5
Valorisation of Woody Biomass
Md Khairul Islam1, Chengyu Dong1, Hsien‐Yi Hsu2, Carol Sze Ki Lin2, and Shao‐Yuan Leu1
1Department of Civil and Environmental Engineering, Hong Kong Polytechnic University, Hung Hom, Hong Kong
2School of Energy and Environment, City University of Hong Kong, Kowloon Tong, Hong Kong
5.1 Generation of Woody Biomass
Woody biomass derived from forest and agricultural residues is an important source of renewable energy and chemicals. According to the Food and Agriculture Organization of the United States (FAO), the global production of wood residues between 1974 and 2016 has increased from 1.85 × 104 tons to 5.69 × 108 tons (Food and Agriculture Organization 2018). Most of the woody biomass has been generated in Asia (51%), America (20%), and Europe (27%) (Food and Agriculture Organization 2018), which is derived from different sources, such as woody product wastes (Figure 5.1a) and yard wastes (Figure 5.1b,c). It contains different parts of woody plants, such as the trunks, branches, and leaves.

Figure 5.1 Examples of woody biomass derived from different sources: (a) woody product wastes; (b) yard wastes; and (c) trimmings.
Living woody plant cells are generated from mitosis division of the apical and lateral meristems (Evert 2006). The apical meristems are located in the tips of the stems, roots, and branches and are responsible for the primary growth, i.e. lengthening of the plants; while the lateral meristems present under the bark of roots, stem, and branches are responsible for the secondary growth, that is, thickening of the plant. The stem, or the trunk, is currently the most useful part of the woody plant. The tree trunk consists of four segments: bark, phloem, vascular cambium, and the secondary xylem (Harada and Côté Jr 1985). Bark protects the plant from mechanical and biological attacks. The thin layer of cells between the phloem and the xylem is the vascular cambium, which divides the wood trunk into xylem in the inner side and phloem in the outer. Phloem is the living tissue that stores and transports nutrients to different parts of the plants, and the xylem is produced by the multiplication of meristematic cells in the vascular cambium (Lachaud et al. 1999).
5.2 General Classification and Properties of Woods
Woody materials are most commonly classified based on the seed structure and reproduction system of the plants (Figure 5.2). Hardwood, known as angiosperm, originates from deciduous trees such as oak, maple, eucalyptus, and beech. Softwood, or gymnosperm, originates from coniferous trees, such as pine, spruce, Douglas fir, and redwood. Hardwood and softwood vary in appearance and have different functional plant cells. In softwood, approximately 90% of the wood volume comprises tracheids, which transport liquid and provide mechanical support to the tree. In hardwood, fibers provide the mechanical support while vessels transport liquid throughout the plant. The transformation and storage of nutrients is performed by parenchyma cells in both softwood and hardwood (Daniel 2009).
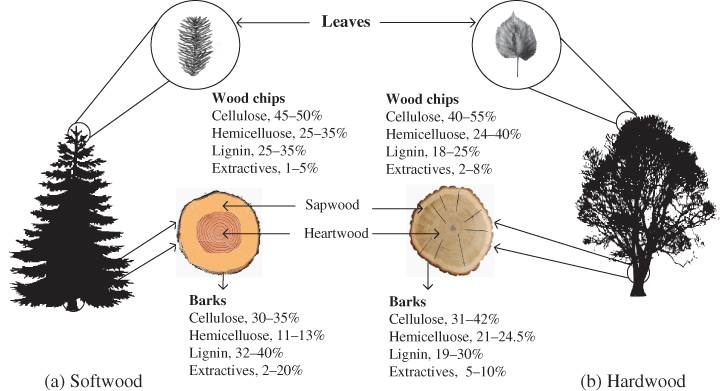
Figure 5.2 Structure and chemical compositions of softwood and hardwood.
Source: Adapted from Kumar and Sharma (2017) and Popa (2015).
Wood consists of elongated cells (Fujita and Harada 2000) in the longitudinal direction of the stem, and grows radially as concentric rings. The inner part of the wood ring is heartwood and the outer parts are sapwood, of which the types and contents of basic chemicals are provided in Figure 5.2. Heartwood consists of entirely dead tissues and provides mechanical support to the trees. The dead cells in heartwood contain deposited extractives, which improve biological resistance and darken the color of the heartwood. Transportation and storage of water, mineral and nutrients take place in the sapwood, although most cells in the sapwood are dead. The living cells of sapwood provide protection against microbial attacks. When the living cells in sapwood die, the sapwood is gradually converted into heartwood with aging.
5.3 Wood Chemistry
Wood is composed of various heterogeneous chemical constituents. Cellulose, hemicellulose, and lignin are the main constituents. These building block chemicals are heterogeneously distributed throughout the wood cell walls (Figure 5.3), which vary significantly among different species. Although the cellulose contents in hardwood and softwood are almost same, the softwood contains more lignin and less hemicellulose than hardwood (Kibblewhite and Brookes 1976; Alén 2011). Low molecular weight compounds such as extractives and some inorganics are nonstructural constituents of wood.
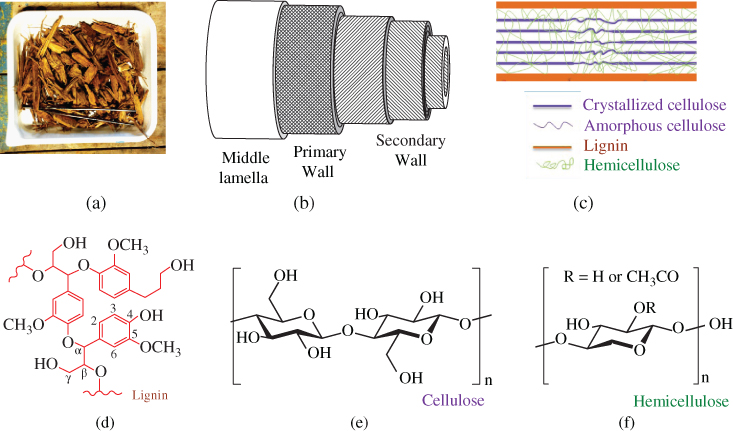
Figure 5.3 Schematic illustration of the physicochemical structure of woody plant cell walls at various scales: (a) wood chips; (b) cell wall structure; (c) chemical composition of the secondary wall; (d) basic structures of lignin; (e) cellulose; and (f) hemicellulose.
5.3.1 Cellulose
Cellulose is the most abundant and widely applied biopolymer in nature. Glucose monosaccharide is the basic unit of cellulose, and units connect together with 1‐4, β‐β‐glucosidic linkages to form a large polymer. The degree of polymerization of the wood cellulose molecule ranges from 800 to 10 000 (Klemm et al. 2005), which is about four to five times higher than artificial polyester and nylon 66 polymers (Papadopoulou et al. 2016). This high degree of polymerization provides cellulosic fiber with superior tensile strength and toughness for advance applications. Engineered nanofibrillated cellulose (NFC) and microfibrillated cellulose (MFC) have high strength and stiffness, and thus can be used to enhance the gas barrier properties of film and the mechanical strength of paper‐based products (Osong et al. 2016). The linear cellulose molecules have a strong tendency to form intra‐ and intermolecular hydrogen bonds due to the presence of strongly electronegative oxygen atoms, making this hydrophilic biopolymer an outstanding candidate for cleaning and packaging. The bundles of cellulose molecules aggregate to form microfibrils, a basic unit of cellulose fibers. Cellulose fibers are formed by crystalline, highly ordered arrangements of microfibrils, or amorphous, less ordered arrangements of microfibrils (Xiang et al. 2016). This characteristic makes cellulose a lightweight fiber with excellent tensile strength. The strong hydrogen bonding also imparts the cellulose molecules with high tensile strength and insolubility in most solvents. However, the cellulose is soluble in strong mineral acid solution, in which cellulose molecules are completely hydrolyzed to glucose.
5.3.2 Hemicelluloses
Hemicelluloses are hetero‐polysaccharides of hexose and pentose sugar. Hemicelluloses are less ordered than cellulose and more easily dissolved in acids. The degree of polymerization of hemicelluloses is usually less than 300. This component easily hydrolyzes to monomer sugars in dilute mineral acids at low temperature (Zhang et al. 2006). The monomers are easily digestible and fermentable by various micro‐organisms to produce desired products (Kumar et al. 2018). Lower energy consumption in the fractionation of hemicellulose sugars is a key factor for the production of platform chemicals like succinic acid, levulinic acid, xylitol, fumaric acid, malic acid, 2,5‐furan dicarboxylic acid, 3‐hydroxy propionic acid, aspartic acid, glucaric acid, glutamic acid, and arabinitol (Kumar et al. 2018). There are some basic differences between the hemicellulose structure in hardwood and softwood. Hardwood hemicellulose is mainly composed of glucuronoxylan and glucomannan, while softwood hemicelluloses are mainly made up of galactoglucomannan and arabinoglucuronoxylan. Combinations of different types and amounts of hexoses and pentoses in hemicellulose show different digestibility or fermentability characteristics during fermentation in a biorefinery (Wang et al. 2014).
