CONTENTS
51.3 Generic Design Approach and Potential Applications
51.3.1 Synthesis of ZnO Nanowires
51.3.2 Characterization and Surface Studies
51.3.3 Development of the ZnO Nanowire-Based p-Nitrophenol Sensor
One-dimensional (1D) and quasi-1D systems, such as nanowires, nanobelts, and nanotubes, have been the focus of intensive research due to their unique physical and chemical properties. These novel and interesting characteristics have been shown to stem primarily from their nanometer size resulting in quantum confinement of their electronic wavefunctions. Pertaining to nanometer dimensions, these quasisystems manifest additional surface-related properties, which have opened new frontiers of research in sensing [1, 2 and 3], energy [4], catalysis [5], photovoltaics [6], and bionanotechnology [7].
Recently, considerable research effort has been directed toward zinc oxide (ZnO) nanostructures, due to their superior electronic, optical, piezoelectric, and thermal properties, which have provided a new perspective in nanoelectronics, optoelectronics, and sensor development [8, 9 and 10]. ZnO is a wide bandgap (3.37 eV) semiconductor, which has been particularly interesting to the research community due to its large exciton binding energy (60 meV) that facilitates room-temperature lasing action based on the exciton recombination. Owing to such excellent optical properties, ZnO nanostructures have versatile applications in short-wavelength-based optoelectronic devices, such as light emitting diodes (LEDs), laser diodes, nanocatalysis, OLED-based displays, and optoelectronic chemical sensors in particular [11, 12, 13, 14, 15, 16, 17 and 18]. The aforementioned applications require ZnO nanostructures in different morphologies, such as nanowires, nanorods, and nanobelts, which must be subjected to postsynthesis procedures to achieve the desired functionality. High-aspect-ratio morphologies of ZnO are desirable due to their large surface-to-volume ratio, which facilitates enhanced surface interactions. Therefore, it is rational to expect surface-related effects to be crucial for any electronic or optoelectronic devices based on ZnO nanowires. The technological relevance of surface functionalized ZnO nanowires has already been demonstrated in sensing devices, dye-sensitized solar cells (DSSCs) for efficient light harvesting, and other hybrid photovoltaic devices [19, 20 and 21]. The polar nature of ZnO and the ability to maintain high-symmetry c-axis orientation during growth has also facilitated the growth of densely packaged nanowire arrays, which are promising candidates for nonlinear optical sensing devices. Furthermore, at the nanowire surface, there is a loss of symmetry, which is expected to increase the nonlinear optical response of these nanowires as compared to the bulk. The functionalization of these nanowires with additional organic or inorganic compounds should therefore allow for tailoring of the surface for its nonlinear signal, thereby enabling the design of efficient nanoscale optical sensors.
Although these quasi-1D ZnO nanowires cover a breadth of applications, their deployment in the fabrication of different types of chemical and biological sensors is pertinent to the present-day scenario. Novel sensors for detecting hazardous compounds need to be developed due to the rapidly evolving military, DHS (Department of Homeland Security), and NIH (National Institutes of Health) requirements. The potential of ZnO nanowires for chemical gas sensing, biological moiety detection, and environmental probes has already been demonstrated [22, 23, 24 and 25]. ZnO nanowires operating as a single nanowire probe [26] or in field effect transistor (FET) [27] mode have provided sensitive detection up to ppb ranges. The advantages of using ZnO nanowires as sensing elements are manifold. First, rapid progress made in synthesis methods for ZnO nanostructures and the ability to perform controlled synthesis make them a cost-effective choice for developing sensing platforms. Second, the ZnO nanowire surface is resistant to atmospheric oxidation due to the inherent oxide structure of the material, which provides robustness and high chemical stability. Third, the surface of the nanowire can be tailored for high selectivity toward specific bioanalytes through surface functionalization techniques. Finally, the nanowires in array morphology provide high permeability for analyte molecules, which decreases the detection time and enhances sensitivity [28,29]. Furthermore, the diameter of these nanostructures is comparable to the biomolecules being detected, which effectively makes them an excellent transducer for producing signals to interface with macroscopic instruments. Owing to the aforementioned properties and advantages, ZnO nanowires have emerged as excellent candidates for fabricating biosensor devices with a wide spectrum of applicability.
