![]()
Explanations always go in one direction, from the complex to the simple and, in particular, toward what is less distinctly human.
—T. H. JONES
We’ve just looked at how reductionist design leads to reductionist answers and excludes the true nature of biological complexity. Now it’s time to revel in that mind-boggling complexity, specifically when it comes to nutrition.
In this chapter I want to introduce you to an old friend of mine: an enzyme called mixed function oxidase (MFO), which ultimately converted me from a reductionist to a wholist.1 Sharing more about the function of enzymes, those amazingly complex and powerful molecules responsible for every chemical reaction that goes on in our bodies, is the best way I can think of to show you the complexity of nutrition’s effect on health—and the inadequacy of the reductionist model of scientific inquiry to address it.
MY MFO BACKSTORY: PEANUTS AND LIVER CANCER
As I mentioned in the book’s introduction, my first official research project as a professor at Virginia Tech back in 1965 was to analyze peanut samples for the presence of the cancer-causing chemical aflatoxin (AF).2 A product of the mold Aspergillus flavus,3 AF had recently been shown to be a very potent liver carcinogen for laboratory rats.4 On the list of America’s most popular foods, peanuts rank somewhere up there with milk and T-bone steaks. They’re what help keep hands busy at cocktail parties; they’re half of that most beloved of lunchbox sandwiches, the PB&J. So the possibility of a mold-produced carcinogen in peanuts was a dreadful thought. The other troubling aspect of these findings was that the amounts of AF required for liver cancer in rats appeared to be exceptionally low, possibly making AF the most potent chemical carcinogen ever discovered, at least for rats.5
My team’s task was to learn something about the climatic and geographic conditions that fostered Aspergillus flavus growth. We studied several edible plants, but focused specifically on peanuts.
Shortly thereafter, the dean who hired me at Virginia Tech, Charlie Engel, asked me to join him in developing the nationwide childhood nutrition program in the Philippines in collaboration with Manila’s Department of Health—a project funded by USAID. One of our main goals was to identify a source of protein for these children that could be grown locally and relatively inexpensively. The obvious answer, at least to us, would have been peanuts. They’re high in protein, most kids love them, and they grow like crazy in a wide variety of climates and settings. There was just one problem: AF.
Before we could grow peanuts to solve the protein gap, we had to understand and solve the potential AF contamination problem. Because of my earlier experience with AF, that became my assignment. After setting up and equipping an analytical laboratory in Manila, I then began with my colleagues in the Philippines to explore the chief food sources of AF consumption. Were peanuts the main source of contamination? What about other foods? Did the people eating AF-contaminated foods really get more liver cancer? If so, what could we do to eliminate AF, or neutralize its negative effects, so we could use peanuts as a cost-effective protein source for the poor?
We started by collecting peanut products from the marketplace. Shelled peanuts, the more expensive product purchased by the affluent (our original samples came from a cocktail party at the U.S. Embassy!) were clean, with little or no AF. In contrast, peanut butter, a cheaper product especially consumed in urban centers like Manila, was heavily contaminated. All of the twenty-nine peanut butter samples we initially collected contained AF, with an average of 500 parts per billion (ppb),6 but with exceptional levels as high as 8,600 ppb.7 These findings were alarming because, at that time, the U.S. FDA had proposed an upper limit of 30 ppb as a “safe” level in human food (later revised downward because even lower levels were shown to cause serious toxicity and cancer in rats, rainbow trout, and very young ducklings).8
To learn the reasons for this huge discrepancy in AF levels between whole cocktail peanuts and peanut butter, I joined the Philippines’ FDA Commissioner in a visit to a peanut butter manufacturing plant. The answer was easy to see. In the manufacturing plant, whole peanuts in their shells were placed onto one end of a conveyer belt, which moved past a line of workers; at the end of the moving belt, the peanuts were delivered into a grinder and a big cooking pot. As the peanuts passed by the workers, they handpicked kernels for the cocktail peanuts, leaving the rest to be dumped into the grinder and cook pot to make peanut butter. The good, attractive kernels went into the cocktail jars, the bad into the peanut butter tank. By “bad,” I mean the discolored, often shriveled kernels—the ones most likely to be infected with the fungus. These kernels, we learned when we tested them, contained AF in concentrations as high as two million parts per billion, meaning that even a single fungus-contaminated kernel could spoil an entire batch of peanut butter and easily push AF levels over the allowable limit.9
