Use of Vertically Aligned Carbon Nanotubes for Electrochemical Double-Layer Capacitors |
CONTENTS
35.2 Capacitive Behavior of VACNTs
35.5 Applications and Concluding Remarks
The demand for, and consumption of, global energy is continually increasing across a world whose population will grow to nearly 9 billion people in the next couple of years. It is poised to continue its steady increase in the next several decades. The climate change and the decreasing availability of fossil fuels have hastened the development of satisfying sustainable and renewable energy technologies. Since electrical energy storage systems such as batteries and capacitors are considered as the most critical link between the ever-increasing renewable energy supply and demand, a lot of work has been done to improve their efficiency and performance. Further, the advancement of micro/nano-electro-mechanical devices for telecommunication and biomedical applications initiates the demand for reliable lightweight electrical energy storage systems with small form factors. To fulfill all these requirements, the development of new materials with an exceptional specific surface area and excellent electrochemical properties has become the focus of much research effort.
Electrical energy can be stored either chemically, for example, batteries, or physically, for example, capacitors. Chemically, the electrical energy is converted into chemical energy via Faradaic reduction and oxidation (redox) reactions. Although the energy conversion from electrical to chemical and vice versa is thermodynamically reversible in principle, in practice, such conversion often involves some degree of irreversibility due to the electrode–electrolyte interphase changes during the charging and discharging processes [1]. Therefore, the charge/discharge rates of batteries are very low and their lifecycle is limited to only several thousand cycles. However, depending on the types of batteries, they can have a very high gravimetric energy density of up to 200 Wh/kg [2].
In contrast to batteries, capacitors store electrical energy physically via non-Faradaic electrostatic process. Owing to the absence of phase changes due to redox reactions, the charging and discharging processes are highly reversible. Therefore, capacitors in general have an exceptional cyclability, which leads to a very stable behavior over a very long lifecycle and very high charge/discharge rates. However, the gravimetric energy density of capacitors is typically very low, because it is limited by the accessible surface area of the electrodes.
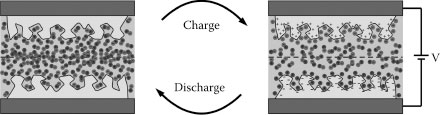
Great efforts have been made in the last few decades to combine the high energy-storage capability of conventional batteries and the high-power-delivery capability of conventional solid-state capacitors, resulting in the invention of electrochemical double-layer capacitors (EDLCs). EDLCs are electrical energy storage devices that utilize the highly reversible electrostatic accumulation of ions of the electrolytes on the surface of the active electrode materials. When an EDLC is charged, cations accumulate on the surface of the negatively polarized electrode, creating a capacitor-like electrical double-layer separation. Similarly, an electric double layer is also formed on the surface of the positively polarized electrode due to the accumulation of anions. When an EDLC is discharged, cations and anions of the electrolytes can be transported back from the surface of the electrodes in a very fast response time (Figure 35.1). Therefore, EDLCs are capable of providing short high-energy bursts that neither batteries nor solid-state capacitors can provide efficiently, with a remarkably long lifecycle [1,3]. Such capability has been used to provide high-power pulses for a few seconds, for a wide range of applications such as electric transportation technology, emergency backup power, consumer electronics, such as laptops or cell phones, medical electronics, and military devices, including communication devices, spacecraft probes, and missile systems [4,5].
The gravimetric-specific double-layer capacitance (CG) of an EDLC is dictated by the dielectric constant of the electrolyte (εe) and the accessible surface area of the electrodes (A) according to the following relation:
(35.1) |
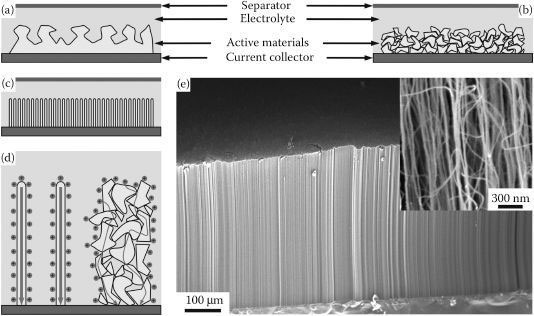
where ε0 is the vacuum dielectric constant, ddl is the electrical double-layer separation thickness, and m is the mass of active electrode materials. Obviously, active electrode materials with a highspecific surface area are the key component to achieve a high-specific capacitance. To date, activated carbons (ACs) have been the most widely used active electrode materials in commercially available EDLCs (Figure 35.2a and b). ACs are known to have a very high-specific surface area, up to 2000 m2/g [3,6], due to their tremendous network of micropores (pore size: <2 nm). However, the electrolyte accessibility of the micropores dominated network is considerably poor such that an electric double layer cannot be formed on most of the micropores. Thus, the ACs-based EDLCs usually exhibit unsatisfactory capacitance, which is about 10–20% of the theoretical values [2].
