Chapter 1. Introduction to the Resistance Problem
In this chapter, we define terms and provide an overview of antibiotic resistance. One of the key problems is that as a global community we have not considered antibiotics as a resource to be actively protected.1 Consequently, we use antibiotics in ways that directly lead to resistance. Changing those ways requires an understanding of antibiotic principles. We begin with a brief description of MRSA to illustrate a bacterial-based health problem.
MRSA Is Putting Resistance in the News
MRSA is the acronym for methicillin-resistant Staphylococcus aureus. (Acronyms are usually pronounced letter by letter, as in DNA; scientific names are always italicized; after an initial spelling of the entire name, the first name is often abbreviated by its first letter.) S. aureus is a small, sphere-shaped bacterium (see Figure 1-1) that causes skin boils, life-threatening pneumonia, and almost untreatable bone infections. It often spreads by skin-to-skin contact, shared personal items, and shared surfaces, such as locker-room benches. When the microbe encounters a break in the skin, it grows and releases toxins.
Figure 1-1. Staphylococcus aureus. Scanning electron micrograph of many MRSA cells at a magnification of 9,560 times.

Public Health Image Library # 7821; photo credit, Janice Haney Carr.
Sixty years ago, S. aureus was very susceptible to many antibiotics, including penicillin. Susceptibility disappeared, and the pharmaceutical industry produced increasingly potent antibiotic derivatives. Among these was methicillin, which overcame resistance to penicillin. But in 1960, one year after the introduction of methicillin, MRSA was recovered in the United States. As the resistant bacterium spread through hospitals, surgical procedures and long-term use of catheters became more dangerous. MRSA also caused pneumonia, commonly following influenza, and recently skin infections caused by MRSA captured public attention. In one newspaper account,2 pimples on a newborn baby were found to contain MRSA. Antibiotics cleared the infection; however, a month later, the father found boils on his own leg that contained MRSA. Treatment cleared the boils, but they came back. The mother developed mastitis during breast feeding that required a 2-inch incision into her breast to drain the infection. About a year later, an older child developed an MRSA boil on his back. The family is now constantly on alert for MRSA, trying to wash off the bacteria before the microbes find a break in the skin.
Community-associated MRSA has its own acronym (CA-MRSA) to distinguish it from the hospital-associated form (HA-MRSA). Many community-associated S. aureus strains are members of a group called USA300, which now accounts for half of the CA-MRSA infections. The strain causes necrotizing (flesh-eating) skin infection, pneumonia, and muscle infection. In 2005, MRSA accounted for more than 7 million cases of skin and soft tissue infection seen in outpatient departments of U.S. hospitals.3 As expected, CA-MRSA strains are moving into hospitals. In a survey of U.S. hospitals taken from 1999 through 2006, the fraction of S. aureus that was resistant to methicillin increased 90%, almost entirely from an influx of CA-MRSA.4
Although many infections tend to occur in persons having weakened immune systems, MRSA can infect anyone. For example, healthy young adults tend to be susceptible to a lethal combination of influenza and MRSA pneumonia. In Chapter 7, “Transmission of Resistant Disease,” we describe occurrences of CA-MRSA infection among athletes. Fortunately, most of these dangerous CA-MRSA strains are still susceptible to several antibiotics; however, that susceptibility may soon disappear.
HA-MRSA has been a problem in hospitals for years; in many countries, it is getting worse. For example, in the United States, MRSA climbed from 22% of the S. aureus infections in 1995 to 63% in 2007 (from 1999 through 2005, it increased 14% per year).5 From 2000 to 2005, MRSA helped double the number of antibiotic-resistant infections in U.S. hospitals, which reached almost a million per year or 2.5% of hospitalizations.6 In the United States, more persons now die each year from MRSA (17,000) than from AIDS.