With additional funding from the National Health Institute, I then did a quick survey of possible consumers of AF and learned that, just like in the United States, children ate most of the peanut butter in the Philippines. Because I assumed that virtually all commercially sold peanut butter was contaminated, my coworkers and I then visited homes to ask whether they customarily ate peanut butter and, if so, whether we could purchase any partly emptied jars for AF analysis. We also asked the mother in the household for an estimate of when and how much peanut butter had been consumed in the previous twenty-four to forty-eight hours, and from this I estimated actual AF consumption. We also collected urine specimens from each family member so that, for future follow-up studies, we might be able to measure some product of AF in the urine as a reliable marker of AF ingestion.10
I therefore had estimates both of AF consumption and excretion and was able to show that AF metabolites only appeared in the urine samples of those individuals consuming the AF-contaminated peanut butter.11 We also found that consumers of AF-contaminated foods were excreting AF metabolites in their urine that proved carcinogenic12 to animal test subjects.13
MFO, AF, AND CANCER
Throughout this research period, I continued to believe, as other researchers did, that AF might be an important carcinogen for humans. But I also understood that this very potent animal carcinogen had not yet been shown to be a human carcinogen—at least not in an independent manner. We knew at that time, for example, that the mouse, unlike the rat, was not susceptible to AF carcinogenicity,14 and if these closely related species responded to AF in totally opposite ways, one susceptible and one resistant, it was not unreasonable to assume that humans might also be resistant as well. Clearly we still had a lot to learn about AF’s connection to cancer: was it relevant to humans, and if so, what was the causal mechanism?15
In exploring these questions, I started with the assumption that the MFO enzyme was involved because evidence suggesting its relationship to AF and cancer already had been published by a research group in England.16 It showed that MFO was responsible for converting AF into not one but several less carcinogenic products that were excreted in milk and urine. The more efficiently MFO functioned (i.e., the more “active” it was), the more AF was detoxified, suggesting that increasing MFO activity might lower the risk of liver cancer.
At around the same time, researchers were discovering that MFO’s activity could be modified—sped up, slowed down, and altered in other ways—by certain agents, like drugs.17 In my laboratory, we were finding that increasing dietary protein increased MFO activity.18 Perhaps, we thought, protein could be used to supercharge MFO and stop cancer in its tracks.
Then I stumbled upon that 1968 report from India I mentioned in chapter three that showed what appeared to be the opposite: namely, that higher dietary protein increased AF-induced tumor development.19 That couldn’t be! Protein, everyone’s favorite nutrient, could cause cancer? And the protein they used was casein, the principal protein in the healthiest drink there was: cow’s milk. I needed to learn more about this finding and either reproduce it or refute it as a fluke.
At the same time I was discovering an equally unsettling fact about childhood liver cancer in the Philippines: it occurred with much higher frequency not necessarily in the children who consumed greater quantities of AF, but in children from wealthier families, the ones who ate more protein and more “high quality” animal protein. The Indian protein/tumor study and the Philippine animal protein/cancer connection were starting to shake my world. Did more protein prevent cancer or cause cancer?
The possible key to solving this mystery was MFO, the startling enzyme that was now implicated both in the initiation of liver cancer by AF and in the detoxification and disposal of AF from the body. What was going on? Did dietary protein speed up MFO’s conversion of AF into nontoxic water-soluble metabolites? Or did it activate AF into nasty carcinogenic metabolites? Or both? We suspected we were on to something much bigger than just a way to neutralize or promote AF-induced liver cancer. We theorized that MFO might be a key factor in turning cancer on and off not just in the liver, but possibly also in other tissues in the human body.
