Chapter 9
Signs of Life
How Will We Find E.T.?
Once the invention of the telescope showed that the Earth is but one world among many, it opened the serious prospect of life on other planets. The reconnaissance within our solar system has revealed some tantalizing hints but no definitive evidence so far. Now that we are on the verge of finding extrasolar worlds with conditions hospitable for life, the question has gained a new urgency. Guided by remote observations of the Earth, clues about how the solar system’s three large rocky planets have evolved, and theoretical models of planets in other stellar environments, scientists are figuring out how best to search for extraterrestrial life. The quest has brought together researchers of different stripes to launch the field of astrobiology. It seems likely that our first glimpse of life elsewhere will be in the form of an ambiguous spectral imprint—one that does not distinguish between lowly bacteria and superior intelligence. But it could also be in the form of a dramatic radio signal, of clear technological origin. Either way, contact (in the loose sense of the word) will be a significant moment not only for science but for all of us. And that moment is closer than ever.
Mass Delusion
By the early nineteenth century, there was widespread belief in life beyond the Earth. It was in this environment that John Herschel, son of William, set sail from England for South Africa with his family and a 20-foot-long refracting telescope in 1833. He might have been prompted by a confluence of events: the failure of his bid to become the president of the influential Royal Society, the death of his mother, and the completion of the Cape of Good Hope observatory that offered an unprecedented opportunity to explore the southern skies. Their ship reached South Africa the following January.
On August 25, 1835, the New York Sun carried the first installment in a series of six articles titled “Great Astronomical Discoveries Lately Made by Sir John Herschel at the Cape of Good Hope,” evidently reprinted from the Edinburgh Journal of Science and reported by his colleague Dr. Andrew Grant. The article explained at great length how the younger Herschel had designed “a telescope of vast dimensions and an entirely new principle,” built it with the patronage of the Royal Society and the Duke of Sussex, and transported it to South Africa. The first installment offered a preview of the stunning breakthroughs Herschel had made with regard to the planets and the Moon and mentioned almost in passing that he “has affirmatively settled the question whether this satellite be inhabited, and by what order of things.” The sales of the Sun shot up pretty much overnight. The next installments did not disappoint the paper’s readers: there were vivid descriptions of rushing rivers, lush forests, and exotic life forms on the Moon, including blue unicorns, two-legged beavers, and intelligent “man-bats.”
Herschel did make wonderful astronomical observations from South Africa—of double stars, nebulae, star clusters, and Halley’s comet during its 1835 appearance. But the New York Sun series was, of course, an elaborate hoax. There was no Edinburgh Journal of Science or a Dr. Andrew Grant. Herschel himself did not learn about the hoax until much later. The story was the brainchild of the Sun’s ambitious publisher Benjamin Day, who wanted to move papers, and was likely written by a Cambridge-educated reporter named Richard Adams Locke, who intended to poke fun at serious speculations about extraterrestrial life in popular science books of the time. One best-selling volume titled The Christian Philosopher, or the Connexion of Science and Philosophy with Religion by the Scottish church minister and zealous science promoter Thomas Dick, first published in 1823 and reprinted several times, had claimed that the Moon harbored billions of inhabitants. Many of the Sun’s readers, and even some Yale College scholars, apparently failed to recognize the articles as satire, however. The paper never quite confessed to its transgression but did run a column in its September 16 issue discussing the possibility that the story was a hoax. Even after that, the Sun retained its popularity: its publisher had orchestrated the first major fake news story of modern times and profited handsomely from it.
Next came “Mars Fever.” It started in 1877, when the Italian astronomer Giovanni Schiaparelli reported observing a network of canali, translated as canals into English, on the Red Planet. Others claimed to see seas or lakes at the intersection of these canals, and even changing patterns of vegetation with the seasons. (Perhaps not coincidentally, it was a time of great canal building on Earth.) Some interpreted the features as the irrigation network of an advanced civilization on Mars. Percival Lowell, scion of a prominent Boston family, took this view seriously, and built a private observatory near Flagstaff, Arizona, dedicated to the study of Mars. Other observers disputed the existence of canals. By the early twentieth century, it was clear that the reported features had simply been an optical illusion, perhaps fed by the overactive imagination of Lowell and others.
