can be neglected. As a result, overall mass-transfer coefficients ![]() and
and ![]() are measured based on the gas phase or liquid phase. This method is used in heat transfer, where overall heat-transfer coefficients are measured based on inside or outside areas instead of film coefficients.
are measured based on the gas phase or liquid phase. This method is used in heat transfer, where overall heat-transfer coefficients are measured based on inside or outside areas instead of film coefficients.
The overall mass transfer ![]() is defined as
is defined as
Equation 10.4-10
![]()
where ![]() is based on the overall gas-phase driving force in kg mol/s · m2 · mol frac, and
is based on the overall gas-phase driving force in kg mol/s · m2 · mol frac, and ![]() is the value that would be in equilibrium with xAL, as shown in Fig. 10.4-2. Also,
is the value that would be in equilibrium with xAL, as shown in Fig. 10.4-2. Also, ![]() is defined as
is defined as
Equation 10.4-11
![]()
where ![]() is based on the overall liquid-phase driving force in kg mol/s · m2 · mol frac and
is based on the overall liquid-phase driving force in kg mol/s · m2 · mol frac and ![]() is the value that would be in equilibrium with yAG.
is the value that would be in equilibrium with yAG.
2. Equimolar counterdiffusion and/or diffusion in dilute solutions
Equation (10.4-2) holds for equimolar counterdiffusion, or, when the solutions are dilute, Eqs. (10.4-8) and (10.4-2) are identical:
Equation 10.4-2
![]()
From Fig. 10.4-2,
Equation 10.4-12
![]()
Between the points E and M the slope m' can be given as
Equation 10.4-13
![]()
Solving Eq. (10.4-13) for (yAi − ![]() ) and substituting into Eq. (10.4-12),
) and substituting into Eq. (10.4-12),
Equation 10.4-14
![]()
Then, on substituting Eqs. (10.4-10) and (10.4-2) into (10.4-14) and canceling out NA,
Equation 10.4-15
![]()
The left-hand side of Eq. (10.4-15) is the total resistance based on the overall gas driving force and equals the gas film resistance ![]() plus the liquid film resistance
plus the liquid film resistance ![]() .
.
In a similar manner, from Fig. 10.4-2,
Equation 10.4-16
![]()
Equation 10.4-17
![]()
Proceeding as before,
Equation 10.4-18
![]()
Several special cases of Eqs. (10.4-15) and (10.4-18) will now be discussed. The numerical values of ![]() and
and ![]() are very roughly similar. The values of the slopes m' and m'' are very important. If m' is quite small, so that the equilibrium curve in Fig. 10.4-2 is almost horizontal, a small value of yA in the gas will give a large value of xA in equilibrium in the liquid. The gas solute A is then very soluble in the liquid phase, and hence the term
are very roughly similar. The values of the slopes m' and m'' are very important. If m' is quite small, so that the equilibrium curve in Fig. 10.4-2 is almost horizontal, a small value of yA in the gas will give a large value of xA in equilibrium in the liquid. The gas solute A is then very soluble in the liquid phase, and hence the term ![]() in Eq. (10.4-15) is very small. Then,
in Eq. (10.4-15) is very small. Then,
Equation 10.4-19
![]()
and the major resistance is in the gas phase, or the “gas phase is controlling.” The point M has moved down very close to E, so that
Equation 10.4-20
![]()
Similarly, when m" is very large, the solute A is very insoluble in the liquid, ![]() becomes small, and
becomes small, and
Equation 10.4-21
![]()
The “liquid phase is controlling” and xAi ≅ ![]() . Systems for absorption of oxygen or CO2 from air by water are similar to Eq. (10.4-21).
. Systems for absorption of oxygen or CO2 from air by water are similar to Eq. (10.4-21).
3. Diffusion of A through stagnant or nondiffusing B
For the case of A diffusing through nondiffusing B, Eqs. (10.4-8) and (10.4-14) hold and Fig. 10.4-3 is used:
Equation 10.4-8
![]()
Equation 10.4-14
![]()
We must, however, define the equations for the flux using overall coefficients as follows:
Equation 10.4-22
![]()
The bracketed terms are often written as follows:
Equation 10.4-23
![]()
where Ky is the overall gas mass-transfer coefficient for A diffusing through stagnant B and Kx the overall liquid mass-transfer coefficient. These two coefficients are concentration-dependent. Substituting Eqs. (10.4-8) and (10.4-22) into (10.4-14), we obtain
Equation 10.4-24
![]()
where
Equation 10.4-25
![]()
Similarly, for ![]() ,
,
Equation 10.4-26

where
Equation 10.4-27
![]()
It should be noted that the relations derived here also hold for any two-phase system, where y stands for one phase and x for the other phase. For example, for the extraction of the solute acetic acid (A) from water (y phase) by isopropyl ether (x phase), the same relations will hold.
EXAMPLE 10.4-2. Overall Mass-Transfer Coefficients from Film CoefficientsUsing the same data as in Example 10.4-1, calculate the overall mass-transfer coefficient Solution:
From Fig. 10.4-4,
From Example 10.4-1,
Using Eq. (10.4-25),
Then, using Eq. (10.4-24),
Solving,
This, of course, is the same flux value as was calculated in Example 10.4-1 using the film equations. |
4. Discussion of overall coefficients
If the two-phase system is such that the major resistance is in the gas phase, as in Eq. (10.4-19), then to increase the overall rate of mass transfer, efforts should be centered on increasing the gas-phase turbulence, not the liquid-phase turbulence. For a two-phase system where the liquid film resistance is controlling, turbulence should be increased in this phase to increase rates of mass transfer.
To design mass-transfer equipment, the overall mass-transfer coefficient is synthesized from the individual film coefficients, as discussed in this section.
Generally, when the major resistance to mass transfer is in the gas phase, the overall mass transfer coefficient ![]() or the film coefficient
or the film coefficient ![]() is used. An example would be absorption of ammonia from air to water. When the major resistance is in the liquid phase, as in absorption of oxygen from air by water,
is used. An example would be absorption of ammonia from air to water. When the major resistance is in the liquid phase, as in absorption of oxygen from air by water, ![]() or
or ![]() is used.
is used.