5.3.3 Lignin
Lignin is an amorphous, hydrophobic biopolymer constructed by three monolignols (Table 5.1): p‐hydroxyphenyl (H‐lignin), syringyl (S‐lignin), and guaiacyl (G‐lignin), which is the main renewable source of aromatic chemicals (Sjostrom 2013). Lignin has many remarkable properties, like high resistance to chemical, biological, and thermal degradation. The presence of multiple functional groups in the matrix makes lignin a promising alternative for carbon‐based materials (Ho‐Yin et al. 2019). Lignin has exhibited superior functionality in UV absorbance, amphiphilicity, and anti‐oxidation (Tian et al. 2017). The application of lignin and its derivatives in adhesives and green composites have been reported recently (Espinoza‐Acosta et al. 2018; Faris et al. 2016). Owing to its low cytotoxicity and pH controllable properties, lignin has also been considered for bioimaging and drug delivery systems (Dai et al. 2017). The exact linkages between the building block components are very complex and have not yet been completely decoded (Özparpucu et al. 2017), but a few major linkages have been confirmed through investigating the dissociated monomers after digestion. β‐aryl (β‐o‐4) and α‐aryl (α‐o‐4) ether linkages are the representative chemical linkages in the plant cell wall, accounting for at least 43–50% and 6–8% (in number) in softwood, respectively (Li et al. 2015). The potential to cleave the two linkages in or outside the phenolic structures are process‐specific.
Table 5.1 Lignin monomers and their composition (Pinkert et al. 2011).
| Name | Structure | Softwood | Hardwood |
| p‐hydroxyphenyl (H‐lignin) | 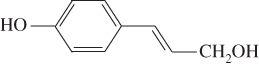 |
<5% | 0–8% |
| Syringyl (S‐lignin) | 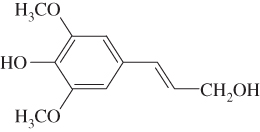 |
Trace amounts | 46–75% |
| Guaiacyl (G‐lignin) | 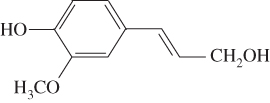 |
>95% | 25–50% |
Lignins are generally classified as hardwood lignin, softwood lignin, and grass lignin. Hardwood lignins are composed of syringyl and guaiacyl units, which originate from trans‐sinapyl alcohols and transconiferyl alcohol respectively. Softwood lignin, on the other hand, is mainly composed of guaicyl, which comes from trans‐coniferyl alcohol. The p‐hydroxyphenyl unit of grass lignin is derived from trans‐p‐coumaryl alcohol and two foregoing precursors. Native lignins are insoluble in water and acids, but isolated lignins show the maximum solubility in different organic solvents such as dimethyl sulfoxide, acetone, dioxane, tetrahydrofuran, and so on. Isolated lignins can be classified as milled wood lignin, kraft lignin, alkali lignin, and enzymatically liberated lignin.
5.3.4 Extractives
Extractives are various low molecular weight organic compounds that are present in small fractions but with high functionality, and therefore with high potential for different uses. They have been used in recent years for pharmaceuticals (Nair et al. 2015; Constable et al. 2007), food additives (Moure et al. 2006), and chemical reagents (Eriksson et al. 2018; Miranda et al. 2017; Mattos et al. 2016). Extractives are bio‐synthesized as secondary metabolites and protect plants from different insects and microbial attacks (Gierlinger et al. 2004; Ayvaz et al. 2010). Although wood contains less than 10% extractives, they greatly contribute to the physical properties of wood (e.g., color and odor). Extractive content determines the tensile strength, density, elasticity, permeability, flammability, and wettability of wood (Taylor et al. 2007; Jankowska et al. 2018). The major chemicals of wood extractives can be grouped as terpenoids, phenolics, alkaloids, fats, and waxes. The composition of extractives largely varies in different wood species and in the different parts (i.e., stem, leaves, barks, and roots) of the same wood species. The functions of extractives differ significantly, and different extractives can be discovered in different morphological areas of the plant. For instance, resin acids in the resin canals of plants protect wood from microbial attack; while fats and waxes exist in ray parenchyma tissue and act as a power source of woody plants. The majority of wood‐based extractives are low boiling point organic compounds, while a small amount of inorganic extractives has also been reported.
5.3.4.1 Terpenoids
Terpenes and terpenoids are collectively known as isoprenoids because both are derived from isoprene units (C5H8). Terpenes are a pure hydrocarbon having low molecular weight, whereas terpenoids contain functional groups like carboxyl, carbonyl, and hydroxyl groups. To avoid complexity, terpenes and their derivatives are collectively known as terpenoids. Depending on the number of isoprene units in the structure, terpenes and terpenoids are classified into mono‐, sesqui‐, di‐, tri‐, and polyterpenoids.
Myrcene, limonene, β‐phellandrene, α‐pinene, β‐pinene, 3‐carene, camphene, borneol, and hinokitiol are dominant volatile monoterpenoids and can be easily recovered as turpentine by simple steam distillation. Monoterpenoids impart an odor to the wood and are mainly found in softwood oleoresin. Although monoterpenoids are not usually found in hardwood, tropical hardwood oleoresin contains a few of these chemicals as minor ingredients (Sjostrom 2013; Zhu et al. 2011).
Diterpenoids are the most important constituents of oleoresin canal and can be categorized into acyclic, bicyclic, tricyclic, tetracyclic, and macrocyclic terpenoids. Diterpenoids are found mainly in softwoods in the form of resin acids. Geranyl‐linalool, β‐epimanool, cis‐abienol, manoyloxide, pimaral, cembrene, lambertianic acid, communic acid, and mercusic acid are the most common resin acids (Sjostrom 2013; Zhu et al. 2011).
Triterpenoids are oxygenated derivatives and are classified as tetracyclic lanostane, pentacyclic lupine, and pentacyclic oleanane derivatives. Triterpenoids and steroids are structurally similar and both synthesized from squalene. The difference in synthesis pathways result in different structures. The most prominent triterpenoids in wood are sitosterol, sitostanol, citrostadienol, botulin, and serratenediol; which are important raw materials for wood‐based chemicals (Sjostrom 2013; Zhu et al. 2011).
5.3.4.2 Fats and Waxes
The lipophilic materials in hardwood parenchyma cells are predominantly fats and waxes, whereas the softwood parenchyma resin contains mainly fats. Wood contains more than 30 types of saturated and unsaturated fatty acids, alcohols, and alkanes. Palmitic, stearic, arachidic, behenic, lignoceric, oleic, linoleic, linolenic, pinolenic, and eicosatrienoic acids are the common fatty acids found in woody plants (Sjostrom 2013).
5.3.4.3 Phenolic Compounds
Heartwood and bark contain thousand types of phenolic and complex polyphenol compounds. Phenolics protect wood plants from fungal and microbial attack. Simple phenolics, stilbenes, lignans, flavonoids, and tannins are a major class of phenolics found in plants (Sjostrom 2013).
5.4 Chemical Composition Analysis
Chemical composition analysis is often conducted to evaluate the true value of the waste‐derived biomass. The analytical procedure for structural and non‐structural components of wood is demonstrated in Figure 5.4.
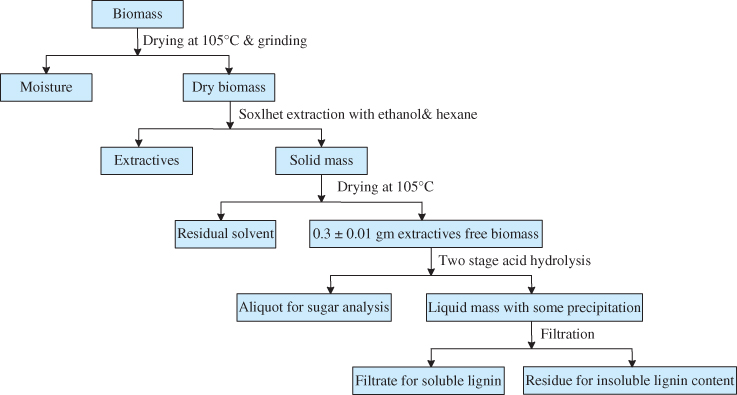
Figure 5.4 Chemical composition analysis scheme of lignocellulosic biomass.
