CONTENTS
9.3 Generation of Nanoparticles of Organic Semiconductors
9.4 Organic Semiconductor-Based Sensors
9.5 Humidity Sensing Applications of Nanoparticles of Organic Semiconductors
9.5.1 Generation of Nanoparticles of CoPc
9.5.2 Device Fabrication and Characterization
9.5.3 Humidity-Dependent Capacitance
The synthesis and characterization of organic semiconductors are receiving great attention because of their potential use in a wide range of electronic and photonic devices, such as junction diodes, organic field effect transistors, memories, solar cells, organic light-emitting diodes, optical power-limiting, radio frequency identification tags (RFIDs), sensors, and so on [1, 2, 3, 4, 5, 6 and 7]. Most of the devices are based on the thin films of organic semiconductors, and expensive equipment is required for depositing their thin films because of the poor solubility of many important families of organic semiconductors in common organic solvents. Solution-processable organic semiconductors are potential candidates for the low-cost, large-area, and large-scale production of many electronic and photonic devices. Using pulsed laser ablation of organic semiconductors in polar solvents, dispersions of the nanoparticles of these materials can be produced [8] and their thin films can be deposited employing the low-cost deposition techniques such as drop casting, dipping, spin casting, inkjet printing, and so on.
The synthesis and characterization of nanoscaled materials have received much more attention because of their unique properties and applications. Most of these studies have been devoted to the nanoparticles of metals and inorganic semiconductors. A large number of organic semiconductors and their derivatives have been synthesized and many are being synthesized every year but little is known about the organic semiconducting nanomaterials and their potential applications. Devices fabricated using nanoparticles have exhibited better performance. Using chemically synthesized nanoparticles of cobalt phthalocyanine, a highly responsive glucose biosensor has been reported [9]. It is expected that the other devices fabricated using nanoparticles of organic semiconductors will exhibit better performance.
A wide variety of materials are used as sensors for capturing physical, chemical, or biological stimuli and converting them to measurable output signals. Nanomaterials bring many advantages to sensor technology and can be used to overcome many limitations of bulk material-based sensors. They are attractive because of their small characteristic size and correspondingly large surface-to-volume ratio. Nanomaterials increase a sensor’s active surface area and generate novel interfaces, thus improving the sensitivity, response, and recovery times. In recent years, there has been great progress in the application of nanomaterials in sensor technologies. In these applications, nanomaterials such as metal nanoparticles, nanostructured metal oxides, and carbon nanotubes have been been actively investigated.
Humidity measurement and control are of great importance for many industrial, medical, agricultural, and domestic applications [10 and references therein]. Therefore, many relative humidity (RH) sensors using various technologies and active materials have been fabricated and investigated. Recently, we have investigated the sensing potential of bulk organic semiconducting thin films [11, 12 and 13]. Organic semiconductors have been observed to show a large change in their electrical properties with variation in RH. In this chapter, a brief introduction of the organic semiconductors is given. Methods of generation of the nanoparticles of organic semiconductors are mentioned and the PLA technique for the generation of the nanoparticle dispersion of organic semiconducting materials is briefly described. Bulk and nanoscaled organic semiconductor-based sensors are discussed. The potential of the nanoparticle dispersion of organic semiconductors for humidity sensor applications is demonstrated. The fabrication, study, and results of a capacitive-type humidity sensor fabricated using nanoparticles of organic semiconductors are presented.
Organic semiconductors are multifunctional materials because of their capability of exhibiting a variety of properties, such as charge transport, photoconductivity, electroluminescence, and so on. The electrical and optical properties of organic semiconductors are highly dependent on ambient conditions, which makes them very promising for the development of various types of sensors, such as temperature, light, humidity, and so on. Phthalocyanines belong to a class of organic semiconductors and have been extensively employed in the fabrication of organic and organic–inorganic devices [14, 15 and 16]. They exhibit good thermal and chemical stability [17,18]. Phthalocyanines can be easily deposited as thin films by thermal evaporation without dissociation. Therefore, many studies have been carried out on phthalocyanine-based thin-film devices [19, 20 and 21].