MRSA in hospitals is largely an infection-control problem, that is, control requires keeping the organism from spreading from one patient to another, and if possible, keeping it out of the hospital entirely. Neither is easy. For many years, the Dutch have had an aggressive screening program for incoming patients. They isolate persons who test positive for MRSA and treat them with antibiotics that still work with S. aureus. Entire wards of hospitals are closed for cleaning when an MRSA case is found, and colonized healthcare workers are sent home on paid leave until they are cleared of the bacterium. The cost is about half that required to treat MRSA blood-stream infections;7 consequently, the effort is thought to be cost-effective.
Until recently, many U.S. hospitals took a different approach: MRSA infections were considered part of the cost of doing business. Holland is a small country that can implement specialized care—the United States has a much higher incidence of MRSA. Nevertheless, in 2007, a Pittsburgh hospital reported that it had adopted the Dutch method. The hospital saved almost $1 million per year by screening patients and by insisting on more intensive hand-washing protocols for hospital staff.8 Other U.S. hospitals are reconsidering their own stance.
Individual consumers will begin to search for hospitals having low MRSA incidence. That search will be easier when hospitals publish their drug-resistant infection statistics. Some states now require reporting of MRSA to health departments; consequently, the numbers are being collected. As an added incentive for MRSA control, some insurance carriers refuse to cover hospital costs when a patient contracts MRSA while there. Hospitals have responded by setting up antibiotic oversight committees to help keep resistance under control.
Humans Live with Many Pathogens
MRSA is one type of pathogen, the collective word applied to microbes and viruses that cause disease. (The term microbe includes bacteria, some types of fungi, and protozoans.) Each type of microbe has a distinct lifestyle. Bacteria are single-celled organisms that reproduce by binary fission; each cell grows and then divides to form two new cells. Bacteria cause many of the diseases that make headlines: tuberculosis, flesh-eating disease, and anthrax. Pathogenic fungi include yeasts and molds. Yeasts are single-celled, whereas molds tend to grow as thread-like structures composed of many cells. (Some pathogenic fungi switch between the forms in response to the environment.) Yeasts and molds cause pneumonia, and in immuno-suppressed persons yeasts and molds can cause deadly systemic infections. Pathogenic protozoans, such as the types that cause malaria, are single-celled microbes that are often spread by insect bites. In tropical and subtropical regions, protozoan diseases are among the major killers of humans. Protozoa and helminths (worms) are usually called parasites rather than pathogens due to their larger size. In Antibiotic Resistance, we do not distinguish between pathogens and parasites, because antibiotics are used for maladies caused by parasites as well as by pathogens.
Viruses differ qualitatively from the cellular organisms just mentioned. Viruses cannot reproduce outside a host cell. They require the machinery of a living cell to make new parts. Indeed, one could argue that viruses are not alive even though they are composed of the same types of molecules found in microbes, plants, and animals. Another feature of viruses is that they are generally much smaller than microbes: An electron microscope is required to see most virus particles, whereas a light microscope is adequate for microbes.
Many microbes and viruses are found in and on our bodies (see Box 1-1). Some are beneficial; others are harmful. Some pathogens only occasionally cause infectious symptoms. For example, Mycobacterium tuberculosis enters a dormant state in most persons it infects, with a minority of infected persons exhibiting symptoms. However, immune deficiency enables M. tuberculosis to exit dormancy and cause disease. Other serious diseases arise from microbes, such as the yeast Candida albicans, that ordinarily live harmlessly in or on humans. This organism causes vaginitis with healthy women and more serious disease with immune-compromised patients.
Pathogens that normally grow only inside humans often have effective means of transmission. Mycobacterium tuberculosis and influenza virus are two that spread through air; Vibrio cholerae, the cause of cholera, contaminates drinking water; and many digestive tract pathogens move with contaminated food. (Salmonella typhi, the bacterium that causes typhoid fever, is an example.) Many other pathogens are spread by insects and ticks. Among these are the protozoans responsible for sleeping sickness and malaria, the bacteria that cause plague and typhus, and many types of viruses, such as the agent of yellow fever. Avoiding contact with pathogens is exceedingly difficult.