5.4.1 Structural Carbohydrates and Lignin
The analytical procedures for structural carbohydrates and lignin have been well‐documented by the National Renewable Energy Laboratory (NREL) of the US Department of Energy (Sluiter et al. 2008). The basic concept of this process is to dissociate the polysaccharides in the plant cell wall into monomers without promoting the formation of dehydration byproducts, such as furfural, hydromethoxy furfural, and levulinic acid. The dissociation of polysaccharides is carried out in two stages: (i) hydrolysis with strong sulfuric acid (72 wt%) at near‐room temperature, and (ii) high‐temperature secondary hydrolysis after diluting the primary hydrolysis slurry to 4 wt% of sulfuric acid. During primary hydrolysis, polysaccharides are completely separated from the lignin whereas secondary hydrolysis depolymerizes oligomers to monomer sugars (Bhagia et al. 2016). The fundamentals behind the NREL procedure were developed on the assumption of low proteins and lipids. This technique cannot be applied precisely for high lipid, protein, and pectin‐containing biomass, such as grass, leaves, and vegetable samples.
5.4.2 Extractives
Extractives can be analyzed by soaking the biomass samples in polar and nonpolar organic solvents, such as methanol, ethanol, acetone, hexane, benzene, and diethyl ether, at a desired temperature with continuous solvent refluxing. Soxhlet extraction is the most effective method of extractive separation from plants, and is reported comprehensively (Ramluckan et al. 2014; Schwanninger and Hinterstoisser 2002).
5.5 Pretreatment
Conversion of woody biomass into useful products relies on a serious of complex processes, such as pretreatment, hydrolysis, fermentation, and separation. Pretreatment aims to alter and modify the basic structure of lignocellulosic biomass for functional application. The main purpose of pretreatment is to increase the biomass conversion yield by increasing the enzyme accessibility to the pretreated substrate. Lignocellulosic biomass is a complex matrix of the introduced building block chemicals, which provide strong recalcitrance to cellulose degradation (Himmel et al. 2007). Woody biomass contains more lignin and possesses greater recalcitrance than herbaceous biomass. To overcome the biomass recalcitrance, it is necessary to fractionate all the components at the very beginning of the bioconversion process. Different pretreatment techniques have been designed to remove the recalcitrance of lignocellulosic biomass (Park et al. 2017; Baker et al. 2017; Zhao et al. 2017; Brosse et al. 2009). Pretreatment removes lignin and hemicellulose, and increases the surface area and digestibility of cellulose. It can also prevent the degradation of pentose sugar and minimize the formation of inhibitory components for fermentation. Physical, biological, and chemical methods have been reported for the pretreatment of plant biomass, while biological pretreatment is less preferred industrially due to its low efficiency and processability (Abd‐Aziz et al. 2018; Mussatto 2016). Physical pretreatment like steam explosion is usually used for woody biomass, but the process alone has not been extensively used commercially in comparison with chemical pretreatment approaches (Zhu and Pan 2010).
The most extensively studied chemical pretreatments utilize a wide range of chemicals at elevated temperature (Wang et al. 2018; Terán Hilares et al. 2017). Acid, alkali, organosolv, peroxide, sodium hypochlorite, sodium sulfide, ozone, and ammonia have all been used in the chemical pretreatments (Leu and Zhu 2013). We classify here the major chemical pretreatment concepts into three categories (i.e., organosolv, acid, and alkaline pretreatment), of which the key mechanisms are presented in Figure 5.5.
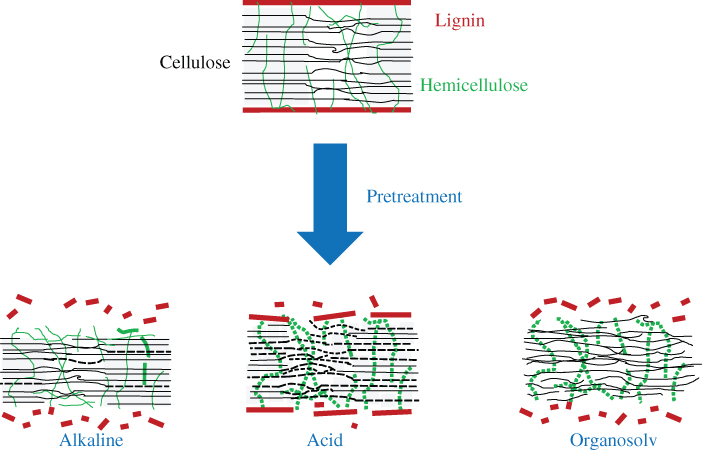
Figure 5.5 Mechanisms of different chemical pretreatments.
The chemical reactions in cleaving the two representative ether linkages under different types of biorefinery processes are illustrated in Figure 5.6. The presented model lignin structures include two G‐lignins linked at the α‐o‐4 position. The monolignol at the bottom right contains a –OH group linking to its phenolic structure at the 4th position while its α‐ and β‐ positions both linked with other lignins. The monolignol at the upper left links with other lignins at the 4th and β‐ positions, but its α‐ position links only with a –OH group. The concept diagram demonstrates the major functions and limitations of applied reagents and/or catalysts to cell wall decomposition during thermochemical processes. Cleavage of the two linkages under an alkaline pretreatment process is shown in Figure 5.6a. At elevated pH, the cleavage reactions can initiate most rapidly at the –OH group at the 4th position of the phenolic structure, while it can be carried out only very slowly when the 4th position links to another lignin. During the pretreatment process, more –OH structures can be created to continue the cleavage of the linkages. Alkali can also cleave some hydrolysable linkages in lignin and polysaccharides, which result in a reduction in the degree of polymerization and crystallinity of the substrate (Kim et al. 2016). For acid pretreatment processes, bond cleavage can start randomly at either the α‐ or β‐ positions regardless of whether there is a hydroxyl group bonding with the aromatic ring at the 4th carbon. With different reagents the α‐ and β‐ linkages can be cleaved and the original functional groups can be replaced by hydrogen, ethyl, or sulfonate groups. In addition, acid‐based biorefinery processes can effectively cleave glucosidic linkages in hemicelluloses and amorphous cellulose, which further improve the accessibility of enzymes to cellulose to promote enzymatic hydrolysis (Leu and Zhu 2013).
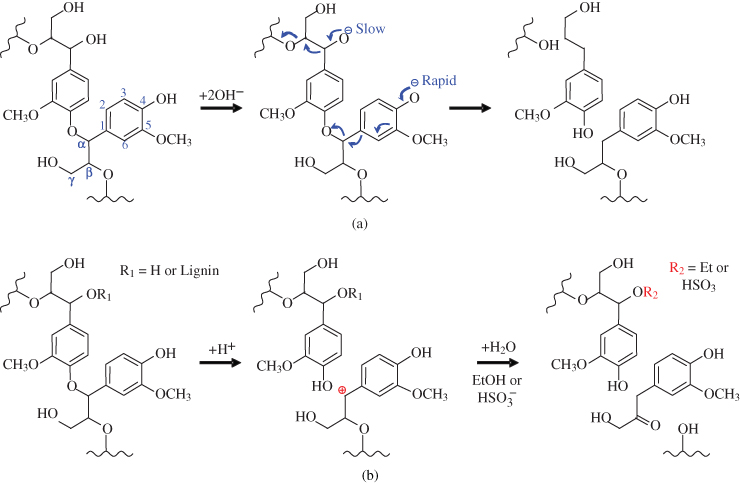
Figure 5.6 (a) Cleavage of α‐o‐4 and β‐o‐4 bonds in an alkaline pretreatment process; (b) cleavage of α‐o‐4 and β‐o‐4 bonds in dilute acid, organosolv, and sulfite pretreatment processes.
Acid pretreatments have been successfully applied to solubilize hemicellulose and efficiently used to remove the recalcitrance of biomass. Both dilute and concentrated H2SO4 acids are most commonly used for pretreatment of lignocellulosic biomass (Mosier et al. 2005). Dilute acid pretreatment processes are more preferable than concentrated acid processes because concentrated acids mediate the generation of fermentation inhibitors (Taherzadeh and Karimi 2008). Acid pretreatment can be done at low temperatures (<120°C) for longer retention times or at high temperatures (>180°C) for shorter retention times. Other acids such as nitric acid, hydrochloric acid, phosphoric acid, acetic acid, and formic acid are also successfully studied in biomass pretreatment (Gámez et al. 2006; Rodrıguez‐Chong et al. 2004).
Alkali preferentially removes lignin over hemicellulose from biomass (Leu and Zhu 2013). Alkali reacts with acetyl and uronic acid groups in the hemicellulose and thus increases the accessibility of enzymes (Chang and Holtzapple 2000). The hydrolysis of ester linkages more effectively removes lignin with minimal cellulose and hemicellulose solubility (Sun and Cheng 2002). NaOH is more effective than other alkalis in reducing cellulose crystallinity and degree of polymerization, and altering the structure of lignin (Kumar and Wyman 2009).