This paradoxical protein effect hinted at what we eventually found to be the case: MFO responds to the foods that we eat every day. Certain diets turn MFO into a highly efficient cancer-fighting machine; other diets send MFO into a frenzy that produces carcinogenic by-products.
To understand how this is possible, we need to look at nutrition and how it affects enzymes more generally. Not only will we resolve the MFO–AF paradox, we’ll also see how reductionist nutritional thinking simply can’t handle the question—and thereby misses the most powerful lever we possess in our effort to eradicate cancer.
THE BIOCHEMICAL BASIS OF NUTRITION
If you took high school biology, you probably spent some time memorizing bits of a chart of aerobic respiration known as the Krebs cycle. That chart, if it didn’t put you to sleep first, probably gave you the idea that nutrition is a very linear process. From the inputs of carbohydrates, fats, and proteins, the cells in the body predictably extract energy, produce a myriad collection of useful metabolites, and release leftover carbon dioxide and water. The arrows that connect different steps in the process seem authoritative, as if the described step always happens in precisely the same way every place, every time, under every condition. While this model is useful for understanding the basics, it doesn’t reliably correspond to reality. Nutrition is far more complex than a static diagram might imply.
Nutrients generally do not follow a single predictable path after they enter the trillions of cells in our bodies. In most cases, the potential route a nutrient can take once it enters the body branches out, directly or indirectly, into multiple pathways of products (metabolites), with each pathway possibly branching out into still more pathways. Furthermore as these pathways develop, they may lead to many different kinds of activities or functions, like mobilization of energy and repair of damaged cells. The dominant pathways end up determining to a great extent whether we enjoy health or suffer disease. Understanding metabolism is not just a matter of following a nutrient down a large number of independent pathways, however. As these pathways branch out, their integration with one another seems endless.
Maps of these metabolic mazes decorate the walls of many research facilities; your high school Krebs cycle chart is just a highly pared-down version of part of one of them. I’ve been in this research business long enough that I’ve been able to watch the emergence of one of the most complex of these maps, which began many years ago as the glucose–metabolism network of reactions that produces energy shown in Figure 7-1. (This particular chart, which does an excellent job of displaying the complexity of intermediary metabolism, is the work of Dr. William L. Elliott [HealthBuilding.com].) The earliest version of this map was most helpful as I taught biochemistry during the 1960s and 1970s at Virginia Tech’s Department of Biochemistry and Nutrition. It took me at least a dozen lectures in a basic biochemistry course merely to describe the series of reactions that lead from glucose to the circular Krebs cycle at the bottom of the chart, primarily representing the extraction of energy from glucose.
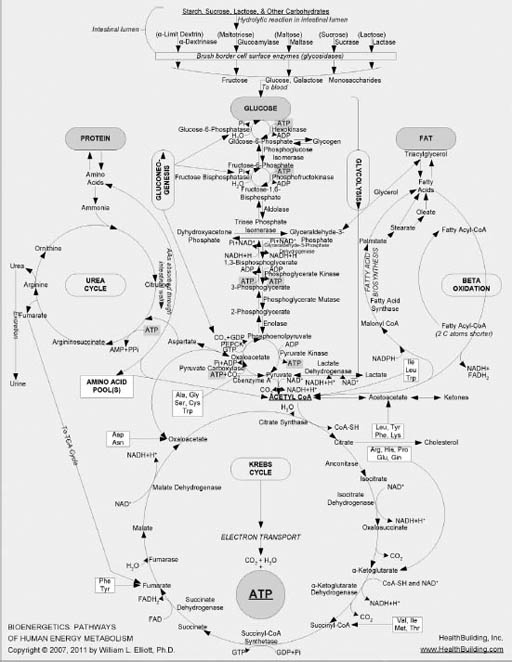
FIGURE 7-1. Chart mapping glucose metabolism and other metabolic pathways20
Complicated, right? But the map I used in class only scratched the surface of what we know now about glucose’s metabolic pathway. Over time, more clusters of metabolic reactions were added to that initial map, including segments on protein, fat, and nucleic acid metabolism. It wasn’t long before so many reactions had been added, and the font size had become so small for reasonably sized paper, that it was clear that no more could be added and still be readable by the naked eye. The metabolic cartographers began creating entire atlases of cellular metabolism, with what had once been simple reactions now meriting several pages of diagramming to account for updated discoveries.