Compared to ACs, carbon nanotubes (CNTs) have a relatively lower specific surface area since their porous network is dominated by mesopores (pore size: 2–50 nm) and macropores (pore size: >50 nm). However, these mesopores and macropores are large enough to be accessed freely by ions of the electrolyte such that their intrinsic capacitance is higher than that of ACs (Figure 35.2c). CNTs are basically 1D graphitic structure made of rolled-up graphene sheets. They possess an exceptionally high surface-to-volume ratio, that is, an extremely high surface area available for chemisorptions or physisorptions in a small form factor, with an aspect ratio of up to 320 million. Owing to their sp2 carbon hybridization, the electronic transport along their main axis is almost perfectly ballistic, which means that the electrons flow directly without being scattered (Figure 35.2d). Therefore, the electrical conductivity of CNTs is truly exceptional and much superior to the currently available electronic materials [7]. CNTs are also known to have an excellent electrochemical stability, as well as exceptional thermal and mechanical properties.
FIGURE 35.1 Schematic of an EDLC in charged and discharged states. In an EDLC, charge is stored electrostatically in the form of reversible ion adsorption on the surface of the active materials. When the EDLC is charged, cations accumulate on the surface of the negatively polarized electrode creating a capacitor-like electrical double-layer separation. Similarly, an electric double layer is also formed on the surface of the positively polarized electrode due to the accumulation of anions.
FIGURE 35.2 Schematic construction of an EC, which typically comprises of active electrode materials attached to current collectors, electrolytes, and a separator. Compared to bulk graphitic carbons (a), randomly oriented microporous ACs (b) and VACNTs (c) are more preferably used as active electrode materials because of their high specific surface area and optimal pore size distribution. Between these two high specific surface area materials, CNTs have a higher electrical conductivity which may lead to a smaller IR drop (d). Lowmagnification (e) and high-magnification (inset) electron microscopy images of a vertically aligned carbon nanotube array on a current collector.
Among diverse types of CNTs, vertically aligned CNTs (VACNTs) oriented perpendicularly to the current collectors are considered to be well suited to be used as active electrode materials in EDLCs (Figure 35.2e). Since the physical properties of VACNTs, such as the packing density, number of walls, and length, can be varied by changing the growth conditions, the porosity of VACNTs can be tuned such that their porous network is dominated by mesopores while maximizing their specific surface area. In addition, a low contact resistance between the VACNTs and the current collector can be maintained such that the overall IR drop can be lowered to achieve an even higher power density than that of the ACs-based EDLCs.
VACNTs are normally grown using thermal chemical vapor deposition (CVD) on silicon substrates with carbon-containing gas and hydrogen as the precursor gases at an elevated temperature. Typically, the substrates are precoated with a thin-layer transition metal catalyst of iron (Fe), nickel (Ni), or cobalt (Co), along with a thin supporting layer of aluminum oxide (Al2O3) serving as a buffer layer between the catalyst and the substrate. Methane (CH4), acetylene (C2H2), or ethylene (C2H4) is usually used as the carbon containing a precursor gas. Alternatively, a floating catalyst method may also be used for growing VACNTs. Here, the substrate is exposed to a mixture of catalyst vapor and carbon-containing precursor gas, which is typically a mixture of ferrocene (Fe(C5H5)2) and xylene (C8H10) at an elevated temperature. These VACNTs are then transferred onto metal current collectors using conductive epoxies or low melting temperature metal alloys [8,9]. It is also possible to grow VACNTs directly on conducting metal substrates, such as Ni, tungsten (W), and aluminum (Al) foil. However, the fabrication process for such direct growth typically involves the plasma-enhanced CVD (PECVD) or low-pressure CVD (LPCVD) method [10,11].
According to Equation 35.1, the dielectric constant of the electrolyte is another key component to achieve the high-specific capacitance of EDLCs. Since it is assumed that all ions are in the solvated state, the dielectric constant of the electrolyte is dominated by the dielectric constant of the solvent. Typically, protic solvents such as water, hydrogen cyanide (HCN), and formic acid (FA) have a very high dielectric constant due to the existence of strongly structured hydrogen bonds. On the other hand, aprotic solvents such as propylene carbonate (PC), acetonitrile (ACN), and dimethyl formamide (DMF) typically have a lower dielectric constant than that of the protic solvents, although it is still higher than that of the unstructured solvents due to the presence of strong dipole–dipole interactions. Water, for instance, has a dielectric constant of 80°C at 25°C, while PC has a dielectric constant of 64.