Antibiotics Block Growth and Kill Pathogens
Antibiotics are drugs, taken orally, intermuscularly, or intravenously, that counter an infection. They include agents such as penicillin, tetracycline, ciprofloxacin, and erythromycin. Common bacterial diseases treated with antibiotics are tuberculosis and gonorrhea. Fungal and protozoan diseases are also treatable, but with agents specific for these organisms. (The biochemistry of fungi and protozoa differs substantially from that of bacterial cells.) Antiviral agents constitute a third set of specialized compounds. In general, little cross-reactivity exists among the categories, that is, agents used for fungi do not cure infections caused by viruses, bacteria, or protozoa. However, the principles underlying action and resistance are the same; consequently, in Antibiotic Resistance we lump all these agents together as antibiotics. Combining all the agents into a single category risks confusion, because the public has been told repeatedly not to use antibiotics for viral diseases. In this instruction, antibiotics are equated to antibacterials, and indeed antibacterials should not be used for viral infections. But the world is changing. We now have many antiviral and antifungal agents that are just as antibiotic as penicillin. The important issue is to identify principles that enable experimental data obtained with one agent to be used for making decisions with another. Such a cross-disciplinary effort is facilitated by having a general term (antibiotic); we use specific terms, such as antibacterial and antiviral, only when we need to distinguish the agents.
In molecular terms, antibiotics are small molecules that interfere with specific life processes of pathogens. Antibiotics generally enter a pathogen, bind to a specific component, and prevent the component from functioning. In cases of lethal antibacterials, treatment leads to formation of toxic reactive oxygen species that contribute to bacterial death. Not all antibiotics kill pathogens. Indeed, many of the older drugs only stop pathogen growth. Nevertheless, they can be quite effective because they give our natural defense systems time to remove the pathogens.
Antibiotics have been called magic bullets and miracle drugs because they quickly cure diseases that might otherwise cause death. When penicillin first became available in the middle of World War II, it gave life to soldiers who were otherwise doomed by infection of minor wounds. Penicillin was so valuable that urine was collected from treated soldiers and processed to recover the drug. Now antibiotics enable many complicated surgeries to be performed without fear of infection. Developments in molecular biology have even enabled pharmaceutical companies to design antibiotics that work against viruses. Among the more striking examples are antibiotics that attack the human immunodeficiency virus (HIV): They reduce the viral load and relieve many symptoms of HIV disease.
Broad-Spectrum Antibiotics Also Perturb Our Microbiomes
Our bodies contain trillions of bacteria that have evolved to live in humans. More than 38,000 different species live in the human digestive tract, and bacteria occupy at least 20 distinct niches on our skin. The microbes carried by each host are collectively called a microbiome. Humans have evolved to take advantage of the bacteria, and the bacteria gain advantage from us. Box 1-2 describes examples relating to obesity and pain. Some bacteria help humans digest food, whereas others protect from particular pathogens. For example, acid-producing bacteria in the vagina keep yeast populations in check. The complex ecosystem of the digestive tract protects humans from Clostridium difficile, the cause of a serious form of diarrhea and bowel inflammation. An unwelcome consequence of antibiotic treatment is the death of much of our microbiome, which can enable resistant pathogen populations to expand.
Antibiotic Resistance Protects Pathogens
Antibiotic resistance is the capability of a particular pathogen population to grow in the presence of a given antibiotic when the antibiotic is used according to a specific regimen. Such a long, detailed definition is important for several reasons. First, pathogens differ in their susceptibility to antibiotics; thus, pathogen species are considered individually. Second, resistance to one antibiotic may not affect susceptibility to another. This means that the antibiotics must also be considered separately. Third, dose is determined as a compromise between effectiveness and toxicity; dose can be changed to be more or less effective and more or less dangerous. Consequently, the definition of resistance must consider the treatment regimen.
Control of infection caused by a resistant pathogen requires higher doses or a different antibiotic. If neither requirement can be met, we have only our immune system for protection from lingering disease or even death. Indeed, infectious diseases were the leading cause of death in developed countries before the discovery of antibiotics. (They still account for one-third of all deaths worldwide.)
