Historical overview
In recent years there has been an increasing interest in the development of micro-scale robotic systems. Researchers have explored numerous ways and techniques to provide systems capable of performing micro-scale tasks. Many micro-scale systems have been biologically inspired or based. For effective micro-scale systems, it is critical to understand cellular mechanics and their interaction with low Reynolds number environments. Cellular swimming has led researchers to mimic biological motors such as flagella for bacteria-inspired microrobots. Others have used biological phenomena and external stimuli for micro-scale robotic systems such as magnetotactic bacteria. Using bacteria as well as other microorganisms as a power source for a microrobot has also been investigated. Furthermore, mathematical modeling has been used to characterize cell behavior for control of microbiorobotic systems. Microbiorobotics has vast potential for creating robust micro-scale robotic systems.
Low Reynolds number swimming
The first step to building micro-scale robotic systems is to understand fluid mechanics at low Reynolds numbers. In low Reynolds number environments, viscosity is the dominating force, as inertia plays no role whatsoever. Motion at very low Reynolds number is entirely determined by the forces exerted on the organism at that moment and by nothing in the past [1]. Purcell's scallop theorem says that to achieve propulsion at low Reynolds numbers, reciprocal motion cannot be used. Purcell describes reciprocal motion as, “I change my body into a certain shape and then I go back to the original shape by going through the sequence in reverse. So, if the animal tries to swim by a reciprocal motion, it can't go anywhere.” [1] The scallop theorem forms the basis of aquatic locomotion for micro-swimming devices [2].
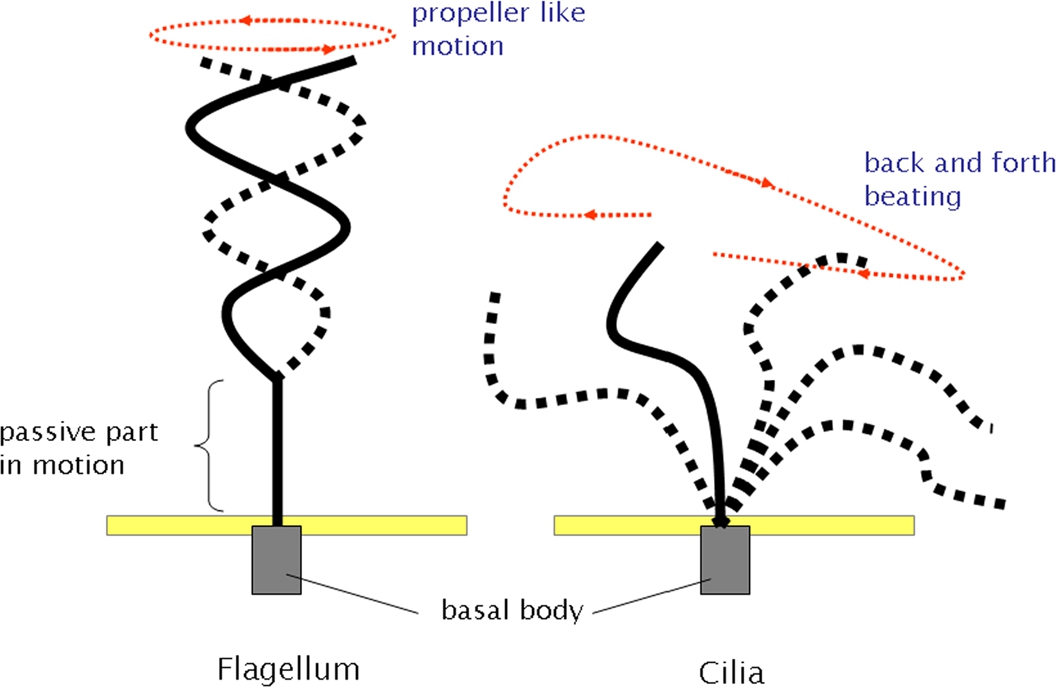
To create efficient propulsion at low Reynolds numbers, the mechanics of biological motors such as flagella and cilia have been examined. Many bacteria, such as Escherichia coli and Salmonella, have several flagella attached at points distributed over the surface of the cell. The flagella, which are typically helical in shape, rotate in a corkscrew-like motion. While bacteria are swimming, the flagella come together in a synchronous flagellar bundle, which propels the cell [3]. For microorganisms with cilia, such as Tetrahymena pyriformis and Paramecium, the locomotive cilium motion can be described in terms of two swimming strokes, effective (forward) and recovery (back). The cilia are aligned in arrays along the cell body and beat in a phase relationship with neighboring cilia [4]. The mechanics of flagella and cilia are shown in Fig. 1. These biological motors are efficient in low Reynolds number environments and are a source of inspiration for microbiorobotics.
