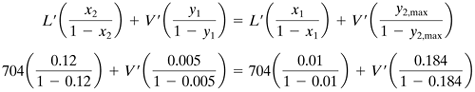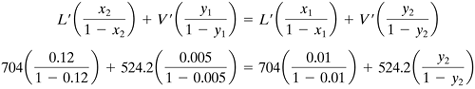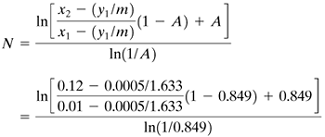12.7. CONTINUOUS MULTISTAGE COUNTERCURRENT EXTRACTION
12.7A. Introduction
In Section 12.5 single-stage equilibrium contact was used to transfer solute A from one liquid to another liquid phase. To transfer more solute, single-stage contact can be repeated by bringing the exit L1 stream into contact with fresh solvent V2, as shown in Fig. 12.5-6. In this way a greater percentage removal of solute A is obtained. However, this is wasteful of the solvent stream as well as giving a dilute product of A in the outlet solvent extract streams. In order to use less solvent and to obtain a more concentrated exit extract stream, countercurrent multistage contacting is often employed.
Figure 12.7-7. Extraction-tower flows: (a) process flow and material balance for countercurrent extraction tower, (b) operating line for minimum solvent flow for tower and actual operating line.

Many of the fundamental equations for countercurrent gas absorption and rectification are the same as or similar to those used in countercurrent extraction. Because of the frequently high solubility of the two liquid phases in each other, the equilibrium relationships in extraction are more complicated than in absorption and distillation.
12.7B. Continuous Multistage Countercurrent Extraction
1. Countercurrent process and overall balance
The process flow for this extraction process is the same as given previously in Fig. 10.3-2 and is shown in Fig. 12.7-1. The feed stream containing the solute A to be extracted enters at one end of the process and the solvent stream enters at the other end. The extract and raffinate streams flow countercurrently from stage to stage, and the final products are the extract stream V1 leaving stage 1 and the raffinate stream LN leaving stage N.
Making an overall balance on all N stages,
Equation 12.7-1
![]()
where M represents total kg/h (lbm/h) and is a constant, L0 the inlet feed flow rate in kg/h, VN+1 the inlet solvent flow rate in kg/h, V1 the exit extract stream, and LN the exit raffinate stream. Making an overall component balance on component C,
Equation 12.7-2
![]()
Combining Eqs. (12.7-1) and (12.7-2) and rearranging,
Equation 12.7-3
![]()
A similar balance on component A gives
Equation 12.7-4
![]()
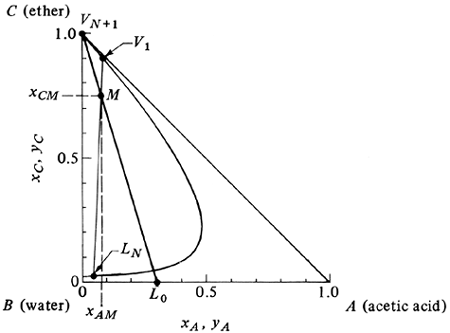
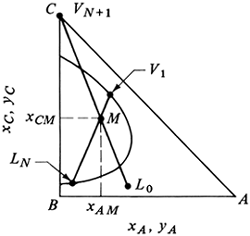
Equations (12.7-3) and (12.7-4) can be used to calculate the coordinates of point M on the phase diagram, which ties together the two entering streams L0 and VN+1 and the two exit streams V1 and LN. Usually, the flows and compositions of L0 and VN+1 are known and the desired exit composition xAN is set. If we plot points L0, VN+1, and M as in Fig. 12.7-2, a straight line must connect these three points. Then LN, M, and V1 must lie on one line. Moreover, LN and V1 must also lie on the phase envelope, as shown. These balances also hold for lbm and mass fraction, kg mol and mol fractions, and so on.
Figure 12.7-2. Use of the mixture point M for overall material balance in counter current solvent extraction.
