Magnetic nanoparticles and cancer
S. Bucak and C.L. Altan, Yeditepe University, Istanbul, Turkey
Abstract
Cancer, being the number one cause of death, is a disease that becomes even more widespread as the population’s life expectancy increases. Chemotherapy drugs that are designed to destroy the cancer cells, inevitably kill also the healthy cells. Without being targeted or controlled release, these drugs are administered in high doses which make them highly toxic. Magnetic nanoparticles (MNPs) are currently being investigated for their versatile use in drug targeting, therapy, and diagnostics. In this chapter the toxicity of magnetic particles and types of MNPs and their synthesis methods are shortly summarized. As different types of MNPs are employed in this research field, we focused the chapter to magnetite (Fe3O4) nanoparticles. Research in the field of magnetic drug delivery is summarized based on the type of magnetic carrier. Hyperthermia, which is the destruction or reduction of tumor by procuring heat at the tumor site, can be achieved by the application of an external magnetic field, inducing heat generation around the MNPs. This makes MNPs invaluable in cancer therapy, and the type of MNPs along with suitable coatings and targeting ligands used in hyperthermia are summarized. Cancer diagnostics is another field where MNPs find diverse use. MNPs are employed as contrast agents in magnetic resonance imaging (MRI), not only to image the existing tumors but also for post therapy monitoring in stem cell transplantation. By summarizing the work done in this field, MNPs, with ease of surface modification, low toxicity, and magnetic properties are shown to have great potential in novel therapies against cancer.
Keywords
Magnetic nanoparticles; drug delivery; magnetic drug delivery; hyperthermia; MRI
Introduction
Each year, more than 14 million people are diagnosed with and more than 8 million lose their lives because of different types of cancer. About 24.6 million are still alive among those who have been diagnosed in the past 5 years. In the developing countries, the risk of cancer is predicted to grow until 2020 (~15 million patients), whereas in developed countries this number is expected to remain stable [1]. On the other hand, recent scientific studies give rise to the emergence of novel treatment methods that lead to an increasing amount of patients who had successful treatments in consequence of early diagnosis. However, cancer is still the number one cause of death in the world. Currently, surgery, chemotherapy, immunotherapy, and radiation therapy are the most used treatment methods for cancer but none still offers a true total recovery. In many cases, surgical eradication or radiation treatments are not feasible. On the other hand, many chemotherapeutic drugs are cytotoxic and distributed within the whole body causing toxicity along with serious side effects so that it becomes impossible for the patient to use them [2–4]. These cytotoxic drugs also attack normal and healthy cells in addition to the primary target during the treatment process.
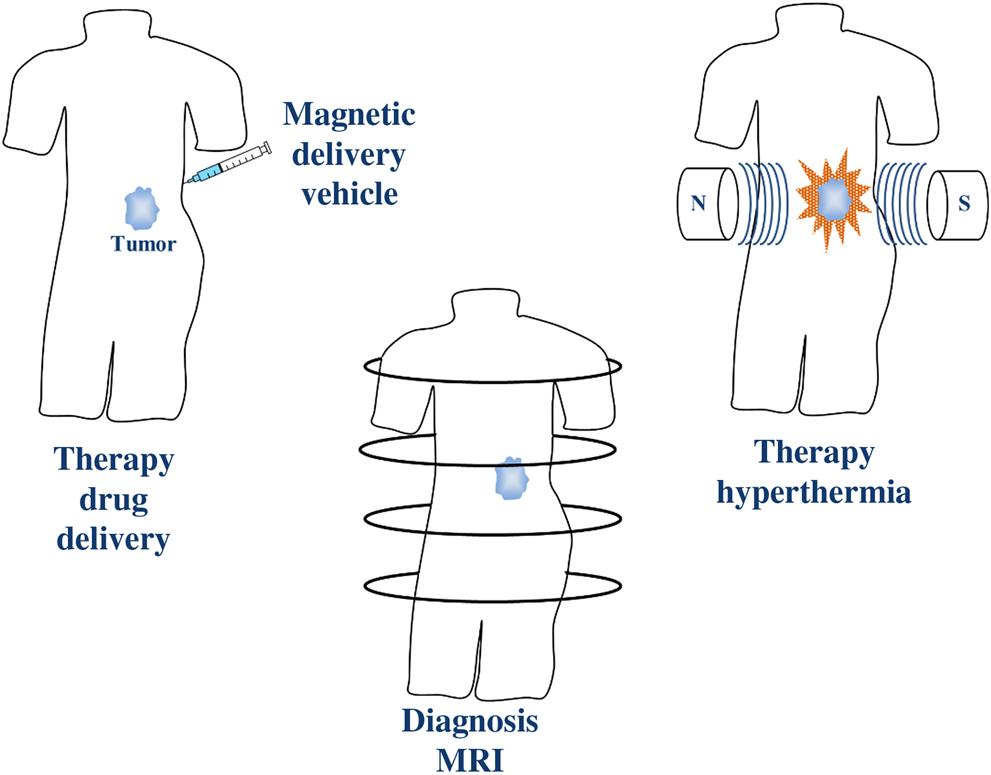
Within the last two decades, magnetic nanoparticles (MNPs) have gained a lot of attention in biomedical applications such as magnetic resonance imaging (MRI) [5–7], tissue repair [8,9], immunoassay [10,11], detoxification of biological fluids [12], magnetic hyperthermia [13–17], drug delivery [18–24], and cell and protein separation [25,26] due to their biocompatibility, facile surface modification, and unique magnetic properties. One of the most important features of MNPs is the possibility of manipulation in the presence of a magnetic field gradient. The other advantages of MNPs in biomedical applications are that they can be used for visualization as in MRI, guided or targeted to a specific location by the application of an external magnetic field followed by induced drug delivery or hyperthermia as a result of heating in an alternating magnetic field causing depredation of cancer cells (Fig. 6.1).
MNPs, although may contain different elements such as cobalt and nickel, are generally composed of iron oxides. In biomedical applications, magnetite (Fe3O4) is the most preferred type of iron oxide due to its excellent magnetic properties. It consists of both iron (II) and iron (III) ions and it is the most magnetic, naturally occurring mineral. There are various synthesis methods for the preparation of magnetite nanoparticles which are capable of controlling the particle size ranging from a few nanometers up to tens of nanometers [5,8]. The effective use of magnetite nanoparticles in biomedical applications depend on the size, morphology, and most importantly the magnetic properties of the particles. As the formation mechanism affect these properties, it is necessary to decide the suitable synthesis method for the preparation of magnetite nanoparticles that can be used in specific biomedical applications in order to obtain the most feasible magnetization values along with narrow size distribution thus have uniform physical and chemical properties. Among a variety of synthesis methods, chemical coprecipitation is the most used wet chemical route due to its simplicity and facile reaction kinetics [27]. The essential motive for the use of this method is the ability to synthesize relatively smaller MNPs (5–20 nm) which have superparamagnetic properties. As a result of this property, the organization of particles can be manipulated in nanofluids and particles align themselves according to the applied external magnetic field direction while in the absence they do not have any remaining magnetization. Coprecipitation method can also be performed in the presence of suitable surface active agents and the size, colloidal stability and magnetic properties of the particles can easily be modified. These superparamagnetic nanoparticles have wide applications from magnetic drug targeting (MDT) to contrast enhancement in MRI. Another aqueous synthesis method for the preparation of different magnetic iron oxide nanoparticles is partial oxidation by which larger (~50 nm) single-domain particles that have ferrimagnetic properties can be synthesized [28–33]. As these particles exhibit permanent magnetic properties, it is generally challenging to obtain colloidal stabilization in dispersions. However, due to their enhanced magnetic properties, these particles are suitable candidates for many biomedical applications such as magnetic hyperthermia and cell separations.
As the size of MNPs are smaller or similar to those of a cell, a protein, etc., they can bind to drugs, proteins, enzymes, antibodies, or nucleotides and target a specific region within the body by using an external magnetic field [34]. Furthermore, MNPs can also be functionalized with biological molecules to enhance interaction or binding to an organ, a tissue, or a tumor which provides a controllable tagging [35–37].