Taxis of microorganisms
For the control of microbiorobots for micro-scale applications, external stimuli can be utilized. Many biological microorganisms respond to stimuli, such as magnetotaxis (magnetic fields), galvanotaxis (electric fields), phototaxis (light), and chemotaxis (chemicals). Based on the characterized behavior of microorganisms, these taxes can be applied to produce a desired response from the micro-scale robot. Responses from microbiorobots can be used as a method for chemical detection [5] or the manipulation of objects [6].
Magnetotaxis is used to change the direction of locomotion in motile organisms by inducing a magnetic stimulus [7,8]. Two different magnetotactic mechanisms, polar and axial, are found in different magnetotactic bacteria strains. Strains that swim in only one direction along the magnetic field are polar magnetotactic. These magnetotactic bacteria always swim towards either the north or south direction of the magnetic field, and will only reverse direction if exposed to a more powerful magnetic field. They always move in the same magnetic direction, relative to the dominant field. Axial magnetotactic bacteria move along either direction of magnetic field lines with frequent reversals of swimming direction and make no distinction between north and south poles. The magnetic field provides only an axis of motility for axial magnetotactic bacteria, while both an axis and a direction are specified for polar magnetotactic bacteria. These two magnetotactic mechanisms can be utilized to control micro-scale robotic systems.
Electrical current can be used to produce directional movement of motile cells; this is known as galvanotaxis. Bacteria strains will only swim in one direction, either towards the anode or cathode. When the electric field is reversed the cell will turn around, so that the same end of the cell is leading towards the new anode or cathode [9]. Researchers have established which direction bacteria strains, Escherichia coli and Salmonella, will swim based on surface structure [10]. Rough bacteria swam towards the anode, while smooth bacteria moved toward the cathode. Galvanotaxis has been shown to be applicable to numerous microorganisms [11,12].
Phototaxis is the movement of an organism in response to light, which can vary with light intensity and direction. The organism's reaction to light can either be negative or positive. Negative phototaxis causes swimming away from the light source, while in positive phototaxis movement occurs towards light. There are two types of positive phototaxis that are observed in bacteria, scotophototaxis and true phototaxis. Scotophototaxis is a phenomenon found underneath a microscope. Once the bacterium moves outside the illuminated area, it reverses direction and reenters the light. In true phototaxis cells follow a gradient of increasing light intensity [13]. Phototactic responses are observed in many microorganisms such as Serratia marcescens [14] and Tetrahymena pyriformis [12]. Similar to phototaxis, chemotaxis can either be positive (chemoattractants) or negative (chemorepellents) based on the cell's motility response to a chemical concentration gradient. Chemotaxis is more commonly used for research in biology and medicine, as there are some disadvantages for controlling organisms as microbiorobots. There is a significant delay in response and release time when compared to taxes such as photo, galvano, and magnetic. Also, there are challenges in the introduction and removal of chemicals as well as the creation of fluidic disturbances.
Artificial bio-inspired microrobots
Microrobots have vast potential in many engineering applications, such as micromanipulation, microassembly, and minimally invasive procedures. However, locomotion at the microscopic level is challenging. One source of inspiration for microfluidic propulsion has been found in motile organelles, such as flagella [15] and cilia [16]. The capabilities of these organelles at low Reynolds numbers are intriguing to researchers in the design of microrobots.
In recent years, mimicking flagella for purposes such as biomedical applications have been studied extensively [17]. It has been shown that helical propellers can be manufactured at the microscale [18–21]. A notable example of the helical swimmers is the helical micro- and nanopropellers which were fabricated using the nano-GLAD technique [22]. Magnetic fields rotate the swimmers to produce propulsive forces [20]. It was demonstrated that the micro- and nanopropellers can be controlled in 3D space and fluids with complex rheological properties such as biological fluids. These propellers are the smallest known helical swimmer with a length scale down to a few hundred nanometers which are small enough to be controllably navigated through the macromolecular mesh of biological fluids or gels [23]. Nanopropellers had been shown to move through hyaluronan solutions, which is an important step toward the goal of operating micro- and nanorobots inside biological media and living organisms [23].