ZnO nanowires not only possess a large number of surface sites, owing to their nanoscale morphology, but they also provide a favorable microenvironment for retaining the bioactivity of the functionalized enzyme or antigens on the nanowire surface. The high isoelectric point (IEP) (~9.5) of these nanowires helps in immobilizing antigens with low IEP. Furthermore, the high electron mobility concomitant with the biocompatible nature of ZnO nanowires has led to a considerable thrust in the development of mediator-less implantable biosensors. Despite the potential of ZnO nanowires for biosensing applications, the literature on the subject is far from comprehensive. However, the research is catching up in the light of new challenges and development in existing biotechnology and therapeutics. Lee and coworkers demonstrated the use of the ZnO/Si surface acoustic wave (SAW) device for detecting prostate-specific antigen (PSA) antibody–antigen immunoreaction as a function of PSA concentration [30]. The immobilization of PSA on the SAW device leads to a shift in resonance frequency, which can be related to the amount of PSA detected. A detection limit of 2–10,000 ng/mL was reported. Cai and coworkers focused on the development of a pH nanosensor based on ZnO [31]. The large surface-to-volume ratio was observed to enhance the diffusion time of the analyte, concomitantly increasing the response time of the sensor. In another recent study by Ahmad and coworkers, the pH sensitivity and biochemical sensing capability of ZnO nanostructures were reported. Pt-ZnO nanospheres on a glass carbon electrode were utilized for estimating the amount of cholesterol in a solution by Ahmad and coworkers [32]. A sensitivity of 1886.4 mA M−1 cm−2 was reported. Glucose is yet another compound whose concentration in blood plays a crucial role in diseases, such as diabetes and endocrine disorder. There is compelling empirical evidence that suggests that ZnO nanowires can be appropriately tailored to provide sensitive and selective detection of glucose in blood serum. In a recent study, ZnO 1D nanostructures have been investigated as a suitable candidate for glucose sensing with a limit of detection of 1 µM [33]. Good anti-interference ability and prolonged shelf life were also observed. Gu and coworkers developed ZnO nanowire-based H2O2 sensors by alternate immobilization of poly(sodium 4-styrenesulfonate) (PSS) and horseradish peroxidase on a nanowire surface [34]. A wide linear range and a low detection limit up to 1.9 µM were deduced. In another recent study, Ibupoto and coworkers utilized ZnO nanorods immobilized with penicillinase enzyme through N-5-azido-2-nitrobenzoyloxysuccinimide (ANB-NOS) cross-linking molecules, and estimated the detection limit of penicillin at 100 µM [35].
The literature surveyed above only serves to corroborate the argument that much more exhaustive research needs to be conducted to obtain complete benefits of ZnO nanowire-based biosensors. This could be ascertained through a detailed investigation of the surface chemistry, mechanistic studies, and biochemistry of the receptors to be immobilized on the nanowire surface. In the forthcoming sections, we would outline a generic design approach for developing a ZnO nanowire-based biosensor. Through the oleic acid–ZnO nanowire model system, steps for successful synthesis, characterization, and surface functionalization will be outlined. Subsequently, the discussion would be culminated in a prospective application for developing a selective and sensitive p-nitrophenol biosensor.