As summarized earlier, with excellent magnetic properties which allow their manipulation upon application of an external magnetic field and with tunable sizes that determine their type of magnetism (para-, super-, or ferri-), high surface areas, and functionable surfaces, MNPs lend themselves for various biomedical applications. One very important aspect that should be carefully examined is the biocompatibility of these particles. Most studies in the literature investigate the in vitro toxicity of these particles; however, their applicability depends on their in vivo toxicity [38]. In vitro cytotoxicity test are performed on different cell lines where their viability in the presence of different doses of MNPs are investigated over a short period of time [39,40]. In vivo toxicity test involve the exploration of the distribution of these particles in different tissues and organs [38,41]. An excellent review on the toxicity of these particles is recently published where work in this field is very well organized [42]. In the biomedical field MNPs of a great variety of sizes (few nanometers to micrometers) are employed. These particles may be bare or coated with a variety of coatings such as citric acid, oleic acid, lipids, dextrans, other sugar-based materials, polymers such as PEG, PAA, and PEI or biopolymers such as chitosan and gum arabic or they may be embedded in biodegradable polymers such as PLLA and PLGA, or maybe formulated as hydrogels or microcapsules [5,8,27]. Depending on the application, different doses and exposure times are required. In addition the type of cells where the target is greatly varies. Considering all applications and types of MNPs used, it is impossible to come to a general conclusion about the safety of these particles. In 1996, FDA approved the use of SPIONs (superparamagnetic iron oxide nanoparticles) as MRI agents. However, based on published results in the literature, it is evident that cytotoxicity depends on:
• The cell type exposed to particles where some cell lines are found to be less tolerant [40].
• The type of coating, despite various reports there is somewhat a common understanding that bare particles are least tolerated [43,44].
• The dose, where particles of whichever type is found to be toxic above a threshold concentration, that concentration being dependent of the particle itself and the type of cell line used [38,44].
• The size, which dictates where these particles will be accumulated and how long they remain in circulation, even along with aspect ratio where high aspect ratio provides longer circulation type and allows better targeting to the desired area [45–47].
• The surface charge, where positively charged particles show greater toxicity [48].
• The stiffness of the material where soft matter is much more tolerated than the hard one, suggesting hydrogels and polymer particles with embedded magnetic material to be more compatible [49].
Based on this knowledge, although very promising, each magnetic delivery system should be evaluated in and of itself before being further developed as drug delivery vehicles, contrast agents, or other potential biomedical applications.
Magnetic Drug Targeting
Therapeutic drugs are mainly administered intravenously; thus these agents do not specifically concentrate to the area of interest and instead disperse throughout the whole body causing enhanced toxicity with several side effects [50]. The essential disadvantage of most cancer treatment methods, especially of chemotherapy, is the affection of healthy cells along with the primary target [23]. Additionally the majority of traditional chemotherapeutic agents have poor aqueous solubility, selectivity, and pharmacokinetic variability [51]. Site-directed drug targeting is a possible antitumor treatment that can be applied to direct and concentrate the drug locally or regionally at the tumor site by using nanoparticles [19,52,53]. The conjugation of drug can be applied directly onto the surface of the nanoparticles or both entities can be encapsulated within a coating shell from which latter possesses the advantage of higher entrapment efficiency and stability [54]. Following the targeting of the carrier that is loaded with the drug, the corresponding release is performed in the vicinity of tissue of interest or target cells by the change in pH [55–58], temperature [56,59,60], or as a consequence of enzymatic activity [61,62], etc. [23,63]. The core benefit of this method is the possibility of designing specific drug delivery systems by considering the properties of the tumor environment such that the anticancer drugs may be targeted directly to the tumor site and affect only the cancer cells leading to the reduction or total elimination of the side effects [23,50,64]. Moreover, it is possible to deal with lower and sufficient doses of the cytotoxic compounds within the body [24].
The administration of designed drug delivery systems is still investigated from which intravenous (iv) administration and intraarterial (ia) administration are the prominent methods [65]. The advantage of intraarterial administration over conventional intravenous administration is the injection of drug-loaded nanocarrier in the vicinity of the tumor consequently preventing the uptake of nanoparticles by the reticuloendothelial system (RES) [66] before being functional at the tumor site [67]. However, nothing more than solitary tumors can be addressed by the intraarterial administration, which complicates its use in spreading tumors after metastasis.