From a capacitance point of view, the use of aqueous alkaline or acidic electrolytes usually leads to a higher specific capacitance than that of organic electrolytes. However, at a high operating potential, discharge of H2 from the solvent is very likely to happen. Thus, to prevent decomposition due to the electrolysis of water (E° = 1.23 V), the operating potential for aqueous electrolytes is limited to 1 V [2,3,12]. From an energy density and power density perspective, the use of nonaqueous solvents is more favored due to the lack of electrochemically active H atoms such that a higher operating potential of 2.5–3.5 V is achievable. The gravimetric energy density (EG) stored by EDLCs is given by the following relation:
(35.2) |
where V is the operating potential of EDLCs. The gravimetric power density, PG, of EDLCs is given by the following relation:
(35.3) |
where R is the equivalent series resistance (ESR) measured from the IR drop of EDLCs. Therefore, a threefold increase in operating potential achieved using nonaqueous electrolytes will result in an order of magnitude increase in stored energy for the same capacitance value. However, it is important to note that the resistivity of most nonaqueous electrolytes is substantially larger than that of aqueous electrolytes, which results in a higher ESR.
Among a few available nonaqueous electrolytes that can be reliably used to obtain a high value of specific capacitance as well as energy density and power density, a mixture of tetraalkylammoniumtetrafluoroborate (Et4NBF4) in PC is the most common choice. Although Et4NBF4/PC electrolytes are currently more expensive than their aqueous counterparts, they offer a better stability at a higher operating voltage and at a larger temperature range. Et4NBF4/PC electrolytes are also considered to be relatively safe due to their high boiling point and low toxicity.
35.2 CAPACITIVE BEHAVIOR OF VACNTS
Cyclic voltammetry is a very common and widely used electrochemical characterization technique, because of its ability and effectiveness to quickly observe an electrochemical behavior over a wide potential range. It is basically a cyclic measurement of potential-dependent current when the potential is varied linearly across the potential window at a constant scan rate. Since, in principle, capacitors are free from Faradaic redox reactions, the plot of current versus potential (called voltammogram) of ideal capacitors is perfectly rectangular. The gravimetric specific capacitance (CG) of an ideal capacitor is given by the following relation:
(35.4) |
where I is the recorded response current and dV/dt is the scan rate. Since, in reality, Faradaic processes are always involved and the ESR is always nonzero, the voltammogram shape of an EDLC is trapezoidal with nonconstant current during the linear potential sweep. Hence, the gravimetric specific capacitance of an EDLC is given by the following relation:
(35.5) |
where IC and ID are the recorded response current during the charge and discharge state, respectively, and V1 and V2 are the lower and upper limits of the potential window, respectively.
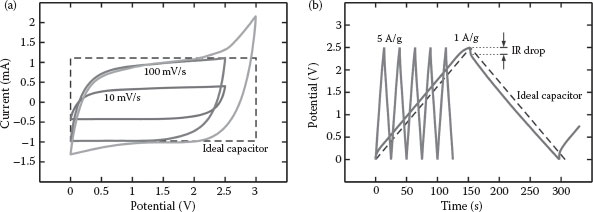
The cyclic voltammograms of VACNTs in 1 M Et4NBF4/PC in a two electrodes configuration at various scan rates show a smooth and symmetrical shape over a potential range of 0–2.5 V (Figure 35.3a). Such featureless voltammograms indicate the absence of Faradaic reaction during the charge and discharge cycles. A linear increase of response current during the linear potential sweep implies a nonzero finite ESR of the VACNT-based EDLCs. A relatively sharp transient response when the potential sweep changes sign indicates a rapid charge storage and delivery kinetic of the VACNTs. As per the calculations in Equation 35.5, the average gravimetric specific capacitance of VACNTs at a scan rate of 100 mV/s is about 31 F/g.
FIGURE 35.3 Cyclic voltammograms of VACNTs in 1 M Et4NBF4/PC electrolyte at scan rates of 10 and 100 mV/s in two electrodes configuration (a). The smooth, symmetrical shape of the voltammograms indicates the absence of a Faradaic behavior during the charge and discharge cycles. Ideally, the Et4NBF4/PC electrolyte has a voltage window of 3 V. However, a significant increase of current at a potential higher than 2.5 V indicates undesirable Faradaic losses. Galvanostatic charge/discharge curves of a vertically aligned carbon nanotubes array in a 1 M Et4NBF4/PC electrolyte at a gravimetric current density of 1 and 5 A/g in two electrodes configuration (b). Although the shape of the charge/discharge curves is almost perfectly triangular, the presence of IR drop caused by undesirable Faradaic losses is observable.
At a larger potential range of 0–3 V, the Faradaic behavior of VACNTs becomes more pronounced. At such a high potential, the response current increases exponentially, suggesting the occurrence of potential-dependent redox reactions where the relative quantity of the active redox species is dependent on the potential. Since, in principle, the Et4NBF4/PC electrolyte is stable at a potential of up to 3 V, the observed potential-dependent redox reactions may be caused by the discharge of electroactive species from the VACNTs.