51.3 GENERIC DESIGN APPROACH AND POTENTIAL APPLICATIONS
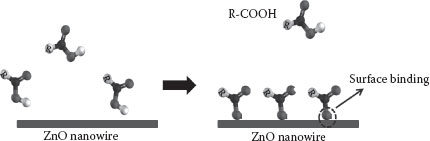
As outlined in the previous sections, to effectively engineer the surface of ZnO nanowires for biological sensing, an in-depth understanding of the surface phenomenon and interactions is crucial. Therefore, we have reported the immobilization of a common organic compound, oleic acid, on a ZnO nanowire surface. We synthesized ZnO nanowires in a customized chemical vapor deposition (CVD) furnace and established the morphology and composition of the synthesized nanowires through scanning electron microscopy (SEM), transmission electron microscopy (TEM), and x-ray diffraction (XRD). Thereafter, the ZnO nanowires were modified with oleic acid and the nature of bonding and surface orientation of oleic acid molecules on nanowire surface was determined with surface-sensitive characterization techniques of Raman and Fourier transform-infrared (FT-IR) spectroscopies. Photoluminescence (PL) measurements on modified and unmodified ZnO nanowires showed changes in peak heights based on which a prospective mechanism of sensing oleic acid on a ZnO nanowire surface is proposed. Oleic acid is a surfactant that has been shown to attract different moieties present in biologically important molecules [36]. It is believed that modifying the ZnO nanowire surface with oleic acid can improve the detection limits of these moieties to the molecular level. Hence, we chose this model system to demonstrate the capability of covalent functionalization of the ZnO nanowire surface with an organic moiety, as pertinent to previous biosensor research. The most crucial factor in designing a sensitive and selective ZnO-based biosensor is the appropriate choice of an antigen/receptor, which is capable of altering either optical or electrical characteristics of the ZnO nanowire postgrafting. This inorganic–organic heterostructure must be capable of producing a measurable response to an analyte stimulus to ascertain sensitive detection. Now, let us focus on the ZnO nanowire–oleic acid model system to highlight significant steps in a ZnO nanowire-based biosensor fabrication. The schematic of the idea is presented in Figure 51.1.
FIGURE 51.1 Schematic representation of nanowire-based detection. (A. Gupta et al., Zinc oxide nanowires for biosensing applications, Proc. 11th IEEE International Conference on Nanotechnology, August 15–18, Portland, Oregon, pp. 1615–1618. © (2011) IEEE. With permission.)
51.3.1 SYNTHESIS OF ZNO NANOWIRES
The ZnO nanowires were synthesized on the ZnO (0001) substrate using the vapor liquid solid (VLS) mechanism widely published in the literature [37,38]. In brief, ZnO substrates were coated with gold (4 nm) by sputtering (Shirley Sputtering System), which serves as a template for the growth of ZnO nanowires during the VLS process. Precursor powders of ZnO (99.9%, from J. T. Baker) and graphite (99%, from Alfa Aesar) were homogenously mixed in a 1:1 ratio and introduced in a customized CVD furnace at 950°C. Mixed gas (2% O2 + Ar) was utilized as a carrier medium.Using an optimal flow rate and a growth time of 30 min, dense nanowire growth was observed.
51.3.2 CHARACTERIZATION AND SURFACE STUDIES
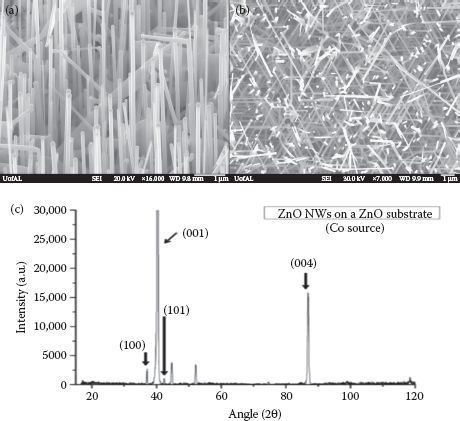
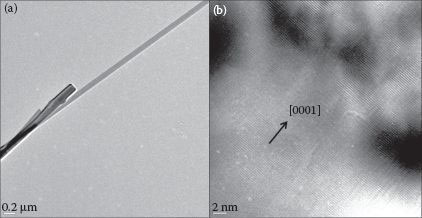
The morphology and crystal structure of the synthesized nanowires were determined by FE-SEM (JEOL 7000), x-ray diffraction (Bruker-AXS), and TEM (Technai). Figure 51.2a and b shows the SEM images of ZnO nanowires on the ZnO substrate along with the XRD spectrum of the nanowires, as synthesized by the CVD process. It can be observed that the nanowires are vertically aligned with respect to the substrate and are laterally branched close to the tip. While the vertical alignment is due to the absence of lattice mismatch between the nanowires and the substrate, hierarchical growth could be due to the high supersaturation of Zn vapors in the furnace as suggested by Zhang and coworkers [39]. Figure 51.2c corresponds to the XRD spectrum of the as-synthesized ZnO nanowires, which indicates preferential growth along the [0001] direction as the (001) peak is sufficiently high in intensity than other peaks, consistent with the documented literature. In addition, the hexagonal wurtzite structure of the synthesized nanowires can be confirmed by the superimposition of standard spectra (ICDD PDF# 01-089-0510) over the obtained spectrum. This observation can be further supported by the TEM analysis of the nanowires as presented in Figure 51.3.