After the administration, the targeting of drugs by using carriers can be accomplished by either passive or active targeting. For the passive targeting, the nanoparticles make use of the specific properties of the tumor environment and penetrate into the tumor by leaky vascularization as a result of enhanced permeability and retention (EPR) effect [68,69] while healthy tissues possess a barrier of endothelial cells that prevent nanoparticle extravasation [70]. This method of targeting has limited applications as a result of inefficient diffusion of drugs, nonuniform nature of tumor vessels, and the lack of EPR effect in certain types of tumors [68]. On the other hand, active targeting is based on guiding the carriers to the tumor site by the help of specific ligands that have affinity solely on the cells in question [69]. In order to target a drug conjugated nanoparticle to a desired site in the body by active targeting, often folic acid or an antibody is attached to the surface of the nanocarrier which contains the drug. Once in the system, the nanocarrier is designed for preferential binding at the tumor site due to the presence of folic acid or antibody. Although this route is frequently encountered in the literature, it has limitations in vivo due to low binding affinity.
Finally, the drug carrier should be eliminated from the system with minimum side effects following the release of the drug by metabolic activities and excretion [54].
As an alternative, externally applied magnetic field driven active drug targeting, or briefly MDT, is one of the novel route which improves the localization of the drug that is attached to an MNP by using an external magnetic field consequently concentrating the drug at the tumor site [19,52,71–73] (Fig. 6.2). Generally the magnetic carriers are composed of iron oxide cores for which magnetite is the most preferred one due to its convenient magnetic properties and a biocompatible shell or matrix for the encapsulation of therapeutic drugs. These magnetic cores not only allow for the magnetic field targeting of the entity but also bring the possibility of detection in an MRI scan [74]. Although the magnetic particles that are forming the magnetic core of the drug delivery system are required to have strong magnetic properties for convenient manipulation in the presence of an external magnetic field, it is necessary to prevent clogging of small capillaries due to coagulation which consequently impel the application of superparamagnetic particles rather than ferrimagnetic ones having remnant magnetization even in the absence of a magnetic field [22]. Additionally, for the optimum resistance to the renal clearance, particles having sizes larger than 10 nm are reported to be useful in targeted drug delivery for increased blood circulation time after intravenous administration [75].
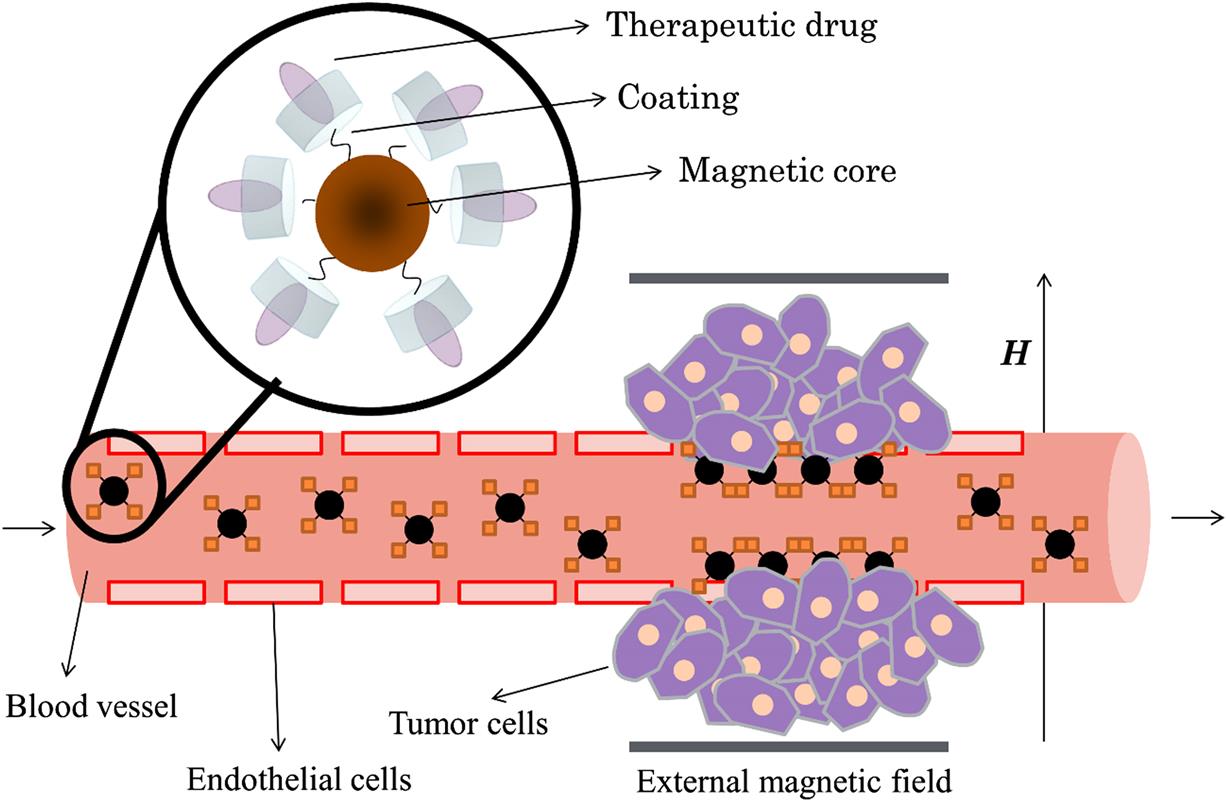
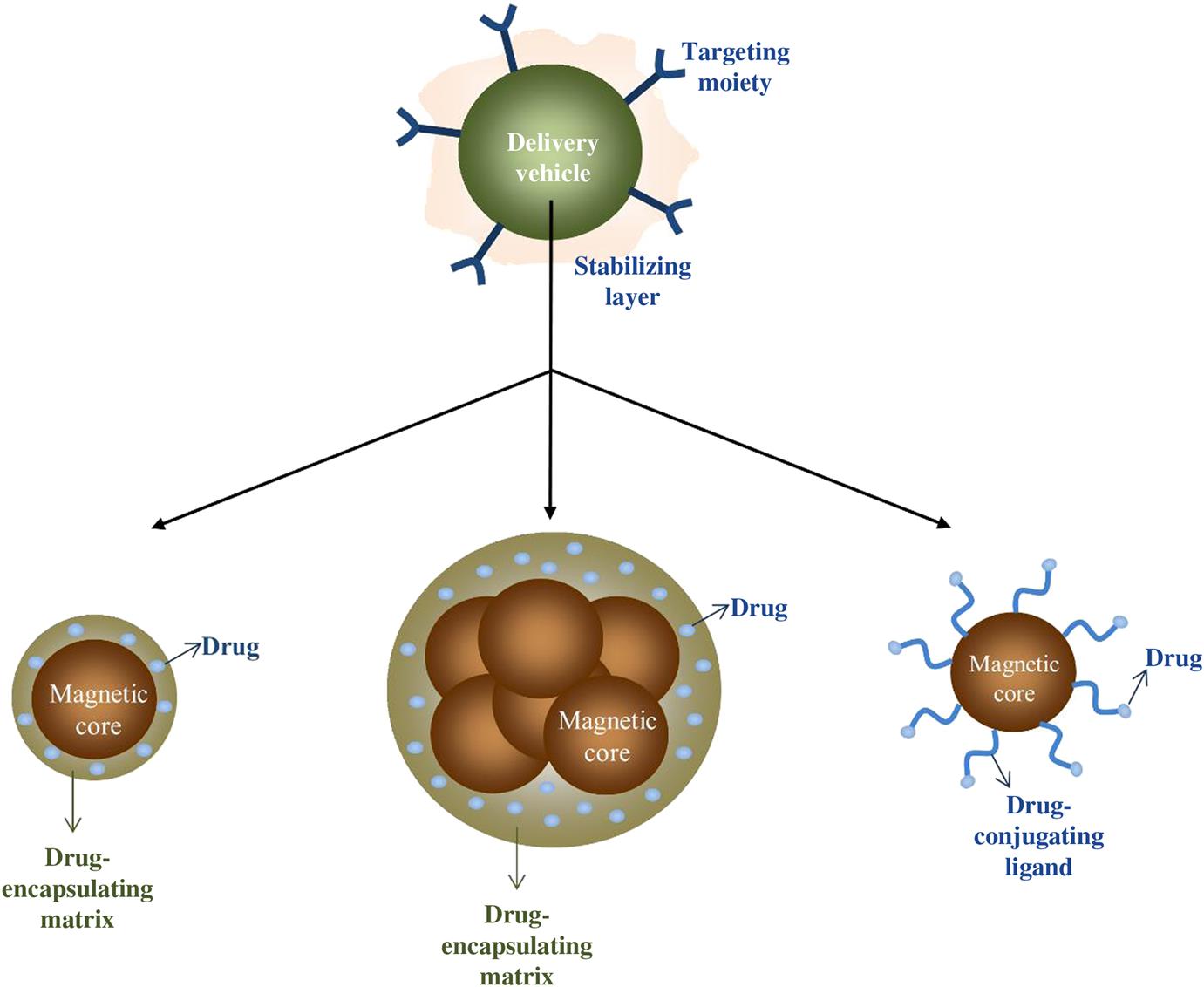
Similar to many other applications, there are several factors to take into consideration such as the size of MNPs (magnetic properties), characteristics of the applied magnetic field [76], binding efficiency of the drug, and physiological factors [38] when designing an effective MDT system. As in the case of many other applications, a suitable coating or a matrix is essential for the stability of magnetic particles in order to prevent agglomeration which may cause embolization of capillary vessels [23] (Fig. 6.3). This outer shell or matrix not only enhances the functionality of magnetic particles by the encapsulation of the drug and/or binding molecules but also delays the uptake by the mononuclear phagocyte system [77]. For the surface functionalization of magnetic particles that are used in magnetic drug delivery systems, several surface active agents and polymers are reported up to date such as PVA [78], PEG [79,80], chitosan [81,82], PEI [83], and starch [6,20,71,84]. These entities can further be functionalized with antibodies [85], folic acid [86], aptamers [87], etc. [6] for enhanced binding capabilities. Liposomes [6,88,89], as in the case of magnetic hyperthermia, dextran [6,60], and silica [90–92] shells may also be used for the encapsulation of magnetic particles and the therapeutic agents. However, it should be noted that any and every additional surface attachment have detractive effect over the magnetic properties of the core [93].
Although iron oxides are frequently encountered in magnetic drug delivery, several other metals are also reported. These metals can either be incorporated into the crystal structure or used to obtain a core–shell structure. For example, cobalt nanoparticles are incorporated in order to enhance the magnetic properties of the carrier. On the other hand, gold can be used for the direct functionalization of iron oxide surfaces to facilitate certain binding processes. Although the existence of gold on the surface of the magnetic core reduces the magnetic properties slightly, it has shown great improvement in surface binding of some antibodies. Additionally, gold surfaces are facilely functionalized therefore, gold-coated core–shell iron oxide nanoparticles are widely employed in magnetic drug delivery.
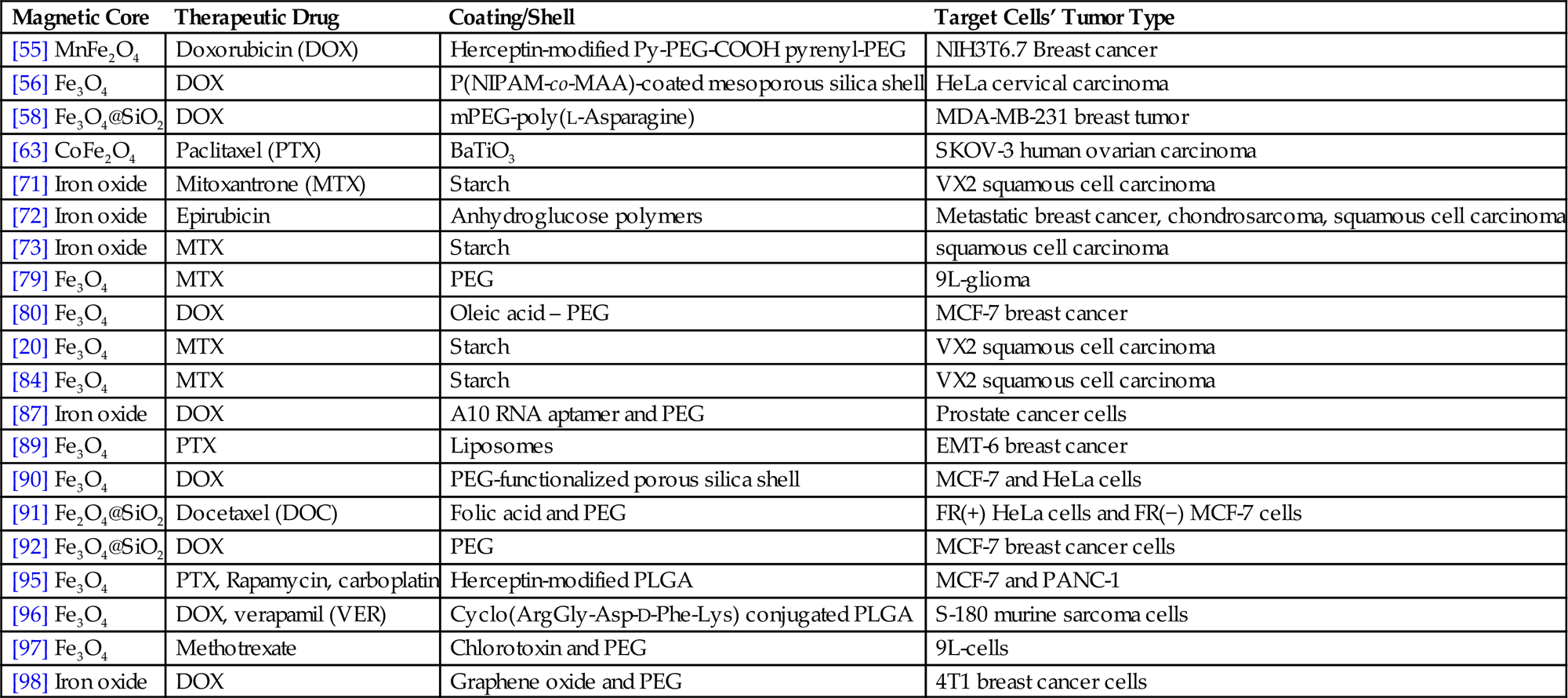
Actually, the concept of using magnetic particles for targeting dates back to couple of decades. For instance, Senyei et al. encapsulated doxorubicin in an albumin matrix containing magnetite particles and targeted these microspheres at a specific area in an in vitro analogue of the human circulatory system by using an applied magnetic field in 1978 [21]. On the other hand, Mosbach and Schroder reported in 1979 the localization of albumin-coupled magnetic starch microspheres in the ear of rabbits to which an external magnetic field (0.7 T) is applied after injection to the opposite ear after 10 min [94]. One of the leading preclinical studies for the application of MDT was reported by Lubbe et al. where an amino sugar containing positively charged anthracycline drug epirubicin is chemically bound to MNPs functionalized with anionic phosphate groups and concentrated locally in tumors by means of an external magnetic field [52]. This study paved the path for several scientists to use MNPs in drug delivery systems some of which are summarized in Table 6.1.