The cyclic voltammograms of VACNTs also show a dependency of the response current on the scan rate. An increase of response current with an increasing scan rate implies that the electrochemical characteristic of VACNTs is dominated by their capacitive behavior [13,14]. Note that the relation between the specific capacitance of VACNTs and the scan rate follows a power law, where CG scales as dV/dt−1/2. This suggests a large variation in the diffusion path length of the ions in the electrolyte, which may be caused by the presence of a complex porous network with various pore sizes in the VACNTs.
The capacitive behavior of active electrode materials can also be probed using galvanostatic charge/discharge analysis at a constant current density, where the response potential is recorded and plotted against the time. For an ideal capacitor, the shape of the potential versus time curve is perfectly triangular with constant slopes showing a perfect capacitive behavior. Since, in reality, the ESR is always nonzero, the charge/discharge curves of an EDLC are typically curved. An IR drop can be obviously seen from the charge/discharge curves when the ESR is significantly high.
It is possible for a VACNT-based EDLC to have a high ESR. Factors that influence the ESR of an EDLC include the internal resistance of active electrode materials, contact resistance between active electrode materials and current collectors, wettability of active electrode materials by the electrolytes, and conductivity of the electrolyte. Since the internal resistance of VACNTs is typically very low, such a high ESR may be attributed to a poor wettability by the electrolyte and a poor contact to the current collector. The slightly curved and nontriangular shape of galvanostatic charge/discharge curves of VANCTs shows a nonzero ESR (Figure 35.3b). The ESR of VACNTs is measured to be ~10 Ω, which is considered reasonable, although it is relatively higher than that reported by previous studies [8,15,16].
Gravimetric specific capacitance can be calculated directly from the charge/discharge curves using Equation 35.4, where the scan rate, dV/dt, is measured from the slope of the discharge curves. At a current density of 5 A/g, the gravimetric specific capacitance of VACNTs is about 25 F/g. A much higher gravimetric specific capacitance of about 61 F/g can be reached at a lower current density of 1 A/g. Clearly, the gravimetric specific capacitance of VACNTs increases as the applied current density decreases, and the relation between these two parameters follows an inverse law, where CG scales as (I/m)−1. Again, this finding suggests the presence of a complex porous network with a large number of micropores.
Oxidation processes have been known for decades as one of the most credible methods to increase the specific surface area of carbon-based active electrode materials. When an oxidation process is performed on carbon-based active electrode materials, oxygen atoms attack the defect sites on the surface of the materials, resulting in the removal of impurities and modification of their porous structures. Oxygen is readily physisorbed by carbons as molecular O2 whenever they are exposed to air or any oxygen containing gas. In addition, chemisorptions of oxygen during the oxidation process result in the presence of oxygenated groups on the surface of the materials. ACs are typically made by selective oxidation of carbon-rich organic precursors [3], resulting in a substantial increase of the surface area and a better pore size distribution.
Carbon-based active electrode materials, including VACNTs, can be oxidized using several known methods such as the liquid-phase or gas-phase oxidation and plasma treatments. Liquid-phase oxidations are carried out by exposing these materials to strong acids and oxidants, including potassium chlorate (KClO3), potassium permanganate (KMnO4), sodium nitrate (NaNO3), phosphoric acid (H3PO4), nitric acid (HNO3), and sulfuric acid (H2SO4). It is important to note that in addition to the highly corrosive nature of these chemicals, the oxidation processes usually involve the generation of toxic and explosive gases [17]. Liquid-phase oxidations are also considered impractical for oxidizing VACNTs, because the capillary forces induced by these liquid chemicals are very likely to destroy the vertical alignment of the VACNTs. Hence, the gas-phase oxidations and plasma treatments are more favorably used for oxidizing VACNTs.
Gas-phase oxidations using oxygen (O2), ozone (O3) or carbon dioxide (CO2) gas, as well as water vapor (H2O) at an elevated temperature are considered a safer, more convenient and more practical way compared to their liquid-phase counterparts, especially in large industrial-scale production.
Compared to both the aforementioned methods, oxygen plasma treatments are able to oxidize carbon-based active electrode materials in a much faster way without sacrificing safety, convenience, and practicality. However, oxygen plasma treatments are more commonly used in smallscale production.
The presence of oxygenated groups on the surface of ACs or oxidized VACNTs should influence their electrical properties and electrochemical characteristics. Since pristine VACNTs are technically nonpolar, the presence of polar oxygenated groups on their surfaces should improve their wettability in highly polar electrolytes. On the other hand, the presence of such groups may also negatively affect the ESR, the self-discharge and cyclability characteristics as well as the ion adsorption behavior of the VACNTs.