Organic semiconductors are π-conjugated systems and can be classified into two broad categories of low-molecular-weight compounds and long-chain polymers. Low-molecular-weight compounds comprise the families of azo dyes, perylenes, phthalocyanines, porphyrins, and so on. Numerous organic devices have been fabricated and studied using these materials. Recently, the devices fabricated using nanomaterials of low-molecular-weight organic semiconductors have shown better performance; therefore, it is expected that, in the future, there will be more activities in the synthesis and characterization of low-molecular-weight organic semiconducting nanomaterials.
9.3 GENERATION OF NANOPARTICLES OF ORGANIC SEMICONDUCTORS
The formation of nanoscaled organic semiconductors has recently attracted a great deal of interest due to the possibility of tailoring the specific material function depending on the particle size [8], their role in the modification of optical and electrooptical properties [22], their potential impact in several attractive economic fields [23], and so on. Various methods such as reprecipitation, miniemulsion, direct condensation of organic vapor, precipitation by solvent displacement with or without subsequent microwave irradiation, supercritical fluids, vapor deposition, laser ablation, template-based approaches, and so on have been developed to prepare the nanoparticles of organic semiconductors [22, 23, 24 and 25].
Laser ablation in liquids has received much attention as a very simple nanoparticle production technique with some remarkable features, such as the synthesis of nanoparticle dispersions in different liquids, stability of the dispersions, and control of the shape and size. Using laser ablation in a liquid, nanoparticles of a wide range of materials, such as metals, metal oxides, organic/inorganic semiconductors, and so on, are obtainable in different liquids. Nanoparticle synthesis using this technique involves a large number of parameters, such as laser wavelength, laser energy, pulse width, repetition rate, liquid type, and liquid composition [26]. By varying these parameters, the size, shape, and morphology of the nanostructured materials can be controlled. In this method, a pulsed laser beam with enough energy falls onto a solid target in a transparent liquid, and a high-temperature (about 6000 K), high-pressure (about 1 GPa), and short-lived plasma plume comprising nanoparticles of the target material is produced on the solid–liquid interface [27]. Many studies regarding the generation and evolution of the laser plasma plume have been carried out. This plasma plume quickly cools and a dispersion of the nanoparticles of the target material is formed.
9.4 ORGANIC SEMICONDUCTOR-BASED SENSORS
Organic sensors offer great potential for the applications in security, industry manufacturing, environmental monitoring, and medical science. Besides the advantages of easy processing, low cost, and compatibility with plastic substrates, another important advantage of organic-based sensors is that the organic compounds can also be chemically functionalized. This property makes them more favorable, thereby improving the selectivity of the sensor. Compared to the traditional detection methods, organic sensors have higher selectivity, sensitivity, and lower cost [28,29]. Pure polymer, polymer blends, and polymer–inorganic composites have also been studied for the purposes, resulting in different degrees of advancements in this area [30, 32 and 33]. In organic sensors, analyte molecules diffuse into the organic film and interact with the organic material. The analyte molecules can have various effects on organic film, such as doping of carrier density, dipole-induced trapping, and retardation of charges [34]. Those interactions alter the conductivity, threshold voltages, and mobility of devices and hence change the current flow through the thin films between electrodes. Several mechanisms of sensing such as capacitance [35, 36 and 37], electrical resistance [29], and surface acoustic wave (SAW) [38,39] have been proposed.
Capacitive sensors work at either change in dielectric permittivity or induced polarization in the organic film. For instance, water molecules are bound at suitable sites in organic materials during the adsorption and desorption processes in capacitive humidity sensors. Since there exists a big difference between the dielectric constant of water (~80) and organic materials (~5), the water molecules adsorbed in the film influence the dielectric constant greatly. If the electrodes are similar to the parallel plates, the principle of operation of the capacitive-type humidity sensor is based on a familiar expression defined as C = (εA)/d, where C is the capacitance, A is the electrode area, d is the distance between the two electrodes, and ε is the permittivity of the sensing material, which is a function of RH. With the adsorption of humidity into the film or the desorption of moisture from the film, the dielectric constant of the sensing organic semiconductor changes; therefore, a variation of capacitance as a result of a change in the dielectric constant of the sensing film is measured.
Phthalocyanines represent a large family of organic semiconducting materials with high chemical and thermal stability. Using these compounds, numerous organic electronic devices have been fabricated. The interaction of various analytes with the surfaces of the thin films of these compounds produces reversible changes in their physical properties. By measuring these changes using different technologies, such as electrical, optical, and so on, their potential for numerous sensors has been studied.