Table 6.1
Summary of Selected In Vivo and In Vitro Studies Involving Magnetic Drug Delivery Systems
| Magnetic Core | Therapeutic Drug | Coating/Shell | Target Cells’ Tumor Type |
| [55] MnFe2O4 | Doxorubicin (DOX) | Herceptin-modified Py-PEG-COOH pyrenyl-PEG | NIH3T6.7 Breast cancer |
| [56] Fe3O4 | DOX | P(NIPAM-co-MAA)-coated mesoporous silica shell | HeLa cervical carcinoma |
| [58] Fe3O4@SiO2 | DOX | mPEG-poly(L-Asparagine) | MDA-MB-231 breast tumor |
| [63] CoFe2O4 | Paclitaxel (PTX) | BaTiO3 | SKOV-3 human ovarian carcinoma |
| [71] Iron oxide | Mitoxantrone (MTX) | Starch | VX2 squamous cell carcinoma |
| [72] Iron oxide | Epirubicin | Anhydroglucose polymers | Metastatic breast cancer, chondrosarcoma, squamous cell carcinoma |
| [73] Iron oxide | MTX | Starch | squamous cell carcinoma |
| [79] Fe3O4 | MTX | PEG | 9L-glioma |
| [80] Fe3O4 | DOX | Oleic acid – PEG | MCF-7 breast cancer |
| [20] Fe3O4 | MTX | Starch | VX2 squamous cell carcinoma |
| [84] Fe3O4 | MTX | Starch | VX2 squamous cell carcinoma |
| [87] Iron oxide | DOX | A10 RNA aptamer and PEG | Prostate cancer cells |
| [89] Fe3O4 | PTX | Liposomes | EMT-6 breast cancer |
| [90] Fe3O4 | DOX | PEG-functionalized porous silica shell | MCF-7 and HeLa cells |
| [91] Fe2O4@SiO2 | Docetaxel (DOC) | Folic acid and PEG | FR(+) HeLa cells and FR(−) MCF-7 cells |
| [92] Fe3O4@SiO2 | DOX | PEG | MCF-7 breast cancer cells |
| [95] Fe3O4 | PTX, Rapamycin, carboplatin | Herceptin-modified PLGA | MCF-7 and PANC-1 |
| [96] Fe3O4 | DOX, verapamil (VER) | Cyclo(ArgGly-Asp-D-Phe-Lys) conjugated PLGA | S-180 murine sarcoma cells |
| [97] Fe3O4 | Methotrexate | Chlorotoxin and PEG | 9L-cells |
| [98] Iron oxide | DOX | Graphene oxide and PEG | 4T1 breast cancer cells |
For instance, Chen et al. encapsulated doxorubicin-conjugated magnetite nanoparticles within a porous silica shell and further attached a layer of PEG onto the matrix. It is reported that DOX release is performed through the porous silica matrix by a diffusion-controlled process and the existence of PEG at the outer shell prevent recognition by RES thus increase the duration of DOX administration at the targeted site [90].
In another study, Singh et al. proposed an alternative system which is capable of targeting both hydrophobic (paclitaxel, rapamycin) and hydrophilic (carboplatin) drugs where superparamagnetic magnetite nanoparticles are embedded in a polylactide-co-glycolide matrix (PLGA-MNPs). Additionally a targeting ligand Herceptin was conjugated in order to enhance the active targeting properties. Their results illustrated the encapsulation of approximately 82% of hydrophobic and 47% of hydrophilic drugs within PLGA-MNPs followed by a successful 85% release after 3 weeks. In addition, Herceptin conjugation on the surface of PLGA-MNPs was shown to enhance the selectivity while the overall targeting lead to a cytotoxic activity in MCF-7 and PANC-1 cells in in vitro studies [95].
It is also possible to use a dual-drug delivery systems, which assemble different therapeutic drugs of diverse features such as antitumor and antiangiocardiopathy activities in the same matrix [96].
In another study a specific peptide, chlorotoxin (CTX) which is used in combination with iodine for the targeting of radiation to tumor cells is conjugated with iron oxide nanoparticles due to its subfunction in affinity for specific types of tumors (NP-CTX) [99]. The drug delivery system that contains the chemotherapeutic drug methotrexate (MTX) is shown an enhanced accumulation in glioma and medulloblastoma cells in in vitro studies for MRI applications. In vivo, a retention time of at least 2 weeks for the chemotherapeutic agent is also demonstrated for a 9L xenograft tumor-bearing mice [97].
In recent years, the application of two-dimensional graphene and its corresponding composite nanomaterials rather than conventional systems in MDT was reported by some authors for their excellent mechanical, thermal, and structural properties. For instance, Ma et al. illustrated the promising use of PEG-functionalized graphene oxide-superparamagnetic iron oxide hybrid nanocomposite (GO-IONP-PEG) in both MDT and MRI. The GO-IONP-PEG was shown to be effective as T2 contrast agent in in vivo MRI of tumor-bearing mice as well as in in vitro direct targeting of DOX to the murine breast cancer 4T1 cells [98].
In another study, Tietze et al. investigated the effect of administration type over in vivo biodistribution of Mitoxantrone (MTO) after MDT at the targeted tumor tissue which is implanted in the limbs of rabbits. In order to analyze the biodistribution, MTO was both administered alone and with superparamagnetic particles intravenously in the presence and absence of an external magnetic field. Additionally, MTO-loaded superparamagnetic particles were also administered by intraarterial injection with an external magnetic field. Their results indicated that MDT after intraarterial injection resulted in a considerable accumulation (fourfold) of MTO at the tumor site in comparison to liver and kidneys while intravenous administration led to the accumulation of MTO in kidneys (>75%) decreasing the doses below 1% at the tumor site [67]. In line with this study, tumor entrapment of PEI-modified MNPs were resulted in an extraordinary 30-fold increase after intraarterial administration followed by magnetic targeting when compared with intravenous administration [83].
Along with its advantages, MDT has also some drawbacks which affect its clinical applications. One of them is again the systemic distribution of therapeutic drugs. These drugs are targeted to the tumor site by magnetic cores (particles) under the influence of a high-gradient magnetic field, in the absence those do not have any retainer force for stabilization at the target site which still causes some systemic distribution [24]. On the other hand, magnetic field intensity may not be sufficient and limiting to affect sites rather than near-surface targets and also cause accumulation of magnetic particles at another location where the magnetic field gradient is relatively more perceived [24,100].
In principle, it can be said that in the design of MNPs in drug delivery, particles with high magnetization cores that already exhibit low cytotoxicity, should be individually coated or embedded in a matrix that can encapsulate the drug, or the drug should be conjugated to the particle, after which the drug-encapsulating entity can be made more biocompatible and their retention in circulation can be enhanced by the addition of some stabilizers and they can be further directed to the tumor site by being functionalized by targeting moieties in addition to being targeted by the application of an external magnetic field. The ability of being targeted bimodally, makes MNPs rise in novel drug delivery applications.