Previously reported studies suggest that the specific capacitance of the VACNTs can be increased dramatically by the presence of oxygenated groups [18,19]. The presence of such oxygenated groups improves VACNTs’ wettability in highly polar electrolytes, especially in aqueous electrolytes, by increasing the number of contact sites with ions of the electrolytes [20]. This, in turn, improves the pore access and increases the wetted surface area of the VACNT–electrolyte interface, which ultimately amplifies the total area of the electric double layer. Therefore, the average gravimetric specific capacitance of VACNT-based EDLCs caused by the presence of the electric double layer increases as the average oxygen/carbon atomic ratio (O/C ratio) of the VACNTs increases. Note that the O/C ratio represents the surface concentration of oxygenated groups on the VACNTs and is measured by elemental analysis using energy-dispersive x-ray spectroscopy (EDX), x-ray photoelectron spectroscopy (XPS), or electron energy loss spectroscopy (EELS).
There is no doubt that the presence of oxygenated groups improves VACNTs’ wettability, which in turn contributes to the increase of their capacitance. Nevertheless, such a significant increase in capacitance may also be attributed to the occurrence of fast Faradaic redox reactions. Basically, oxygenated groups, whether acidic, basic, neutral, or amphoteric oxides, may act as pseudocapacitive materials attached to the surface of VACNTs. Like in batteries, Faradaic redox reactions allow a much higher capacitance due to interphase changes during the charging and discharging processes. Oxygenated groups, such as hydroxyl (C─OH) and carbonyl (C═O), are known to be electroactive and may involve in redox reactions of oxygen as follows [18,21, 22 and 23]:
(35.6) |
(35.7) |
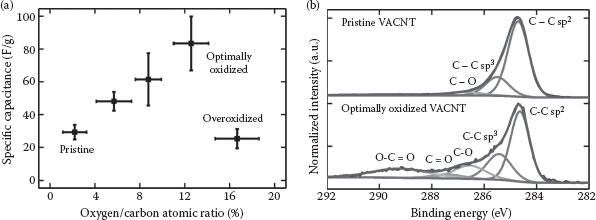
For pristine VACNTs, their capacitive behavior is dominated by the non-Faradaic electric double-layer behavior. However, a nonzero O/C ratio of the pristine VACNTs also means that Faradaic redox reactions are also involved during the charging and discharging cycles, although their contribution is limited. As the O/C ratio of the VACNTs increases due to a prolonged oxidation process, the contribution of Faradaic redox reactions relative to the non-Fardaic electric double layer to the overall capacitance is also expected to increase. Indeed, the specific capacitance of VACNTs increases almost linearly from about 29 F/g to about 83 F/g as the average oxygen/carbon atomic ratio (O/C ratio) increases from about 2% for the pristine VACNTs to about 13% for the well oxidized ones (Figure 35.4a). Note that these capacitances are measured at a current density of 5 A/g.
FIGURE 35.4 Specific capacitance of VACNT-based supercapacitors in 1 M Et4NBF4/PC as a function of their oxygen/carbon atomic ratio. Specific capacitance is obtained from galvanostatic charge/discharge analysis at a current density of 5 A/g. X-ray photoelectron spectroscopy spectra of the pristine and optimally oxidized VACNTs showing a significant increase in surface concentration of C─O, C═O, and O─C═O bonds (b).
XPS analysis before and after the oxidation process shows the evolution of oxygenated groups attached to the VACNTs (Figure 35.4b). Deconvolution of the C 1s XPS spectra of both pristine and oxidized VACNTs shows five distinct peaks associated with C─C sp2 (284.5 ± 0.1 eV, FWHM 0.9 eV), C─C sp3 (285.5 ± 0.1 eV, FWHM 1.3 eV), C─O (286.5 ± 0.1 eV, FWHM 1.4 eV), C═O (287.4 ± 0.2 eV, FWHM 1.4 eV), and O─C═O (289.1 ± 0.4 eV, FWHM 1.7 eV) [20,24,25]. For pristine VACNTs, the presence of a strong C─C sp2 peak and a mild C─C sp3 peak indicates a relatively high-quality graphitic structure with limited defects and disorders. In addition, a very weak C─O peak indicates the presence of hydroxyl or epoxide groups at the basal planes of CNTs. Note that a pristine VACNT is not free from the hydroxyl or epoxide groups, although their concentration is extremely low.
As expected, the surface concentration of the hydroxyl or epoxide groups increases as VACNTs undergo a prolonged oxidation process. An increase in the intensity of the C═O peak can also be observed, suggesting an increase in the surface concentration of carbonyl groups at the edge boundaries and defect sites of VACNTs. Similarly, an increase in the intensity of the O─C═O peak indicates an increase in the surface concentration of the ester or carboxyl groups at the edge boundaries and defect sites of VACNTs. Further, an increase in the intensity of the C─C sp3 peak indicates a considerable increase of defect density induced by oxygen uptake, producing a strong disruption to the π-bond network of the VACNTs.