9.5 HUMIDITY SENSING APPLICATIONS OF NANOPARTICLES OF ORGANIC SEMICONDUCTORS
To investigate the sensing potential of the nanoparticles of organic semiconductors, the nanoparticle dispersion of organic semiconductor CoPc was generated using PLA. Using this dispersion, a surface-type humidity sensor was fabricated and its humidity-dependent capacitance was measured.
9.5.1 GENERATION OF NANOPARTICLES OF COPC
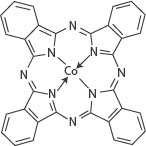
The molecular structure of the organic semiconductor cobalt phthalocyanine (CoPc) is shown in Figure 9.1. It was prepared in accordance with the method of Sakamoto and Ohno, with minor modifications [40].
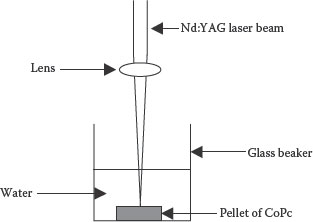
The synthesized CoPc was dried for 24 h under a 200 W tungsten filament lamp and a pellet was formed using a press. A nanoparticle dispersion of CoPc was produced using the PLA technique. A schematic representation of the experimental setup used is shown in Figure 9.2. In this method, a pellet of CoPc was placed under a thin layer of deionized water in a beaker and exposed to a nanosecond Nd:YAG laser radiation working at 532 nm and 10 Hz. Operating the laser for 30 min, a colloidal solution of the nanoparticles of CoPc was obtained.
FIGURE 9.1 Molecular structure of CoPc. M.H. Sayyad et al., Production and characterization of nanoparticle dispersions of organic semiconductors for potential applications in organic electronics, Proceedings of the 11th IEEE International Conference on Nanotechnology, August 15–18, Portland, Oregon, pp. 193–196, © (2011) IEEE. With permission.)
FIGURE 9.2 Setup for the synthesis of nanoparticles of solid targets using pulsed laser ablation in liquid medium. M.H. Sayyad et al., Production and characterization of nanoparticle dispersions of organic semiconductors for potential applications in organic electronics, Proceedings of the 11th IEEE International Conference on Nanotechnology, August 15–18, Portland, Oregon, pp. 193–196, © (2011) IEEE. With permission.)
9.5.2 DEVICE FABRICATION AND CHARACTERIZATION
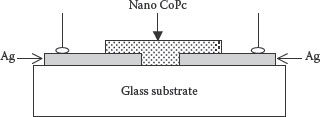
The Ag/NanoCoPc/Ag-type capacitive humidity sensor was fabricated by drop casting the nanoparticle dispersion of CoPc. The 100-nm-thick silver electrodes were deposited on a glass substrate with a gap of 50 μm by the thermal vacuum evaporation technique using a mask. The electrodes were deposited at a rate of 0.2 nm/s and a vacuum of 10−5 mbar. The structure of the fabricated device is shown in Figure 9.3.
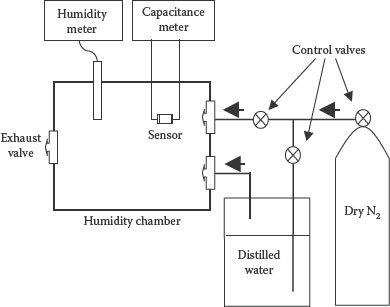
The humidity-dependent capacitance was investigated using a homemade experimental setup shown in Figure 9.4. It consists of a humidifier and a measurement chamber. The RH in the chamber was obtained by bubbling dry nitrogen in the humidifier containing distilled water. The percentage of RH inside the measurement chamber was controlled by two valves. The RH and capacitance were measured using commercial digital meters. During capacitive measurements, the frequency was kept at 1 kHz.
FIGURE 9.3 Cross-sectional view of the Ag/NanoCoPc/Ag surface-type sensor. M.H. Sayyad et al., Production and characterization of nanoparticle dispersions of organic semiconductors for potential applications in organic electronics, Proceedings of the 11th IEEE International Conference on Nanotechnology, August 15–18, Portland, Oregon, pp. 193–196, © (2011) IEEE. With permission.)
FIGURE 9.4 Experimental setup for humidity-dependent capacitance measurements.
