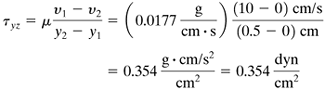2.4. VISCOSITY OF FLUIDS
2.4A. Newton's Law and Viscosity
When a fluid is flowing through a closed channel such as a pipe or between two flat plates, either of two types of flow may occur, depending on the velocity of this fluid. At low velocities the fluid tends to flow without lateral mixing, and adjacent layers slide past one another like playing cards. There are no cross currents perpendicular to the direction of flow, nor eddies or swirls of fluid. This regime or type of flow is called laminar flow. At higher velocities eddies form, which leads to lateral mixing. This is called turbulent flow. The discussion in this section is limited to laminar flow.
A fluid can be distinguished from a solid in this discussion of viscosity by its behavior when subjected to a stress (force per unit area) or applied force. An elastic solid deforms by an amount proportional to the applied stress. However, a fluid, when subjected to a similar applied stress, will continue to deform, that is, to flow at a velocity that increases with increasing stress. A fluid exhibits resistance to this stress. Viscosity is that property of a fluid which gives rise to forces that resist the relative movement of adjacent layers in the fluid. These viscous forces arise from forces existing between the molecules in the fluid and are similar in character to the shear forces in solids.
The ideas above can be clarified by a more quantitative discussion of viscosity. In Fig. 2.4-1 a fluid is contained between two infinite (very long and very wide) parallel plates. Suppose that the bottom plate is moving parallel to the top plate and at a constant velocity Δνz m/s faster relative to the top plate because of a steady force F newtons being applied. This force is called the viscous drag, and it arises from the viscous forces in the fluid. The plates are Δy m apart. Each layer of liquid moves in the z direction. The layer immediately adjacent to the bottom plate is carried along at the velocity of this plate. The layer just above is at a slightly slower velocity, each layer moving at a slower velocity as we go up in the y direction. This velocity profile is linear, with y direction as shown in Fig. 2.4-1. An analogy to a fluid is a deck of playing cards, where, if the bottom card is moved, all the other cards above will slide to some extent.
Figure 2.4-1. Fluid shear between two parallel plates.

It has been found experimentally for many fluids that the force F in newtons is directly proportional to the velocity Δνz in m/s and to the area A in m2 of the plate used, and inversely proportional to the distance Δy in m. Or, as given by Newton's law of viscosity when the flow is laminar,
Equation 2.4-1
![]()
where μ is a proportionality constant called the viscosity of the fluid, in Pa · s or kg/m · s. If we let Δy approach zero, then, using the definition of the derivative,
Equation 2.4-2
![]()
where τyz = F/A and is the shear stress or force per unit area in newtons/m2 (N/m2). In the cgs system, F is in dynes, μ in g/cm · s, νz in cm/s, and y in cm. We can also write Eq. (2.2-2) as
Equation 2.4-3
![]()
where τyz is in units of lbf/ft2.
The units of viscosity in the cgs system are g/cm · s, called poise or centipoise (cp). In the SI system, viscosity is given in Pa · s (N · s/m2 or kg/m · s):

Other conversion factors for viscosity are given in Appendix A.1. Sometimes the viscosity is given as μ/p, kinematic viscosity, in m2/s or cm2/s, where ρ is the density of the fluid.
EXAMPLE 2.4-1. Calculation of Shear Stress in a LiquidReferring to Fig. 2.4-1, the distance between plates is Δy = 0.5 cm, Δνz = 10 cm/s, and the fluid is ethyl alcohol at 273 K having a viscosity of 1.77 cp (0.0177 g/cm · s).
Solution: We can substitute directly into Eq. (2.4-1) or we can integrate Eq. (2.4-2). Using the latter method, rearranging Eq. (2.4-2), calling the bottom plate point 1, and integrating, Equation 2.4-4
Equation 2.4-5
Substituting the known values, Equation 2.4-6
To calculate the shear rate dνz/dy, since the velocity change is linear with y, Equation 2.4-7
For part (b), using lb force units and the viscosity conversion factor from Appendix A.1,
Integrating Eq. (2.4-3), Equation 2.4-8
Substituting known values into Eq. (2.4-8) and converting Δνz to ft/s and Δy to ft, τyz = 7.39 × 10−4 lbf/ft2. Also, dνz/dy = 20 s−1. For part (c), Δy = 0.5/100 = 0.005 m, Δνz = 10/100 = 0.1 m/s, and μ = 1.77 × 10−3 kg/m · s = 1.77 × 10−3 Pa · s. Substituting into Eq. (2.4-5),
The shear rate will be the same at 20.0 s−1. |
2.4B. Momentum Transfer in a Fluid
The shear stress τyz in Eqs. (2.4-1)–(2.4-3) can also be interpreted as a flux of z-directed momentum in the y direction, which is the rate of flow of momentum per unit area. The units of momentum are mass times velocity in kg · m/s. The shear stress can be written
Equation 2.4-9
![]()
This gives an amount of momentum transferred per second per unit area.