These comprehensive maps became more and more specialized and fragmented in a way that graphically symbolizes how reductionism, by pushing for ever smaller and more specific pieces of information, loses sight of the whole. Researchers spent years, even decades, working on just one or two reactions. Gradually, insets of insets of insets emerged on the map, as our probes of knowledge went ever deeper into cellular metabolism and grew ever less able to see the intelligence and power of the whole system.
A phrase with the same root as reductionism is “reductio ad absurdum,” or following a concept to the point of absurdity. Remember Figure 7-1’s complex chart showing glucose metabolism? You can see an updated version in Figure 7-2.
Scientists have gone even deeper than this. Figure 7-3 shows the complexity involved in just a very small section of that map, blown up for visibility.
And the more comprehensive metabolic map in Figure 7-2 is only an infinitesimally small portion of all the reactions in each of our hundred trillion cells.
I emphasize this metabolic complexity so you can see just how impossible it is to fully understand the way our bodies react to the foods we eat and the nutrients they contain. Explaining nutrient function by only one or even a couple of these reactions is not sufficient. Once consumed, nutrients interact with one another and with other food-borne chemicals within an enormous maze of metabolic reactions located in these hundred trillion cells. No single reaction or single mechanism accounts for an individual nutrient’s effect. Every nourishing nutrient and related food chemical enters cellular metabolism and gets metabolized into multiple products via highly integrated pathways just as complex as those as shown in Figures 7-1 to 7-3.
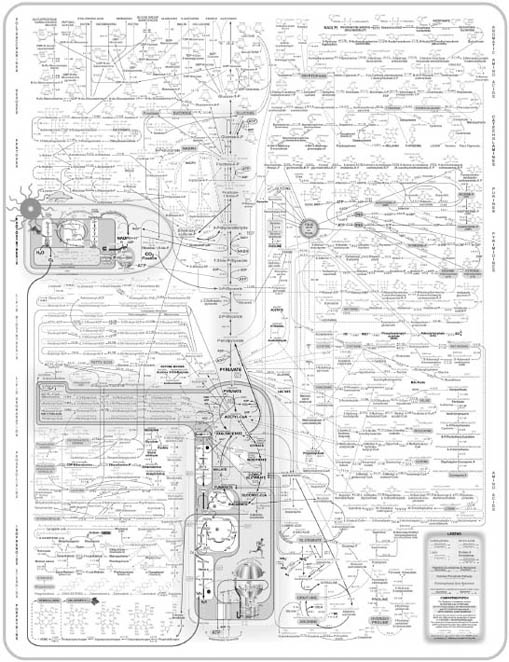
FIGURE 7-2, Expanded chart mapping glucose metabolism and other metabolic pathways
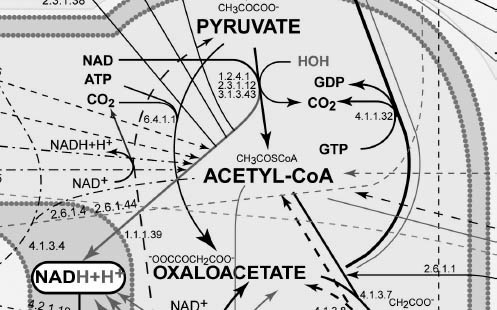
FIGURE 7-3. Expanded inset of Figure 7-2
The fact that each nutrient passes through such a maze of reaction pathways suggests that each nutrient also is likely to participate in multiple health and disease outcomes. The one nutrient/one disease relationship implied by reductionism, although widely popular, is simply incorrect. Every nutrient-like chemical that enters this complex system of reactions creates a rippling effect that may extend far into the pool of metabolism. And with every bite of food we eat, there are tens and probably hundreds of thousands of food chemicals entering this metabolism pool more or less simultaneously.