Hyperthermia
Hyperthermia is an alternative therapeutic method for the treatment of cancer by procuring heat at the tumor site. In hyperthermia, the cancer cells are affected adversely by enhancing the temperature of a region of the body locally (41–46°C) resulting in the partial or complete destruction of the tumor by either a programmable (apoptosis) or a premature death (necrosis) of malignant cells as a consequence of mechanisms such as protein denaturation, protein folding, and DNA cross-linking [14,101–105]. Hyperthermia can be applied locally, regionally, or to whole body considering the conditions and dissemination of cancer [106]. Magnetic hyperthermia is applied by using suitable MNPs where the reorientation of the magnetization of MNPs is achieved in the presence of an external magnetic field. This continuous reversal of magnetization process causes losses and heat generation thus a local temperature increase which results in death of cancer cells or increases susceptibility for the complementary treatment methods such as surgery, chemotherapy, radiation therapy, and gene therapy [107,108]. Previous studies showed that there is a promising progression in the treatment of cancer as a result of the combination of these therapeutic methods [109–112]. There are several advantages of using MNPs in hyperthermia such as targeting the particles to the tumor site by the application of an external field which can then be applied as AMF in order to stimulate the heating. It is also possible to encapsulate drugs to magnetic carriers to facilitate effective drug delivery systems as well as enhancing the treatment efficiency of hyperthermia. The effective use of MNPs in magnetic hyperthermia depends on the intensity and the frequency of the applied magnetic field, exposure time as well as the concentration, size, morphology, and magnetic properties of the nanoparticles [8,113–115]. There exists several in vitro and in vivo studies related with magnetic hyperthermia are performed at various conditions from the pioneering studies to this date [14,15,17,107,116–121]. Essentially, the magnetic field frequencies and intensities are indicated to be in the range of f=0.05–1.2 MHz and H=0–15 kA/m while the concentration of the MNPs is about 5–10 mg/cm3 of tumor [18,122]. The heating ability of MNPs in magnetic hyperthermia is given by the specific absorption rate (SAR), which is the heating power obtained per unit mass of particles which is also referred in some studies as the specific loss power (SLP) or the specific power loss. SAR is given by
where C is the specific heat capacity of the sample and ΔT/Δt defines the temperature enhancement per unit time [120]. The heating ability of the MNPs is proposed to be due to the combination of Néel relaxations and Brownian relaxation processes, which are the physical rotations of particles within the nanofluid and the rotation of magnetic moments with in the particles, respectively [18,107,123]. An intrinsic parameter, the intrinsic loss power is also recommended as it excludes the extrinsic factors such as the frequency and the intensity of the applied magnetic field [124]. Consequently, for the optimum application of MNPs in magnetic hyperthermia, it is desired to achieve an effective temperature increase by using minimum amount of MNPs for which the SLP must be sufficiently high. One of the most challenging problems of magnetic hyperthermia is the difficulty in selective targeting or homogeneous distribution of MNPs at the tumor site. An alternative approach is to target surface functional MNPs to the tumor site using an external magnetic field in order to increase the local concentration [125]. In addition, functionalized MNPs may be modified in such a way that a chemotherapeutic drug is also incorporated to the entity for the direct administration to the tumor site [126,127].
Although there are quite different magnetic materials proposed for the application of magnetic hyperthermia, overall inclination is the use of biocompatible (nontoxic) magnetic iron oxides, magnetite (Fe3O4), and maghemite (γ-Fe2O3) [105]. For magnetic hyperthermia applications initially the use of large multidomain particles or smaller superparamagnetic particles was preferred. However, it was later on shown that the most advanced SLP may be achieved by the use of magnetic particles in the transition range from superparamagnetic to ferrimagnetic properties where heating arises as a result of hysteresis losses due to magnetic domain wall displacements or Ne´el relaxations in the presence of an AC magnetic field [16,120]. There are also contrary reports stating that superparamagnetic nanoparticles give higher SAR [120]. On the other hand, the maximum SLP (1 kW/g) is revealed for bacterial magnetosomes where the particle size is around 30–35 nm [128]. As the isolation of these perfect uniform MNPs from the bacteria is compelling, studies focus on obtaining similar single-domain MNPs that are readily stabilized in suspensions for magnetic hyperthermia applications [32]. Although obtaining the maximum amount of SAR with MNPs is an essence, it is a challenge for in vitro and clinical studies to target these specific particles and obtain a uniform distribution throughout the tumor. A review of experimentally determined, both in vivo and in vitro, SAR/SLP for different nanoparticle systems and magnetic field properties is given in Table 6.2.
Table 6.2
Summary of SAR/SLP for Different Magnetic Nanoparticle Systems
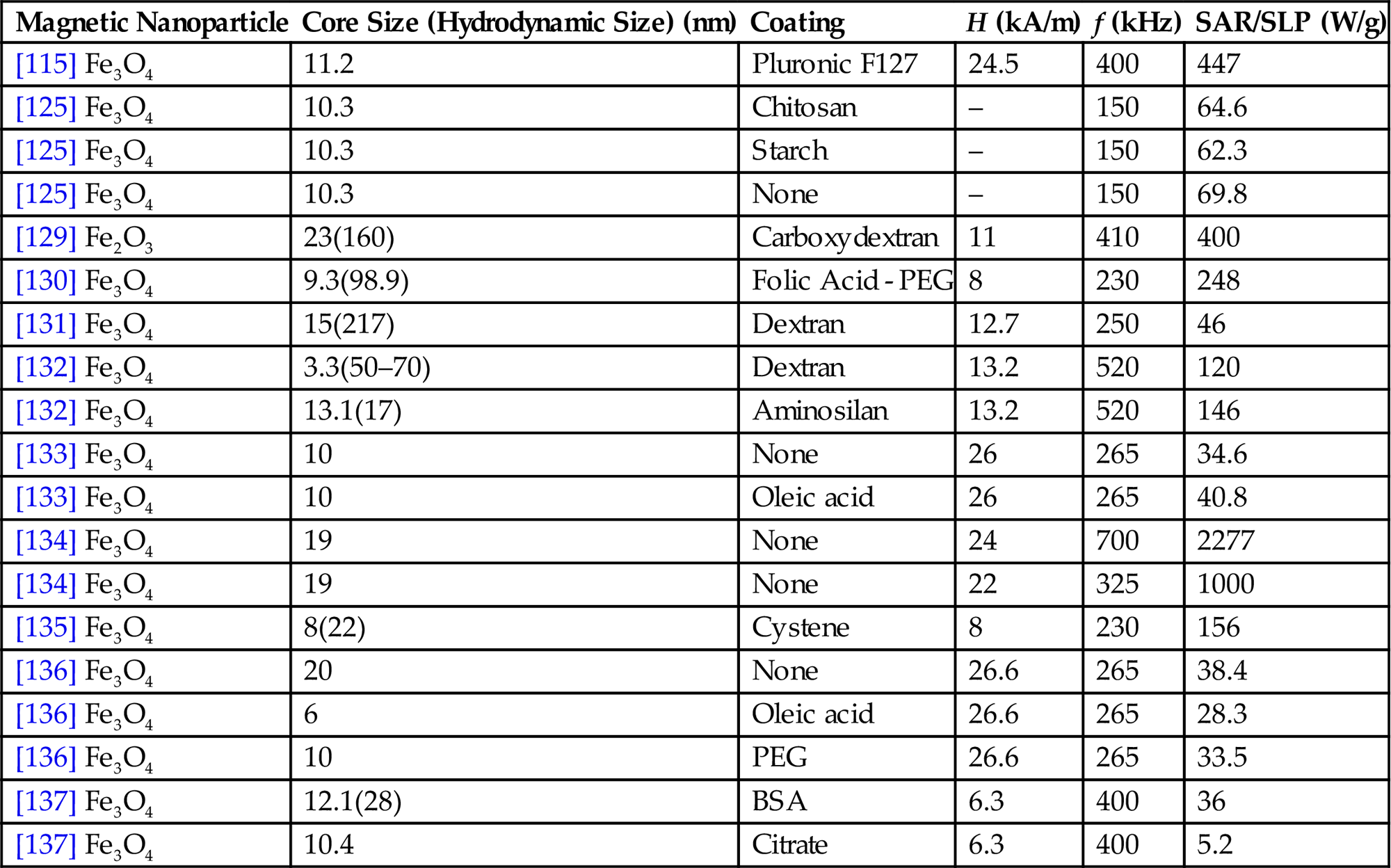
| Magnetic Nanoparticle | Core Size (Hydrodynamic Size) (nm) | Coating | H (kA/m) | f (kHz) | SAR/SLP (W/g) |
| [115] Fe3O4 | 11.2 | Pluronic F127 | 24.5 | 400 | 447 |
| [125] Fe3O4 | 10.3 | Chitosan | – | 150 | 64.6 |
| [125] Fe3O4 | 10.3 | Starch | – | 150 | 62.3 |
| [125] Fe3O4 | 10.3 | None | – | 150 | 69.8 |
| [129] Fe2O3 | 23(160) | Carboxydextran | 11 | 410 | 400 |
| [130] Fe3O4 | 9.3(98.9) | Folic Acid - PEG | 8 | 230 | 248 |
| [131] Fe3O4 | 15(217) | Dextran | 12.7 | 250 | 46 |
| [132] Fe3O4 | 3.3(50–70) | Dextran | 13.2 | 520 | 120 |
| [132] Fe3O4 | 13.1(17) | Aminosilan | 13.2 | 520 | 146 |
| [133] Fe3O4 | 10 | None | 26 | 265 | 34.6 |
| [133] Fe3O4 | 10 | Oleic acid | 26 | 265 | 40.8 |
| [134] Fe3O4 | 19 | None | 24 | 700 | 2277 |
| [134] Fe3O4 | 19 | None | 22 | 325 | 1000 |
| [135] Fe3O4 | 8(22) | Cystene | 8 | 230 | 156 |
| [136] Fe3O4 | 20 | None | 26.6 | 265 | 38.4 |
| [136] Fe3O4 | 6 | Oleic acid | 26.6 | 265 | 28.3 |
| [136] Fe3O4 | 10 | PEG | 26.6 | 265 | 33.5 |
| [137] Fe3O4 | 12.1(28) | BSA | 6.3 | 400 | 36 |
| [137] Fe3O4 | 10.4 | Citrate | 6.3 | 400 | 5.2 |