EXAMPLE 12.7-1. Material Balance for Countercurrent Stage ProcessPure solvent isopropyl ether at the rate of VN+1 = 600 kg/h is being used to extract an aqueous solution of L0 = 200 kg/h containing 30 wt % acetic acid (A) by countercurrent multistage extraction. The desired exit acetic acid concentration in the aqueous phase is 4%. Calculate the compositions and amounts of the ether extract V1 and the aqueous raffinate LN. Use equilibrium data from Appendix A.3. Solution: The given values are VN+1 = 600, yAN+1 = 0, yCN+1 = 1.0, L0 = 200, xA0 = 0.30, xB0 = 0.70, xC0 = 0, and xAN = 0.04. In Fig. 12.7-3, VN+1 and L0 are plotted. Also, since LN is on the phase boundary, it can be plotted at xAN = 0.04. For the mixture point M, substituting into Eqs. (12.7-3) and (12.7-4), Equation 12.7-3
Figure 12.7-3. Method to perform overall material balance for Example 12.7-1.
Equation 12.7-4
Using these coordinates, the point M is plotted in Fig. 12.7-3. We locate V1 by drawing a line from LN through M and extending it until it intersects the phase boundary. This gives yA1 = 0.08 and yC1 = 0.90. For LN a value of xCN = 0.017 is obtained. By substituting into Eqs. (12.7-1) and (12.7-2) and solving, LN = 136 kg/h and V1 = 664 kg/h. |
2. Stage-to-stage calculations for countercurrent extraction
The next step after an overall balance has been made is to go stage by stage to determine the concentrations at each stage and the total number of stages N needed to reach LN in Fig. 12.7-1.
Making a total balance on stage 1,
Equation 12.7-5
![]()
Making a similar balance on stage n,
Equation 12.7-6
![]()
Rearranging Eq. (12.7-5) to obtain the difference Δ in flows,
Equation 12.7-7
![]()
This value of Δ in kg/h is constant, and for all stages,
Equation 12.7-8
![]()
This also holds for a balance on component A, B, or C:
Equation 12.7-9
![]()
Combining Eqs. (12.7-8) and (12.7-9) and solving for xΔ,
Equation 12.7-10

where xΔ is the x coordinate of point Δ.
Equations (12.7-7) and (12.7-8) can be written as
Equation 12.7-11
![]()
From Eq. (12.7-11), we see that L0 is on a line through Δ and V1, Ln is on a line through Δ and Vn+1, and so on. This means Δ is a point common to all streams passing each other, such as L0 and V1, Ln and Vn+1, LN and VN+1, and so on. The coordinates for locating this Δ operating point are given for xCΔ and xAΔ in Eq. (12.7-10). Since the end points VN+1, LN or V1, and L0 are known, xΔ can be calculated and point Δ located. Alternatively, the Δ point is located graphically in Fig. 12.7-4 as the intersection of lines L0V1 and LNVN+1.
Figure 12.7-4. Operating point Δ and number of theoretical stages needed for countercurrent extraction.

In order to step off the number of stages using Eq. (12.7-11) we start at L0 and draw the line L0ΔΔ, which locates V1 on the phase boundary. Next a tie line through V1 locates L1, which is in equilibrium with V1. Then line L1ΔΔ is drawn giving V2. The tie line V2L2 is drawn. This stepwise procedure is repeated until the desired raffinate composition LN is reached. The number of stages N required to perform the extraction is thus obtained.
EXAMPLE 12.7-2. Number of Stages in Countercurrent ExtractionPure isopropyl ether of 450 kg/h is being used to extract an aqueous solution of 150 kg/h with 30 wt % acetic acid (A) by countercurrent multistage extraction. The exit acid concentration in the aqueous phase is 10 wt %. Calculate the number of stages required. Solution: The known values are VN+1 = 450, yAN+1 = 0, yCN+1 = 1.0, L0 = 150, xA0 = 0.30, xB0 = 0.70, xC0 = 0, and xAN = 0.10. The points VN+1, L0, and LN are plotted in Fig. 12.7-5. For the mixture point M, substituting into Eqs. (12.7-3) and (12.7-4), xCM = 0.75 and xAM = 0.075. The point M is plotted and V1 is located at the intersection of line LNM with the phase boundary to give yA1 = 0.072 and yC1 = 0.895. (This construction is not shown. See Example 12.7-1 for construction of lines.) Figure 12.7-5. Graphical solution for countercurrent extraction in Example 12.7-2.