METABOLISM AND ENZYMES
Metabolism is the sum total of all the chemical reactions in the body that sustain life. When you think of the billions of reactions that occur all the time, you might wonder how we have enough energy in our bodies to get anything else done. After all, every one of those chemical reactions requires energy. And since one of the main outputs of metabolism is usable energy for the body, it’s crucial that the energy produced be greater—by a wide margin—than the energy expended to produce it. Fortunately, we’ve evolved molecules whose main job is to significantly lower the energy required for chemical reactions within the body. These molecules are called enzymes.
I used enzymes, earlier, to help explain why a part cannot be fully understood outside the context of its system as a whole, an idea that should become even more clear as we look further at the role they play in the body. Enzymes are large protein molecules, present in all our cells, that, through a series of reactions, turn one thing (say, a sugar molecule), called a substrate, into another (say, a glucose-related chemical the body uses to synthesize fat), called a product or metabolite. Think of enzymes as large, fully-automated factories. Imagine inserting a small log (the substrate) into one end of a huge factory building and, at the exit end, collecting a nicely designed salad bowl (the product). You could turn the log into a salad bowl by hand, of course, but it would require much more time and labor. The factory dramatically increases the efficiency of the transformation. Enzymes do the same inside cells, converting substrates into products very quickly while using very little energy. The reactions enzymes cause (the word biologists use is that they catalyze reactions) rarely, if ever, occur without the assistance of an enzyme. If they do, the rate of reaction—the speed with which the reaction occurs—is a minuscule fraction of what is possible when an enzyme is involved, and the amount of energy required is much higher.
Comparatively speaking, enzymes are very large. An enzyme molecule might be 10,000 to 20,000 times the size of a substrate molecule that it processes—hence the visual of the factory and the log. Figure 7-4 shows a substrate, A, being converted to a product, B. But most reactions do not occur in isolation. They connect with follow-on reactions, like the one in Figure 7-4 where B (now the substrate) is converted to C (the new product). Enzyme 1 converts A to B, while enzyme 2 converts B to C.
A given enzyme can function at different levels of potency based on supply (the amount of substrate available) and demand (the amount of product already in the cell). Just as factory assembly lines can move quickly or slowly based on the supply of raw materials and the demand for finished goods, enzymes adjust the speed at which they convert substrate to product (known in the trade as its activity). In fact, an enzyme can even reverse reactions to return a product to its substrate. In short, enzymes control whether reactions occur and, if so, how fast and in which direction.
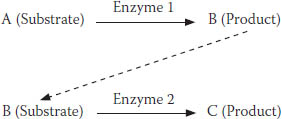
FIGURE 7-4. A simple enzyme reaction
When they initially form, enzymes appear as linear chains of amino acids, carefully arranged in sequences dictated by DNA. But because amino acids have chemical and physical affinities for each other, the chain folds onto itself (as in Figure 7-5), creating a three-dimensional shape the same way a very long string of magnetic beads might.