For a couple of decades, there has been countless studies on magnetic hyperthermia both in vitro and in vivo all of which pave the way of using MNPs in human patients [138]. For the determination of suitable conditions for the application of magnetic hyperthermia in vivo or in clinical studies, it is necessary to analyze the performance of the method in vitro where SLP of MNPs is attempted to be optimized by the variation of parameters such as concentration of MNPs, specific properties of the applied magnetic field, and tested cell types, etc by applying induction heat studies and calorimetric methods [15]. At the same time, for these studies several surface active agents such as dextran [139], lauric acid [140], silane [132], cationic liposomes [141], oleic acid [133], folic acid [130], and citric acid [142] are incorporated onto the surface of MNPs in order to improve the colloidal stability as magnetic particles tend to agglomerate and enhance the surface functionality. It was shown in vitro that cellular uptake of superparamagnetic magnetite nanoparticles that were functionalized with dextran or aminosilane groups in glioblastoma cells was substantially higher as in normal cells which shows the possibility of active targeting without a tagging ligand [132]. Additionally, it was shown that reproduced cancer cells concurrently contain MNPs that were taken by predecessor cells such that consequent MFH is possible without additional targeting of MNPs [14]. On the contrary, another report showed that nanoparticles had significantly taken up by normal tissue cells as well as tumor cells after iv administration of magnetite nanoparticles into a tail vein of rats having mammary tumors, indicating the importance of ligand labeling [117]. In a similar manner, the effect of superparamagnetic magnetite nanoparticles in a dextran shell over the death rate of dendritic cells in the presence of an alternating magnetic field was investigated by Asin et al. for determining the optimum particle concentration as well as exposure time and field amplitude. Their results specifically point out the necessity for the proper choice of magnetic field properties for magnetic hyperthermia applications [131]. Ghosh et al. represented that oleic acid and PEG-coated superparamagnetic magnetite nanoparticles achieve up to 65% reduction in the human breast cancer cells (MCF-7) under heating conditions [136]. Guardia et al. also reported the hyperthermia performance of PEG-functionalized MNPs in the size range between 13 and 40 nm as a function of magnetic field intensity and frequency. Their results indicated one of the highest values of SAR (2452 W/g) at 520 kHz and 29 kA/m and almost 50% mortality of KB cancer cells at 43°C after 1 h of treatment in vitro [134]. Similarly, heating ability that is comparable to that of magnetosomes are obtained for cysteine-coated superparamagnetic magnetite nanoparticles [135]. Samanta et al. reported that protein (BSA) passivated superparamagnetic magnetite nanoparticles cause total destruction of HeLa cells after 45 min exposure to an alternating magnetic field (400 kHz and 6.3 kA/m) [137]. Differently, Saavedra et al. proposed the advantage of using unique coating agents that have ordered pore structures on the multimodal therapies. Results indicated the internalization of maghemite nanoparticles within a mesa-porous silica matrix by human lung carcinoma cells. It was further shown that magnetic microspheres may be used for heat treatments leading to a decrease in the cell viability by AMF exposure [143]. In vivo, essentially, the application of magnetic nanofluids in hyperthermia applications is performed via injection into the tumor site or by iv administration [144]. One of the most important necessity for the efficient application of magnetic hyperthermia is obtaining adequate particle concentration and uniform distribution at the tumor site which is still a challenge to overcome [145]. The first application of magnetic particles for hyperthermia in vivo was in 1957 when Gilchrist et al. performed selective heating of lymph nodes by using magnetite [116]. This study was followed by countless experimental studies at various conditions. Jordan et al, who illustrated the heating of superparamagnetic dextran coated magnetite nanoparticles that were injected into mammary carcinomas of mice [119]. Magnetic cationic liposomes (magnetoliposomes) were also injected into rat glioma and shown to be potentially effective by both destroying the tumor cells and inducing an immune response [146]. In another study, for hamster osteosarcoma, these liposomes were shown to regress the tumor almost 100% in the presence of AMF where no relapse was observed after 3 months [147]. Hayashi et al. addressed the low accumulation in tumors due to the size of superparamagnetic nanoparticles and synthesized clustered folic acid and PEG-coated MNPs to prevent leakage from fenestrated capillaries in tumors and enhance SAR of individual particles. Their results indicated a local accumulation of MNPs in tumor tissues of mice and a 6°C difference in the temperature of tumor with respect to the surrounding tissues at 20 min after treatment [130]. In 2007 the first application of magnetic hyperthermia for the treatment of glioblastoma was successfully presented by Jordan et al. in human patients by direct injection of superparamagnetic magnetite nanoparticles (15 nm and 112 mg/mL) to multiple tumor sites that were exposed to an alternating magnetic field up to 13.5 kA/m at 100 kHz [138]. On the other hand, Lee et al. performed in vivo hyperthermia treatment with cancer cells (U87MG) and compared the effect of chemotherapeutic drug doxorubicin with magnetic thermal induction by nanoparticles at the same concentration over tumor destruction. After 18 days the tumor was completely eliminated for the group where magnetic hyperthermia was applied with core–shell nanoparticles. On the other hand, initially regressed tumor size for the group treated with doxorubicin come up with four times its original figure after 26 days [148]. Finally, Balivada et al. reported that even microgram amounts of core–shell porphyrn-labeled dopamine-anchored Fe/Fe3O4 nanoparticles is sufficient to cause an antitumor effect on melanoma in mice with relatively short repetitive exposure time (10 min) [149].
As summarized in this section, hyperthermia is a unique application of MNPs, as the local increase in temperature is achieved upon application of an external magnetic field. Due to the need of particles with high specific adsorption rates, choice of magnetic core is very important. Specific binding of magnetite to the tumor site achieved by targeting is favored to homogeneously distribute the particles around the tumor to obtain a homogeneous heating profile. Similar drug targeting strategies can be utilized as summarized in “Magnetic Drug Targeting” section to simultaneously delivery drugs to the tumor site to enhance the efficiency of hyperthermia. The predominantly in vitro success of the materials at this point, only points to their potential in vivo applications where by better distribution and retention of particles at the tumor site, clinical studies will soon flourish.
Magnetic Resonance Imaging
MRI is a widely used technique in the imaging of soft tissue, which in principle is based on Nuclear Magnetic Resonance (NMR). When placed in a magnetic field, different atomic nuclei absorb and emit electromagnetic radiation which is in the radio frequency. Hydrogen atoms, which are most abundant in living organisms predominantly in the form of water or hydrocarbon (i.e., fat or sugar), are often used to generate a detectable signal. Due to the presence of hydrogen atoms in the soft tissue, MRI technique is often used to detect tumors, hemorrhages, etc.
There are mainly two types of relaxations, T1 which is longitudinal is the time for the nucleus to relax back to its thermal equilibrium after being probed by electromagnetic radiation. The other type of relaxation is T2 which is transverse and refers to loss of phase coherence.
In obtaining images, different contrast modes can be used depending on the target tissue. In some cases, more information can be revealed using either T1- or T2-weighed images, whereas mostly a combination serves best. Often a contrasting agent is administered before imaging to shorten the relaxation times and obtain better images. Gadolinium-based contrast agents are most widely used [150–152], although some superparamagnetic iron oxides are also FDA approved and are currently being used. Gadolinium-based contrast agents which are paramagnetic shorten the T1 relaxation, whereas MNPs lower the T2 signal, enhancing the contrast, resulting in higher resolution images. As we are focusing on MNPs in this chapter, extensive work with gadolinium-based contrast agents will not be discussed.