The lines L0V1 and LNVN+1 are drawn and the intersection is the operating point ΔΔ as shown. Alternatively, the coordinates of ΔΔ can be calculated from Eq. (12.7-10) to locate point ΔΔ. Starting at L0 we draw line L0ΔΔ, which locates V1. Then a tie line through V1 locates L1 in equilibrium with V1. (The tie-line data are obtained from an enlarged plot such as the bottom of Fig. 12.5-3.) Line L1ΔΔ is next drawn locating V2. A tie line through V2 gives L2. A line L2ΔΔ gives V3. A final tie line gives L3, which has gone beyond the desired LN. Hence, about 2.5 theoretical stages are needed. |
3. Minimum solvent rate
If a solvent rate VN+1 is selected at too low a value, a limiting case will be reached, with a line through ΔΔ and a tie line being the same. Then an infinite number of stages will be needed to reach the desired separation. The minimum amount of solvent has been reached. For actual operation a greater amount of solvent must be used.
The procedure for obtaining this minimum is as follows. A tie line is drawn through point L0 (Fig. 12.7-4) to intersect the extension of line LNVN+1. Other tie lines to the left of this tie line are drawn, including one through LN to intersect the line LNVN+1. The intersection of a tie line on line LNVN+1 which is nearest to VN+1 represents the ΔΔmin point for minimum solvent. The actual position of ΔΔ used must be closer to VN+1 than Δmin for a finite number of stages. This means that more solvent must be used. Usually, the tie line through L0 represents the ΔΔmin.
12.7C. Countercurrent-Stage Extraction with Immiscible Liquids
If the solvent stream VN+1 contains components A and C and the feed stream L0 contains A and B, and if components B and C are relatively immiscible in each other, the stage calculations may be made more easily. The solute A is relatively dilute and is being transferred from L0 to VN+1.
Referring to Fig. 12.7-1 and making an overall balance for A over the whole system and then over the first n stages,
Equation 12.7-12
![]()
Equation 12.7-13
![]()
where L'= kg inert B/h, V' = kg inert C/h, y = mass fraction A in V stream, and x = mass fraction A in L stream. This Eq. (12.7-13) is an operating-line equation whose slope ≅ L'/V'. If y and x are quite dilute, the line will be straight when plotted on an x-y diagram.
The number of stages are stepped off as shown previously for cases of distillation and absorption, as shown in Fig. 10.3-4.
If the equilibrium line is relatively dilute, then since the operating line is essentially straight, the analytical Eqs. (10.3-21)–(10.3-26) given in Section 10.3D can be used to calculate the number of stages.
EXAMPLE 12.7-3. Extraction of Nicotine with Immiscible Liquids.An inlet water solution of 100 kg/h containing 0.010 wt fraction nicotine (A) in water is stripped with a kerosene stream of 200 kg/h containing 0.0005 wt fraction nicotine in a countercurrent-stage tower. The water and kerosene are essentially immiscible in each other. It is desired to reduce the concentration of the exit water to 0.0010 wt fraction nicotine. Determine the theoretical number of stages needed. The equilibrium data are as follows (C5), with x the weight fraction of nicotine in the water solution and y in the kerosene:
Solution: The given values are L0 = 100 kg/h, x0 = 0.010, VN+1 = 200 kg/h, yN+1 = 0.0005, xN = 0.0010. The inert streams are
|
Making an overall balance on A using Eq. (12.7-12) and solving, y1 = 0.00498. These points on the operating line are plotted in Fig. 12.7-6.
Figure 12.7-6. Solution for extraction with immiscible liquids in Example 12.7-3.

Since the operating-line equation is for dilute solutions, a straight line is drawn. The equilibrium data are plotted and the line is slightly curved.
