Chapter 2. Working with Pathogens
Differences among the various pathogens require distinct management strategies, particularly when considering antibiotic therapy. We begin by considering how to detect and count microbes. Then we briefly discuss criteria for establishing causal relationships between putative pathogens and disease. The chapter concludes with a central point for resistance: infections contain large numbers of pathogens that must be considered as heterogeneous populations (populations of susceptible cells containing small subpopulations of resistant mutants).
Pathogens Are a Diverse Group of Life Forms
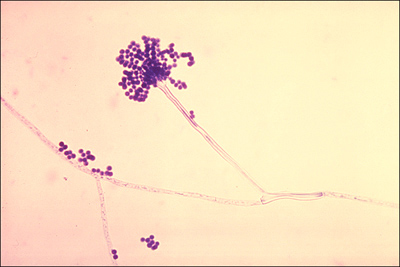
Pathogens fall into three general types: 1) bacteria, which lack a clearly defined nucleus; 2) fungi, protozoa, and helminths, whose cells have a distinct nucleus and are biochemically similar to human cells; and 3) viruses, which are inert molecules when outside their host cells. Single-celled organisms, such as bacteria, are sometimes thought to lead simple lives: they grow and then divide to form two new cells. Upon closer examination we see that some form spores that permit survival in extreme environments, and many have ways to sense and respond to population density. Fungi such as yeasts are also single-celled organisms, whereas those called molds form filamentous networks and specialized fruiting bodies that produce spores. The spores drift through the air until they land on a suitable nutrient surface. There, they germinate, forming filamentous hyphae. The opportunistic pathogenic yeast Candida albicans also generates hyphae upon infection. Protozoa are a third type of single-celled organism. Parasitic protozoa often have complex life cycles in which some forms grow in insect vectors, whereas quite different forms live in our bodies. Helminths are multicellular worms that invade our bodies; pinworm and hookworm are examples. Viruses lack the molecular machinery for independent life, but when they penetrate our cells, they can force the cells to make viral components. Those components assemble to form progeny virus particles that are then released to infect other host cells. A common feature of these diverse life forms is their ability to multiply inside our bodies and form large populations.
Pathogen Numbers Are Measured by Microscopy and by Detecting Growth
To understand and control pathogens, we must have a way to count them—we need to know whether an antibiotic reduces pathogen numbers. When bacteria are placed on a glass microscope slide, stained with a dye, and viewed through a microscope, they appear as tiny spheres or rods, depending on the species. Most bacteria are surrounded by a protective cell wall. The structure of the cell wall and its ability to take up a particular stain separates bacteria into two general types. One group is called Gram-positive and the other Gram-negative in honor of Christian Gram, the inventor of the stain. These two bacterial groups, which have evolved along separate paths, often differ in their response to antibiotics. Fungal cells and protozoa are much larger than bacteria and are easily observed by light microscopy; most viruses are submicroscopic.
Situations exist in which microscopy is used routinely for diagnosis of disease. One example concerns tuberculosis. With this bacterial disease, samples of sputum (mucus and fluids coughed up from lungs) are stained in a way that distinguishes M. tuberculosis from other bacteria. This microscopic diagnosis is rapid, low-tech, and inexpensive. Unfortunately, microscopy does not detect all cases of tuberculosis, in part because the sample is small. Thus, other detection techniques, such as culture methods that enable bacteria to reproduce, are also important.
Most microbes are so small that little detail is seen by light microscopy. For detail and to see small pathogens such as viruses, we turn to electron microscopy. Electron beams have a shorter wavelength than visible light, which enables resolution of much smaller objects. Although electron microscopy has been a powerful research tool, the methods are too cumbersome and the instruments are too expensive for routine measurement of pathogen numbers.
Another way to “see” microbes is to allow them to grow and divide on a solid surface, such as agar. The cells pile on top each other, and when millions are present in the same spot, they form a visible colony (see Figure 2-1). Because all cells in a colony derive from a single cell, they represent a clone. We can estimate the number of cells deposited on an agar plate by counting the number of colonies that form.
