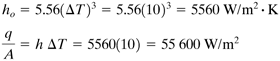4.8. BOILING AND CONDENSATION
4.8A. Boiling
1. Mechanisms of boiling
Heat transfer to a boiling liquid is very important in evaporation and distillation as well as in other kinds of chemical and biological processing, such as petroleum processing, control of the temperature of chemical reactions, evaporation of liquid foods, and so on. The boiling liquid is usually contained in a vessel with a heating surface of tubes or vertical or horizontal plates which supply the heat for boiling. The heating surfaces can be heated electrically or by a hot or condensing fluid on the other side of the heated surface.
In boiling, the temperature of the liquid is the boiling point of this liquid at the pressure in the equipment. The heated surface is, of course, at a temperature above the boiling point. Bubbles of vapor are generated at the heated surface and rise through the mass of liquid. The vapor accumulates in a vapor space above the liquid level and is withdrawn.
Boiling is a complex phenomenon. Suppose we consider a small heated horizontal tube or wire immersed in a vessel containing water boiling at 373.2 K (100°C). The heat flux is q/A W/m2; ΔT = Tw − 373.2 K, where Tw is the tube or wire wall temperature; and h is the heat-transfer coefficient in W/m2 · K. Starting with a low ΔT, the q/A and h values are measured. This is repeated at higher values of ΔT and the data obtained are plotted as q/A versus ΔT, as shown in Fig. 4.8-1.
Figure 4.8-1. Boiling mechanisms for water at atmospheric pressure, heat flux vs. temperature drop: (A) natural convection, (B) nucleate boiling, (C) transition boiling, (D) film boiling.

In the first region A of the plot in Fig. 4.8-1, at low temperature drops, the mechanism of boiling is essentially that of heat transfer to a liquid in natural convection. The variation of h with ΔT0.25 is approximately the same as that for natural convection to horizontal plates or cylinders. The very few bubbles formed are released from the surface of the metal and rise without appreciably disturbing the normal natural convection.
In the region B of nucleate boiling for a ΔT of about 5–25 K (9–45°F), the rate of bubble production increases so that the velocity of circulation of the liquid increases. The heat-transfer coefficient h increases rapidly and is proportional to ΔT2 to ΔT3 in this region.
In the region C of transition boiling, many bubbles are formed so quickly that they tend to coalesce and form a layer of insulating vapor. Increasing the ΔT increases the thickness of this layer and the heat flux and h drop as ΔT is increased. In the region D of film boiling, bubbles detach themselves regularly and rise upward. At higher ΔT values radiation through thevapor layer next to the surface helps increase the q/A and h. Similar-shaped curves are obtained for other shapes of surfaces (M1).
The curve of h versus ΔT has approximately the same shape as in Fig. 4.8-1. The values of h are quite large. At the beginning of region B in Fig. 4.8-1 for nucleate boiling, h has a value of about 5700–11 400 W/m2 · K, or 1000–2000 btu/h · ft2 · °F, and at the end of this region h has a peak value of almost 57 000 W/m2 · K, or 10 000 btu/hr · ft2 · °F. These values are quite high, and in most cases the percent resistance of the boiling film is only a few percent of the overall resistance to heat transfer.
The regions of commercial interest are the nucleate and film-boiling regions (P3). Nucleate boiling occurs in kettle-type and natural-circulation reboilers.
2. Nucleate boiling
In the nucleate-boiling region, the heat flux is affected by ΔT, pressure, nature and geometry of the surface and system, and physical properties of the vapor and liquid. Equations have been derived by Rohesenow et al. (P1). They apply to single tubes or flat surfaces and are quite complex.
Simplified empirical equations for estimating the boiling heat-transfer coefficients for water boiling on the outside of submerged surfaces at 1.0 atm abs pressure have been developed (J2).
For a horizontal surface (SI and English units),
Equation 4.8-1