9.5.3 HUMIDITY-DEPENDENT CAPACITANCE
Humidity can affect human comfort, numerous material properties, and industrial processes. It is used to represent the amount of water vapor in the air and is defined in terms of RH, specific humidity, and absolute humidity.
Absolute humidity measures the water vapor content in absolute terms, that is, how much water vapor is present, without any concern about the maximum amount the air could carry. The RH is the type mostly used in conventional humidity meters. The RH is the ratio of partial water vapor pressure (pw) in an air–water mixture at any temperature to the saturated vapor pressure (ps) of water at the same temperature. RH as a percentage is defined as [41]
(9.1) |
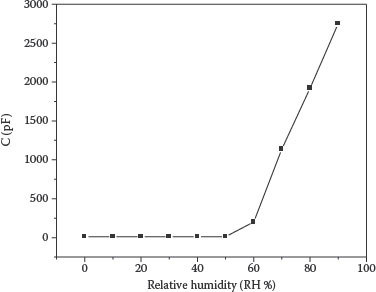
Figure 9.5 shows the variation in the capacitance of the Ag/NanoCoPc/Ag sensor with an RH at room temperature and under dark conditions. It is observed that in the range 0–55% RH, the capacitance of the sensor does not change but above 55% RH, it increases significantly with a corresponding increase in humidity. Similar behavior has been reported in the case of a polypyrrole-based sensor [42] and our earlier studies of porphyrin-based sensors [29,43].
This type of capacitance variation with the increase in humidity can be explained on the basis of water adsorption and dielectric physics theory [42]. According to the adsorption theory, the adsorption of water molecules on the sensing material takes place during the two stages of chemisorption and physisorption. When the sensor is exposed to humidity, the formation of a chemisorbed layer starts. The capacitance of the sensor does not change until the complete chemisorbed layer is formed. The threshold RH at which the chemisorbed layer is completed has been observed to be dependent upon the material type, film surface morphology, and film thickness. When the chemisorbed layer is completed with an increase in further humidity, many more physisorbed layers are formed; owing to the formation of these additional layers on the surface of a sensitive material, the capacitance of the sensor increases.
FIGURE 9.5 Capacitance–humidity relationship for the Ag/NanoCoPc/Ag sensor. M.H. Sayyad et al., Production and characterization of nanoparticle dispersions of organic semiconductors for potential applications in organic electronics, Proceedings of the 11th IEEE International Conference on Nanotechnology, August 15–18, Portland, Oregon, pp. 193–196, © (2011) IEEE. With permission.)
According to the dielectric physics theory, the capacitance of an ideal capacitor is independent on the frequency [42]. At low RH, the capacitor exhibits ideal behavior. This shows that the amount of water vapor adsorbed by the sensing material is not large enough. At higher RH, as more water molecules are adsorbed, the leak conduction appears in the sensing material; therefore, the expression of the capacitance of the sensor could be written as [42]
(9.2) |
where C0 is the capacitance of the ideal capacitor, ε* is the complex dielectric constant, εr is the relative dielectric constant of the ideal capacitor, ε0 is the vacuum capacitance constant, ω is the frequency, and γ is the leak conductance. Using this equation, the increase in capacitance of the sensor with the increase in RH can be explained. The leak conductance γ is directly proportional to the RH, so the capacitance of the sensor is directly proportional to the RH. Thus, the capacitance of the sensor increases with an increase in humidity. We also see from the expression that the capacitance is inversely proportional to ω. Therefore, the sensor is expected to show better performance at low frequencies. At low frequencies, the carriers have enough time to respond and the capacitance is greatly affected. At higher frequencies, the change of the electric field becomes too fast for the carriers to follow and the capacitance is not affected significantly by RH.
Response and recovery times are the most important parameters for all humidity sensors. Response/recovery time is defined as the time taken by a capacitive sensor to attain 90% of the total capacitance change. The response and recovery times were measured using the method described in Ref. [43]. The response time of the sensor was measured from 30% to 90% RH at room temperature. In this method, the response time is measured by quickly moving the sensor from an RH level of 30% to 90% and the recovery time is measured by moving very quickly from a humidity level of 90% RH, maintained in a closed chamber, to an open atmosphere at a humidity level of 30% RH. Both the response and recovery times for the Ag/NanoCoPc/Ag sensor were observed for approximately less than 5s, which is much less than the times reported for the capacitive humidity sensors based on other nanostructured materials [44,45] and many commercially available humidity meters. Therefore, this sensor can be used for the fabrication of a fast hygrometer for the measurement of high RH where the commercially available humidity sensors are either too slow or incapable of working [44].