The first actual detection of extraterrestrial life, if and when it happens, is indeed likely to come from telescopic observations of a distant world. But it almost certainly won’t consist of sightings of winged humanoids or even lush vegetation. Instead, we will probably find signs of life imprinted on a remote planet’s feeble light by spreading that light into a spectral rainbow of colors.
Turning Homeward
What would we look for? Carl Sagan, the accomplished planetary scientist, best-selling author, and host of the wildly successful television series Cosmos, tried to find the answer by turning that question on its head. As the Galileo spacecraft few past our planet in 1990 for a little gravitational kick on its way to Jupiter, he persuaded NASA to train its instruments on our own “pale blue dot.” He wanted Galileo to address a crucial issue: is there life on Earth? Sagan didn’t intend this as a tongue-in-cheek question. For him it was a serious scientific query, with far-reaching implications. He wanted to investigate whether signs of life on Earth can be detected unambiguously from afar. It makes sense to use our planet as a benchmark, because it is the only one we know for sure is teeming with life.
As a necessary but not sufficient indication of life, Sagan and his colleagues decided to look for an atmosphere in severe chemical disequilibrium. Joshua Lederberg, a Nobel Prize–winning molecular biologist, first proposed the criterion back in 1965. James Lovelock, best known for his Gaia hypothesis of the Earth as one tightly integrated ecosystem, made the concept more concrete by pointing out that the presence of a huge amount of oxygen (O2) in the Earth’s atmosphere together with methane (CH4) implies disequilibrium. Chemically, that shouldn’t happen. The two molecules destroy each other, so their coexistence suggests continuous production, presumably by living organisms on the surface. One has to be careful with this line of argument, though. For example, ozone (O3) in the Earth’s upper atmosphere is not in equilibrium either, but that’s because of chemical reactions driven by ultraviolet radiation from the Sun, not directly due to biological processes. (This ozone layer shields life on Earth from harmful UV rays.) It is a useful concept nonetheless, if applied carefully in the context of a particular planetary environment. As Sagan’s team wrote in its Nature paper on the Galileo experiment, “Life is the hypothesis of last resort,” once we eliminate alternative explanations.
The spacecraft’s instrument suite found evidence of water in several forms: spectral features indicating ice cover near the south pole and water vapor all over the atmosphere plus light refection levels hinting at large bodies of liquid water (i.e., oceans) on the surface. It also detected oxygen in abundance. Oxygen can be produced by solar ultraviolet light breaking water (H2O) molecules apart, allowing the light hydrogen atoms to escape into space. Some of that oxygen should react with elements like silicon and iron and become bound up in the Earth’s crust. Yet the Earth has a much larger fraction of oxygen in the atmosphere than either Venus or Mars. “Galileo’s observations of O2 thus at least raise our suspicions about the presence of life,” Sagan and his colleagues wrote.
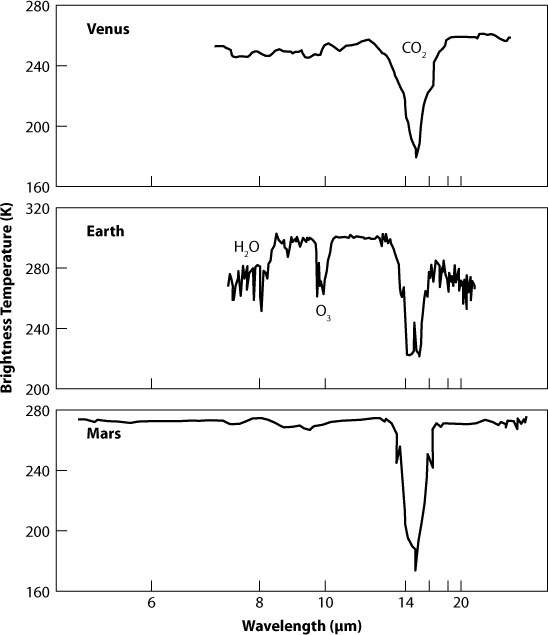
Figure 9.1. Features due to water, ozone, and carbon dioxide in the spectrum of the Earth. Credit: C. A. Beichman (Jet Propulsion Laboratory)
The detection of methane, from its distinct finger-print in the near-infrared part of the spectrum, provided a more compelling case. As Lovelock had pointed out, methane reacts quickly with oxygen to make water and carbon dioxide, so there shouldn’t be a single molecule of it left. Clearly something is pumping methane into the atmosphere much faster than it is removed by these reactions, at a prodigious rate of some 500 billion kilograms every year. That something is life—specifically, methane bacteria, rice paddies, cows, and fossil fuel burning in the case of the Earth. The presence of nitrous oxide (N2O), albeit in smaller quantities, also implied biology—bacteria and algae that convert nitrates in soil and oceans into N2O. Sagan’s team concluded: “Galileo found such profound departures from equilibrium that the presence of life seems the most probable cause.” The clinchers came in the form of a prominent feature in the red part of the visual spectrum, presumably from a “light-harvesting pigment” (i.e., chlorophyll in plants), and radio signals strongly suggestive of an artificial origin. The latter, by the way, was the only indication Galileo picked up of a technological civilization.
