Coal Types and the Production and Trade in Coal
Coal was formed from vegetation that grew on the earth in pre-historic times. Since it was created by photosynthesis, driven by the energy from the sun, it can be viewed as a stored form of solar energy. There are a variety of coals, ranked by their carbon and water content. Anthracite has the highest carbon content, up to 92%, and the lowest water content. The most abundant coals are bituminous coals which also have relatively high carbon content and low levels of water. Lignites, brown coal and peat all have much lower carbon content and more moisture. Global known coal reserves are around 900,000 Mtonnes. These are widely dispersed across most continents. The largest global producer of coal is China which produces nearly four times as much as the next largest producer, the USA. Global annual production is close to 8000 Mtonnes. Coal is relatively expensive to transport and most coal is used close to the point of production. However some coal is traded internationally. Coal as produced may contain high levels of impurity. Some of these can be removed by physical cleaning, increasing its energy content and reducing transportation costs.
Keywords
Fossil fuel; photosynthesis; anthracite; bituminous coal; lignite; peat; heat content; coal reserves; coal production; coal trade; coal cleaning; physical cleaning; moisture reduction; ash removal; fluid-based separation; chemical cleaning
Coal is a type of fossil fuel that contains the remains of vegetation that grew on the earth’s surface during prehistoric times. This prehistoric vegetation absorbed energy from sunlight and used it to drive the photosynthesis reaction which converts carbon dioxide and water into glucose, the first stage in the cycle converting solar energy into chemical energy. In consequence, coal can be viewed as a form of stored solar energy.
Most plants decay when they die and are recycled through the soil. However under certain conditions the plant material does not decay but both builds up and become buried. It is this material, after hundreds of millions of years, that becomes coal and other fossil fuels. The type of coal that is produced depends on a variety of factors including the age of the deposit, the type of vegetation that has been buried and the temperature and pressure experienced by the vegetation at the depth at which it is buried.
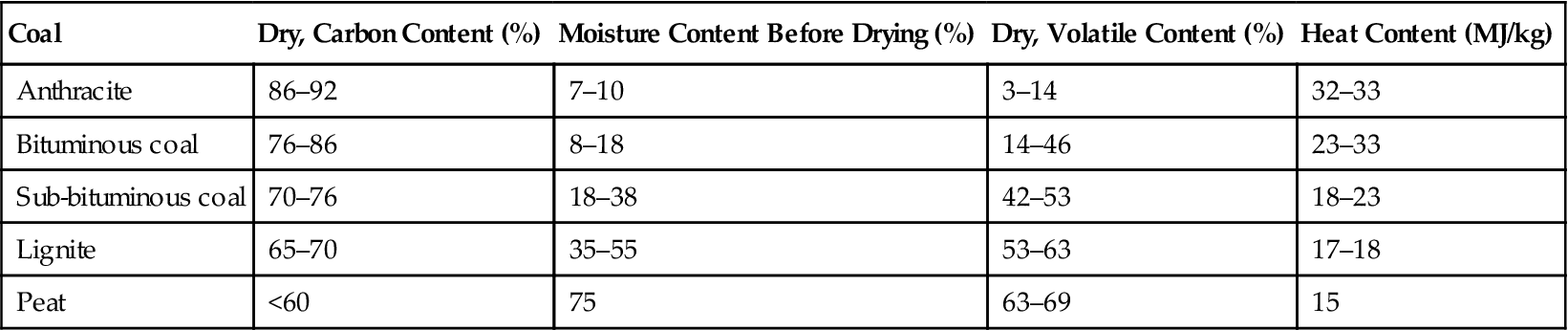
Coals are ranked according to the amount of carbon they contain and their water content. High-ranking coals have a high carbon content and low water content, while low-ranking coals contain less carbon and high levels of moisture. The ranges of each of these for the main coal classes are shown in Table 2.1. The proportion of volatile material also varies from coal to coal. The physical properties of the different coals affect their suitability for use in power plants.