VACNTs with an excessive surface concentration of the oxygenated groups due to over-oxidation are most likely to exhibit high rates of self-discharge [26]. During the charging process, dissociation of the −OH groups may occur, leading to the formation of −H or −O free radicals. These free radicals may generate molecular H2, O2, or H2O2, which are typically involved in self-discharge processes. Moreover, these oxygenated groups are also known to be active catalysts for the electrochemical decomposition of electrolytes [21,26], resulting in a high leakage current, a poor cyclability, and a very short lifecycle. Furthermore, over-oxidized VACNTs are expected to have a lower electrical conductivity than the pristine ones due to the disappearance of their π-bond network. All the above-mentioned factors contribute to a significant deterioration in capacitance for over-oxidized VACNTs, which is proven by a decrease of specific capacitance of VACNT-based EDLCs from more than 80 F/g to about 25 F/g as the O/C ratio by increases about 17% (Figure 35.4).
As mentioned earlier, capacitors, including EDLCs, in principle, have an excellent stability to be used for millions of cycles at a very high charge/discharge rate. Such capability arises from the absence of Faradaic redox reactions that eliminates the electrode–electrolyte interphase changes. Although, in reality, limited Faradaic redox reactions are involved, the pristine VACNT-based EDLCs are able to withstand more than 120,000 charge/discharge cycles at a high current density of 10 A/g without any significant degradation in capacitance. Note that there exists a transient condition such that they need to be cycled for several charge/discharge cycles before reaching their maximum capacitance.
The optimally oxidized VACNTs are able to withstand about the same number of charge/discharge cycles at a moderate current density of 10 A/g while only losing 10% of their original capacitance. On the other hand, the over-oxidized VACNTs are only capable of withstanding less than 25,000 charge/discharge cycles before losing more than 15% of their original capacitance. Such fast degradation in capacitance is actually expected from VACNTs that have been exposed to a prolonged oxidation process. These VACNTs have an excessive surface concentration of oxygenated groups involved in Faradaic redox reactions such that they exhibit high rates of self-discharge, high ESR, and poor cyclability. Nonetheless, their lifecycle is still much longer than that of typical batteries.
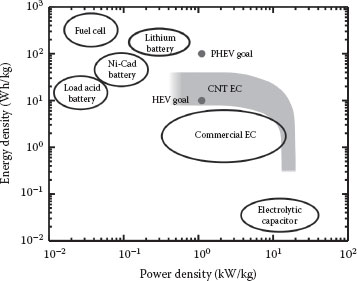
As mentioned earlier, the performance of VACNT-based EDLCs can be improved by an effective utilization of the oxidation process. Basically, when VACNTs have the right surface concentration of oxygenated groups, they have a relatively high gravimetric specific capacitance in nonaqueous electrolytes. Using Equations 35.2 and 35.3, such capacitance can be translated into gravimetric energy density and power density. Optimally oxidized VACNT-based EDLCs typically exhibit a gravimetric energy density of 16 Wh/kg at a gravimetric power density of 6 kW/kg, or a gravimetric energy density of 7 Wh/kg at a gravimetric power density of 12 kW/kg (Figure 35.5). These findings exceed the gravimetric energy density of currently available AC-based EDLCs [27,28], or even other CNT-based EDLCs [10,15,26,29,30].
The performance of VACNT-based EDLCs can be further improved by incorporating pseudocapacitive materials onto the CNTs. Pseudocapacitive materials, including metal oxides and conducting polymers, have been extensively studied in the past to increase the gravimetric specific capacitance of ACs. Ruthenium oxides (RuO2), cobalt oxides (Co3O4), manganese oxides (MnO2), and titanium dioxide (TiO2) are the most common metal oxides to be used along with VACNTs due to their unique oxidation states that are accessible at relatively low potential and simplicity in the fabrication process [3,22]. Conducting polymers such as polyaniline (PANI), polypyrrole (PPy), and polythiophene (PT) have been used in many electrochemical applications because of their compatibility with CNTs and inherent high specific capacitance [3,22]. However, owing to the presence of Faradaic redox reactions, both metal oxides and conductive polymers cannot be cycled fast enough and often suffer from lack of stability. Gravimetric energy densities of about 32 and 22 Wh/kg have been achieved in the past using the Ni(OH)2/CNTs composite and PANI/CNTs composite, respectively [31,32].
FIGURE 35.5 Ragone plot for CNT-based ECs compared to currently available energy storage systems and performance targets of hybrid electric vehicles (HEV) and plug-in hybrid electric vehicles [1]. The energy density and power density of both CNT-based ECs are calculated based on the mass of active electrode materials only.
Improvement to the performance of VACNT-based EDLCs can also be achieved by incorporating other microporous carbons onto the CNTs and using ionic liquid electrolytes. A gravimetric energy density of about 50 Wh/kg at a power density of about 22 kW/kg has been achieved using AC-CNT composites in the ionic liquid electrolyte [6]. Ionic liquid electrolytes, such as the 1-ethyl-3-methylimidazoliumbis(trifluoromethylsulfonyl)imide (EMIM-Tf2N), are nonvolatile room-temperature solvent-free electrolytes with a large electrochemical window. A further improvement to the performance of VACNT-based EDLCs can be achieved by optimizing their physical properties, especially their packing density. A gravimetric energy density of about 70 Wh/kg at a power density of about 24 kW/kg has been achieved in the past using compacted VACNTs [16]. These compacted VACNTs have a packing density 10 times higher than that of the common as-grown VACNTs.