FIGURE 51.2 (a) Tilted and (b) top view of ZnO nanowires synthesized on a ZnO substrate. (c) XRD spectrum of nanowires showing preferential c-axis growth. (A. Gupta et al., Zinc oxide nano-wires for biosensing applications, Proc. 11th IEEE International Conference on Nanotechnology, August 15–18, Portland, Oregon, pp. 1615–1618. © (2011) IEEE. With permission.)
FIGURE 51.3 (a) TEM image of the as-synthesized ZnO NW; (b) HR-TEM image showing the [0001] growth direction of an NW. (A. Gupta et al., Zinc oxide nano-wires for biosensing applications, Proc. 11th IEEE International Conference on Nanotechnology, August 15–18, Portland, Oregon, pp. 1615–1618. © (2011) IEEE. With permission.)
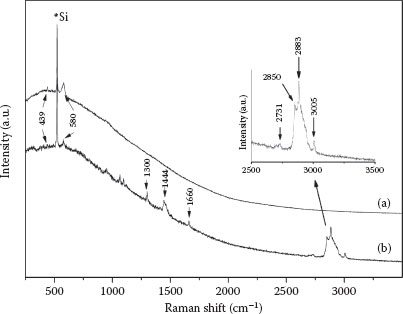
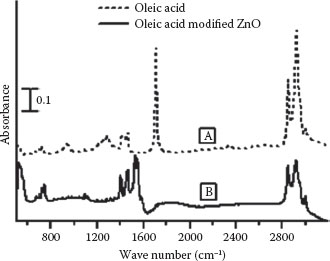
After characterization, the ZnO nanowires as-synthesized on the ZnO substrate were dipped in oleic acid solution (1% v/v in hexane) for 15 min to functionalize the ZnO nanowire surface. Thereafter, the specimens were copiously rinsed in pure hexane to remove residual oleic acid. It is essential to remove unadsorbed oleic acid to prevent erroneous signal generation during subsequent characterization. The nature of bonding and orientation of oleic acid on a nanowire surface were determined by comparing unmodified and modified ZnO nanowires through Raman (JobinYvon, HR800 UV) and Fourier transform–infrared spectroscopy. Figure 51.4a shows the Raman spectrum of the as-synthesized ZnO nanowires. The characteristic ZnO peaks were observed at 439 (E2 mode) and 580 cm−1 (A1(LO) mode), which serve as a reference to further modification procedures [40]. Figure 51.4b is the Raman spectrum of the oleic acid-modified ZnO nanowires. It can be seen that the spectrum has peaks corresponding to oleic acid in addition to the characteristic ZnO peaks. This observation is suggestive of possible modification of the nanowire surface by oleic acid, but the nature of carboxylate bonding is ambiguous. Accordingly, complementary evidence was obtained through FT-IR. Figure 51.5a is the FT-IR spectrum of 1% oleic acid (v/v) in hexane. Peaks at 937 and 1464 cm−1 correspond to the out-of-plane and in-plane OH deformations, respectively. The broad peak at 1284 cm−1 can be attributed to the C–O stretch, while the intense peak at 1710 cm−1 is C∙= O. Small peaks at 2570 and 2673 cm−1 indicate the oleic acid dimer formation. The group of peaks from 2825 to 2950 cm−1 is from CH2 and CH3 asymmetric and symmetric vibration stretches and are typical of long-chain organic compounds. The weak peak at 3005 cm−1 is the C–H stretch of (cis)alkene [41]. Figure 51.5b shows the modified ZnO nanowire spectrum. It can be observed that the changes in the spectrum are primarily due to the features associated with the carboxylic acid group, leading to bound carboxylate. The absence of peaks at 2570 and 2673 cm−1 rules out any possibility of oleic acid