The results of Sagan’s experiment were reassuring about our prospects for detecting signs of life, or “bio-signatures,” from afar. But in many ways it was too easy. The spacecraft was barely 1,000 kilometers from Earth at its closest approach, so its images revealed continents, oceans, the Antarctic polar cap, and changing cloud patterns. Our first views of an extrasolar Earth, on the other hand, will be limited to a speck of light at best, glimpsed from many light-years away. That means we will have to settle for interpreting a planet’s emission averaged over an entire hemisphere.
A Little Help from the Moon
Luckily, there is a way to mimic that eventuality without ever leaving our planet, thanks to earthshine. It’s the glow of sunlight reflected by the Earth and falling on the unlit part of the Moon, easily visible during the Moon’s crescent phase. Also known as the “ashen glow” or “the old Moon in the new Moon’s arms,” it was Leonardo da Vinci who figured out the cause of this phenomenon back in 1510. Since the Moon is not a good mirror, its refection back to us blends together light from different parts of the Earth. Thus, by taking a spectrum of earthshine, scientists get an integrated hemispherical view of the home planet, akin to a spectrum we would take someday of a distant alien world. There is a slight complication, however: earthshine is contaminated by solar and lunar spectra, since it is sunlight reflected by both the Earth and the Moon. Subtracting light from the illuminated lunar crescent, which also contains the solar and lunar spectra, allows scientists to isolate the Earth’s contribution.
A few years ago, two teams of astronomers made careful observations of earthshine, to look for signs of life in its midst. Wesley Traub of the NASA Jet Propulsion Laboratory and Neville Woolf of the University of Arizona led one team, while Luc Arnold of the Observatoire de Haute Provence and Jean Schneider of the Observatoire de Paris led the other. Sure enough, both the American and French teams identified spectral features due to candidate biosignatures like water vapor, oxygen, and ozone. What’s more, the earthshine spectrum rises toward the blue, because molecules in the Earth’s atmosphere scatter blue light more efficiently than red light. (The sky appears blue to us for the same reason.) That’s why Sagan’s “pale blue dot” is a fitting moniker.
Astronomers have also made a tentative detection of the so-called red-edge signature of chlorophyll. Plants absorb visible light and use that energy for photosynthesis. But just beyond 0.7 microns, which is about the longest wavelength our eyes can see, they become highly reflective, sending back nearly half the incoming light. That leap in reflectivity appears as a sharp rise in the red part of the spectrum. In fact, Earth-observing satellites like Landsat use it to map changes of the forest cover in the Amazon, for example, by taking images in two bands that fall on either side of the red edge. In the hemisphere-averaged spectrum of earthshine, the red-edge signature is diluted by those vast areas on the planet either devoid of vegetation (e.g., oceans, deserts) or hidden beneath clouds, making it tougher to detect. Giovanna Tinetti of University College London has estimated that at least 20 percent of a planet’s surface must be covered by plants and free from clouds for the vegetation’s imprint to show up in the global spectrum.
The spectrum of the Earth has not remained the same over its 4.5-billion-year lifetime. At the earliest times, absorption features due to carbon dioxide were likely the most prominent, before much of that carbon was tied up in carbonate minerals like limestone and dolomite. Sometime later, methane would have built up in the atmosphere, though it is difficult to determine whether the dominant source of it at the time was biological (i.e., methane-fixing bacteria) or not (i.e., outgassing from midocean ridge volcanoes). For that reason, methane by itself is “an ambiguous signature,” according to James Kasting of Pennsylvania State University. “If we were to find an Earth-like planet rich in methane, there would be a big debate as to whether or not it harbors life,” he added. But scientists point out that that other biosignatures would have been detectable with reasonable confidence for about half the Earth’s history, much longer than it has harbored intelligent life. For example, oxygen and ozone became abundant a little over 2 billion years ago, with the rise of photosynthetic bacteria and plants. Our planet’s spectrum has exhibited chlorophyll’s traces since the first land plants evolved 450 million years ago. As Woolf put it, “Any advanced civilization that cared to inquire would know that life has been present on Earth for a very long time.”