35.5 APPLICATIONS AND CONCLUDING REMARKS
Although the gravimetric energy density of VACNTs-based EDLCs is relatively higher than that of commercially available EDLCs, it is still much lower when compared with that of batteries, especially lithium-ion batteries [23,25]. Therefore, it is unlikely for EDLCs to substitute batteries as the main electrical energy storage devices. However, since EDLCs in general have a much better lifecycle and gravimetric power density, they can complement the operation of batteries in hybrid systems [4]. In such systems, an EDLC acts as a buffer to uptake and deliver high power on demand that certainly cannot be handled by batteries. Thus, the battery’s energy can be delivered at the capacitor’s rate [1].
These EDLC–battery hybrid systems are useful in many modern applications, particularly for small mobile or wireless devices such as mobile phones, wireless sensors, portable computers, and wearable drug delivery systems. These modern devices do not require a lot of energy to be delivered continuously but, instead, very short high-power low-energy bursts. Using batteries for repeated delivery of high-power bursts will quickly degrade the lifecycle of the batteries. Furthermore, most users do not want to wait for hours to fully charge these devices. Hence, hybridization with EDLCs will improve the charging rate of batteries as well as their lifecycle.
EDLC–battery hybrid systems can also be utilized in green transportation and renewable energy applications. Using these systems, as opposed to purely battery-based energy storage systems, the charging/discharging time of midsized electric cars may be cut down from several hours to less than an hour. In addition, faster acceleration, higher top speed, and better regenerative braking can all be accommodated by these hybrid systems. EDLC–battery hybrid systems will also allow an efficient production of renewable energy from the sun, wind, or ocean tides and currents, by stabilizing the generated power before being stored.
At the moment, the biggest limiting factor in producing VACNT-based EDLCs is their extremely high price. However, CNTs and VACNTs in particular are expected to be much cheaper in the near future due to a significant increase in supplies. In addition, since no corrosive liquids need to be used as electrolytes, cheap lightweight materials such as aluminum, polypropylene, and polyimide can be used for current collectors, separators, and cases, respectively. The small ecological footprint of VACNT-based EDLCs and their lack of toxic materials should also be highlighted. Finally, VACNT-based EDLCs are relatively safe since they do not have reactivity issues associated with lithium, such as leakages or even explosions.
This work was supported by The Office of Naval Research under Grant number N00014-11-1-0031 and The Fletcher-Jones Foundation under Grant number 9900600. The authors gratefully acknowledge support and infrastructure provided for this work by the Charyk Laboratory for Bioinspired Design, the Kavli Nanoscience Institute (KNI), and the Molecular Materials Research Center (MMRC) at the California Institute of Technology.
1. Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications (Kluwer Academics/Plenum Publisher, New York, 1999).
2. Liu, C., Yu, Z., Neff, D., Zhamu, A., and Jang, B.Z. Graphene-based supercapacitor with an ultrahigh energy density, Nano Letters, 2010, 10(12), 4863–4868.
3. Simon, P. and Gogotsi, Y. Materials for electrochemical capacitors, Nature Materials, 2008, 7(11), 845–854.
4. Miller, J.R. and Simon, P. Electrochemical capacitors for energy management, Science, 2008, 321(5889), 651–652.
5. Miller, J.R. and Burke, A.F. Electrochemical capacitors: Challenges and opportunities for real-world applications, Electrochemical Society Interface, 2008, 17, 53–57.
6. Lu, W., Hartman, R., Qu, L., and Dai, L. Nanocomposite electrodes for high-performance supercapacitors, The Journal of Physical Chemistry Letters, 2011, 2(6), 655–660.
7. Jorio, A., Dresselhaus, G., and Dresselhaus, M. Carbon nanotubes: Advanced topics in the synthesis, structure, properties and applications (Springer, Berlin, 2007).
8. Kumar, A. Contact transfer of aligned carbon nanotube arrays onto conducting substrates, Applied Physics Letters, 2006, 89(16), 163120.
9. Fu, Y., Qin, Y., Wang, T., Chen, S., and Liu, J. Ultrafast transfer of metal-enhanced carbon nanotubes at low temperature for large-scale electronics assembly, Advanced Materials, 2010, 22(44), 5039–5042.
10. Yoon, B-J., Jeong, S-H., Lee, K-H., Seok Kim, H., Gyung Park, C., and Hun Han, J. Electrical properties of electrical double layer capacitors with integrated carbon nanotube electrodes, Chemical Physics Letters, 2004, 388(1–3), 170–174.
11. Signorelli, R., Ku, D.C., Kassakian, J.G., and Schindall, J.E. Electrochemical double-layer capacitors using carbon nanotube electrode structures, Proceedings of the IEEE, 2009, 97(11), 1837–1847.