In another technique to mimic the spiral swimming of bacteria, it was shown that rigid chains of micro- and nanoparticles can produce propulsion at low Reynolds number when rotated by a magnetic field. These are called particle based microswimmer. When actuated, the microswimmers exhibit a spiral motion resulting in forward propulsion. These microswimmers are fabricated using avidin biotin conjugation and magnetic self-assembly [24]. They can consist of any number of particles with 13 being the longest sustainable chain. The smallest number of particles is three, which makes up an achiral shape with two planes of symmetry. Interestingly, this achiral structure does not require chirality or flexibility to swim [24,25]. Furthermore, these particle-based microswimmers have been demonstrated in autonomous control [26,27] and multiple robot control [28], and can be scaled to nano-size [29].
Biological microrobots
Constructing artificial microrobots creates many challenges for engineers. Due to the differences between the physics of the macro- and micro-scale, it is difficult to design and manufacture microrobots. The costs are great for the microrobots that can be constructed. Also, there is a lack of sufficient power sources for microrobots making them unsuitable for time-consuming tasks. Some researchers have turned to microorganisms, such as magnetotactic bacteria, Serratia marcescens and Tetrahymena pyriformis, to be used as biological microrobots. Biological organisms are easily and cheaply cultured in labs with little equipment. They can draw chemical energy from their environment eliminating the need for external power sources. Microorganisms also respond to various external stimuli allowing them to be controlled as biological microrobots.
Magnetotactic bacteria (MTB) are geomagnetically sensitive and orient themselves along the earth's magnetic field lines. MTB have organelles called magnetosomes that contain magnetic crystals, which allow them to be directed by magnetic fields. Magnetotaxis has been used to show the controllability of MTB by manipulating microbeads [30,31]. MTB has been proposed as a micro-carrier as individual MC-1 bacteria were measured to produce a thrust of 4 pN [32]. Designs to utilize the thrust from a swarm of MTB to provide propulsion and steering for a microrobot have also been presented [33,34]. In this case, MTB are embedded in special reservoirs within the microrobot structure. An embedded microcircuit powered through photovoltaic cells is used to control the swimming direction of the bacteria and consequentially the microrobot.
Another method for using flagellated bacteria as microrobot has been shown using Serratia marcescens. Negative photoresist SU-8 microstructures were fabricated using simple microfabrication techniques. Serratia marcescens are then blotted on the surface of the microstructure, allowing the flagella to randomly propel and rotate the structure without stimulus. Phototactic control is demonstrated by exposing ultraviolet rays to localized regions of the swarm bacteria [14]. Within a few seconds, exposed areas become inactive, eliminating random motion. When combined with galvanotaxis, the microbiorobot's position and orientation can be controlled [11]. The microbiorobot could then be utilized for applications such as single cell manipulation [6]. Recent development had also demonstrated the feasibility to implement autonomous control, including feedback control, and obstacle avoidance [35].
Eukaryotes that are significantly larger than bacteria such as Tetrahymena pyriformis have also been employed as cellular microrobots. Tetrahymena pyriformis uses locomotive cilia for propulsion. Galvanotactic and phototactic control have been validated in past research [12]. Tetrahymena pyriformis is also capable of internalizing magnetic iron-oxide particles using oral cilia located at the anterior part of the cell [36]. After magnetization of the particles, the cell's swimming direction can be controlled using magnetic fields. Using magnetotaxis feedback control with real-time path planning was implemented for micro-scale tasks such as object manipulation or transport [37]. They can also be manipulated en mass using swarm control by global inputs [38,39]. This validates Tetrahymena pyriformis as a useful cellular microrobot.
Conclusion
Much effort has been put into the study of biologically inspired microscale robotic systems. To design a robust system, it is important to understand cellular mechanics as well as the stimuli needed for control of microbiorobots. Both artificial bio-inspired and biological microrobots exhibit great promise. Microbiorobots have the potential to revolutionize many research disciplines including biology and medicine.