Table 2.1
Coal Types1
| Coal | Dry, Carbon Content (%) | Moisture Content Before Drying (%) | Dry, Volatile Content (%) | Heat Content (MJ/kg) |
| Anthracite | 86–92 | 7–10 | 3–14 | 32–33 |
| Bituminous coal | 76–86 | 8–18 | 14–46 | 23–33 |
| Sub-bituminous coal | 70–76 | 18–38 | 42–53 | 18–23 |
| Lignite | 65–70 | 35–55 | 53–63 | 17–18 |
| Peat | <60 | 75 | 63–69 | 15 |
Source: Coal Marketing International.
The hardest of coals is anthracite. This coal contains the highest percentage of carbon (up to 92% dry content) and relatively little volatile matter or moisture. When burnt it produces little ash and relatively low levels of pollution (excluding carbon dioxide). Its energy density is generally higher than other coals at 32–33 MJ/kg. Anthracite is typically slow-burning and often difficult to fire in a power station boiler unless it is mixed with another fuel, and it has traditionally been used for heating rather than industrial use. However, it is becoming more common as a power plant fuel in countries with large reserves, such as Russia and Ukraine, which are switching to anthracite for power generation to free other fuels such as natural gas for export.
While anthracite reserves are important, the most abundant of the coals are the bituminous coals. These coals contain significant amounts of volatile matter. When they are heated they form a sticky mass, from which their name is derived. Bituminous coals normally contain between 76% and 86% carbon. Moisture content is between 8% and 18% and they may contain up to 46% volatile matter by dry weight. They burn easily, especially when ground or pulverized. This makes them ideal fuels for power stations. Bituminous coals are further characterized, depending on the amount of volatile matter they contain, as high (31–46%), medium (22–31%), or low volatile (14–22%) bituminous coals. Some bituminous coals contain high levels of sulfur which can be a handicap for power generation purposes.
A third category, called sub-bituminous coals or soft coals, are black or black-brown. These coals contain between 70% and 86% carbon and 18% to 38% water, even though they appear dry. Volatile content is 42–53%. They burn well, making them suitable as power plant fuels and the sulfur content is low.
The last group of coals that is widely used in power stations is lignites. These are brown rather than black and have a dry carbon content of 65–70%, although this is much lower as mined due to the moisture content of 35–50%. Lignites are formed from plants which were rich in resins and contain a significant amount of volatile material, typically 53–63% when dry. The amount of water in as-mined lignite, and its consequent low carbon content, makes the fuel uneconomic to transport over any great distance. Lignite-fired power stations are usually found adjacent to the source of fuel which needs drying before being burnt in the plant furnaces.
A type of unconsolidated lignite, usually found close to the surface of the earth where it can be strip-mined, is sometimes called brown coal. (This name is common in Germany.) Brown coal has a moisture content of around 45%. Peat is also burned in power plants, though rarely. Peat has a carbon content of under 60% and a moisture content of around 75%. Volatile material makes up 63–69% of the dry weight.
Coal Resources and the Coal Trade
Coal is one of the most widely available fuels. It is found in more than 70 countries according to the World Energy Council in its 2013 Survey of World Energy Resources and is actively mined in 50 countries. This means that most nations either have access to domestic supplies or can purchase it from a close neighbor. Even so, there is an active global trade in coal and this has expanded significantly over the last 20 years.
The amount of coal available for human exploitation is usually quantified in terms of the proven reserves from different countries and regions. This figure represents the known accessible reserves. The total quantities within the earth may be significantly higher. At the end of 2013, the total proven reserves were 891,531 Mtonnes as shown in Table 2.2.
Table 2.2
Proven Coal Reserves at the End of 20132
| Region | Anthracite and Bituminous Coal Reserves (million tonnes) | Sub-bituminous and Lignite Reserves (million tonnes) | Total Reserves (million tonnes) | Reserve/Production Ratio |
| North America | 112,835 | 132,253 | 245,088 | 250 |
| Central and South America | 7282 | 7359 | 14,641 | 149 |
| Europe and Eurasia | 92,557 | 217,981 | 310,538 | 254 |
| Middle East and Africa | 32,722 | 214 | 32,936 | 126 |
| Asia Pacific | 157,803 | 130,525 | 288,328 | 54 |
| World total | 404,199 | 488,332 | 891,531 | 113 |

Source: BP.