Equation 4.8-2
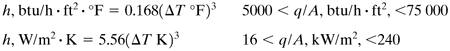
Equation 4.8-3

Equation 4.8-4

where ΔT = Tw - Tsat K or °F.
If the pressure is p atm abs, the values of h at 1 atm given above are multiplied by (p/1)0.4. Equations (4.8-1) and (4.8-3) are in the natural convection region.
For forced convection boiling inside tubes, the following simplified relation can be used (J3):
Equation 4.8-5

where p in this case is in kPa (SI units) and psia (English units).
3. Film boiling
In the film-boiling region, the heat-transfer rate is low in view of the large temperature drop used, which is not utilized effectively. Film boiling has been subjected to considerable theoretical analysis. Bromley (B3) gives the following equation to predict the heat-transfer coefficient in the film-boiling region on a horizontal tube:
Equation 4.8-6

where kν is the thermal conductivity of the vapor in W/m · K, ρν the density of the vapor in kg/m3, ρl the density of the liquid in kg/m3, hfg the latent heat of vaporization in J/kg, ΔT = Tw - Tsat, Tsat the temperature of saturated vapor in K, D the outside tube diameter in m, μν the viscosity of the vapor in Pa · s, and g the acceleration of gravity in m/s2. The physical properties of the vapor are evaluated at the film temperature of Tf = (Tw + Tsat)/2, and hfg at the saturation temperature. If the temperature difference is quite high, some additional heat transfer occurs by radiation (H1).
EXAMPLE 4.8-1. Rate of Heat Transfer in a Jacketed KettleWater is being boiled at 1 atm abs pressure in a jacketed kettle with steam condensing in the jacket at 115.6°C. The inside diameter of the kettle is 0.656 m and the height is 0.984 m. The bottom is slightly curved but it will be assumed to be flat. Both the bottom and the sides up to a height of 0.656 m are jacketed. The kettle surface for heat transfer is 3.2-mm stainless steel with a k of 16.27 W/m · K. The condensing-steam coefficient hi inside the jacket has been estimated as 10 200 W/m2 · K. Predict the boiling heat-transfer coefficient ho for the bottom surface of the kettle. Solution: A diagram of the kettle is shown in Fig. 4.8-2. The simplified equations will be used for the boiling coefficient ho. The solution is trial and error, since the inside metal surface temperature Tw is unknown. Assuming that Tw = 110°C,
Substituting into Eq. (4.8-2),
To check the assumed Tw, the resistance Ri of the condensing steam, Rw of the metal wall, and Ro of the boiling liquid must be calculated. Assuming equal areas of the resistances for A = 1 m2, then by Eq. (4.3-12),
The temperature drop across the boiling film is then
Hence, Tw = 100 + 5.9 = 105.9°C. This is lower than the assumed value of 110°C. For the second trial, Tw = 108.3°C will be used. Then, ΔT = 108.3 − 100 = 8.3°C and, from Eq. (4.8-2), the new ho = 3180. Calculating the new Ro = 31.44 × 10-5, and
and
This value is reasonably close to the assumed value of 108.3°C, so no further trials will be made. |
4.8B. Condensation
1. Mechanisms of condensation
Condensation of a vapor to a liquid and vaporization of a liquid to a vapor both involve a change of phase of a fluid with large heat-transfer coefficients. Condensation occurs when a saturated vapor such as steam comes in contact with a solid whose surface temperature is below the saturation temperature, to form a liquid such as water.
Normally, when a vapor condenses on a surface such as a vertical or horizontal tube or other surface, a film of condensate is formed on the surface and flows over the surface by the action of gravity. It is this film of liquid between the surface and the vapor that forms the main resistance to heat transfer. This is called film-type condensation.
Another type of condensation, dropwise condensation, can occur, where small drops are formed on the surface. These drops grow and coalesce, and the liquid flows from the surface. During this condensation, large areas of tube are devoid of any liquid and are exposed directly to the vapor. Very high rates of heat transfer occur on these bare areas. The average coefficient can be as high as 110 000 W/m2 · K (20 000 btu/h · ft2 · °F), which is five to 10 times larger than film-type coefficients. Film-condensation coefficients are normally much greater than those in forced convection and are on the order of magnitude of several thousand W/m2 · K or more.
Dropwise condensation occurs on contaminated surfaces and when impurities are present. Film-type condensation is more dependable and more common. Hence, for normal design purposes, film-type condensation is assumed.
2. Film-condensation coefficients for vertical surfaces
Film-type condensation on a vertical wall or tube can be analyzed analytically by assuming laminar flow of the condensate film down the wall. The film thickness is zero at the top of the wall or tube and increases in thickness as it flows downward because of condensation. Nusselt (H1, W1) assumed that the heat transfer from the condensing vapor at Tsat K, through this liquid film, and to the wall at Tw K was by conduction. Equating this heat transfer by conduction to that from condensation of the vapor, a final expression can be obtained for the average heat-transfer coefficient over the whole surface.
In Fig. 4.8-3a, vapor at Tsat is condensing on a wall whose temperature is Tw K. The condensate is flowing downward in laminar flow. Assuming unit thickness, the mass of the element with liquid density ρl in Fig. 4.8-3b is (δ − y)(dx · 1)ρl. The downward force on this element is the gravitational force minus the buoyancy force, or (δ - y)(dx) × (ρl - ρν)g, where ρv is the density of the saturated vapor. This force is balanced by the viscous-shear force at the plane y of μl (dv/dy) (dx · 1). Equating these forces,
Equation 4.8-7
![]()
Figure 4.8-3. Film condensation on a vertical plate: (a) increase in film thickness with position, (b) balance on element of condensate.