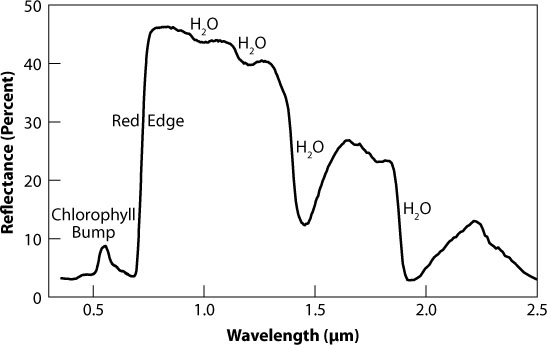
Figure 9.2. The so-called red edge, due to chlorophyll in the Earth’s spectrum. Credit: S. Seager (MIT) et al.
Earth’s surface brightness varies dramatically from place to place: shiny snow-covered mountain slopes reflect much more light than deep dark oceans. In fact, astronomers have managed to measure how the spectrum of earthshine changes as different areas of the planet rotate into and out of view. For instance, it is brighter when the large landmass of central Asia faces the Moon than when the Pacific Ocean points moonward. If we monitor the spectrum of an extrasolar Earth, we might espy similar brightness and color variations even if the planet is seen as a mere point of light. Cyclical variations due to continents and oceans, or at least steady cloud patterns, coming into view could permit astronomers to infer the length of that planet’s day (i.e., rotation period). Model calculations by Eric Ford of the University of Florida, Sara Seager of MIT, and their colleagues suggest that a planet could vary in brightness by up to a factor of 2 over the course of a day, depending on ice and cloud cover. On top of the daily cycle, the brightness of earthshine also fluctuates by about 10 percent due to changing weather. Detecting similar fluctuations would indicate weather on an alien world. With precise observations over time at multiple wavelengths, we might even infer seasonal variations.
Scientists got to do a trial run in 2008, when the Deep Impact spacecraft looked back at the Earth from tens of millions of kilometers away, after it had completed its original mission to a comet. Nicolas Cowan, then a graduate student at the University of Washington in Seattle, and his colleagues used the probe’s cameras to observe the Earth in seven colors over the course of a day as different parts of the planet rotated into view. Then they shrank each image into a single point of light, to mimic future observations of extrasolar planets, and measured how its brightness changed with time. The researchers saw variations of 15–30 percent, depending on the wavelength, and were able to infer the presence of land and oceans. They also found signs of something in the atmosphere moving independently of the Earth’s rotation, based on observations on two different days—evidence of clouds. What’s more, the variations in cloud cover suggested the presence of a liquid near its vapor temperature; based on the Earth’s temperature, they concluded the liquid had to be water.
Gathering Momentum
These remote observations of the Earth and inferences about biosignatures detectable from afar constitute aspects of the emerging field of astrobiology. It is a multi-disciplinary endeavor bringing together geochemists, molecular biologists, astronomers, and planetary scientists to investigate issues of life and its habitats. The ultimate goal of astrobiology, of course, is to detect and characterize extraterrestrial life. In the meantime, some researchers have made great strides in expanding our view of habitable environments by identifying life forms that survive, or even thrive, under what we would consider rather harsh conditions on Earth. Some of these “extremophile” organisms live near superheated volcanic vents on the deep ocean floor, while others consider caves dripping with sulfuric acid home. Yet others live kilometers beneath the ice sheets of Greenland and Antarctica. Their survival, with little or no energy from the Sun, makes it conceivable that microbes could exist below the surface of Mars, Jupiter’s icy satellite Europa, or even Saturn’s geyser moon Enceladus. Robotic landers on Mars have already dug up topsoil to search for signs of present or past life. None has been found yet, but recent revelations about ancient surface water, from NASA’s Mars Reconnaissance Orbiter and other spacecraft, and present-day methane, first from ground-based telescopes and later from the European Space Agency’s Mars Express, have added to the excitement.