dimerization. It can also be observed that the C∙= O band at 1710 cm−1, C–O stretch at 1284 cm−1, and the out-of-plane O–H deformation mode are no longer present. Instead, new features indicative of the carboxylate species appear. Peaks at 1407 and 1589 cm−1 can be attributed to COO–symmetric and asymmetric stretching, respectively. The remaining unidentified peaks are characteristic of the long hydrocarbon chain as mentioned before. All peaks beyond 2800 cm−1, associated with CH3 and CH2 as well as C∙= C modes beyond 2800 cm−1, remain in the same position pre- and postmodification of ZnO nanowires with oleic acid.
FIGURE 51.4 (a) Raman spectrum of as-synthesized ZnO nanowires. (b) Raman spectrum of modified ZnO nanowires. (A. Gupta et al., Zinc oxide nano-wires for biosensing applications, Proc. 11th IEEE International Conference on Nanotechnology, August 15–18, Portland, Oregon, pp. 1615–1618. © (2011) IEEE. With permission.)
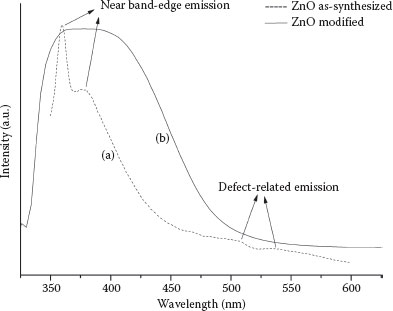
After the immobilization of oleic acid molecules on the ZnO nanowires surface, we tested the PL behavior of unmodified and modified ZnO nanowires. Figure 51.6a shows the PL spectrum of assynthesized nanowires. Weak peaks at 510 and 536 nm can be attributed to the defect-related emission, and prominent peaks at 360 and 380 nm are due to the onset of excitonic band-edge-related emission. When the nanowire surface sites are occupied with oleic acid molecules, two significant changes occur in the spectrum. In Figure 51.6b, the defect-related peaks disappear, which could be due to the occupation of surface sites by oxygen from oleic acid, which otherwise would have contributed to visible luminescence. Furthermore, the band-edge-related emission peaks merge and display a slight red-shift that can be attributed to the surface adsorption of oleic acid molecules. Both these effects could be due to the surface interaction between the ZnO nanowire and oxygen atoms from oleic acid. Although the role of oxygen in ZnO PL is still debated, it can nonetheless serve as a functional tool for quantification and characterization. For developing biosensors based on ZnO nanowires, emission characteristics, both band-edge and defect-related, can together serve as a fingerprint to identify different biological moieties.
FIGURE 51.5 FT-IR spectra of (a) oleic acid 1% (v/v) in hexane. (b) Oleic acid-modified ZnO nanowires. (A. Gupta et al., Zinc oxide nano-wires for biosensing applications, Proc. 11th IEEE International Conference on Nanotechnology, August 15–18, Portland, Oregon, pp. 1615–1618. © (2011) IEEE. With permission.)
FIGURE 51.6 (a) PL spectrum of as-synthesized ZnO NWs. (b) PL spectrum of modified ZnO NWs. (A. Gupta et al., Zinc oxide nano-wires for biosensing applications, Proc. 11th IEEE International Conference on Nanotechnology, August 15–18, Portland, Oregon, pp. 1615–1618. © (2011) IEEE. With permission.)