Using laser ablation, nanoparticle dispersions of almost all the organic semiconductors and conjugated polymers can be synthesized. Their morphological, spectroscopic, and electrical characterization can help in the identification of their potential applications in numerous areas. Using the technique of PLA, the nanoparticle dispersion of an organic semiconductor CoPc is produced in deionized water and the potential of these nanoparticle dispersions for the fabrication of an organic nanoparticle-based sensor is demonstrated. The sensor has been observed to be very sensitive and quicker in response than the commercially available low-cost sensors. This study demonstrates the great potential of the nanodispersion of CoPc in water, prepared using laser ablation, for the fabrication of a low-cost, very sensitive, and fast humidity sensor for the measurement of high RH. Owing to the high surface-to-volume ratio, the nanostructured materials are potential candidates for gas sensors. Therefore, it is proposed that the nanoparticle dispersion of CoPc and other active organic semiconductors may also be used as active materials in gas sensors.
1. Z. Ahmad and M. H. Sayyad, Extraction of electronic parameters of Schottky diode based on an organic semiconductor methyl-red, Physica E, 41, 631–634, 2009.
2. S.M. Khan, M. H. Sayyad, and K. S. Karimov, Investigation of temperature-dependent electrical properties of p-VOPc/n-si heterojunction under dark conditions, Ionics, 17, 307–313, 2011.
3. M.H. Sayyad, Z. Ahmad, Kh. S. Karimov, M. Yaseen, and M. Ali, Photo organic field effect transistor based on a metallo-porphyrin, Journal of Physics D (Applied Physics), 42, 105112, 2009.
4. Z. Ahmad, P. C. Ooi, K. C. Aw, and M. H. Sayyad, Electrical characteristics of poly(methylsilsesquioxane) thin films for non-volatile memory, Solid State Communications, 151, 297–300, 2011.
5. S.M. Khan, M. Kaur, J. R. Heflin, and M. H. Sayyad, Fabrication and characterization of ZnTPP:PCBM bulk heterojunction (BHJ) solar cells, Journal of Physics and Chemistry of Solids, 72, 1430–1435, 2011.
6. Z. Ahmad, M. H. Sayyad, M. Yaseen, K. C. Aw, M. M. Tahir, and M. Ali, Potential of 5,10,15,20-Tetrakis(3/,5/-di-tertbutylphenyl)porphyrinatocopper(II) for a multi-functional sensor, Sensors and Actuators B: Chemical, 155, 81–85, 2011.
7. F. Aziz, M. H. Sayyad, K. Sulaiman, B. H. Majlis, Khassan S. Karimov, Z. Ahmad, and G. Sugandi, Influence of humidity conditions on the capacitive and resistive response of an Al/VOPc/Pt co-planar humidity sensor, Measurement Science and Technology, 23, 014001, 2012.
8. J. Hobley, T. Nakamori, S. Kajimoto, M. Kasuya, K. Hatanaka, H. Fukumura, and S. Nishio, Formation of 3,4,9,10-perylenetetracarboxylicdianhydride nanoparticles with perylene and polyyne byproducts by 355 nm nanosecond pulsed laser ablation of microcrystal suspensions, Journal of Photochemistry and Photobiology A: Chemistry, 189, 105–113, 2007.
9. K. Wang, J.-J. Xu, and H.-Y. Chen, A novel glucose biosensor based on the nanoscaled cobalt phthalocyanine–glucose oxidase biocomposite, Biosensors and Bioelectronics, 20, 1388–1396, 2005.
10. Z. Chen and C. Lu, Humidity sensors: A review of materials and mechanisms, Sensor Letters, 3, 274–295, 2005.
11. M. Saleem, M. H. Sayyad, K. S. Karimov, M. Yaseen, and M. Ali, Synthesis and application of Ni(II) 5,10,15,20-tetrakis(4′-isopropylphenyl) porphyrin in a surface-type multifunctional sensor, Physica Scripta, 82, 015703–015708, 2010.