Some would say that astrobiology had a dubious debut on the world stage in 1996, when a team of researchers announced that a meteorite from Mars collected in Antarctica contained fossils and other evidence of nano-bacteria —a claim now disputed by many scientists. Soon after, the field received a huge boost from NASA, when the space agency developed a “national astrobiology roadmap,” set up a network of research groups across the country and committed tens of millions of dollars in annual funding. A slew of Mars missions and extrasolar planet discoveries have fueled the field’s growth in no small measure. Today a major astrobiology conference can easily attract over 500 researchers.
The purview of astrobiologists includes planning for future space missions, such as NASA’s Terrestrial Planet Finder and ESA’s Darwin (see chapter 7). Scientists need to evaluate the relative significance of various planetary biosignatures before agreeing on the most promising observing strategies. Using earthshine as a benchmark does have a severe drawback: it is pretty much limited to wavelengths of visible light and a bit of near-infrared. That’s because the Earth’s atmosphere absorbs mid-infrared radiation before it reaches the ground. Besides, the Moon shines brightly at these longer wavelengths, swamping any Earth signal. Space-borne telescopes, on the other hand, could exploit the mid-infrared regime, especially because the brightness contrast between a terrestrial planet and its host star is much more favorable at those wavelengths. What’s more, many of the relevant molecules, such as oxygen, ozone, methane, water, and carbon dioxide, all exhibit prominent features in that spectral range.
Not surprisingly, among the most reliable biosignatures identified by a NASA-commissioned study are oxygen (O2), a by-product of photosynthesis by plants on Earth, and its cousin, ozone (O3), which is actually easier to detect in a spectrum even though it is much less abundant. Oxygen molecules do not stay single for long; they combine easily with other compounds, such as silicon in rocks, in a process known as oxidation. “So, to sustain a large amount of oxygen in a planet’s atmosphere, there needs to be continuous production,” explained Wesley Traub of the Jet Propulsion Laboratory. And life as we know it is an excellent source of oxygen.
Does that mean the detection of oxygen or ozone in a planetary spectrum is a foolproof indication of life? Not necessarily, says Penn State’s James Kasting, who is a member of the NASA working group on biosignatures and a renowned expert on planetary atmospheres. “We know of nonbiological processes that can also result in an oxygen-rich atmosphere,” he pointed out. One such example is Venus, with its runaway greenhouse effect. The breaking up of water molecules (H2O), followed by the quick escape of hydrogen into space, continuously replenishes the oxygen in its atmosphere. Another example would be an ice-covered planet massive enough to hold its oxygen. The frozen crust would prevent the oxygen from interacting with minerals on the ground, thus retaining it in the atmosphere. Although scientists need to guard against such false positives, the identification of either oxygen or ozone in an exoplanet spectrum would be “very exciting and significant,” as the NASA biosignature report concludes. The likelihood of their biological origin would be bolstered greatly if a gas like methane, which reacts strongly with oxygen and removes it from the atmosphere, were also present.
The search for life as we know it is also a search for liquid water. So detecting high levels of water vapor in a terrestrial planet’s atmosphere would be good news. At least in the case of an old rocky world, it would indicate large reservoirs of surface water for continuous replenishment. Otherwise, the water molecules in the atmosphere would have been broken apart by stellar ultraviolet rays long ago.
Chris Chyba, who did his PhD under Carl Sagan and is now a professor at Princeton, for one, believes that unambiguous detection of life via remote sensing will be extremely difficult. Separating true biosignatures from those that could also arise from nonbiological processes is far from trivial, he thinks, especially if scientists have little information about the surface conditions of a planet. “But that doesn’t mean we shouldn’t be trying to do it,” said Chyba.
Perhaps the most convincing evidence for life on a distant world would be finding multiple biosignatures. One possibility would be to detect oxygen or ozone along with liquid water, coupled with high levels of carbon dioxide (exhaled by animals) or methane (released on Earth by bacteria in rice paddies and cow dung). “The general consensus is that if we find several of these bio-signatures simultaneously, it will be a very strong indicator of life’s presence,” said Malcolm Fridlund, project scientist for Darwin at ESA. Of course, finding those signatures would not distinguish between primitive bacteria and complex aliens. That would require a different kind of sign.