51.3.3 DEVELOPMENT OF the ZNO NANOWIRE-BASED p-NITROPHENOL SENSOR
In the previous section, a generic approach for synthesizing a ZnO nanowire-based biosensor was highlighted. It is possible to extend this approach for sensing desired analytes by performing the functionalization of the nanowire surface through the appropriate receptor. In one of our recent studies, we studied the surface chemistry of the 1-pyrenebutyric acid (PyBA) receptor for developing the p-nitrophenol sensor [42]. Nitrophenols are a class of organic compounds that are generated as degradation products of many organophosphorus compounds (OPs). OP compounds are key components of the modern agricultural industry and find application in the manufacture of herbicides, insecticides, and so on. Although, these compounds do not get accumulated in biological systems, they exhibit high toxicity and are purported to have carcinogenic behavior on human subjects [43]. To develop the aforementioned ZnO nanowire-based sensor, the receptor was immobilized onto the ZnO nanowire surface through covalent grafting. Subsequently, vapor phase detection of p-nitrophenol was envisaged up to 20 ppb. We believe that the synergistic utilization of optical and electrical characteristics of the ZnO nanowire–PyBA system could lead to an optoelectronic device platform for selective and selective determination of p-nitrophenol.
Based on the above findings and previous empirical evidence, it can be deduced that ZnO nanowires coupled with a suitable receptor or antigen has great potential for detecting a wide spectrum of biologically important compounds. To achieve this, however, rigorous research related to all fundamental aspects of biochemistry, surface phenomenon, and functionalization is required. ZnO nanostructures have the required capabilities to provide a sensitive and selective detection platform, and the imminent needs of biodetection, imaging, and therapeutics are expected to provide the much-needed thrust to the ZnO-based nanosensor technology research.
Approved for public release; distribution unlimited. Review completed by the AMRDEC Public Affairs Office June 13, 2012; FN5869.
1. T. Asefa, C. T. Duncan, K. K. Sharma, Recent advances in nanostructured chemosensors and biosensors, Analyst, 10, 1980–1990, 2009.
2. S. Börner, R. Orghici, S. R. Waldvogel, U. Willer, W. Schade, Evanescent field sensors and the implementation of waveguiding nanostructures, Appl. Opt., 48, B183–B189, 2009.
3. Y. Cui, Q. Wei, H. Park, C. M. Lieber, Nanowire nanosensors for highly-sensitive, selective and integrated detection of biological and chemical species, Science, 293, 1289–1292, 2001.
4. C. B. Murray, D. J. Norris, M. G. Bawendi, Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites, J. Am. Chem. Soc., 115, 8706–8715, 1993.
5. M. C. Daniel, D. Astruc, Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size related properties, and applications towards biology, catalysis and nanotechnology, Chem. Rev., 104, 293–346, 2004.
6. B. Tian, X. Zheng, T. J. Kempa, Y. Fang, N. Yu, G. Yu, J. Huang, C. M. Lieber, Coaxial silicon nanowires as solar cells and nanoelectronic power sources, Nature, 449, 885–889, 2007.
7. C. M. Niemeyer, Nanoparticles, proteins, and nucleic acids: Biotechnology meets materials science, Angew. Chem. Int. Ed., 40, 4128–4158, 2001.
8. D. H. Cobden, Nanowires begin to shine, Nature, 409, 32–33, 2001.
9. Y. Cui, C. M. Lieber, Functional nanoscale electronic devices assembled using silicon nanowire building blocks, Science, 291, 85–853, 2001.
10. R. J. Tonucci, B. L. Justus, A. J. Campillo, C. F. Ford, Nanochannel array glass, Science, 258, 783–785, 1992.
11. E. Comini, G. Faglia, G. Sberveglieri, Z. Pan, Z. L. Wang, Stable and high-sensitive gas sensors based on semiconducting oxide nanobelts, Appl. Phys. Lett., 81, 1869–1871, 2002.
12. Q. Wan, K. Yu, T. H. Wang, C. L. Lin, Low-field electron emission from tetrapod-like ZnO nanostructures synthesized by rapid evaporation, Appl. Phys. Lett., 83, 2253–2255, 2003.