Figure 2-1. Bacterial colonies. A small drop of Escherichia coli culture was spread (streaked) on a portion of an agar plate. Then a sterile wire loop was drawn across a small region where cells had been placed; streaking the loop across a clean portion of the agar served to dilute the culture so that individual colonies would be seen. The plate was then incubated at 37°C and photographed.
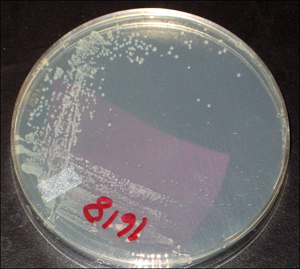
Photo credit: M. Malik and X. Zhao, Public Health Research Institute.
Liquid cultures of bacteria often contain hundreds of millions of cells per milliliter. Such dense cultures contain too many cells to count as colonies if all were placed on an agar plate and allowed to grow. (The colonies would grow together and form a lawn.) To solve this problem, we dilute the culture before spreading a small drop over the agar surface. By knowing 1) the extent of dilution, 2) the volume of diluted culture applied to the agar, and 3) the number of colonies that form, we can calculate the number of cells present in the original sample. That number is expressed as colony-forming units per milliliter. This measure is used to evaluate antimicrobial action.
Pathogen culturing methods are frequently used to evaluate antibiotic action, as detailed in Box 2-1. Some antibiotics block pathogen growth, whereas others also kill cells. To measure effects on growth, the antibiotic is present in the culture medium throughout the experiment. To measure lethal action, the microbial culture is exposed to the antibiotic for a specific time, and then the number of surviving cells is determined by growth into colonies on drug-free agar.
The general idea behind counting bacterial colonies also applies to certain viruses. Virus measurement depends on the capability of host cells to grow as a layer on a solid surface. If enough cells are placed on the surface, their growth covers the entire surface. With bacteria, such growth is called a lawn; with human or animal cells, it is called confluent growth (growth of some cell types stops when cells touch each other). If a virus that kills cells is mixed with the host cells before a lawn forms or confluent growth occurs, the virus quickly kills the growing cells. Many progeny virus particles are released, and they infect nearby host cells. A zone of cell death spreads over the surface from the point of initial infection. Eventually uninfected cells surrounding the death zone stop growing due to lack of nutrients or contact inhibition. Then the viruses no longer establish a productive infection. The result is a visible hole (plaque) inside a flat mass of host cells (see Figure 2-3). By counting plaques of diluted virus samples, we can estimate the number of infectious virus particles (plaque-forming units) initially present.
Figure 2-3. Detection of bacteriophage. An agar plate is shown on which Escherichia coli was spread over the entire plate. At the same time, dilutions of a bacteriophage preparation were placed on the agar as drops. During incubation at 37°C, the bacteria grew into a confluent lawn except where they were killed by the phage. Large clear regions occurred where large numbers of phage were deposited. On the left are small clear zones where only single phage particles were initially present. Each of these multiplied and gave rise to a plaque. (Top row left shows seven plaques.)
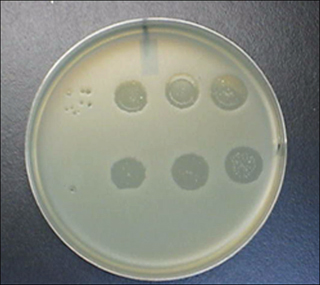
Photo credit: J. Qiao and X. Zhao, Public Health Research Institute.
Not all viruses form plaques. For example, some tumor viruses convert normal human and animal cells into tumor cells that continue to grow and divide after normal cells in the culture stop. In cases where normal cells cover the surface of a Petri dish and stop growing, tumor cells continue to pile on top of each other. The pile of tumor cells is called a focus. Foci can be seen and counted using a low-power microscope. The concentration of tumor viruses is then estimated by knowing the extent of dilution and volumes of virus samples applied to the Petri dish. Some other viruses cause cells to fuse into giant cells called syncytia that can also be seen and counted using a low-power microscope. Thus, the biological properties of pathogens are used to estimate their numbers.