FIGURE 7-5. Computer-developed model of the enzyme cyclic ADP ribose hydrolase (CD38)
This folding gives enzymes one way to vary their activity: they simply change shape. This enzymatic shapeshifting is crucial because it changes the enzyme’s chemical and physical properties in ways that alter its ability to modify reaction rates. Many scientists who study enzymes wax poetic about the incomprehensible speed with which enzymes configure themselves to perform their tasks. Here’s a typical entry, from the New World Encyclopedia:
For an enzyme to be functional, it must fold into a precise three-dimensional shape. How such a complex folding can take place remains a mystery. A small chain of 150 amino acids making up an enzyme has an extraordinary number of possible folding configurations: if it tested 1,012 different configurations every second, it would take about 1,026 years to find the right one. . . . Yet, a denatured enzyme can refold within fractions of a second and then precisely react in a chemical reaction. . . . [I]t demonstrates a stunning complexity and harmony in the universe.21
The author cites numbers for a relatively small (by enzyme standards) hypothetical molecule in his attempt to describe the indescribable. The rapidity with which an enzyme responds (from a limp linear chain to a precise glob ready to do its business, in fractions of a second) is phenomenal. The chemical variety of substrates that can be metabolized by a single active enzyme is likewise phenomenal. And the large number of factors capable of modifying enzyme structure, amount, and activity is equally phenomenal.
Inherent in this discussion is the intimate connection between nutrient metabolism and the world of enzymes. Enzyme-catalyzed reactions, infinite in number and infinitely networked, are controlled by nutrients and related compounds, which also are infinite in number. Although nutrients control enzymes, enzymes also act on nutrients to manufacture endless products that are then used in the body as well as for the proper functioning of the body.
THE MFO PARADOX
Which brings us, finally, back to MFO and the role it plays in cancer formation.
Unavoidably, I’ve had to summarize, truncate, and simplify our research and findings here—the topic is just too extensive and too technical to explain in a single chapter. My goal here, after all, is not to turn you into an MFO expert. Rather, in sharing the tale of my fifty-plus-year research journey with MFO, I hope to give you a better understanding of how animal protein affects cancer formation, and a deeper appreciation of how the complexity of MFO eloquently testifies to a wholistic, not reductionist, view of nutrition and health.
MFO is a particularly complex enzyme that metabolizes many chemicals, some normally present in the body and others the body might never have encountered previously. Located largely but not exclusively in the liver, MFO metabolizes steroid-type hormones (e.g., sex hormones like estrogens and androgens, and stress hormones), fatty acids (i.e., precursors to chemicals that support the immune and neurological systems), and cholesterol (involved in cardiovascular disease and the building of cell membranes), among other chemicals, into substances that are closer to the state in which our bodies will ultimately use them. MFO also detoxifies foreign chemicals, rendering them capable of being readily excreted in the urine.
Very early in my research career I was taught that AF (like other carcinogens) is converted by the MFO enzyme to a less toxic metabolite that is excreted in the urine and feces, as is shown in Figure 7-6.
But this model was clearly too simple. For one thing, the Indian researchers I mentioned earlier, who in 1968 published their finding that a high-protein (20 percent) diet increased AF-initiated liver tumors in rats,22 previously had shown that this same high-protein diet actually decreased the immediate toxicity of AF when it is administered at very high doses.23 The results were a paradox that the traditional model of AF metabolism didn’t account for.
Suspecting MFO as the key to resolving this paradox, my lab started by establishing that the high-protein diet increased MFO enzyme activity in rats,24 meaning that the more dietary protein the rat consumed, the faster AF (specifically, the parent substrate, AFB1) was detoxified. This was the finding that made sense, but it ran counter to the Indian researchers’ observation25 that cancer increased with a high-protein diet.
![]()
FIGURE 7-6. Presumed model for MFO conversion of AF
One possibility we considered was that the MFO enzyme might be producing two kinds of metabolites: one that was less toxic than AF and safely excreted, and one that was more toxic than AF that gave rise to cancer. But why would an enzyme do such a strange and contradictory thing? Even though it seems strange, it was a real possibility in our minds; for a long time, before this and before the MFO enzyme was discovered, scientists thought that many chemical carcinogens initiated cancer only after they were “activated” by enzymes, and so a chemical like AF producing a more toxic metabolite sounded very possible.