12. M.H. Sayyad, F. Aziz, K. S. Karimov, M. Saleem, Z. Ahmad, and S. M. Khan, Characterization of vanadylphthalocyanine based surface-type capacitive humidity sensors, Journal of Semiconductors, 31, 114002, 2010.
13. M.H. Sayyad, Z. Ahmad, M. Yaseen, K. C. Aw, M. M. Tahir, and M. Ali, Potential of 5,10,15,20-Tetrakis(3/,5/-di-tertbutylphenyl)porphyrinatocopper(II) for a multifunctional sensor, Sensors and Actuators B: Chemical, 155, 81–85, 2011.
14. M. Biancardo and F. C. Krebs, Battery effects in organic photovoltaics based on polybithiophene, Solar Energy Materials and Solar Cells, 92, 353–356, 2008.
15. G. Liang, T. Cui, and K. Varahramyan, Electrical characteristics of diodes fabricated with organic semiconductors, Microelectronic Engineering, 65, 279–284, 2003.
16. J. Portier, J. H. Choy, and M. A. Subramanian, Inorganic–organic-hybrids as precursors to functional materials, International Journal of Inorganic Materials, 3, 581–592, 2001.
17. A.S. Riad, S. M. Khalil, and S. Darwish, Effect of temperature on photoconduction and low frequency capacitance measurements on β-CuPc photovoltaic cells, Thin Solid Films, 249, 219–223, 1994.
18. T. G. Abdel-Malik and G. A. Cox Charge transport in nickel phthalocyanine crystals. I. Ohmic and space-charge-limited currents in vacuum ambient Journal of Physics C: Solid State Physics, 10, 63–74, 1997.
19. M. M. El-Nahass, K. F. Abd-El-Rahman, and A. A. A. Darwish, Fabrication and electrical characterization of p-NiPc/n-Si heterojunction, Microelectronics Journal, 38, 91–95, 2007.
20. A.C. Varghese and C. S. Menon, Electrical properties of nickel phthalocyanine thin films using gold and lead electrodes, Journal of Materials Science: Materials in Electronics, 17, 149–153, 2006.
21. O.V. Molodtsova, T. Schwieger, and M. Knupfer, Electronic properties of the organic semiconductor hetero-interface CuPc/C60, Applied Surface Science, 252, 143–147, 2005.
22. Z. Jia, D. Xiao, W. Yang, Y. Ma, J. Yao, and Z. Liu, Preparation of perylene nanoparticles with a membrane mixer, Journal of Membrane Science, 241, 387–392, 2004.
23. D. Oliveira, K. Baba, J. Mori, Y. Miyashita, H. Kasai, H. Oikawa, and H. Nakanishi, Using an organic additive to manipulate sizes of perylene nanoparticles, Journal of Crystal Growth, 312, 431–436, 2010.
24. X. Xu and L. Li, Organic semiconductor nanoparticle film: Preparation and application smart nanoparticles technology, A. A. Hashim, Ed., In Tech Janeza Trdine 9, 51000 Rijeka, Croatia, 2012.
25. Y.S. Zhao, H. Fu, A. Peng, Y. Ma, D. Xiao, and J. Yao, Low-dimensional nanomaterials based on small organic molecules: Preparation and optoelectronic properties, Advanced Materials, 20, 2859–2876, 2008.
26. F. Barreca, N. Acacia, E. Barletta, D. Spadaro, G. Currò, and F. Neri, Small size TiO2 nanoparticles prepared by laser ablation in water, Applied Surface Science, 256(21), 6408–6412, 2010.
27. P. Liu, W. Cai, L. Wan, M. Shi, X. Luo, and W. Jing, Fabrication and characteristics of rutile TiO2 nanoparticles induced by laser ablation, Transactions of Nonferrous Metals Society of China, 19, Supplement 3(0), s743–s747, 2009.
28. M.H. Sayyad, M. Shah, K. S. Karimov, Z. Ahmad, M. Saleem, and M. M. Tahir, Fabrication and study of NiPc thin film based surface type photocapacitors, Journal of Optoelectronics and Advanced Materials, 10, 2805–2810, 2008.
29. M. Saleem, M. H. Sayyad, Kh. S. Karimov, M. Yaseen, and M. Ali, Cu(II) 5,10,15,20-tetrakis(4-isopropylphenyl) porphyrin based surface-type resistive–capacitive multifunctional sensor, Sensors and Actuators B, 137(2), 442–446, 2009.