13. B. Y. Oh, M. C. Jeong, T. H. Moon, W. Lee, J. M. Myoung, J. Y. Hwang, D. S. Seo, Transparent conductive Al-doped ZnO films for liquid crystal displays, J. Appl. Phys., 99, 124505–124509, 2006.
14. K. Nomura, H. Ohta, K. Ueda, T. Kamiya, M. Hirano, H. Hosono, Thin-film transistor fabricated in single-crystalline transparent oxide semiconductor, Science, 300, 1269–1272, 2003.
15. T. Yoshida, H. Minoura, Electrochemical self-assembly of dye-modified zinc oxide thin films, Adv. Mater., 12, 1219–1222, 2000.
16. K. Önenkamp, R. C. Word, C. Schlegel, Vertical nanowire light-emitting diode, Appl. Phys. Lett., 85, 6004–6006, 2004.
17. H. Nanto, T. Minami, S. Takata, Zinc-oxide thin-film ammonia gas sensors with high sensitivity and excellent selectivity, J. Appl. Phys., 60, 482–484, 1986.
18. M. H. Sarvari, H. Sharghi, Zinc oxide (ZnO) as a new, highly efficient, and reusable catalyst for acylation of alcohols, phenols and amines under solvent free conditions, Tetrahedron, 61, 10903–10907, 2005.
19. M. Law, L. E. Greene, J. C. Johnson, R. Saykally, P. D. Yang, Nanowire dye-sensitized solar cells, Nat. Mater., 4, 455–459, 2005.
20. D. C. Olson, Y. J. Lee, M. S. White, N. Kopidakis, S. E. Shaheen, D. S. Giney, J. A. Voigt, J. W. P. Hsu, Effect of polymer processing on the performance of poly(3-hexylthiophene)/ZnO nanorod photovoltaic devices, J. Phys. Chem. C, 111, 16640–16645, 2007.
21. P. Ravirajan, A. M. Peiro, M. K. Nazeeruddin, M. Graetzel, D. D. C. Bradley, J. R. Durrant, J. Nelson, Hybrid polymer/zinc oxide photovoltaic devices with vertically oriented ZnO nanorods and an amphiphilic molecular interface layer, J. Phys. Chem. B, 110, 7635–7639, 2006.
22. P. C. Chen, S. Sukcharoenchoke, K. Ryu, A. Gomez, A. Badmaev, C. Wang, C. Zhou, 2,4,6-Trinitrotoluene (TNT) chemical sensing based on aligned single-walled carbon nanotubes and ZnO nanowires, Adv. Mater., 22, 1900–1904, 2010.
23. S. Das, J. P. Kar, J. H. Choi, T. I. Lee, K. J. Moon, J. M. Myoung, Fabrication and characterization of ZnO single nanowire-based hydrogen sensor, J. Phys. Chem. C, 114, 1689–1693, 2010.
24. Y. Gui, C. Xie, J. Xu, G. Wang, Detection and discrimination of low concentration explosives using MOS nanoparticle sensors, J. Hazard. Mater., 164, 1030–1035, 2009.
25. A. Choi, K. Kim, H. I. Jung, S. Y. Lee, ZnO nanowire biosensors for detection of biomolecular interactions in enhancement mode, Sens. Actuat. B Chem., 148, 577–582, 2010.
26. L. Liao, H. B. Lu, J. C. Li, C. Liu, D. J. Fu, Y. L. Liu, The sensitivity of gas sensor based on single ZnO nanowire modulated by helium ion radiation, Appl. Phys. Lett., 91, 173110–3, 2007.
27. Z. Fang, J. G. Lu, Gate-refreshable nanowire chemical sensors, Appl. Phys. Lett., 86, 12, 2005.
28. F. Patolsky, B. P. Timko, G. Yu, Y. Fang, A. B. Greytak, G. Zheng, C. M. Lieber, Detection, stimulation, and inhibition of neuronal signals with high-density nanowire transistor arrays, Science, 313, 1100–1104, 2006.