Organosolv techniques are mainly used for the extraction of high‐quality lignin. Ethanol, methanol, propanol, butanol, butanediol, ethylene glycol, glycerol, acetic acid, acetone, and tetrahydrofuran are most commonly used in combination with inorganic acids, salts, or alkali catalysts (Zhao et al. 2009; Bajpai 2016). Organic solvents are most effective in lignin degradation than other chemical pretreatments. Lignin removal increases the accessible surface of substrate for enzyme saccharification (Agbor et al. 2011). Pretreatment temperature, reaction time, solvent concentration, and catalyst concentration greatly determine the crystallinity, fiber length, and surface area of pretreated biomass. The effects of the different catalysts such as H2SO4, NaOH, and MgSO4 have been studied on ethanol organosolv of pine. The results showed that H2SO4 is the most effective catalyst for pine (J.Y. Park et al. 2010; N. Park et al. 2010). The combination of ethanol organosolv to ball milling increased the lignin separation and glucose yield and decreased the pretreatment severity of organosolv (Hideno et al. 2013). Ionic liquid and deep eutectic solvents are recyclable organic compounds, and have been widely exploited as solvents for pretreatment of lignocellulose before further processing to energy and chemicals (Moniruzzaman and Goto 2018; Kanbayashi and Miyafuji 2016). Presence of solvent in the substrate reduces the enzyme activity of cellulose hydrolysis (Mosier et al. 2005). Removal and recovery of solvent also reduces the operational cost of the process (Sun and Cheng 2002). Organic solvents should be carefully handled because they are inflammable and can cause explosions.
5.6 Saccharification and Fermentation
Saccharification is the conversion process to break down polysaccharides into monomeric sugars (Zhu et al. 2017). Acid hydrolysis and enzymatic hydrolysis are commonly applied procedures for saccharification of lignocellulosic biomass. Acid hydrolysis can directly depolymerize the polysaccharide chains in the lignocellulosic biomass without any pretreatment, which can effectively release more than 90% of monomer sugars from the feedstock (Lavarack et al. 2002; Frederick Jr et al. 2008). However, concentrated acid hydrolysis can further react with the dissolved substrates (i.e., carbohydrates), resulting in high concentrations of fermentation‐inhibiting products, such as organic acids, furfural, and hydroxymethylfurfural (HMF) (Badger 2002).
Enzymatic hydrolysis is currently the most widely applied/discussed approach for biomass conversion. This process accounts for approximately 27% of the total operation cost of the overall bioconversion process, and therefore needs to be optimized with the pretreatment processes for cost reduction (Bbosa et al. 2018). Saccharification efficiency can be affected by enzyme‐related (Gupta et al. 2016) and substrate‐related factors (Leu and Zhu 2013). The mechanisms of the interactions between dissociated and residual components to enzyme activities are the functions of the biomass and pretreatment process. Figure 5.7a–d shows some images of the pretreated substrate before and after enzymatic hydrolysis. The surface of woody biomass before pretreatment (Figure 5.7a) is smooth, with the inner surface well protected from hydrolysis. The pretreatment by ethanol, alkaline, and SPORL opens the plant cell wall (red arrow) and creates different levels of dissociation (Figure 5.7b–d, respectively). With increased severity of pretreatment conditions and degree of lignin removal, the damage in the cell wall become visible, together with lignin precipitation on the substrate surface (Figure 5.7d). The cell wall structure is further decomposed after hydrolysis, while enzymes may be captured under a scanning electron microscope (SEM) within 24 hours of hydrolysis (arrow mark in Figure 5.7e). The cell wall can be completely decomposed after enzymatic hydrolysis (Figure 5.7f).
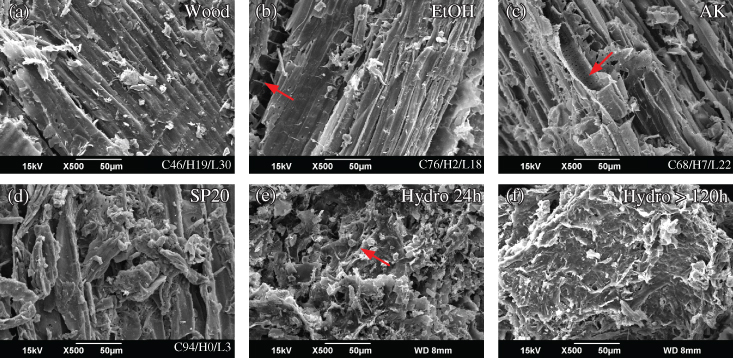
Figure 5.7 SEM images of substrate at different stages of pretreatment or hydrolysis (see text).
Enzyme hydrolysis of biomass suffers with a low reaction rate due to the high crystallinity of cellulose, and less accessible surface area for enzyme and non‐productive binding to cellulase enzyme (Dadi et al. 2006). Enzymatic hydrolysis requires pretreatment to break the regular structure of cellulose and to increase surface area for enzyme access (Badger 2002). The yield of enzymatic hydrolysis increased from 20% to 90% after proper pretreatment of biomass (Table 5.2). The efficiency of both acid and enzyme hydrolysis depends on the crystallinity, molecular structure, degree of polymerization, surface area, swelling, lignin content, and hemicellulose content (Dadi et al. 2006). The extent of hydrolysis to be done depends on the targeted chemicals. Complete hydrolysis is necessary to produce ethanol. On the other hand, complete hydrolysis reduces the efficiency of production of butanol and lactic acid from the biomass.
Table 5.2 Pretreatment conditions and saccharification yield for different biomass feedstocks.
| Composition of raw substrate | Composition of pretreated substrate | |||||||||||||
| Feedstocks | Solvents (%) | Catalyst (%) | Solid/liquid | Cellulose | Hemi. | Lignin | Temp. (°C) | Time (min) | Cellulose | Hemi. | Lignin | Enzyme load (FPU) | SED (%) | Ref. |
| Lodgepole pine | 65 EtOH | 1.1 H2SO4 | 0.14 | 49.0 | 21.7 | 26.4 | 170 | 60 | 74.8 | 3.58 | 17.59 | 20 | 100 | Del Rio et al. (2010) |
| Lodgepole pine | 65 BuOH | 1.1 H2SO4 | 0.14 | 49.0 | 21.7 | 26.4 | 170 | 60 | 74.64 | 2.35 | 18.25 | 20 | 90 | |
| Pitch pine | 50 EtOH | 1 MgCl2 | 0.1 | 44 | 30 | 26 | 210 | 10 | 46 | 0 | 25 | 61.1 | N. Park et al. (2010) | |
| Pitch pine | 50 EtOH | 2 NaOH | 0.1 | 44 | 30 | 26 | 210 | 20 | 34 | 0 | 12 | 85.4 | ||
| Hemp hurd | 45 MeOH | 3 H2SO4 | 0.04 | 43 | 21 | 23 | 165 | 20 | 48 | 18 | 22 | 67.9 | Gandolfi et al. (2014) | |
| Eucalyptus | 25w EtOH | 1 CH3COOH | 0.2 | 42.2 | 34.1 | 28.1 | 200 | 60 | 83 | 3.5 | 13.5 | 9.5 | ∼100 | Teramoto et al. (2009) |
| Japanese cypress | 50 EtOH | 0.4 HCl | 0.18 | 41.5 | 25 | 32 | 170 | 45 | 75 | 0 | 25 | 22.8 | ∼70 | Hideno et al. (2013) |
| Liriodendron tulipifera | 50w EtOH | 1 NaOH | — | 41.1 | 21.2 | 21.4 | 140 | 50 | 60 | 22.3 | 20 | 67 | Koo et al. (2012) | |
| Miscanthus | 80 EtOH | 1 H2SO4 | 49.09 | 24.18 | 25.11 | 170 | 60 | 48.09 | 8.5 | 22.01 | 45 | Obama et al. (2012) | ||
| Norway spruce | 65 EtOH | — | 0.07 | 38.8 | 22.2 | 26.4 | 210 | 15 | 35.7 | 2.9 | 3.9 | 15 | 87 | Agnihotri et al. (2015) |
| Empty palm fruit branch | 78 FA | — | 0.1 | 37.01 | 15 | 17.4 | 107 | 90 | 82.44 | 5.6 | 4.91 | 15 | 99 | Cui et al. (2014) |
| Willow wood | 55w EtOH | 0.1 H2SO4 | 0.10 | 32.9 | 14.2 | 28.5 | 187 | 180 | 46.6 | 12.2 | 26.6 | 33 | 87 | Huijgen et al. (2011) |
| Bamboo | 75 EtOH | 2 H2SO4 | 0.2 | 41.3 | 24 | 22.8 | 180 | 60 | 89.7 | 6.4 | 3.3 | 15 | 83.4 | Li et al. (2012) |
| Alfalfa stem | 9w PAA | — | 0.16 | 30.2 | 18 | 15.2 | 100 | 120 | 30.1 | 16.3 | 14.9 | 60 | 47 | Xu and Tschirner (2011) |
| Crofton wood | 40w PAA | — | 0.25 | 37.6 | 22.4 | 16.4 | 90 | 90 | 73 | 33.27 | 1 | 20 | 38 | Zhao et al. (2008) |
| Siam wood | 50w PAA | — | 0.25 | 41 | 22.3 | 20.7 | 90 | 90 | 64.6 | 26.7 | 6.1 | 10 | 70 | Zhao et al. (2010) |
| Oil palm trunk | 7 H2SO4 | 4 Na2SO3 | 0.2 | 38.1 | 24.4 | 21.4 | 170 | 30 | 68.8 | 4.9 | 30.1 | 15 | 66 | Noparat et al. (2017) |
BuOH, butanol; EtOH, ethanol; FA, formaldehyde; MeOH, methanol; PAA, peracetic acid; SED, substrate enzyme digestibility.