Table 2.2 also shows a breakdown of proven reserves by region. The largest reserves, 310,538 Mtonnes, are found in Europe and Eurasia. However, 70% of this coal is sub-bituminous and lignite and only 30% is harder coals such as anthracite and bituminous coal. The second-largest regional reserves, 288,328 Mtonnes are found in the Asia Pacific region, which includes China and India, both of which have large deposits. The coal in this region is roughly 55% anthracite and bituminous coal and 45% sub-bituminous and lignite. North America has total reserves of 245,088 Mtonnes, with 54% lignite and sub-bituminous and 46% harder coals. The two other regions in Table 2.2, Central and South America, and the Middle East and Africa, both have much smaller reserves.
The current level of coal global reserves is predicted to last for 113 years at the rate of extraction found across the world today. However, there are large regional variations. European and North American reserves are expected to last for around 250 years, while those in the Asia Pacific region are only expected to last for 54 years, a reflection of the vastly differing rates of consumption.
While coal reserves are widely scattered, five countries hold between them 75% of all the deposits. These are the USA (28%), Russia (18%), China (13%), Australia (9%), and India (7%).
Part of the reason for the rapid decline in Asian Pacific coal reserves can be seen in Table 2.3 which lists the top coal-producing nations of the world in 2013. At the top of the list, with by a wide margin the largest global production, is China with 3561 Mtonnes or close to 46% of the global total. The second-largest producer, the USA, mined only 904 Mtonnes or roughly one quarter of that mined by China. India, with production of 613 Mtonnes, has the third-largest industry.
Table 2.3
Coal Production by Country in 20133
| Country | Coal Production in 2013 (million tonnes) |
| China | 3561 |
| United States of America | 904 |
| India | 613 |
| Indonesia | 489 |
| Australia | 459 |
| Russian Federation | 347 |
| South Africa | 256 |
| Germany | 191 |
| Poland | 143 |
| Kazakhstan | 120 |
| Rest of the world | 740 |
| World total | 7823 |
Source: International Energy Agency.
Much of the production listed in Table 2.3 is for steam coal but several countries also produce significant quantities of lignite which is used exclusively in local power station. The top lignite producers are Germany, Russia, the USA, Poland, Turkey, and Australia.
The global trade in coal accounts for about 15% of the total coal consumed according to the World Energy Council, with most coal being used in the country where it is produced. Coal has a lower energy density that either oil or natural gas, as shown in Table 2.4. The average energy density of coal is around 24 MJ/kg (although as seen above some coals can have an energy density of up to 33 MJ/kg). In contrast crude oil has an average energy density of 46 MJ/kg and natural gas 54 MJ/kg. This means that coal is more costly to transport than either of the other two fossil fuels. Transportation costs therefore account for a large part of the cost of coal so that according to BP, in 2013, imported Japanese steam coal was 56% more expensive than US Central Appalachian coal.
Table 2.4
Energy Densities for Different Fossil Fuels
| Fossil Fuel | Average Energy Density |
| Coal | 24 MJ/kg |
| Crude oil | 46 MJ/kg |
| Natural gas | 54 MJ/kg |
As a consequence of the cost, the steam coal market (coal for power generation) is divided into two regional markets. One, the Atlantic market, comprises importing nations of Western Europe, principally the UK, Germany, and Spain. The second, the Pacific market, serves the major Asian importers, Japan, South Korea, Taiwan, India, and China.
The largest exporters of coal are Australia and Indonesia. Both are well placed to serve this Pacific market. Russia is another large coal exporter. Its traditional market has been Europe but the country is trying to win a greater share of the Pacific market. The USA, Colombia, and Canada all export coal, much of it to Europe. South Africa is also a major exporter. It is well placed to export to both the Pacific and the Atlantic markets.