Another key to the puzzle was discovered in the early 1970s, when University of Wisconsin Professors Jim and Betty Miller, both distinguished cancer researchers, working with their younger colleague, Colin Garner, obtained some remarkable evidence: MFO’s production of a detoxified metabolite from AF involves forming an extremely reactive intermediate metabolite that initiates cancer.26 In other words, MFO produces two metabolic products from AF: one that is detoxified and excreted, and one that is activated to initiate cancer. It’s as if a tree enters the factory, gets turned into a billy club for a fraction of a second, and only then is transformed into its ultimate shape, a salad bowl.
This intermediate metabolite is known as an epoxide, and it’s thought to exist only for a few milliseconds. Those milliseconds, unfortunately, appear to be long enough to allow the epoxide to bind very tightly to cell DNA and produce a mutation capable of initiating a series of events that lead to cancer.
![]()
FIGURE 7-7. MFO conversion of AF, updated with intermediate product
The updated reaction scheme, showing the intermediate epoxide, is shown in Figure 7-7.
This discovery provided us with a new way of understanding how high dietary protein increased cancer but decreased acute AF toxicity, as first reported by the Indian researchers: when a high-protein diet increased MFO activity, it also increased both the cancer-causing intermediate metabolite and the final, less toxic metabolites.
Another of our key findings that helped explain this paradox: AF, it turns out, is quite toxic in its own right, without requiring activation; it blocks cell respiration, causing cells to die.27 When a high-protein diet increases MFO activity, it detoxifies the AF that causes cell death—which, out of context, seems like a positive effect. But at the same time, it increases production of the epoxide that can initiate cancer—clearly a negative effect.
Our reaction scheme, one more time, updated to summarize the effects of these AF metabolites (the less toxic metabolite and the carcinogenic epoxide) in the presence of a high-protein diet, is shown in Figure 7-8.
Although we thought this was a reasonably good explanation for our paradox, it left a few questions unanswered. The first is the question of why the body produces a cancer-initiating epoxide in the first place. Or more to the point: how did a process that turns a natural but dangerous mold by-product into an equally dangerous cancer-causing substance evolve in the first place?
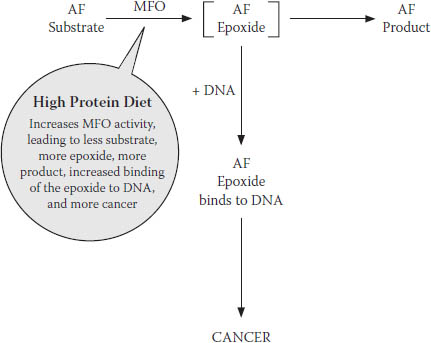
FIGURE 7-8. Final revised model for MFO conversion of AF
I still don’t know the answer to this question. But it does make sense that the body would be willing to tolerate the risk of future cancer in its urgent effort to deal with the immediate threat of cell death posed by AF. Imperfect though it may be, this trade-off clearly proved to be evolutionarily positive, or at least neutral—it couldn’t have contributed negatively to human survival and reproduction, or else it wouldn’t have survived to the present. This suggests that the body may have a self-correcting mechanism to prevent permanent damage from the epoxide. The epoxide has an unusually short life, existing only for fractions of a millisecond, which does not leave much time for damage to occur. It also turns out that water, aided by another enzyme that is close at hand during this process, epoxide hydrolase, can bind with the epoxide to form harmless products that can be excreted—effectively mopping up epoxide before it can damage DNA.
In addition, we also know that the human body has an amazing capacity to repair damaged DNA. If this ability is supported through proper nutrition, most if not all of the damage can be undone long before cancer is initiated.
The second question is why animal protein increases MFO’s activity. A high-animal-protein diet increases a broad array of enzyme activities in the body, of which MFO is only one; animal protein generally puts the body into overdrive. As of this point, we do not yet have an answer for why this occurs. Perhaps in the future we will. In the meantime, the important point is that it does, and that it has a negative effect on our health.