Many pathogens can be handled safely in ordinary microbiology laboratories. To keep from contaminating the cultures, all glassware is sterilized in pressure cookers, as is agar before being placed in Petri dishes. Bacterial cells in colonies are conveniently transferred by touching a colony with the end of a thin wire to pick up some of the cells and then retouching the end of the wire to a clean agar plate or to a liquid growth medium. There, some of the cells fall off the wire and reproduce. To avoid contamination, the wire is sterilized between samples by heating in a flame. Particularly contagious agents are confined to specialized, negative-pressure, biosafety level 3 laboratory rooms where work is performed inside biosafety cabinets. These large, box-like structures have controlled air flow to keep the pathogens inside the cabinet. Workers wear disposable gowns, gloves, and masks. In some cases, full body suits and filtered breathing air is required. As a result of these precautions, laboratory infections rarely occur.
Molecular Probes Can Be Specific and Highly Sensitive
Although tests that require pathogen growth are often easy to perform, they can require considerable time; consequently, physicians frequently prescribe treatment without growing the pathogen and learning the cause of disease. This lack of precision is being corrected by replacement of conventional agar-plate methods with rapid, sensitive nucleic acid tests. For these tests, nucleic acids are extracted from diseased tissue or blood samples, and then they are examined for the presence of a particular pathogen nucleic acid. With DNA, detection begins by forcing apart the two strands of DNA from a patient sample. (Boiling a DNA solution is sufficient to separate the strands, and rapid cooling keeps them from coming back together.) The sample is mixed with a single-stranded DNA probe that is pathogen-specific. Incubation under proper conditions enables the nucleic acid from the laboratory sample, the probe, to bind with single-stranded pathogen DNA obtained from the patient sample. The result is a double-stranded hybrid DNA if the patient sample contains DNA with nucleotide sequences complementary to those in the probe. Formation of duplex DNA containing single-stranded nucleic acids from different sources is called nucleic acid hybridization. Because hybridization occurs only when the nucleotide sequences are complementary, hybridization serves as a specific test for a particular pathogen species. Probes called molecular beacons are available that emit fluorescent light upon hybridization and illumination with visible light (see Figure 8-1).34
One problem with nucleic acid hybridization is that the pathogen nucleic acid may represent only a tiny fraction of the total nucleic acid in the patient sample. (Human DNA may be much more abundant than pathogen DNA.) A method called PCR (see Appendix A, “Molecules of Life,” Box A-3) enables specific portions of pathogen DNA to be amplified millions of times, making detection much easier and more sensitive. In the case of RNA viruses, such as influenza virus, the viral RNA is first converted into a DNA form before carrying out PCR. This conversion process is called reverse transcription.
We know the nucleotide sequences of many pathogen DNAs; consequently, we can make short complementary DNA probes synthetically. That permits us to bypass work with the living microbe. Synthetic nucleic acid probes are becoming increasingly popular for detecting and identifying pathogens quickly. For example, we can now detect HIV shortly after infection, whereas the old antibody tests required more than one month of infection. Examples exist in which we can even tell whether a bacterial pathogen is antibiotic resistant using nucleic acid probes.35,36,37
Koch’s Postulates Help Establish That a Pathogen Causes Disease
Determining whether a particular microbe is actually the cause of a given disease is guided by Koch’s postulates. In the early days of microbiology, when novel bacteria were regularly recovered from diseased persons and animals, Robert Koch proposed a set of rules to help establish causal relationships. His postulates of 1884 are straightforward:
- The microbe must be detected in all host organisms suffering from the disease.
- The microbe must be isolated from a diseased host and grown in pure culture.
- The cultured microbe should cause disease when introduced into a healthy host.
- The microbe must be isolated from the inoculated, diseased experimental host and shown to be identical to the original microbe suspected of causing the disease.
Although his postulates are sometimes taken as the definitive test for causality, they were never taken as absolutes, even by Koch. (Postulate 3 used the word “should” rather than “must” because counter-examples were known.) Moreover, viruses, which had not been discovered when the postulates were published, are notoriously difficult to culture (postulate 2), and for some viral diseases we lack an animal model (postulate 3). Nevertheless, there is little doubt about the viral nature of some diseases.