Integrating and using the boundary condition that ν = 0 at y = 0,
Equation 4.8-8
![]()
The mass flow rate of film condensate at any point x for unit depth is
Equation 4.8-9

Integrating,
Equation 4.8-10
![]()
At the wall, for area (dx · 1) m2, the rate of heat transfer is as follows if a linear temperature distribution is assumed in the liquid between the wall and the vapor:
Equation 4.8-11
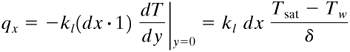
In a dx distance, the rate of heat transfer is qx. Also, in this dx distance, the increase in mass from condensation is dm. Using Eq. (4.8-10),
Equation 4.8-12
![]()
Making a heat balance for dx distance, the mass flow rate dm times the latent heat hfg must equal the qx from Eq. (4.8-11):
Equation 4.8-13

Integrating, with δ = 0 at x = 0 and δ = δ at x = x,
Equation 4.8-14
![]()
Using the local heat-transfer coefficient hx at x, a heat balance gives
Equation 4.8-15
![]()
This gives
Equation 4.8-16
![]()
Combining Eqs. (4.8-14) and (4.8-16),
Equation 4.8-17
![]()
By integrating over the total length L, the average value of h is obtained as follows:
Equation 4.8-18

Equation 4.8-19

However, for laminar flow, experimental data are about 20% above Eq. (4.8-19).
Hence, the final recommended expression for vertical surfaces in laminar flow is (M1)
Equation 4.8-20

where ρl is the density of liquid in kg/m3 and ρν that of the vapor, g is 9.8066 m/s2, L is the vertical height of the surface or tube in m, μl is the viscosity of liquid in Pa · s, kl is the liquid thermal conductivity in W/m · K, ΔT = Tsat − Tw in K, and hfg is the latent heat of condensation in J/kg at Tsat. All physical properties of the liquid except hfg are evaluated at the film temperature Tf = (Tsat + TW)/2. For long vertical surfaces the flow at the bottom can be turbulent. The Reynolds number is defined as
Equation 4.8-21
![]()
Equation 4.8-22
![]()
where m is total kg mass/s of condensate at tube or plate bottom and Г = m/πD or m/W. The NRe should be below about 1800 for Eq. (4.8-20) to hold. The reader should note that some references define NRe as Г/μ. Then this NRe should be below 450.
For turbulent flow for NRe > 1800 (M1),
Equation 4.8-23

Solution of this equation is by trial and error, since a value of NRe must first be assumed in order to calculate h.
EXAMPLE 4.8-2. Condensation on a Vertical TubeSteam saturated at 68.9 kPa (10 psia) is condensing on a vertical tube 0.305 m (1.0 ft) long having an OD of 0.0254 m (1.0 in.) and a surface temperature of 86.11°C (187°F). Calculate the average heat-transfer coefficient using English and SI units. Solution: From Appendix A.2,
Assuming a laminar film, using Eq. (4.8-20) in English as well as SI units, and neglecting ρν as compared to ρl,
Solving, h = 2350 btu/h · ft2 · °F = 13 350 W/m2 · K. Next, the NRe will be calculated to see if laminar flow occurs as assumed. To calculate the total heat transferred for a tube of area Equation 4.8-24
However, this q must also equal that obtained by condensation of m lbm/h or kg/s. Hence, Equation 4.8-25
Substituting the values given and solving for m,
Substituting into Eq. (4.8-21),
Hence, the flow is laminar as assumed. |
3. Film-condensation coefficients outside horizontal cylinders
The analysis of Nusselt can also be extended to the practical case of condensation outside a horizontal tube. For a single tube the film starts out with zero thickness at the top of the tube and increases in thickness as it flows around to the bottom and then drips off. If there is a bank of horizontal tubes, the condensate from the top tube drips onto the one below; and so on.
For a vertical tier of N horizontal tubes placed one below the other with outside tube diameter D (M1),
Equation 4.8-26

In most practical applications, the flow is in the laminar region and Eq. (4.8-26) holds (C3, M1).