WHAT MFO TAUGHT ME
What you may have noticed about my initial research into AF’s connection to liver cancer is that its focus on a single MFO-catalyzed reaction was very reductionist, even though I also took into account other straightforward, reductionist reactions that may or may not have been important to whether liver cancer developed. My focus on a single enzyme (MFO) that presumably catalyzed a single reaction, involving a single substrate (AF) and a single outcome (liver cancer), was naïve to the extreme, and my later search for the mechanism to explain the effect of dietary protein on cancer would prove to be far more complex than a simple MFO-dependent reaction. But it was this period of research with MFO that first forced an awareness of the mind-numbing biological complexity of the body that I had not fully comprehended beforehand.
Consider just a few examples of the complexity MFO presents. First, the MFO enzyme itself is architecturally very complicated. It’s comprised of three main components—really a system more than a single protein-based enzyme. In our research, we investigated the contributions of each of these components to the overall enzyme activity by isolating and reconstituting them into different combinations.28 We also examined these combinations under the influence of dietary protein feeding.29 Each combination exhibited a different MFO activity—a broad continuum of endless complexity. With just a small chemical nudge here or there, MFO and other enzyme molecules can change their shapes and thereby alter their reaction rates—all within time frames too short to document or estimate.
Second, MFO is only one in a series of enzymes, all more properly understood as systems, and changing the activity of one enzyme in this series almost always influences other enzymes in that same series. When a substrate produces a product, it may, for example, prompt the synthesis of another downstream enzyme to assist in subsequent reactions, and/or send a signal back upstream to the enzyme that initiated the first reaction to slow things down. In AF catalysis, as mentioned, epoxide hydrolase allows the MFO-generated epoxide to bond to water.30 Further down the line, the detoxified AF metabolite may be bonded to a variety of products to expedite their excretion31 from the body. Enzymes and their reactions are extensively and unavoidably interdependent.
Third, MFO metabolizes an incredible variety of native and foreign chemicals. Most intriguing, it can rapidly adjust to metabolize even synthetic chemicals never before seen in nature or encountered by the body. It’s as if MFO were a factory that can reconfigure itself instantly, turning out salad bowls one second and framing timber the next—a truly remarkable feat.
HOMEOSTASIS: THE BASIS OF HEALTH
We talk in nutritional science about something called homeostasis, the body’s tendency to always work toward maintaining a stable, functional equilibrium. This is true within bodily systems, from electrolyte balance to body temperature to pH balance, as well as between bodily systems. And this careful balance is what we call health.
Within cells, homeostasis is largely managed by a highly responsive array of enzymes—tens of thousands of them—working together in concert in a hundred trillion cells, all in communication with one another. And the resources they use to maintain homeostasis—to maintain health—are the foods we eat. That’s why nutrition, viewed wholistically, is the crucial factor in health. When we eat the right foods, our bodies naturally tend toward homeostasis. Rather than something that needs to be wheedled and coaxed out of countless reductionist interventions, health “just happens” in spite of—or, more likely, because of—the inherent complexity of body chemistry.
MFO catalyzes so many different kinds of chemicals that it is uniquely vulnerable to changes in our diets. Even relatively modest changes lead to measurable differences, as my team witnessed when we tried to pin down its effect on cancer. When we eat the right foods, MFO moves us toward homeostasis. When we don’t, MFO may contribute to disease. And MFO is just one of the 100,000 or more enzymes that contribute to the function of the human body; the chemicals we’ve discussed here are only a few of the substrates, intermediate metabolites, and products—whose total is larger than anyone can estimate—that interact in our body on a daily basis.
My work with MFO helped me see that each of us is an exceptionally dynamic system, one that changes every nanosecond of our lives with incredible rapidity and order in a symphony extraordinaire. This symphony is no less remarkable just because we’ve discovered and named some of the enzymes and other metabolic “tools” the body uses to manage and control its behavior. And that biological complexity must be acknowledged as the cornerstone of our approach to health. Unfortunately, reductionist science has become so besotted with the growing amount of that complexity it has managed to name, that it all but ignores the relationships between those elements that are the heart of homeostasis and health.