Antibiotic resistance is a natural consequence of evolution. Microbes, as is true for all living organisms, use DNA molecules to store genetic information. (Some viruses use RNA rather than DNA; both acronyms are defined in Appendix A, “Molecules of Life.”) Evolution occurs through changes in the information stored in DNA. Those changes are called mutations, and an altered organism is called a mutant. Therefore, an antibiotic-resistant mutant is a cell or virus that has acquired a change in its genetic material that causes loss of susceptibility to a given antibiotic or class of antibiotics.
Antibiotic-resistant pathogens need not arise only from spontaneous mutations—bacteria contain mechanisms for moving large pieces of DNA from one cell to another, even from one species to another. This process, called horizontal gene transfer (see Chapter 6, “Movement of Resistance Genes Among Pathogens”), enables resistance to emerge in our normal bacterial flora and move to pathogens. It is part of the reason that excessive antibiotic use and environmental contamination are so dangerous.
A pathogen is considered to be clinically resistant when an approved antibiotic regimen is unlikely to cure disease. We quantify the level of pathogen susceptibility through a laboratory measure called minimal inhibitory concentration (MIC), which is the drug concentration that blocks growth of a pathogen recovered from a patient. (Pathogen samples taken from patients are called isolates.) A pathogen is deemed resistant if the MIC for the drug exceeds a particular value set by a committee of experts. Clinicians call that MIC value an interpretive breakpoint. Infections caused by pathogen isolates having an MIC below the breakpoint for a particular antibiotic are considered treatable; those with an MIC above the breakpoint are much less likely to respond to therapy. The MIC for a given patient isolate, reported by a clinical microbiology laboratory, helps the physician make decisions about which antibiotic to use. For example, if the isolate is resistant to penicillin but susceptible to fluoroquinolones, the physician may choose to prescribe a member of the latter class.
Resistant microbes can spread from one person to another. Consequently, an antibiotic-resistant infection differs qualitatively from a heart attack or stroke that fails to be cured by medicine: Antibiotic resistance moves beyond the affected patient and gradually renders the drug useless, whereas disseminated resistance does not occur with other drugs. Even resistance to anticancer drugs stays with the patient that developed the resistance because cancer does not spread from one person to another. This distinctive feature of antibiotics means that dosing, suitable effectiveness, and acceptable side effects must be decided by different rules than apply for treatment of noncommunicable diseases. The key concept is that using doses that are just good enough to eliminate symptoms may be fine for diseases such as arthritis, but it is an inadequate strategy for infectious diseases. Nevertheless, that strategy has been the norm ever since antibiotics were discovered.
Antibiotic Resistance Is Widespread
The seriousness of antibiotic resistance depends on perspective. For most diseases, we still have at least one effective drug. If we instantly stopped all resistance from increasing, our healthcare system could continue to perform well. But clinical scientists see resistance increasing and call the situation “dire.”13 For some pathogens, such as MRSA and Acinetobacter, physicians are forced to turn to antibiotics abandoned decades ago due to their toxic side effects. Our collective task is to develop attitudes and policies that enable all of us to use antibiotics without causing resistance to increase.
We estimate the extent of the resistance problem by surveillance studies. As pointed out, physicians collect microbial samples from patients and send the samples to clinical laboratories for testing (more than 2 billion per year in the United States14). Pathogens are cultured, and their susceptibility to specific antibiotics is determined (described in Chapter 2, “Working with Pathogens”). Surveillance workers then collect the data and calculate the percentage of the cultures that are resistant. (MIC breakpoints are used as the criterion for resistance.) This percentage, called the prevalence of resistance, indicates whether a particular antibiotic treatment is likely to fail due to pre-existing resistance. Surveillance also reveals trends when samples are obtained over several years from a similar patient population. Seeing the prevalence of resistance increase gives health planners advance warning that a change in treatment regimen is required.