Fermentation is a metabolic process that consumes sugars and produces alcohols or other chemicals depending upon the strains used in the fermentation. Fermentable sugars from the biomass can be converted into different chemicals by different enzymes. In the United States, less than 5% of total chemical consumption is obtained from the fermentation of lignocellulosic biomass. Common fermentation products in the recent literatures include ethanol, butanol, and lactic acid (Macedo and Brigham 2014; Zheng et al. 2015; Wang et al. 2015). The US Department of Energy and Department of Agriculture are committed to replace at least 30% of petroleum consumption with biofuel by 2030 (Perlack 2005). The US Department of Energy (Zhu and Zhuang 2012) reported that the United States currently uses 140 billion gallons of petroleum transportation fuel per year. To replace 30% of petroleum with bioethanol will require about 60 billion gallons of ethanol (US Congress 2005) The US alone can sustainably produce annually 1.366 billion tons of dry biomass, of which 998 million tons is from agricultural residues and 368 million tons from forest wood residues (Somerville 2006). This total biomass can theoretically produce 130 billion gallons of ethanol, which is about 60% of the current petroleum energy used in the US. Moreover, the use of bioenergy alters energy dynamics and reduces greenhouse gas emissions. China has also promised to contribute heavily to biofuel production and utilization (Nguyen et al. 2017). China is expected to replace 30% of total oil imports with cellulosic ethanol by 2050 (Zhao 2015).
5.7 New Functions of Wood Residues
Wood has been found in many ancient and historical structures like Egyptian pyramids, Chinese temples, and tombs, as construction materials. Beside the more traditional uses in construction, furniture, pulp paper, and bioethanol production, wood has been used recently for the manufacture of advanced materials like wood–plastic composites (WPCs) and cellulose nanomaterials. Some modern applications of wood materials are given in Table 5.3. These composite materials have good mechanical properties to replace traditional materials for different construction purposes. Wood extractives are widely used in the pharmaceutical industry because of their medicinal properties.
Table 5.3 Current uses of wood products.
| Byproducts | ||||||
| Wood products | Fraction of wood | Market price, USD/ton | Main use | Type | Amount (%) | References |
| Timber or lumber | ∼ 100 | 250–400 | Construction | Wood powder/barks | 8–10 | Fu et al. (2014); Borri and Corradi (2011) |
| Plywood | 80–90 | 300–500 | Furniture | Wood powder/barks | 12–15 | Safin et al. (2015); Pizzi et al. (1993) |
| Wood–plastic composite | 50–60 | 300–1000 | Decoration | — | Ashori (2008); Nourbakhsh and Ashori (2010) | |
| Fiberboard | 40–50 | 350–800 | Furniture | — | Popovska et al. (2016); Reddy and Yang (2005) | |
| Paper | 30–50 | 600–1000 | Printing | Lignin | 20–25 | M'hamdi et al. (2017); Liao (2005) |
| Nanomaterials | 10–20 | 500–3000 | Nanotechnology | Lignin | 20–25 | Isogai (2013); Sanchez et al. (2011) |
| Chemicals | — | ∼ | Natural feedstock | — | Cherubini and Strømman (2011); Czernik and Bridgwater (2004) | |
| Biofuels | — | 400–600 | Fuels | Lignin | 20–25 | Kalyani et al. (2017); Agarwal (2007) |
5.7.1 Wood–Plastic Composite for Construction Purposes
WPCs are prepared by combining two phases of polymeric matrices: thermosetting or thermoplastic polymer, and wood flour. WPC of desired mechanical properties can be prepared by varying the composition of the matrix phase, reinforcing fiber, coupling agent and lubricant (Leu et al. 2012; Javier et al. 2015). Aras et al. (2015) showed that the mechanical properties of the green materials produced from plastic waste and wood are almost similar to those of commercial wood and formaldehyde‐based plastic.
For sustainable building, and renewable and ecofriendly construction, civil and construction engineers are focusing more on the utilization of woody biomass to produce low‐cost and energy‐efficient materials for construction. Wide varieties of wood and wood‐based composites are also used for both residual and non‐residual construction purposes.
Plywood, composite lumber, wafer board, and flake board are the traditional WPC materials used as building and construction materials. These traditional materials are produced by laminating thin wood sheets or strips of veneers glued together with adjacent layers, and are widely used in furniture and underlayment of floors and roofs. Wood is broken down to small particles and fibers, and then glued together with adhesive for the preparation of particleboard and fibreboard. Some fibreboards can be prepared without any adhesive because one of the major wood components can serve as a binding material.
5.7.2 Cellulose Nanomaterials
Cellulose nanocomposites consist of any reinforcing materials having dimensions less than 100 nm in any direction (Mariano et al. 2014). Cellulose nanocrystals (CNCs) and nanofibrillated cellulose (NFC) are the reinforcing agents in cellulose nanocomposites. The CNC is prepared by treating pulp fiber to break the linkages between cellulose and hemicellulose, and to release crystalline cellulose (Siró and Plackett 2010). The NFC, on the other hand, is produced by the application of shear stress perpendicular to the pulp fiber to break internal linkages of nanofibres (Xu et al. 2013). Because of the low cost, large aspect ratio and fiber entanglement in the final product (Xu et al. 2013), NFC is widely used for the production of cellulose composites (Isogai 2013), such as UV curable acrylic resin, poly‐lactide, polyethylene, poly‐polypropylene, polystyrene, glass fiber, carbon nanotubes, phenol‐formaldehyde resin, latex, nano‐clay and paper.
5.7.3 Wood Extractives
Plant extractives are now being used for the production of flavors, fragrances, oil, and pharmaceutical preparations (Gandini 2008). Paclitaxel is a plant extractive used for the treatment of different types of cancer (Tanaka et al. 2017; Foglietta et al. 2018; Bernabeu et al. 2016). Significant anti‐cancer activity of keratin nanoparticles‐based paclitaxel in breast cancer models has been reported recently (Bernabeu et al. 2016). Paclitaxel loading on keratin nanoparticles increases the solubility and drug delivery of paclitaxel. The traditional use of turpentines as a solvent is substituted by cheap petrochemicals. Terpene and terpenoids can be used as a feedstock to produce rose alcohols, linalool, and nerol. Linalool is a key intermediate in the production of vitamin E (Langenheim 2003). Turpentines form a low molecular weight polymer having better flexibility, and this polymer can be used in papermaking, adhesives, printing inks, and coating (Chen 1992). Yumrutaş et al. (2008) reported that turpentines can be used as an additive for gasoline. Plant tannins contain molecules of complex phenolic structures obtained mainly from the bark and heartwood of the trees. Tannins have been used in the leather industry for the vegetable tanning process; but more convenient synthetic tannin has replaced the use of vegetable tannins (Pizzi 1983).
5.8 Conclusions and Future Trends
Recent development of technology has resulted in dramatic changes in consumer behavior and views of woody products and the forestry industry. While the demand for structural timber remains, the need of paper and related products has continuously decreased, lignocellulosic biomass has been seen as an emerging renewable feedstock to replace petroleum refinery products. With the depletion of fossil fuels, bioenergy and biomaterials will eventually substitute plastics, resins, additives, solvents, carbon fibers, medicine, and/or many other materials. Currently, however, technical and economic challenges still remain, preventing wide acceptance of the second‐generation biorefinery. As the feedstock of the conventional refinery is still sufficient, there is no immediate demand to develop and/or use the related products. Developing technology should focus on assisting municipal waste treatment or handling the byproducts of related industry (e.g., kraft lignin). Accumulation of experience of this application should create useful information for the future development of the biomass biorefinery, and therefore needs to be emphasized and practiced.
Acknowledgement
The authors thank the Hong Kong General Research Fund (15212317), Environment and Conservation Fund (ECF 85/2017), and Sinopec Chemical Commercial Holding Company Limited for financial support. The authors also thank the University Research Facility in Chemical and Environmental Analysis (UCEA) of The Hong Kong Polytechnic University for sample analyses.