For part (a), the number of stages are stepped off, giving N = 4.5 theoretical stages.
For part (b), to calculate the total flow rates LN and V1, the following equations can be solved:

Also, L0 = 100 and VN+1 = 200 kg/h. To use Eq. (10.3-21) the slope of the equilibrium curve must be calculated at the top and bottom of the tower, since the line is not straight. At the top on the equilibrium curve, at the point y1 = 0.00498, the tangent or slope at this point is determined to be m1 = 0.91. At the bottom or dilute end, at xN = 0.0010 on the equilibrium curve, the line is straight and m2 can be calculated from the equilibrium data as m2 = 0.000806/0.001010 = 0.798. Then A1 = L/(mV) = L0/(m1V1) = 100/(0.91 × 200.9) = 0.547. Also, A2 = LN/(m2VN+1) = 99.1/(0.798 × 200) = 0.621. The average value of A = ![]() = 0.583.
= 0.583.
Substituting into Eq. (10.3-22) using m = m2 = 0.798,

N = 4.45 theoretical stages. This compares closely with the graphical-method result of 4.5 theoretical stages.
12.7D. Design of Towers for Extraction
1. Operating-line equation for relatively immiscible liquids
In this case, in Fig. 12.7-7a the liquid feed stream L and the solvent stream V are relatively immiscible in each other and solute A is relatively dilute in both streams. The outlet feed stream is commonly called the raffinate and the outlet solvent stream the extract. For the countercurrent extraction of A from the feed stream L to the solvent stream V, an overall material balance around the dashed-line box gives the following operating line equation:
Equation 12.7-14

Figure 12.9-1. Several typical equilibrium diagrams: (a) case for vertical tie lines and yA =xA, (b) case where yA ≠×xA for tie lines.

where L' = kg feed B/h, V' = kg solvent/h, y = mass fraction A in solvent V stream, and x = mass fraction A in feed L stream.
This operating-line equation will be slightly curved when plotted on an x-y diagram as in Fig. 12.7-7b. Note that units of mole fraction and molar flow rates can also be used. The number of theoretical steps are stepped off as previously shown in Fig. 12.7-6. If the two liquid solvent and feed streams are immiscible in each other, L' and V' are constant.
2. Limiting solvent flows and optimum L'/V' ratios
In an extraction process, the inlet feed flow L2 and its composition x2 are usually set. The exit concentration x1 in the raffinate (L1 stream) is often set by the designer. The inlet concentration y1 of the solvent (V1 stream) is generally fixed by process requirements in regenerating the solvent for recycle back to the tower. Hence, the amount of the entering solvent flow V1 or V' is open to choice.
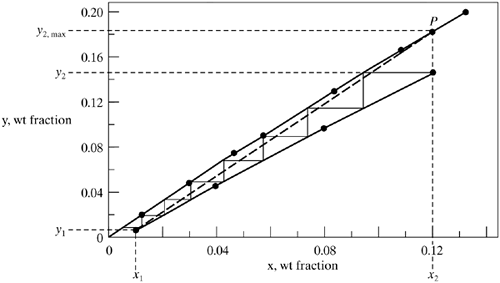
In Fig. 12.7-7b, the concentrations x1, x2, and y1 are set. When the operating line has a maximum slope and touches the equilibrium line at point P, then the solvent flow is at a minimum at . ![]() The value of y2 is at a maximum of y2,max. As in absorption and stripping, the optimum flow rate is taken as 1.2–1.5 times
The value of y2 is at a maximum of y2,max. As in absorption and stripping, the optimum flow rate is taken as 1.2–1.5 times ![]() , with 1.5 normally used (S6). Note that if the equilibrium line is curved and highly concave upward, then, for the minimum value , the operating line is tangent to the equilibrium line instead of intersecting it. Then the optimum is taken as 1.2–1.5 times.
, with 1.5 normally used (S6). Note that if the equilibrium line is curved and highly concave upward, then, for the minimum value , the operating line is tangent to the equilibrium line instead of intersecting it. Then the optimum is taken as 1.2–1.5 times.![]() .