30. M.H. Sayyad, K. ul Hasan, M. Saleem, Kh. S. Karimov, F. A. Khalid, M. Karieva, Kh. Zakaullah, and Z. Ahmad, Electrical properties of poly-N-epoxypropylcarbazole/vanadium pentoxide composite, Eurasian Chemico-Technological Journal, 9(1), 57–62, 2007.
31. K.S. Karimov, K. Akhmedov, I. Qazi, and T. A. Khan, Poly-N-epoxypropylcarbazole complexes photocapacitive detectors, Journal of Optoelectronics and Advanced Materials, 9, 2867–2872, 2007.
32. N. Parvatikar, S. Jain, C. M. Kanamadi, B. K. Chougule, S. V. Bhoraskar, and M. V. N. A. Prasad, Humidity sensing and electrical properties of polyaniline/cobalt oxide composites, Journal of Applied Polymer Science, 103(2), 653–658, 2006.
33. S. Jain, S. Chakane, A. B. Samui, V. N. Krishnamurthy, and S. V. Bhoraskar, Humidity sensing with weak acid-doped polyaniline and its composites, Sensors and Actuators B: Chemical, 96(12), 124–129, 2003.
34. H.E. Katz and J. Huang, Thin-film organic electronic devices, Annual Review of Materials Research, 39(1), 71–92, 2009.
35. C. Roman, O. Bodea, N. Prodan, A. Levi, E. Cordos, and I. Manoviciu, A capacitive-type humidity sensor using crosslinked poly(methyl methacrylate-co-(2 hydroxypropyl)-methacrylate), Sensors and Actuators B: Chemical, 25(13), 710–713, 1995.
36. M. Matsuguchi, S. Umeda, Y. Sadaoka, and Y. Sakai, Characterization of polymers for a capacitive-type humidity sensor based on water sorption behavior, Sensors and Actuators B: Chemical, 49(3), 179–185, 1998.
37. Z. Ahmad, M. H. Sayyad, M. Yaseen, K. C. Aw, M. M. Tahir, and M. Ali, Sensors and Actuators B: Chemical, 155(2), 81–85, 2011.
38. A.E. Hoyt, A. J. Ricco, J. W. Bartholomew, and G. C. Osbourn, SAW sensors for the room-temperature measurement of CO2 and relative humidity, Analytical Chemistry, 70(10), 2137–2145, 1998.
39. C. Caliendo, I. Fratoddi, M. V. Russo, and C. Lo Sterzo, Response of a Pt-polyyne membrane in surface acoustic wave sensors: Experimental and theoretical approach, Journal of Applied Physics, 93(12), 10071–10077, 2003.
40. K. Sakamoto and E. Ohno. Synthesis of cobalt phthalocyanine derivatives and their cyclic voltammograms, Dyes and Pigments, 35(4), 375–386, 1997.
41. J. Fraden, Handbook of Modern Sensors: Physics, Designs, and Applications, 4th Ed., Springer, New York, 2010, pp. 445–459.
42. T. Zhang et al., Analysis of dc and ac properties of humidity sensor based on polypyrrole materials, Sensors and Actuators B, 131(2), 687–691, 2008.
43. M. H. Sayyad et al., Synthesis of Zn(II) 5,10,15,20-tetrakis(4-isopropylphenyl) porphyrin and its use as a thin film sensor, Applied Physics A, 98(1), 103–109, 2010.
44. W.-P. Chen, Z.-G. Zhao, X.-W. Liu, Z.-X. Zhang, and C.-G. Suo, A capacitive humidity sensor based on multi-wall carbo nanotubes (MWCNTs), Sensors and Actuators B, 149(1), 136–142, 2010.
45. L. Xiaowei, Z. Zhengang, L. Tuo, and W. Xin, Novel capacitance-type humidity sensor based on multi-wall carbon nanotube/SiO2 composite films, Journal of Semiconductors, 32(3), 034006–034011, 2011.
46. M.H. Sayyad, F. Wahab, Z. Ahmad, M. Shahid, J. A. Chaudry, and M. A. Munawar, Production and characterization of nanoparticle dispersions of organic semiconductors for potential applications in organic electronics, Proceedings of the 11th IEEE International Conference on Nanotechnology, August 15–18, Portland, Oregon, pp. 193–196, 2011.