References
- Abd‐Aziz, S., Ibrahim, M.F., and Jenol, M.A. (2018). Biological pretreatment of lignocellulosic biomass for volatile fatty acid production. In: Emerging Areas in Bioengineering, vol. 1 (ed. H.N. Chang), 191–201. Wiley‐VCH Verlag.
- Agarwal, A.K. (2007). Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Prog. Energy Combust. Sci. 33 (3): 233–271.
- Agbor, V.B., Cicek, N., Sparling, R. et al. (2011). Biomass pretreatment: fundamentals toward application. Biotechnol. Adv. 29 (6): 675–685.
- Agnihotri, S., Johnsen, I.A., Bøe, M.S. et al. (2015). Ethanol organosolv pretreatment of softwood (Picea abies) and sugarcane bagasse for biofuel and biorefinery applications. Wood Sci. Technol. 49 (5): 881–896.
- Alén, R. (2011). Biorefining of Forest Resources. Paperi ja Puu Oy.
- Aras, U., Kalaycıoğlu, H., Yel, H., and Bitek, G. (2015). Effects of ammonium nitrate on physico‐mechanical properties and formaldehyde contents of particleboard. Procedia Social Behav. Sci. 195: 2130–2134.
- Ashori, A. (2008). Wood–plastic composites as promising green‐composites for automotive industries! Bioresour. Technol. 99 (11): 4661–4667.
- Ayvaz, A., Sagdic, O., Karaborklu, S., and Ozturk, I. (2010). Insecticidal activity of the essential oils from different plants against three stored‐product insects. J. Insect Sci. 10 (1): 21.
- Badger, P. (2002). Ethanol from cellulose: a general review. In: Trends in New Crops and New Uses, vol. 1 (eds. J. Janick and A. Whipkey), 17–21. ASHS Press.
- Bajpai, P. (2016). Pretreatment of Lignocellulosic Biomass for Biofuel Production. Springer.
- Baker, P., Charlton, A., and Hale, M. (2017). Fungal pre‐treatment of forestry biomass with a focus on biorefining: a comparison of biomass degradation and enzyme activities by wood rot fungi across three tree species. Biomass Bioenergy 107: 20–28.
- Bbosa, D., Mba‐Wright, M., and Brown, R.C. (2018). More than ethanol: a techno‐economic analysis of a corn stover‐ethanol biorefinery integrated with a hydrothermal liquefaction process to convert lignin into biochemicals. Biofuels, Bioprod. Biorefin. 12 (3): 497–509.
- Bernabeu, E., Gonzalez, L., Legaspi, M.J. et al. (2016). Paclitaxel‐loaded TPGS‐b‐PCL nanoparticles: in vitro cytotoxicity and cellular uptake in MCF‐7 and MDA‐MB‐231 cells versus mPEG‐b‐PCL nanoparticles and Abraxane®. J. Nanosci. Nanotechnol. 16 (1): 160–170.
- Bhagia, S., Nunez, A., Wyman, C.E., and Kumar, R. (2016). Robustness of two‐step acid hydrolysis procedure for composition analysis of poplar. Bioresour. Technol. 216: 1077–1082.
- Borri, A. and Corradi, M. (2011). Strengthening of timber beams with high strength steel cords. Composites Part B 42 (6): 1480–1491.
- Brosse, N., Sannigrahi, P., and Ragauskas, A. (2009). Pretreatment of Miscanthus × giganteus using the ethanol organosolv process for ethanol production. Ind. Eng. Chem. Res. 48 (18): 8328–8334.
- Chang, V.S. and Holtzapple, M.T. (2000). Fundamental factors affecting biomass enzymatic reactivity. Appl. Biochem. Biotechnol. 84 (1): 5–37.
- Chen, G.‐F. (1992). Developments in the field of rosin chemistry and its implications in coatings. Prog. Org. Coat. 20 (2): 139–167.
- Cherubini, F. and Strømman, A.H. (2011). Chemicals from lignocellulosic biomass: opportunities, perspectives, and potential of biorefinery systems. Biofuels, Bioprod. Biorefin. 5 (5): 548–561.
- Congress, U.S. (2005). Energy Policy Act of 2005. Public Law: 109–158. https://www.govinfo.gov/app/details/PLAW-109publ58 (accessed 5 April 2020).
- Constable, D.J., Jimenez‐Gonzalez, C., and Henderson, R.K. (2007). Perspective on solvent use in the pharmaceutical industry. Org. Process Res. Dev. 11 (1): 133–137.
- Cui, X., Zhao, X., Zeng, J. et al. (2014). Robust enzymatic hydrolysis of formiline‐pretreated oil palm empty fruit bunches (EFB) for efficient conversion of polysaccharide to sugars and ethanol. Bioresour. Technol. 166: 584–591.
- Czernik, S. and Bridgwater, A. (2004). Overview of applications of biomass fast pyrolysis oil. Energy Fuels 18 (2): 590–598.
- Dadi, A.P., Varanasi, S., and Schall, C.A. (2006). Enhancement of cellulose saccharification kinetics using an ionic liquid pretreatment step. Biotechnol. Bioeng. 95 (5): 904–910.
- Dai, L., Liu, R., Hu, L.‐Q. et al. (2017). Lignin nanoparticle as a novel green carrier for the efficient delivery of resveratrol. ACS Sustainable Chem. Eng. 5 (9): 8241–8249.
- Daniel, G. (2009). Wood and fibre morphology. In: Pulp and Paper Chemistry and Technology, vol. 1 (eds. M. Ek et al.), 49–71. de Gruyter.
- Del Rio, L.F., Chandra, R.P., and Saddler, J.N. (2010). The effect of varying organosolv pretreatment chemicals on the physicochemical properties and cellulolytic hydrolysis of mountain pine beetle‐killed lodgepole pine. Appl. Biochem. Biotechnol. 161 (1–8): 1–21.
- Eriksson, D., Arshadi, M., Kataria, R., and Bergsten, U. (2018). Lipophilic extractives in different tree fractions and forestry assortments of Pinus sylvestris due for thinning or final cutting. Scand. J. For. Res.: 1–9.
- Espinoza‐Acosta, J.L., Torres‐Chávez, P.I., Olmedo‐Martínez, J.L. et al. (2018). Lignin in storage and renewable energy applications: a review. J. Energy Chem. 27 (5): 1422–1438.
- Evert, R.F. (2006). Esau's Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development. Wiley.
- Faris, A.H., Rahim, A.A., Ibrahim, M.N.M. et al. (2016). Combination of lignin polyol–tannin adhesives and polyethylenimine for the preparation of green water‐resistant adhesives. J. Appl. Polym. Sci. 133 (20): 43437.
- Foglietta, F., Spagnoli, G.C., Muraro, M.G. et al. (2018). Anticancer activity of paclitaxel‐loaded keratin nanoparticles in two‐dimensional and perfused three‐dimensional breast cancer models. Int. J. Nanomed. 13: 4847.
- Food and Agriculture Organization. 2018. FAOSTAT, Forestry Production and Trade. http://www.fao.org/faostat/en/#data/FO (accessed 22 April 2020).
- Frederick, W. Jr., Lien, S., Courchene, C. et al. (2008). Production of ethanol from carbohydrates from loblolly pine: a technical and economic assessment. Bioresour. Technol. 99 (11): 5051–5057.
- Fu, M., Liu, Y., Li, N. et al. (2014). Application of modern timber structure in short and medium span bridges in China. J. Traffic Transp. Eng. 1 (1): 72–80.
- Fujita, M. and Harada, H. (2000). Ultrastructure and formation of wood cell wall. In: Wood and Cellulosic Chemistry (eds. D.N.‐S. Hon and N. Shiraishi), 1–49. CRC Press.
- Gámez, S., González‐Cabriales, J.J., Ramírez, J.A. et al. (2006). Study of the hydrolysis of sugar cane bagasse using phosphoric acid. J. Food Eng. 74 (1): 78–88.
- Gandini, A. (2008). Polymers from renewable resources: a challenge for the future of macromolecular materials. Macromolecules 41 (24): 9491–9504.
- Gandolfi, S., Ottolina, G., Consonni, R. et al. (2014). Fractionation of hemp hurds by organosolv pretreatment and its effect on production of lignin and sugars. ChemSusChem 7 (7): 1991–1999.
- Gierlinger, N., Jacques, D., Schwanninger, M. et al. (2004). Heartwood extractives and lignin content of different larch species (Larix sp.) and relationships to brown‐rot decay‐resistance. Trees 18 (2): 230–236.
- Gupta, V.K., Kubicek, C.P., Berrin, J.‐G. et al. (2016). Fungal enzymes for bio‐products from sustainable and waste biomass. Trends Biochem. Sci 41 (7): 633–645.