Enhancement of contrast due to the lowering of the T2 signal is directly proportional with the magnetic moment of the material. Magnetite (Fe3O4) is the naturally occurring iron oxide with the highest magnetization. It is also possible to increase the magnetic moment by doping Fe3O4 with other transition elements that are highly magnetic such as Co, Mn, and Ni [153–156] to obtain better contrast agents. For the same purpose, it is also possible to synthesize iron alloys such as FeCo [157,158] or FePt [159,160] to be used for MRI. However, a major issue with particles containing metals other than iron is the cytotoxicity [161,162]. Use of naturally occurring iron-based nanoparticles such as ferritin as an MRI agent is also suggested especially due to its excellent biocompatibility [163]. Carbon nanotubes are also exploited as contrast agents where their surfaces are either decorated with maghemite [164] or magnetite [165,166] nanoparticles; or they are filled with a ferromagnetic material [167]. The main focus of this section will be on the use of iron oxide MNPs as contrast agents for MRI, and other magnetic materials will be excluded.
Clinical studies have been carried out using FDA-approved MNPs. There are very specific contrast agents used to differentiate tumors. Lymph node sizes should be accurately determined to differentiate whether the lymph node is metastatic or not. FDA-approved ferumoxtran (a.k.a. Combidex) which consist of monocrystalline superparamagnetic nanoparticles with a dense dextran derivative coating was used in a Phase III clinical trial on 220 patients where persistently high T2* signal intensities were achieved [168,169]. In another study, same particles were shown to demonstrate high sensitivity and specificity in distinguishing metastatic lymph nodes in patients with renal cell cancer [169]. FDA-approved Resovist which consist of superparamagnetic magnetite particles coated with carboxydextran of about 60 nm is predominantly used for the detection and characterization of especially small focal liver lesions [170]. To cure type 1 diabetes, Islets of Langerhans transplantation has been suggested; however a noninvasive assessment such as MRI to follow the retention of islets is required [171]. In a clinical study, Resovist-labeled islets have been transplanted to patients where early detection of islet loss was monitored [171]. In a Phase III trial, same particles were employed for the visualization of myocardial infarct zone; however, no improvement compared to gadolinum-based contrast agents was achieved [172]. Both Resovist and Feridex (small superparamagnetic iron oxide particles with low-molecular-weight dextran coating of about 150 nm size) were shown to be used for the evaluation of macrophage activities as well as imaging liver [173]. Ultrasmall iron oxide nanoparticles (Sinerem, stabilized with dextran and sodium citrate) were proven useful to visualize normal sized pelvic lymph nodes [174]. Clariscan, which consists of small MNPs with PEG coating, was developed as a gastrointestinal contrast agent, also tried for coronary angiography with reasonably good results [175]. Although once approved, neither of the abovementioned products is now available in the market due to limited applications or safety concerns. On the other hand, GastoMARK (a.k.a. Lumirem) which is a superparamagnetic iron oxide nanoparticle aggregates of 300 nm size with dextran coating is currently used as contrast agent in intestinal movements and pancreas and is well received by patients [176,177].
Despite some practical problems in the use of superparamagnetic iron oxide nanoparticles as MRI contrast agents, a vast number of particles are currently being developed and tested. Even bare magnetite nanoparticles are shown to lower the T2 signal [178], however due to cytotoxicity related to bare particles and lack of colloidal stability resulting in aggregation, brings the necessity to generate particles with different coatings. In order to be applicable as MRI contrast agents, particles should remain in circulation a certain amount of time before being cleared. One of the criteria to ensure that is to synthesize particles that fall within a size range. Particles that are too small (<5 nm) are quickly removed by the kidney, whereas particles of about 20 nm are cleared by the liver. Use of larger particles is even more challenging as they are uptaken by the immune system. It should be noted that the abovementioned sizes are hydrodynamic radia, which refer to the magnetic core and the stabilizing layer around the particles.
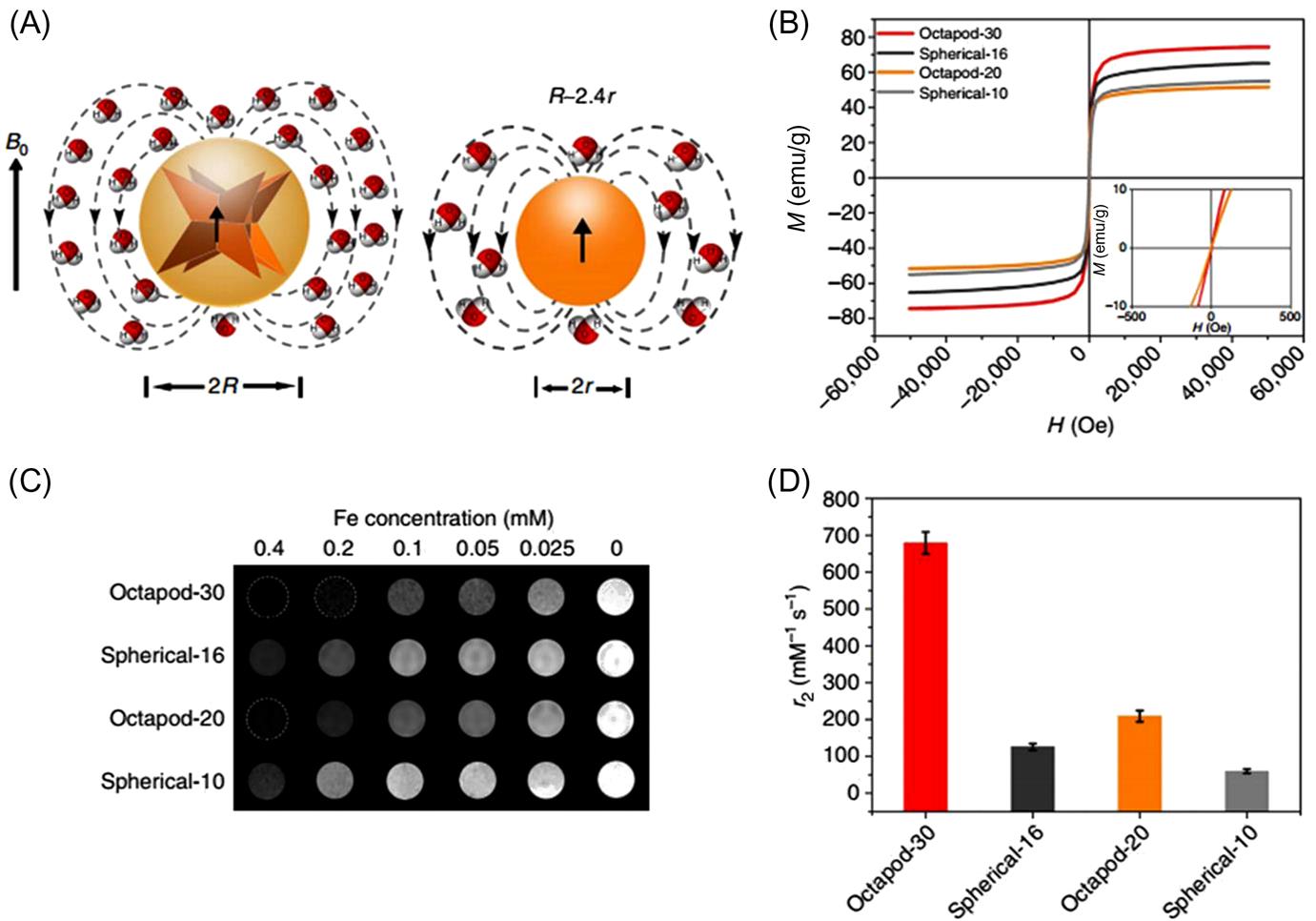
One of the simpler anionic magnetite nanoparticles with an oleic acid double layer were presented as potential MRI contrast agents [179]. Ultrasmall superparamagnetic iron oxide nanoparticles, when synthesized with narrow size distribution and high polymer coverage with low-molecular-weight polyacrylic acid (PAA, MW 5100 Da), exhibited high colloidal stability and dual T1-T2 enhancement [180]. Although endocytosis (cell uptake) of anionic particles is presented [181], cationic nanoparticles, providing better electrostatic interaction with the negatively charged cells, are better uptaken [182]. Ethyl amine–functionalized ultrasmall SPIONs are shown in vitro and in vivo to be good contrast agents [183]. Additionally, octopod magnetite nanoparticles are shown to be more effective as a T2 contrast agents for in vivo imaging than conventional spherical ones, indicating the morphology dependence of T2 relaxivity (Fig. 6.4) [184]. Despite their name as ultrasmall particle, the size of these particles still exceeds the lower size limit for kidney clearance and they are shown to accumulate in the liver, which is the preferred secretion route out of the body for MRI applications.