Virtual worlds
Some scientists worry that life elsewhere would be so fundamentally different that biosignatures identified for terrestrial life might be all but meaningless. Traub agreed: “There’s great danger in generalizing from one example.” On the other hand, he argued, life anywhere may have at least a few things in common. “Despite all our speculations, we haven’t come up with a convincing alternative to water and carbon chemistry as a basis for life. So I see no reason to go looking for more exotic things, at least for now,” he said. Of course, there could well be many forms of life that do not register on a planet’s overall spectrum, such as bacterial communities underground or in deep ocean vents. And it may be difficult to detect biosignatures at the surface—like chlorophyll on Earth—if clouds covered much of the planet.
Coming up with life-bearing worlds different from the present-day Earth is one of the goals of the Virtual Planetary Laboratory (VPL), a NASA-funded multimillion-dollar project led by Vikki Meadows of the University of Washington in Seattle. Her team consists of tens of researchers from disciplines as varied as statistics and biochemistry. They are developing sophisticated computer simulations of the environments and spectra of a broad range of rocky planets with, and without, living organisms. “We are modeling life as we know it—but not necessarily in the balance we have here on Earth,” she explained.
The VPL project’s goal is rather ambitious: to construct the first models of terrestrial planets that combine the effects of—and the interplay between—stellar heat, climate, chemistry, geology, and biology. Once those models are in place, Meadows and her colleagues validate them first by comparison with Venus, Earth, and Mars. Later, they reconstruct what the early Earth would have looked like from a distance, before its atmosphere became rich in oxygen. Then the researchers “play around with the recipe,” trying different combinations of size, composition, and temperature to investigate the effects of these on life and vice versa. To explore the range of temperature at which life might exist, for example, “we’ll model everything from frozen hells to burning hells,” said Meadows.
One question that VPL team members and other scientists have looked at is how Earth-like planets would evolve around different kinds of stars. Using computer codes that calculate the chemistry of terrestrial atmospheres as they interact with incoming starlight, these researchers find big differences between an Earth twin orbiting a K-type star, smaller and cooler than the Sun, and one circling an F-type star, more massive and hotter than the Sun. A K-type star puts out a lot less ultraviolet radiation, so the ozone layer that develops around its planet is thinner, closer to the ground and cooler than the Earth’s. In fact, its ozone layer is much colder than the planetary surface. This temperature contrast results in a deep absorption feature due to ozone in the planet’s spectrum. The reverse is true for an Earth analog next to an F-type host: its ozone layer is denser and hotter than ours, with temperatures nearly the same as on the ground. Given the minimal temperature difference, the imprint of ozone is weak and barely detectable. The bottom line here is that planets around G-type stars like our Sun and somewhat cooler K-type stars are better candidates to look at for the ozone signature than their counterparts orbiting more massive hosts. Luckily, lower-mass stars are more numerous and live longer (see chapter 8).
On Earth, pretty much all life forms—except for some microbe communities sustained by hydrothermal vents or radioactive decay of rocks—rely on energy from the Sun directly or indirectly. Photosynthesis is fundamental to our planet’s ecosystem. The same should hold true for other planets. It is very likely that starlight-processing pigments of some kind, akin to green chlorophyll on our planet, would arise elsewhere, according to Nancy Kiang of the NASA Goddard Institute for Space Studies in New York. That means, in addition to searching for products of photosynthesis like oxygen and ozone as biosignatures, we could also look for the spectral signature of those pigments. Green may not be the dominant color of alien plants, however.
The basic photosynthetic process on Earth uses particles of light, or photons, for the chemical reactions that combine carbon dioxide and water to form simple sugars while releasing oxygen as a by-product. In doing so, the machinery of chlorophyll preferentially absorbs blue and red light, while reflecting green. The blue photons, which have higher energy, are downgraded to lower-energy red photons in a series of steps. That’s because the complex molecules at the reaction center are fine-tuned to use red photons, through evolutionary adaptation. The Sun’s emission peaks at green-yellow, but contains a broad spectrum of light. Water vapor in the Earth’s atmosphere absorbs in the infrared, while oxygen absorbs in the red. The ozone layer blocks pretty much all the ultraviolet and also absorbs weakly across the visible range. As a result, the peak is shifted from yellow to red by the time sunlight reaches the Earth’s surface. So land plants here have adapted to utilize red photons, which are the most common in their environment.