29. N. Kakati, S. H. Jee, S. H. Kim, H. K. Lee, Y. S. Yoon, Sensitivity enhancement of ZnO nanorod gas sensors with surface modification by an InSb thin film, Jpn. J. Appl. Phys., 48, 10500, 2–5, 2009.
30. D. S. Lee, Y. Q. Fu, S. Maeng, J. Luo, N. M. Park, S. H. Kim, M. Y. Jung, W. I. Milne, ZnO Surface Acoustic Wave Biosensor, Inter. Elec. Devices. Meeting, Washington DC, 2007.
31. X. Cai, N. Klauke, A. Glidle, P. Cobbold, G. L. Smith, J. M. Cooper, Ultra-low-volume, real-time measurements of lactate from the single heart cell using microsystems technology, Anal. Chem., 74, 908–914, 2002.
32. M. Ahmad, C. Pan, L. Gan, Z. Nawaz, J. Zhu, Highly sensitive amperometric cholesterol biosensor based on pt-incorporated fullerene-like ZnO nanospheres, J. Phys. Chem. C, 114, 243–250, 2010.
33. M. Ahmad, C. Pan, Z. Luo, J. Zhu, A single ZnO nanofiber-based highly sensitive amperometric glucose biosensor, J. Phys. Chem. C, 114, 9308–9313, 2010.
34. B. X. Gu, C. X. Xu, G. P. Zhu, S. Q. Liu, L. Y. Chen, M. L. Wang, J. J. Zhu, Layer by layer immobilized horseradish peroxidase on zinc oxide nanorods for biosensing, J. Phys. Chem. B, 113, 6553–6557, 2009.
35. Z. H. Ibupoto, S. M. U. Ali, K. Khun, C. O. Chey, O. Nur, M. Willander, ZnO nanorods based enzymatic biosensor for selective determination of penicillin, Biosensors, 1, 153–163, 2011.
36. I. Mahmood, C. Guo, H. Xia, J. Ma, Y. Jiang, H. Liu, Lipase Immobilization on oleic acid − pluronic (L-64) block copolymer coated magnetic nanoparticles, for hydrolysis at the oil/water interface, Ind. Eng Chem. Res., 47, 6379–6385, 2008.
37. P. C. Chang, Z. Fan, J. G. Lu, J. Hong, W. Y. Tseng, ZnO nanowires synthesized by vapor trapping CVD method, Chem. Mat., 16, 5133–5137, 2004.
38. C. Y. Lee, T. Y. Tseng, S. Y. Li, P. Lin, Growth of zinc oxide nanowires on Si (100), Tamkang J. Sci. Eng., 6, 127–132, 2003.
39. Z. Zhang, S. J. Wang, T. Yu, T. Wu, Controlling the growth mechanism of ZnO nanowires by selecting catalyst, J. Phys. Chem. C, 111, 17500–17505, 2007.
40. Y. Huang, M. Liu, Z. Li, Y. Zeng, S. Liu, Raman spectroscopy study of ZnO-based ceramic films fabricated by novel sol–gel process, Mater. Sci. Eng. B, 97, 111–116, 2003.
41. L. J. Bellamy, The Infrared Spectra of Complex Molecules, Halsted, UK, pp. 233–237, 1975.
42. A. Gupta, B. C. Kim, E. Edwards, C. Brantley, P. Ruffins, Synthesis and functionalization study of hierarchical ZnO nanowires for potential nitroaromatic sensing applications, App. Phys. A: Mater. Sci. Process, 107, 709–714, 2012.
43. R. C. Gupta, Toxicology of Organophosphate and Carbamate Compounds, Elsevier Academic Press, USA, 2005.
44. A. Gupta, B. C. Kim, D. Li, E. Edwards, C. Brantley, P. Ruffin, Zinc oxide nanowires for biosensing applications, Proc. 11th IEEE International Conference on Nanotechnology, August 15–18, 2011, Portland, Oregon, pp. 1615–1618.