However the amount of time the particles remain in circulation is also closely related to their coating. To enhance biocompatibility and colloidal stability, as in the case of liposomes, PEGylation of particle surface is a common strategy also for the development of contrast agents. Due to the presence of PEG, particle circulation time in blood increases, making in vivo imaging more viable [185]. Cellular uptake of PEG-modified magnetite particles were achieved using poly-L-lysine transfection agent (cationic, therefore favorable due to induce cellular uptake), exhibiting promising in vivo MRI contrast enhancement [186]. Small superparamagnetic iron oxides when embedded in a shell of cross-linked PEG and polyamino acids (a.k.a. polyion complex vesicles) are shown to perform several times better as MRI contrast agents and detect small tumors [187]. PEG can also be used as an outer corona for drug-loaded particles in their inner shell for simultaneous drug delivery and high T2 relaxivity values [188]. Hydrophobic magnetite nanoparticles embedded in the bilayer of phospholipid liposomes [189] are also developed to obtain better contrast agents, taking advantage of the excellent biocompatibility and stability of liposomes while benefitting from the lipid-enveloped magnetic core.
For highly sensitive MR imaging, prolonged retention in the blood stream as well as accumulation in the tumor area are required. A significant enhancement in magnetic resonance contrast was obtained due to a dense iron oxide core and large cluster size by covalently conjugating β-cyclodextrin to superparamagnetic particles and hosting PEG in its cavity where the cluster size was controlled by particle/PEG ratio [190].
Multicore MNPs of magnetite and maghemite with a diethylaminoethyl-dextran shell were embedded in a gel of petroleum oil and styrenic copolymers at various concentrations and these materials were tested for their suitability for XCT and MRI. Although limited due to the nature of the gel, these materials were acceptable T2 enhancers for MRI [191].
All the abovementioned particles reach the tumor site by passive targeting. In passive targeting, nanoparticles reach and accumulate at the tumor site due to leaky vascularization. The released drug in the blood diffuses into the tumor tissue passively. This brings some inefficiency in targeting, therefore efficient active targeting where MRI agent nanoparticles are coupled with targeting moieties becomes the purpose of cutting edge research.
Folate receptor protein is a protein that is on the surface of cancer cells, therefore is overly expressed at the tumor site. Folate is a vitamin that strongly binds to this protein which makes it a common targeting moiety to accumulate the nanoparticles at the tumor site. Folic acid ligands are conjugated onto the –NH2 groups of magnetite nanoparticles obtaining colloidally stable particles with a core size of 8 nm and a hydrodynamic diameter of about 26 nm. As a result of preferential binding on the tumor cells, the MRI signal is shown to be enhanced, generating a higher contrast between tumor and normal tissue [192]. Magnetite-loaded chitosan particles, when compared with its folic acid counterpart were shown to accumulate better at the tumor site and can be used as an organ-specific MRI contrast agent [193]; however even in the absence of folic acid, magnetite-chitosan particles were also shown to significantly decrease T2 intensity [194]. Carboxymethyl dextran–modified magnetite particles with folic acid were successfully shown to enhance MRI contrast in the detection of KB cells [195]. Magnetite-encapsulated silica particles were conjugated with folic acid and despite the low magnetization values, were shown to be potential candidates as MRI contrast agents [196]. Magnetite nanoparticle surfaces were modified with folate-PEG-caffeic acid–modified magnetite nanoparticles were preferentially taken by a folate receptor overexpressed cancerous cells and the relaxation times increased with particles concentration [197].
Other targeting moieties, such as antibodies can also be conjugated to enhance accumulation at the target site to be used as MRI agents. Specifically image breast cancer tumors, human epidermal growth factor receptor 2 (anti-HER2) antibody was conjugated onto silica-magnetite core–shell particle surfaces and successful binding resulting in MRI detection with better contrast was supported with in vitro and in vivo studies [198].
Liver targeting of MNPs was achieved attaching a liver targeting function mebrofenin on silica-coated magnetite nanoparticles. Effective T2 signal decrease proved these particles as liver targeting contrast agents [199].
Early diagnosis of one of the more aggressive cancers, pancreatic cancer is of utmost importance. Galectin-1 is expressed in pancreatic cancer cells and its natural ligand glycosylated peptides can be attached onto maghemite-embedded recombinant human serum albumin nanoparticles as a targeting moiety. Although MRI contrast enhancement was achieved either way, in the absence of targeting, the particles were mostly found in the liver, whereas the uptake of magnetic particles with the targeting ligand was significant [200].
A specific targeting peptide, chlorotoxin was covalently bound to silica-magnetite core–shell particle surface due to its preferential binding to glicoma cells. As a result of more efficient uptake by the target cells, MRI contrast between glicomas and normal brain tissue was enhanced [201].
To take advantage of the photothermal effect of gold, magnetite nanoparticles were coated with gold and then functionalized with thiol-modified aptamers as the targeting moiety, where they could simultaneously serve as MR agents and used in photothermal therapy [202]. A similar construct with gold-coated magnetite nanoparticles, by the attachment of DNA strands onto the surface, self-assembly to form nanoparticle chains were induced to improve MRI detection modality [203].
MRI is not only used in the detection of tumor in the body but also used for the tracking of cells after stem cell therapies. In stem cell transplantation, noninvasive evaluation of the location and distribution of the stem cells is very important. For the MRI of stem cells, meso-2,3-dimercaptosuccinic acid (DMSA)-coated maghemite nanoparticles of around 30 nm were synthesized. Lowering of T2 signal in vitro studies were in line with successfully higher contrast obtained in vivo [204]. For a similar purpose, single strand DNA was conjugated with 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (DPPE), which is a lipid using PEG as a linker. This lipid got inserted in a cell membrane, leaving ssDNA on the cell surface for conjugation. Once the magnetite nanoparticles surface is modified with the complementary strand, DNA hybridization allow cell labeling where MRI scanning of transplanted cells was possible up to 1 month [205]. Efficient gene delivery and its post therapy monitoring with MRI were simultaneously achieved using cationic polyethylene imine (PEI)–coated chitosan embedded micelles accommodating hydrophobic superparamagnetic iron oxide nanoparticles in their core. The plasmid was adsorbed onto the particle surface due to electrostatic interactions [206].
As seen in previous sections, MNPs have versatile applications for the detection and treatment of cancer. Some particles are developed to serve more than one function. One example to drug delivery in conjunction with MRI is the gelatin-encapsulated magnetite nanoparticles conjugating some drugs onto their surfaces [207]. Doxorubicin (DOX) is an anticancer drug that is frequently used as a modal drug in drug delivery studies as in the following. BSA shell with a PEG corona around MNPs [188], magnetite containing mesoporous silica nanoparticles [208], and superparamagnetic nanoparticles coated by an amphiphilic block copolymer are some examples of simultaneous drug delivery and contrast enhancement systems [209].
Multipurpose nanoparticles for hyperthermia, MRI, and drug delivery were developed by functionalizing magnetite with β-cyclodextrin (β-CD) and coating with a pluronic surfactant. Curcumin was used as a model drug to be delivered from the outer shell of the nanoparticle and the magnetic core provided enhancement of MRI contrast, where the overall particle exhibited better performance in hyperthermia [210]. Hyaluronic acid was used to target CD44-expressed cancer by being conjugated onto magnetite surface and PEG was added to prolong circulation time. In this comparative study better contrast was achieved in the absence of PEG, whereas both types of particles were found to be equally effective in hyperthermia applications [211].
In general, MNPs, especially those with iron oxide cores, with low cytotoxicity, have shown great potential in lowering the T2 signal, enhancing contrast in MR imaging. By enhancing the circulation time, addition of drug molecules, and targeting moieties, these particles are now further optimized to be more frequently used in clinical applications.
To summarize, iron oxide MNPs have shown great promise in cancer research, due to high SAR values which make them valuable for the shrinking or disappearance of tumors as a result of hyperthermia upon application of an alternating current, due to their magnetic core with high magnetic moment which lend these particles as contrast enhancing agents for MRI as well as facilitating their manipulation upon application of an external magnetic field. The relatively facile control over their size, allowing tunable magnetic properties make these particles to partake in versatile applications. Due to the ease of surface functionalization, MNPs can be tailored to be involved in the encapsulation and conjugation of several anticancer drugs while some surface modifications are performed for their prolonged circulation in the blood to become more efficient cancer theranostics agents. Growing research and clinical studies have shown iron oxide nanoparticles to be rather applicable in cancer diagnosis and therapy, hinting future to bring more end-user products with these particles.