Often, the prevalence of resistance is low for many years, and then it increases rapidly (see Figure 1-2). The challenge is to identify resistance problems while prevalence is still low. Then public health measures, such as increasing dose or halting the spread of the pathogen, may stop the increase. Many examples exist in which local outbreaks of resistance have been controlled. However, on a global level no antibiotic has returned to heavy use when resistance became widespread. Instead, the antibiotic is replaced with a more potent derivative.
Figure 1-2. Change in prevalence of methicillin resistance in S. aureus in Great Britain.
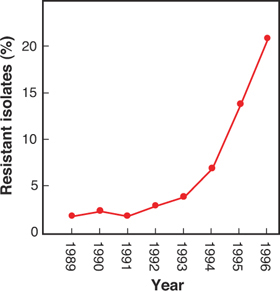
Data replotted from Johnson, A.P. “Antibiotic Resistance Among Clinically Important Gram-Positive Bacteria in the UK.” Journal of Hospital Infection (1998) 40:17–26.
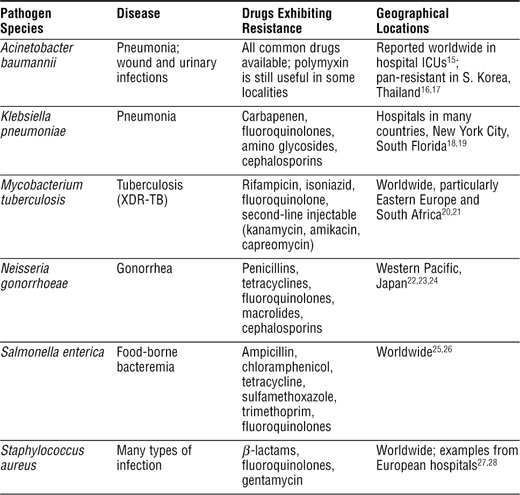
A partial list of major resistance problems is shown in Box 1-3. This list should be considered as a status report that needs to be continually updated, because pathogens are acquiring resistance to more and more antibiotics. It is also important to point out that resistance is generally a local or regional problem. For example, the prevalence of multidrug resistant (MDR) tuberculosis is particularly high in portions of Eastern Europe and South Africa, but in the United States it is rare.
Antibiotic Resistance Is Divided into Three Types
Antibiotic resistance is categorized into several types that require different solutions. One is called acquired resistance. As a natural part of life, mutant cells arise either spontaneously (about one in a million cells per generation) or from the transfer of resistance genes from other microbes (see Chapter 6). When a mutant is less susceptible to a particular antibiotic than its parent, mutant growth is favored during treatment. Eventually, the mutant becomes the dominant member of the pathogen population. One way to slow this process is to limit antibiotic use or use doses that block mutant growth.
When the “acquired” mutant starts to spread from person to person, it causes transmitted or disseminated resistance. In this second type of resistance, the pathogen is already resistant before treatment starts. Disseminated resistance is often highly visible and may elicit immediate action by the healthcare community. Much of that action is aimed at halting transmission.
A third type of resistance involves pathogen species unaffected by particular antibiotics. They are said to be intrinsically resistant. Little can be done about this type of resistance except to develop vaccines and use good infection control practices that keep the pathogens away from us. Most viruses fall in this category.
The Development of New Antibiotics Is Slowing
For many years, pharmaceutical companies developed new antibiotics to replace old ones whose effectiveness was seriously reduced by resistance. The new drugs were often more potent versions of earlier compounds. Unfortunately, finding completely new antibiotic classes becomes progressively more difficult as we exhaust the available drug targets in pathogens. Early in the Twenty-First Century, pharmaceutical companies placed considerable hope on genomic technology as a way to find new bacterial drug targets and thereby new antibiotics. In this approach, computer-based analyses examine the information in bacterial DNA and gene expression profiles to identify potential targets for new antibiotics. So far, that approach has not panned out. At the same time, pharmaceutical executives realized that more money could be made from quality-of-life drugs and drugs for managing chronic diseases. For example, heart disease requires life-long therapy to lower cholesterol. In contrast, antibiotics are administered for only short times. Antibiotics also have a large development cost, almost $1 billion per drug. As a result, many major pharmaceutical companies shut down their microbiology divisions. Small biotech companies are taking on the effort, but we can no longer depend on new compounds to postpone the antibiotic resistance problem.