- Harada, H. and Côté, W. Jr. (1985). Structure of wood. In: Biosynthesis and Biodegradation of Wood Components (ed. T. Higuchi), 1–42. New York: Academic Press.
- Hideno, A., Kawashima, A., Endo, T. et al. (2013). Ethanol‐based organosolv treatment with trace hydrochloric acid improves the enzymatic digestibility of Japanese cypress (Chamaecyparis obtusa) by exposing nanofibers on the surface. Bioresour. Technol. 132: 64–70.
- Himmel, M.E., Ding, S.‐Y., Johnson, D.K. et al. (2007). Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315 (5813): 804–807.
- Ho‐Yin, T., Cheng, S.‐C., Yeung, C.S. et al. (2019). Development of a waste‐derived lignin‐porphyrin bio‐polymer with enhanced photoluminescence at high water fraction with wide pH range and heavy metal sensitivity investigations. Green Chem. 21 (6): 1319–1329.
- Huijgen, W.J., Smit, A.T., Reith, J.H., and Uil, H.D. (2011). Catalytic organosolv fractionation of willow wood and wheat straw as pretreatment for enzymatic cellulose hydrolysis. J. Chem. Technol. Biotechnol. 86 (11): 1428–1438.
- Isogai, A. (2013). Wood nanocelluloses: fundamentals and applications as new bio‐based nanomaterials. J. Wood Sci. 59 (6): 449–459.
- Jankowska, A., Boruszewski, P., Drożdżek, M. et al. (2018). The role of extractives and wood anatomy in the wettability and free surface energy of hardwoods. BioResources 13 (2): 3082–3097.
- Javier, C.‐S., Jorge, D.‐D., Sergio, A.‐R., and Roberto, Z.‐G. (2015). Optimization of the tensile and flexural strength of a wood‐PET composite. Ing., Invest. Tecnol. 16 (1): 105–112.
- Kalyani, D.C., Zamanzadeh, M., Müller, G., and Horn, S.J. (2017). Biofuel production from birch wood by combining high solid loading simultaneous saccharification and fermentation and anaerobic digestion. Appl. Energy 193: 210–219.
- Kanbayashi, T. and Miyafuji, H. (2016). Effect of ionic liquid treatment on the ultrastructural and topochemical features of compression wood in Japanese cedar (Cryptomeria japonica). Sci. Rep. 6: 30147.
- Kibblewhite, R.P. and Brookes, D. (1976). Distribution of chemical components in the walls of kraft and bisulphite pulp fibres. Wood Sci. Technol. 10 (1): 39–46.
- Kim, J.S., Lee, Y., and Kim, T.H. (2016). A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 199: 42–48.
- Klemm, D., Heublein, B., Fink, H.P., and Bohn, A. (2005). Cellulose: fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 44 (22): 3358–3393.
- Koo, B.‐W., Min, B.‐C., Gwak, K.‐S. et al. (2012). Structural changes in lignin during organosolv pretreatment of Liriodendron tulipifera and the effect on enzymatic hydrolysis. Biomass Bioenergy 42: 24–32.
- Kumar, A.K. and Sharma, S. (2017). Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour. Bioprocess. 4 (1): 7.
- Kumar, R. and Wyman, C.E. (2009). Effects of cellulase and xylanase enzymes on the deconstruction of solids from pretreatment of poplar by leading technologies. Biotechnol. Progr. 25 (2): 302–314.
- Kumar, V., Binod, P., Sindhu, R. et al. (2018). Bioconversion of pentose sugars to value added chemicals and fuels: recent trends, challenges and possibilities. Bioresour. Technol. 269: 443–451.
- Lachaud, S., Catesson, A.‐M., and Bonnemain, J.‐L. (1999). Structure and functions of the vascular cambium. C.R. Acad. Sci., Ser. III 322 (8): 633–650.
- Langenheim, J.H. (2003). Plant Resins. Oregon: Timber Press.
- Lavarack, B., Griffin, G., and Rodman, D. (2002). The acid hydrolysis of sugarcane bagasse hemicellulose to produce xylose, arabinose, glucose and other products. Biomass Bioenergy 23 (5): 367–380.
- Leu, S.‐Y. and Zhu, J. (2013). Substrate‐related factors affecting enzymatic saccharification of lignocelluloses: our recent understanding. Bioenergy Res. 6 (2): 405–415.
- Leu, S.‐Y., Yang, T.‐H., Lo, S.‐F. et al. (2012). Optimized material composition to improve the physical and mechanical properties of extruded wood–plastic composites (WPCs). Constr. Build. Mater. 29: 120–127.
- Li, Z., Jiang, Z., Fei, B. et al. (2012). Ethanol organosolv pretreatment of bamboo forefficient enzymatic saccharification. BioResources 7 (3): 3452–3462.
- Li, C., Zhao, X., Wang, A. et al. (2015). Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 115 (21): 11559–11624.
- Liao, S.‐H. (2005). Expert system methodologies and applications—a decade review from 1995 to 2004. Expert Syst. Appl. 28 (1): 93–103.
- Macedo, N. and Brigham, C.J. (2014). From beverages to biofuels: the journeys of ethanol‐producing microorganisms. Int. J. Biotechnol. Wellness Ind. 3 (3): 79–87.
- Mariano, M., El Kissi, N., and Dufresne, A. (2014). Cellulose nanocrystals and related nanocomposites: review of some properties and challenges. J. Polym. Sci., Part B: Polym. Phys. 52 (12): 791–806.
- Mattos, B.D., Lourençon, T.V., Gatto, D.A. et al. (2016). Chemical characterization of wood and extractives of fast‐growing Schizolobium parahyba and Pinus taeda. Wood Mater. Sci. Eng. 11 (4): 209–216.
- M'hamdi, A.I., Kandri, N.I., Zerouale, A. et al. (2017). Life cycle assessment of paper production from treated wood. Energy Procedia 128: 461–468.
- Miranda, I., Sousa, V., Ferreira, J., and Pereira, H. (2017). Chemical characterization and extractives composition of heartwood and sapwood from Quercus faginea. PLoS One 12 (6): e0179268.
- Moniruzzaman, M. and Goto, M. (2018). Ionic liquid pretreatment of lignocellulosic biomass for enhanced enzymatic delignification. Adv. Biochem. Eng./Biotech. 168.
- Mosier, N., Wyman, C., Dale, B. et al. (2005). Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 96 (6): 673–686.
- Moure, A., Gullón, P., Domínguez, H., and Parajó, J.C. (2006). Advances in the manufacture, purification and applications of xylo‐oligosaccharides as food additives and nutraceuticals. Process Biochem. 41 (9): 1913–1923.
- Mussatto, S.I. (2016). Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery. Elsevier.
- Nair, S.G., Shah, J.V., Shah, P.A. et al. (2015). Extractive spectrophotometric determination of five selected drugs by ion‐pair complex formation with bromothymol blue in pure form and pharmaceutical preparations. Cogent Chem. 1 (1): 1075852.
- Nguyen, Q., Bowyer, J., Howe, J., et al. (2017). Global Production of Second Generation Biofuels: Trends and Influences. Minneapolis: Dovetail Partners, Inc. https://dovetailinc.org/upload/tmp/1579558792.pdf (accessed 8 April 2020).
- Noparat, P., Prasertsan, P., Sompong, O., and Pan, X. (2017). Sulfite pretreatment to overcome recalcitrance of lignocellulose for enzymatic hydrolysis of oil palm trunk. Energy Procedia 138: 1122–1127.
- Nourbakhsh, A. and Ashori, A. (2010). Wood plastic composites from agro‐waste materials: analysis of mechanical properties. Bioresour. Technol. 101 (7): 2525–2528.
- Obama, P., Ricochon, G., Muniglia, L., and Brosse, N. (2012). Combination of enzymatic hydrolysis and ethanol organosolv pretreatments: effect on lignin structures, delignification yields and cellulose‐to‐glucose conversion. Bioresour. Technol. 112: 156–163.
- Osong, S.H., Norgren, S., and Engstrand, P. (2016). Processing of wood‐based microfibrillated cellulose and nanofibrillated cellulose, and applications relating to papermaking: a review. Cellulose 23 (1): 93–123.
- Özparpucu, M., Rüggeberg, M., Gierlinger, N. et al. (2017). Unravelling the impact of lignin on cell wall mechanics: a comprehensive study on young poplar trees downregulated for cinnamyl alcohol dehydrogenase (CAD). Plant J. 91 (3): 480–490.
- Papadopoulou, E.L., Pignatelli, F., Marras, S. et al. (2016). Nylon 6, 6/graphene nanoplatelet composite films obtained from a new solvent. RSC Adv. 6 (8): 6823–6831.
- Park, J.‐Y., Shiroma, R., Al‐Haq, M.I. et al. (2010). A novel lime pretreatment for subsequent bioethanol production from rice straw–calcium capturing by carbonation (CaCCO) process. Bioresour. Technol. 101 (17): 6805–6811.