.
3. Analytical equations for number of trays
The analytical equation to calculate the number of theoretical trays N for extraction is the same as Eq. (10.6-8) for stripping in gas–liquid separations and is given as
Equation 12.7-15
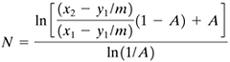
where A = L/mV. The value of A may vary in the tower if the operating and equilibrium lines are curved somewhat. Referring to Fig. 12.7-7b, at the concentrated top of the tower, the slope m2 or tangent at point y2 on the equilibrium line is used. At the bottom or dilute end, the slope m1 at point x1 on the equilibrium line is used. Then A1 = L1/m1V1, A2 = L2/m2
V2, and A = ![]() Also, the dilute m1 is used in Eq. (12.7-15). A useful equation is Eq. (12.7-16), which allows one to calculate the performance of an existing tower when the number of theoretical steps N are known:
Also, the dilute m1 is used in Eq. (12.7-15). A useful equation is Eq. (12.7-16), which allows one to calculate the performance of an existing tower when the number of theoretical steps N are known:
Equation 12.7-16
![]()
12.7E. Design of Packed Towers for Extraction Using Mass-Transfer Coefficients
The use of mass-transfer coefficients in extraction is similar to their use in stripping in absorption. The equations for dilute solutions and essentially immiscible solvents using overall coefficients are essentially identical to those for stripping with dilute solutions as given previously in Section 10.6G as Eq. (10.6-46) and (10.6-47). For extraction,
Equation 12.7-17
![]()
Equation 12.7-18
![]()
If both the operating and equilibrium lines are straight and dilute, the integral in Eq. (12.7-17) can be integrated to give
Equation 12.7-19
![]()
where
Equation 12.7-20
![]()
Equation 12.7-21
![]()
where (y − y*)M is defined in Eq. (10.6-28). The value of the overall number of transfer units NOL can also be determined using Eq. (10.6-53) as
Equation 12.7-22
![]()
EXAMPLE 12.7-4. Extraction of Acetone from Water with Trichloroethane in a TowerAn inlet water solution of 800 kg/h containing 12.0 wt % acetone is extracted with the solvent trichloroethane containing 0.5 wt % acetone in a countercurrent tray tower at 25°C. The solvents water and trichloroethane are essentially immiscible in each other up to a concentration of acetone in water of 27 wt %. The exit concentration in the water (raffinate) stream is set at 1.0 wt % acetone. The equilibrium data are as follows (T3), where x is the weight fraction of acetone in the water solution and y in the trichloroethane solution:
Solution: For part (a), the given values are L2 = 800 kg/h, x2 = 0.12, x1 = 0.01, y1 = 0.005. The inlet stream is
The equilibrium data are plotted in Fig. 12.7-8, and the line is curved somewhat. The operating line for minimum solvent flow is plotted from point x1, y1 to point P, where x2 = 0.12 on the equilibrium line and y2,max = 0.184. Rewriting Eq. (12.7-14) for an overall balance, and substituting the known values,
Solving, V' = For part (b), V' = 1.3
Then, y2 = 0.1486. As before, calculating several points on the operating line, which is curved, for x = 0.04, y = 0.0453; for x = 0.08, y = 0.0977. Plotting these points shows a curved operating line. Stepping off the trays, a total of 7.4 steps are obtained. For part (c), the total flows are first calculated as
The value of m2 is the slope or tangent at point y2 on the equilibrium line, which gives m2 = 1.49. The value of m1 at point x1 at the dilute end on the equilibrium line is, from the first set of equilibrium data points, equal to y/x = 0.0196/0.0120 = 1.633 = m1. Then
Using Eq. (12.7-15),
Then, N = 7.46 theoretical steps. This agrees closely with the graphical value of 7.4. For part (d), Eq. (12.7-19) will not be used, because the operating and equilibrium lines are slightly curved. Using Eq. (12.7-22),
Solving, NOL = 8.09 transfer units. Then, z = HOLNOL = 1.2(8.09) = 9.71 m. |