Coal Processing and Cleaning
Processing coal before combustion offers a way of improving the quality of a coal, both economically and environmentally. Processing can be carried out physically or chemically but the only type of cleaning exploited commercially is physical cleaning. Chemical cleaning processes are under development but have not so far proved cost-effective.
The most well-established methods of coal-cleaning focus on removing excess moisture from the coal, reducing the amount of incombustible material which will remain as ash after combustion and reducing the sulfur content of the coal. Moisture removal reduces the weight and volume of the coal, rendering it more economical to transport and easier to burn. Ash removal also reduces the mass and volume of coal and improves its combustion properties and aids power plant performance.
Moisture is removed from coal by drying. This can simply be solar drying, leaving the coal in the open before transporting it. Drying coal in this way can reduce its mass and increase its energy density, making it relatively cheaper to transport.
The alternative, drying coal by heating, is most often carried out at the power station utilizing waste heat in the plant flue gases. Such a procedure is absolutely essential when burning high-moisture lignites such as brown coal. In this case drying does not affect transportation costs because the fuel has, by this stage, already reached the power station.
Physical separation of ash from coal relies on a difference in density between coal and its common impurities. This is applied quite widely during coal preparation, both before and at a power station.
Ash removal is carried out by crushing the coal into small particles. Incombustible mineral particles are more dense than the coal and can be separated using a gravity-based method, often with air or water as the separation medium. Fluid-based separation may exploit up-currents of air that pass through the crushed coal, sometimes in a fluidized bed. The lighter coal particles are carried away by the fluid for recovery at a later stage, leaving the impurities behind in the fluidized bed. Mixing the crushed coal with water also offers an effective means of separation as the coal particles float and the heavier mineral particles sink. Such treatment will remove some minerals containing sulfur, and can result in a reduction of up to 40% in sulfur dioxide emissions during combustion. (Some sulfur is bound to the carbon in the coal. Such sulfur is not affected by this type of cleaning.) Coal that is cleaned using a wet cleaning process (coal washing) must then be dried before combustion.
The severity of the physical processing is associated with the fineness of the coal. Coal crushing or grinding is needed to release the impurity particles from the fuel. Treatment of fine coal particles tends to be more intense than that of coarse particles. Both processes involve some coal losses, which can vary from between 2% and 15% of the total coal. Rejected coal can still be used in a plant capable of burning low-quality coal such as a fluidized bed furnace.
In addition to the common physical techniques outlined above there are some more specialized techniques being developed. These including using liquid carbon dioxide to selectively trap coal particles from a slurry of coal particles in water, and an electrostatic process in which the differing electrical insulation properties of coal and mineral impurities allow them to be separated after a charge has been applied to both. Cyclones that can selectively separate coal from pyrites, one of the main sources of sulfur in coal, have also been tested, as have magnetic techniques for separation.
There have been attempts to develop more advanced methods for coal treatment employing either higher-temperature processing of the coal or chemical rather than physical processes. These processes, which are aimed at removing polluting impurities in the coal to make it a cleaner fuel to burn, have not so far found commercial application. Coal cleaning processes are also being developed to treat coal wastes that have previously been discarded to make them suitable for combustion.
Chemical cleaning processes often target specific impurities, particularly sulfur. Selective oxidation of the sulfur using specific reagents has been tested, as has a process in which the ground coal is fed into a bath of molten sodium and potassium hydroxide. The latter dissolves much of the impurity material, leaving the coal which can be separated physically from the mixture. Chemical processes such as these and others are feasible but complex and currently appear to be too costly to implement. However they may become cost-effective if they can clean the coal to a sufficient level of purity that no further cleanup to remove sulfur – for example – is required.
Dry coal cleaning was popular in the USA from the 1930s until 1990 but declined after that date. However, there has been a recent revival in interest in coal cleaning as a way of reducing transportation costs. There is also interest in India where some coals are transported up to 1000 km or more. Dry cleaning processes can reduce the ash content in some of these coals from 40% to 30%.