- Park, N., Kim, H.‐Y., Koo, B.‐W. et al. (2010). Organosolv pretreatment with various catalysts for enhancing enzymatic hydrolysis of pitch pine (Pinus rigida). Bioresour. Technol. 101 (18): 7046–7053.
- Park, Y.C., Kim, T.H., and Kim, J.S. (2017). Effect of organosolv pretreatment on mechanically pretreated biomass by use of concentrated ethanol as the solvent. Biotechnol. Bioprocess Eng. 22 (4): 431–439.
- Perlack, R.D. (2005). Biomass as Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion‐Ton Annual Supply. Oak Ridge National Laboratory.
- Pinkert, A., Goeke, D.F., Marsh, K.N., and Pang, S. (2011). Extracting wood lignin without dissolving or degrading cellulose: investigations on the use of food additive‐derived ionic liquids. Green Chem. 13 (11): 3124–3136.
- Pizzi, A. (1983). Tannin‐based wood adhesives,, Chapter 4. In: Wood Adhesives Chemistry and Technology (ed. A. Pizzi), 177–246. New York: Marcel Dekkar.
- Pizzi, A., Valenzuela, J., and Westermeyer, C. (1993). Non‐emulsifiable, water‐based, mixed diisocyanate adhesive systems for exterior plywood. Part II. Theory application and industrial results. Holzforschung Wood Res. Technol. 47 (1): 68–71.
- Popa, V.I. (2015). Wood bark as valuable raw material for compounds with biological activity. Celuloză şi Hârtie 64: 5–17.
- Popovska, V.J., Iliev, B., and Spiroski, I. (2016). Characteristics of medium density fiberboards for furniture production and interior application. South East Eur. J. Archit. Des. 2016: 1–5.
- Ramluckan, K., Moodley, K.G., and Bux, F. (2014). An evaluation of the efficacy of using selected solvents for the extraction of lipids from algal biomass by the soxhlet extraction method. Fuel 116: 103–108.
- Reddy, N. and Yang, Y. (2005). Biofibers from agricultural byproducts for industrial applications. Trends Biotechnol. 23 (1): 22–27.
- Rodrıguez‐Chong, A., Ramı&c.acute;rez, J.A., Garrote, G., and Vázquez, M. (2004). Hydrolysis of sugar cane bagasse using nitric acid: a kinetic assessment. J. Food Eng. 61 (2): 143–152.
- Safin, R., Khasanshin, R.R., Shaikhutdinova, A.R., Ziatdinov, R.R. The technology for creating of decorative plywood with low formaldehyde emission. In IOP Conference Series: Materials Science and Engineering. 2015. IOP Publishing.
- Sanchez, C., Belleville, P., Popall, M., and Nicole, L. (2011). Applications of advanced hybrid organic–inorganic nanomaterials: from laboratory to market. Chem. Soc. Rev. 40 (2): 696–753.
- Schwanninger, M. and Hinterstoisser, B. (2002). Comparison of the classical wood extraction method using a Soxhlet apparatus with an advanced extraction method. Eur. J. Wood Wood Prod. 60 (5): 343–346.
- Siró, I. and Plackett, D. (2010). Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose 17 (3): 459–494.
- Sjostrom, E. (2013). Wood Chemistry: Fundamentals and Applications. Elsevier.
- Sluiter, A., Hames, B., Ruiz, R. et al. (2008). Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure. Golden, CO: National Renewable Energy Laboratory.
- Somerville, C. (2006). The billion‐ton biofuels vision. Science 312 (5778): 1277.
- Sun, Y. and Cheng, J. (2002). Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour. Technol. 83 (1): 1–11.
- Taherzadeh, M.J. and Karimi, K. (2008). Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int. J. Mol. Sci. 9 (9): 1621–1651.
- Tanaka, S., Miyazaki, H., Shiozaki, A. et al. (2017). Cytosolic Cl− affects the anticancer activity of paclitaxel in the gastric cancer cell line, MKN28 cell. Cell. Physiol. Biochem. 42 (1): 68–80.
- Taylor, A.M., Gartner, B.L., and Morrell, J.J. (2007). Heartwood formation and natural durability—a review. Wood Fiber Sci. 34 (4): 587–611.
- Teramoto, Y., Lee, S.‐H., and Endo, T. (2009). Cost reduction and feedstock diversity for sulfuric acid‐free ethanol cooking of lignocellulosic biomass as a pretreatment to enzymatic saccharification. Bioresour. Technol. 100 (20): 4783–4789.
- Terán Hilares, R., Swerts, M.P., Ahmed, M.A. et al. (2017). Organosolv pretreatment of sugar cane bagasse for bioethanol production. Ind. Eng. Chem. Res. 56 (14): 3833–3838.
- Tian, D., Hu, J., Bao, J. et al. (2017). Lignin valorisation: lignin nanoparticles as high‐value bio‐additive for multifunctional nanocomposites. Biotechnol. Biofuels 10 (1): 192.
- Wang, Y., Abdel‐Rahman, M.A., Tashiro, Y. et al. (2014). l‐(+)‐lactic acid production by co‐fermentation of cellobiose and xylose without carbon catabolite repression using Enterococcus mundtii QU 25. RSC Adv. 4 (42): 22013–22021.
- Wang, Y., Tashiro, Y., and Sonomoto, K. (2015). Fermentative production of lactic acid from renewable materials: recent achievements, prospects, and limits. J. Biosci. Bioeng. 119 (1): 10–18.
- Wang, S., Zhao, W., Lee, T.S. et al. (2018). Dimethyl sulfoxide assisted ionic liquid pretreatment of switchgrass for isoprenol production. ACS Sustainable Chem. Eng. 6 (3): 4354–4361.
- Xiang, Z., Gao, W., Chen, L. et al. (2016). A comparison of cellulose nanofibrils produced from Cladophora glomerata algae and bleached eucalyptus pulp. Cellulose 23 (1): 493–503.
- Xu, L. and Tschirner, U.W. (2011). Peracetic acid pretreatment of alfalfa stem and aspen biomass. BioResources 7 (1): 203–216.
- Xu, X., Liu, F., Jiang, L. et al. (2013). Cellulose nanocrystals vs. cellulose nanofibrils: a comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl. Mater. Interfaces 5 (8): 2999–3009.
- Yumrutaş, R., Alma, M.H., Özcan, H., and Kaşka, Ö. (2008). Investigation of purified sulfate turpentine on engine performance and exhaust emission. Fuel 87 (2): 252–259.
- Zhang, P., Hu, H., and Shi, S. (2006). Application of hemicellulose. Tianjin Pap. Mak. 2: 16–18.
- Zhao, J. (2015). Development of China's biofuel industry and policy making in comparison with international practices. Sci. Bull. 60 (11): 1049–1054.
- Zhao, X.B., Wang, L., and Liu, D.H. (2008). Peracetic acid pretreatment of sugarcane bagasse for enzymatic hydrolysis: a continued work. J. Chem. Technol. Biotechnol. 83 (6): 950–956.
- Zhao, H., Jones, C.L., Baker, G.A. et al. (2009). Regenerating cellulose from ionic liquids for an accelerated enzymatic hydrolysis. J. Biotechnol. 139 (1): 47–54.
- Zhao, X., Zhang, L., and Liu, D. (2010). Pretreatment of Siam weed stem by several chemical methods for increasing the enzymatic digestibility. Biotechnol. J. 5 (5): 493–504.
- Zhao, X., Li, S., Wu, R. et al. (2017). Organosolv fractionating pre‐treatment of lignocellulosic biomass for efficient enzymatic saccharification: chemistry, kinetics, and substrate structures. Biofuels, Bioprod. Biorefin. 11 (3): 567–590.
- Zheng, J., Tashiro, Y., Wang, Q., and Sonomoto, K. (2015). Recent advances to improve fermentative butanol production: genetic engineering and fermentation technology. J. Biosci. Bioeng. 119 (1): 1–9.
- Zhu, J. and Pan, X. (2010). Woody biomass pretreatment for cellulosic ethanol production: technology and energy consumption evaluation. Bioresour. Technol. 101 (13): 4992–5002.
- Zhu, J. and Zhuang, X. (2012). Conceptual net energy output for biofuel production from lignocellulosic biomass through biorefining. Prog. Energy Combust. Sci. 38 (4): 583–598.
- Zhu, J., Zhang, X., and Pan, X. (2011). Sustainable Production of Fuels, Chemicals, and Fibers from Forest Biomass. American Chemical Society.
- Zhu, X., Tong, S., Li, X. et al. (2017). Conversion of biomass into high‐quality bio‐oils by degradative solvent extraction combined with subsequent pyrolysis. Energy Fuels 31 (4): 3987–3994.