12. Stoller, M.D., Park, S., Zhu, Y., An, J., and Ruoff, R.S. Graphene-based ultracapacitors, Nano Letters, 2008, 8(10), 3498–3502.
13. Lufrano, F. and Staiti, P. Conductivity and capacitance properties of a supercapacitor based on Nafion electrolyte in a nonaqueous system, Electrochemical and Solid-State Letters, 2004, 7(11), A447–A450.
14. Chen, J.H., Li, W.Z., Wang, D.Z., Yang, S.X., Wen, J.G., and Ren, Z.F. Electrochemical characterization of carbon nanotubes as electrode in electrochemical double-layer capacitors, Carbon, 2002, 40(8), 1193–1197.
15. Niu, C. High power electrochemical capacitors based on carbon nanotube electrodes, Applied Physics Letters, 1997, 70(11), 1480.
16. Futaba, D., Hata, K., Yamada, T., Hiraoka, T., Hayamizu, Y., and Kakudate, Y. Shape-engineerable and highly densely packed single-walled carbon nanotubes and their application as super-capacitor electrodes, Nat. Mater., 2006, 5(12), 987–994.
17. Marcano, D.C., Kosynkin, D.V., Berlin, J.M., Sinitskii, A., Sun, Z., Slesarev, A., Alemany, L.B., Lu, W., and Tour, J.M. Improved synthesis of graphene oxide, ACS Nano, 2010, 4(8), 4806–4814.
18. Lee, S.W., Yabuuchi, N., Gallant, B.M., Chen, S., Kim, B-S., Hammond, P.T., and Shao-Horn, Y. High-power lithium batteries from functionalized carbon-nanotube electrodes, Nat. Nano, 2010, 5(7), 531–537.
19. Hirsch, A. Functionalization of single-walled carbon nanotubes, Angewandte Chem. Int. Ed., 2002, 41(11), 1853–1859.
20. Aria, A.I. and Gharib, M. Reversible tuning of the wettability of carbon nanotube arrays: The effect of ultraviolet/ozone and vacuum pyrolysis treatments, Langmuir, 2011, 27(14), 9005–9011.
21. Hsieh, C.-T. and Teng, H. Influence of oxygen treatment on electric double-layer capacitance of activated carbon fabrics, Carbon, 2002, 40(5), 667–674.
22. Pan, H., Li, J.Y., and Feng, Y. P. Carbon nanotubes for supercapacitor, Nanoscale Res. Lett., 2010, 5(3), 654–668.
23. Lee, S.W., Gallant, B.M., Lee, Y., Yoshida, N., Kim, D.Y., Yamada, Y., Noda, S., Yamada, A., and Shao-Horn, Y. Self-standing positive electrodes of oxidized few-walled carbon nanotubes for light-weight and high-power lithium batteries, Energy Environ. Sci., 2012, 5(1), 5437–5444.
24. Yang, D., Velamakanni, A., Bozoklu, G., Park, S., and Stoller, M. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy, Carbon, 2009, 47(1), 145–152.
25. Byon, H.R., Gallant, B.M., Lee, S.W., and Shao-Horn, Y. Role of oxygen functional groups in carbon nanotube/graphene freestanding electrodes for high performance lithium batteries, Adv. Funct. Mater., 2012 (published online).
26. Pandolfo, A. and Hollenkamp, A. Carbon properties and their role in supercapacitors, J. Power Sources, 2006, 157(1), 11–27.
27. Fernandez, J., Arulepp, M., Leis, J., Stoeckli, F., and Centeno, T. EDLC performance of carbide-derived carbons in aprotic and acidic electrolytes, Electrochim. Acta, 2008, 53(24), 7111–7116.
28. Simon, P. and Burke, A. Nanostructured carbons: Double-layer capacitance and more, Electrochem. Soc. Interface, 2008, 17(1), 38–43.
29. Du, C., Yeh, J., and Pan, N. High power density supercapacitors using locally aligned carbon nanotube electrodes, Nanotechnology, 2005, 16(4), 350.
30. Shah, R., Zhang, X., and Talapatra, S. Electrochemical double layer capacitor electrodes using aligned carbon nanotubes grown directly on metals, Nanotechnology, 2009, 20(39), 395202.
31. Nam, K-W., Kim, K-H., Lee, E-S., Yoon, W-S., Yang, X-Q., and Kim, K-B. Pseudocapacitive properties of electrochemically prepared nickel oxides on 3-dimensional carbon nanotube film substrates, J. Power Sources, 2008, 182(2), 642–652.
32. Mi, H., Zhang, X., An, S., Ye, X., and Yang, S. Microwave-assisted synthesis and electrochemical capacitance of polyaniline/multi-wall carbon nanotubes composite, Electrochem. Commun., 2007, 9(12), 2859–2862.