That wasn’t always the case, because the early Earth’s atmosphere lacked oxygen and ozone. The first photo-synthetic organisms lived under water, which acted as a solvent for biochemical reactions and provided protection from UV rays in the absence of the ozone layer. These bacteria had to use infrared light, filtered through the ocean, so their pigments had to be different too. Later, as oxygen and ozone levels built up, green algae emerged, first in shallow water and eventually on land. Their plant descendents have adapted to the atmosphere’s changing composition, itself primarily the result of photosynthesis. Chlorophyll is customized for present-day conditions on Earth.
Kiang and her collaborators have considered which color pigments would dominate on planets in the habitable zones of stars different from our Sun. Her team was drawn from disciplines as varied as stellar astronomy and biochemistry. The answer depends on the spectrum of light reaching a planet’s surface, which in turn depends mainly on the host-star type. The researchers found that blue photons are the most numerous on planets orbiting hotter F stars, whereas red photons dominate on cooler K stars. Absorption by ozone shifts the peak toward blue in the former and toward red in the latter. On F-star planets, the plant pigments may absorb primarily in the blue, so these alien plants would appear orange or red. If we were to take a spectrum of such a planet from afar, it might show a blue edge, rather than the red edge seen in an earthshine spectrum.
M-type stars, also called red dwarfs, are even cooler and fainter than K stars. That means their planets would receive a lot less light, albeit enough to sustain life, and most photons would arrive in the near-infrared. Under those conditions, the pigments may adapt to utilize the full range of visible and infrared light, reflecting as little as possible, so that their plants might appear black to our eyes. The difficulty is for the first photosynthetic organisms to take hold on such worlds, before an ozone layer develops to protect them from bursts of UV rays that M stars are known to produce often, especially when they are young. Kiang and her colleagues estimated that early microbes would have to be about 9 meters under water to escape the UV fares but still receive enough light for photosynthesis.
If a future telescope reveals a distinct absorption band in the spectrum of a distant rocky world, it just might be due to alien plants. Kiang’s team has given us clues about where in the spectral rainbow that band should appear, depending on the nature of the planet’s host star. But the predictions are not unique, so there may be a debate as to whether a mineral could produce the same imprint.
Some scientists think handedness, or chirality, might constitute a universal beacon for life. Organic molecules have either a left-handed or right-handed orientation, but biochemistry selects for one variety over the other. Dealing with only one version, scientists suspect, is an advantage, if not a necessity, when building complex compounds like DNA and proteins. The same should hold true for life elsewhere. The question is how to espy this subtle characteristic from a distance. It’s fairly easy to detect chirality of purified samples in the lab, as the French microbiologist Louis Pasteur did in 1848 for a compound derived from wine lees by measuring how the electric field of light passing through the material is rotated clockwise or counterclockwise—a phenomenon known as circular polarization. Recently a team led by William Sparks of the Space Telescope Science Institute managed to do the same with photosynthetic microbes. They found a value between 0.1 and 0.01 percent. That would be tough to measure from a distance, though perhaps not impossible with a future telescope, depending on what fraction of an alien planet’s light comes from living organisms.
Discovery Prospects
The detection of biosignatures—whether certain gases or plant pigments—on extrasolar worlds will probably have to await the launch of NASA’s Terrestrial Planet Finder or ESA’s Darwin mission. But if we are extremely lucky, there is a tiny chance of doing it much sooner and a lot more cheaply, according to Michael Jura of the University of California at Los Angeles. In fact, current ground-based telescopes are already up to the task, provided many other “ifs” are satisfied.
When a Jupiter-like planet transits in front of a Sun-like star, it covers about 1 percent of the star’s face, causing an equivalent dip in the star’s brightness (see chapter 5). But what if the parent star is smaller than the Sun, say one-tenth of a solar mass? Such a star, a late-M dwarf, has a diameter almost as small as Jupiter. If such a star harbors a Jupiter-like planet in a tight orbit that happens to be seen edge-on from Earth, we might see the host star undergo a total eclipse, Jura pointed out. What’s more, if the hypothetical system also included a second terrestrial planet, then its feeble reflected light might peek through during the total eclipse. “That would give us a chance to measure its colors in order to look for signatures of life,” said Jura, who is well regarded among his colleagues for thinking outside the box. Of course, as Jura himself conceded, the chances of all the conditions being just right are rather small. To begin with, close-in Jupiters appear to be less common around lower-mass stars. But it is possible that one of the many ongoing planet-transit searches around the world will turn up a near-total eclipse of a small star.