Vaccines Block Disease
Vaccines represent an alternative way to combat microbes and viruses. Vaccines are preparations of attenuated pathogen or noninfectious parts of pathogens. When eaten or injected, vaccines create a protective immune response against a particular pathogen. Some vaccines are so effective that they eliminate a disease, as was the case with smallpox. The absence of disease means no resistance problem. Unfortunately, we have been unable to make effective vaccines for many pathogens, most notably HIV, tuberculosis, and malaria. Moreover, pathogen diversity can generate resistance to a vaccine (see Box 1-4).
Another serious example concerns the pertussis vaccine. Before vaccination began in the 1940s, pertussis (whooping cough) was a major cause of infant death. In the 1990s, pertussis began a resurgence in countries where most of the population had been vaccinated. Some of the resurgence was due to waning vaccine-induced immunity among the elderly, who increasingly were stricken with whooping cough. However, in Holland between 1989 and 2004, a new strain of Bordetella pertussis, the causative agent, replaced the old one among children, and the number of whooping cough cases increased. The new strain appears to be more virulent and produces more toxin than the old one.30
Perspective
Pathogens have attacked humans throughout history. Before the middle of the twentieth century, we relied on our immune systems to survive those attacks. The unlucky and the weak died. Our immune systems were strengthened by improvements in diet, and the frequency of some pathogen attacks was reduced by sanitation and water purification. For other pathogens, vaccines were developed that further decreased the overall burden of infectious disease. Insecticides provided local protection from being bitten by mosquitoes and other disease-carrying vectors. But our fear of pathogens was eliminated only by antibiotics. By taking pills for a few days, we could quickly recover from most bacterial diseases. Resistance is bringing back our fear of the “bugs.”
Many of our resistance problems derive from the cumulative effects of several complex factors. One has been our cavalier attitude. For example, in early 2009, American supermarket chains began to advertise free antibiotics to attract customers. The underlying message was that antibiotics cannot be very valuable and worth protecting. Another factor is lack of stewardship. Drug resistance is discussed widely among health officials, but a coherent plan has not emerged. Hospitals are beginning to oversee their own use, but agricultural and community antibiotic use is largely uncontrolled after the drugs are approved by governmental agencies. For years, medical scientists, notably Fernando Baquero, Stuart Levy, Richard Novick, and Alexander Tomasz, wrote and spoke passionately about the dangers posed by resistance. The medical community now uses education as a strategy to limit antibiotic use. As a part of this effort, the Centers for Disease Control (CDC) formulate and distribute plans for restricting the emergence of resistance in particular environments. In one survey, neonatal intensive care units failed to adhere to the guidelines about 25% of the time.31 Outside hospitals individual patients continue to insist on antibacterial treatments for viral infections, a behavior that stimulates the emergence of resistant bacteria and upsets the balance of microbial ecosystems. In the Latino immigrant community, the prescription process is commonly bypassed.32,33 Thus, the educational effort needs to be intensified. A third factor is the philosophy behind the choice of dosage. Doses are kept low enough to cause few side effects but high enough to block susceptible cell growth or kill susceptible cells. Conditions that block the growth of susceptible cells but not that of mutants are precisely those used by microbiologists to enrich mutants. Conventional dosing strategies lead directly to the emergence of resistance.
Understanding the factors that drive the emergence and dissemination of antibiotic resistance is central to controlling resistance. In the following chapters, we describe how antibiotics are used, how pathogen populations become resistant, and what we as individuals can do about resistance. We begin by considering aspects of pathogen biology relevant to antibiotic treatment.