This can be shown by considering the interaction between two adjacent layers of a fluid in Fig. 2.4-1 which have different velocities, and hence different momentum, in the z direction. The random motions of the molecules in the faster-moving layer send some of the molecules into the slower-moving layer, where they collide with the slower-moving molecules and tend to speed them up or increase their momentum in the z direction. Also, in the same fashion, molecules in the slower layer tend to retard those in the faster layer. This exchange of molecules between layers produces a transfer or flux of z-directed momentum from high-velocity to low-velocity layers. The negative sign in Eq. (2.4-2) indicates that momentum is transferred down the gradient from high- to low-velocity regions. This is similar to the transfer of heat from high- to low-temperature regions.
2.4C. Viscosities of Newtonian Fluids
Fluids that follow Newton's law of viscosity, Eqs. (2.4-1)–(2.4-3), are called Newtonian fluids. For a Newtonian fluid, there is a linear relation between the shear stress τyz and the velocity gradient dνz/dy (rate of shear). This means that the viscosity μ is a constant and is independent of the rate of shear. For non-Newtonian fluids, the relation between τyz and dνz/dy is not linear; that is, the viscosity μ does not remain constant but is a function of shear rate. Certain liquids do not obey this simple law of Newton's. These are primarily pastes, slurries, high polymers, and emulsions. The science of the flow and deformation of fluids is often called rheology. A discussion of non-Newtonian fluids will not be given here but will be included in Section 3.5.
The viscosity of gases, which are Newtonian fluids, increases with temperature and is approximately independent of pressure up to a pressure of about 1000 kPa. At higher pressures, the viscosity of gases increases with increase in pressure. For example, the viscosity of N2 gas at 298 K approximately doubles in going from 100 kPa to about 5 × 104 kPa (R1). In liquids, the viscosity decreases with increasing temperature. Since liquids are essentially incompressible, the viscosity is not affected by pressure.
In Table 2.4-1 some experimental viscosity data are given for some typical pure fluids at 101.32 kPa. The viscosities for gases are the lowest and do not differ markedly from gas to gas, being about 5 × 10−6 to 3 × 10−5 Pa · s. The viscosities for liquids are much greater. The value for water at 293 K is about 1 × 10−3 and for glycerol 1.069 Pa · s. Hence, there are great differences between viscosities of liquids. More complete tables of viscosities are given for water in Appendix A.2, for inorganic and organic liquids and gases in Appendix A.3, and for biological and food liquids in Appendix A.4. Extensive data are available in other references (P1, R1, W1, L1). Methods of estimating viscosities of gases and liquids when experimental data are not available are summarized elsewhere (R1). These estimation methods for gases at pressures below 100 kPa are reasonably accurate, with an error within about ±5%, but the methods for liquids are often quite inaccurate.
| Gases | Liquids | ||||||
|---|---|---|---|---|---|---|---|
| Substance | Temp., K | Viscosity (Pa · s) 103 or (kg/m · s) 103 | Ref. | Substance | Temp., K | Viscosity (Pa · s) 103 or (kg/m · s) 103 | Ref. |
| Air | 293 | 0.01813 | N1 | Water | 293 | 1.0019 | S1 |
| CO2 | 273 | 0.01370 | R1 | 373 | 0.2821 | S1 | |
| 373 | 0.01828 | R1 | Benzene | 278 | 0.826 | R1 | |
| CH4 | 293 | 0.01089 | R1 | ||||
| Glycerol | 293 | 1069 | L1 | ||||
| SO2 | 373 | 0.01630 | R1 | Hg | 293 | 1.55 | R2 |
| Olive oil | 303 | 84 | E1 | ||||