For now, Jura guesses that perhaps only one out of every 10,000 low-mass stars would undergo a total eclipse by a close-in giant planet. Cataloguing and monitoring that many low-mass stars in search of an eclipsing Jupiter with a terrestrial sister is a daunting task. “While the odds of success are highly uncertain and the observations are technically challenging, at least we don’t need to wait a decade to start the search for extrasolar life,” Jura argued.
Our prospects of detecting biosignatures will improve somewhat with the launch of NASA’s James Webb Space Telescope (JWST), scheduled for 2014 (see chapter 7). In principle, it will be capable of looking for imprints of various molecules—like oxygen, ozone, water, and carbon dioxide—in starlight that skims the atmosphere of a large terrestrial planet during transits, the same way that the Hubble and Spitzer space telescopes have already done with transiting Jupiters (see chapter 5). We will need to be rather lucky, though. The transiting super-Earth would have to circle one of the nearest stars, or JWST won’t be able to detect its atmosphere. Even in the best circumstances, it would take many hours of observations to make a detection. The TPF and Darwin missions, which may launch in the next decade, will offer much better odds. These observatories are designed to take pictures and spectra of Earth-like worlds so they do not depend on catching a planet in transit. Besides, they can target more-distant stars, thus a much larger sample of hosts. So these missions offer the best prospects for finding a life-bearing alien world in our lifetime.
Of course, our first indication of life elsewhere could come from an entirely different endeavor: the search for extraterrestrial intelligence, or SETI, through artificial radio (or optical) signals. The modern efforts began in 1960 with Frank Drake, then at the National Radio Astronomy Observatory. He used the 26-meter radio dish at Green Bank, Virginia, to examine two nearby stars for signals at frequencies near that emitted by neutral hydrogen, a reasonable choice because hydrogen is the most common element in the universe. Since then, a number of SETI experiments, growing in scope and sophistication over time, have been undertaken around the world. A NASA-funded SETI program, launched in 1992 with great fanfare, was canceled a year later, after some members of Congress ridiculed it. The Center for SETI Research at the nonprofit SETI Institute in California, led by Jill Tarter, resurrected it in 1995 under the name Project Phoenix with private funding. By now, it has surveyed close to a thousand nearby star systems.
The SETI effort came of age when the first forty-two dishes of the Allen Telescope Array (ATA) were activated in late 2007. Located in the Hat Creek Valley in rural northern California, and funded in part by Microsoft cofounder Paul Allen, the ATA is optimized for SETI but will also be used for conventional radio astronomy. The remote location, shielded by the Cascades, offers some respite from terrestrial radio interference but not from satellites overhead, which makes some frequencies unusable. The array is built relatively cheaply with mass-produced 6-meter dishes and incorporates state-of-the-art digital signal-processing technology. The latter is critical, because it will examine a million stars over the next two decades, monitoring several targets at once and scanning through billions of radio channels. The back end of the telescope needs to keep up with the whopping rate of incoming data, process it rapidly, and fag any unusual signals. Eventually, if funding permits, the ATA will grow to 350 dishes, improving its sensitivity to weaker signals from farther star systems.
In many ways, the search for habitable extrasolar planets and SETI are complementary approaches to addressing the same fundamental question: are we alone? The former may reveal biosignatures elsewhere, but we won’t know whether they indicate slime or civilization. We could then point the SETI receivers at those particular worlds for an exhaustive search for signals of artificial origin. It may be that life is fairly common, but intelligent life is rare. On the other hand, it seems absurd, if not arrogant, to think that we are the only technological civilization in the Galaxy, given 200 billion other suns, the apparent ubiquity of planets, and the cosmic abundance of life’s ingredients. But it’s one thing to guess at probabilities and quite another to have proof.
However it arrives, the first definitive evidence of life—even of primitive life—elsewhere will mark a revolution in science, perhaps only rivaled by Copernicus’s heliocentric theory that dislodged the Earth from the center of the universe or Darwin’s discovery of evolution that suggested all species on our planet, including humans, descended from common ancestors. If life can spring up on two planets independently, why not on a thousand, or even a million, others? The implications of finding out for sure that ours isn’t the only inhabited world are nothing short of astounding: it will trigger paradigm shifts not only in science but also in many other human endeavors, from the arts to religion. We will see ourselves differently. That dramatic moment is no longer a remote possibility: it may well occur in our lifetime, if not during the next decade.