The importance of the postulates is emphasized by a controversy over the viral nature of AIDS. An animal model was not available to establish that the human immunodeficiency virus (HIV) actually causes AIDS, as prescribed by Koch’s postulates. In the late 1980s, Peter Duesberg challenged the prevailing idea that HIV causes AIDS (see Box 2-2).38 If Duesberg were correct, the money being spent to find antiviral agents and vaccines was being wasted. Moreover, the cure for the disease would be changes in behavior and nutrition, not antivirals. Indeed, antivirals used to interrupt the transmission of HIV from mother to a new-born child were said by Duesberg to cause AIDS. Duesberg’s ideas were immediately dismissed by the scientific community, sometimes with strong language, and NIH funding for his work was stopped. However, having a well-known scientist cast doubt on the link between HIV and AIDS provided impetus for South Africa to delay treatment of the virus. Delay is thought to have been costly, in part because South Africa was experiencing an epidemic of tuberculosis,39 a disease that is exacerbated by infection with HIV.
Modern Biology Has Refined Koch’s Postulates
We continue to face new types of infection as we modify our environment. Diseases once restricted to tree-tops (monkeypox) have come to ground as we chop down forests, Lyme disease spreads with deer in backyards that were formerly woods, and hantavirus jumps to humans when rodents invade homes. Today we are considering abnormal proteins (prions) as agents of transmissible spongiform encephalitis (mad cow disease).50 Protein-based diseases require new paradigms, because in all other cases the disease-causing agents contain nucleic acids whose replication is easy to understand. Thus, Koch’s postulates remain relevant.
Many advances have occurred in molecular and cell biology since Koch’s paper. We now have clinical interventions (antibiotics and vaccines) that remove pathogens, and pathogens are known to acquire resistance that overcomes the antibiotics. Both events can correlate with changes in disease, thereby providing evidence that a particular pathogen causes a particular disease. Genomic nucleotide sequence analysis enables molecular ecology and microbial population genetics to contribute to causality arguments that are becoming increasingly complex. Thus, we now have a variety of ways to examine causality (see Box 2-3). At the same time, we are faced with political limitations on use of animals to establish causality (postulates 3 and 4) when a large body of data is already highly supportive. The net result is that a broad approach is used to identify the cause of disease.
Pathogen Studies Focus on Populations
Although infection of individuals can begin with one or a few pathogen cells or virus particles, we are usually concerned with the behavior of large populations. An important property of large microbial populations is that they are not homogeneous, even if they start from a single cell. At any given time, some cells are carrying out different biochemical processes than other cells in the population. Moreover, pathogen populations contain small subpopulations of mutants—between one in a million (10-6) and one in a hundred million (10-8). Mutants are recognized by having properties that differ from those of the bulk population. For example, antibiotic-resistant mutants grow on agar containing antibiotic, whereas wild-type cells do not. Although mutation frequency is a small number, some patients are infected with more than a billion (109) bacterial cells or virus particles, as can be the case with pneumonia, abscesses, tuberculosis, and HIV disease. In such cases, resistant mutants are statistically likely to be present before treatment. Resistant subpopulations, which are discussed in Chapter 10, “Restricting Antibiotic Use and Optimizing Dosing,” create a fundamental problem for conventional therapy strategies.
Bacterial populations also contain a small number of cells (1 in 100,000) that are not readily killed by antibiotic treatment even though they have normal antibiotic susceptibility. These cells are called persisters. Persisters are not resistant, because these survivors of antibiotic treatment have the same antibiotic susceptibility as the starting population when retested. (Resistant mutants would exhibit low susceptibility.) Bacterial persister cells are thought to be in a semi-dormant state that protects them from antibiotic attack. They constitute an important reservoir of bacteria that can regrow after removal of antibiotic.
Another property of many bacterial populations is quorum sensing, a molecular process by which bacterial cells communicate with each other. Quorum sensing involves the release of specific small molecules from bacterial cells and the binding of those small molecules to receptors on bacterial surfaces. When a bacterial culture becomes dense, the concentration of the small molecules becomes high enough to trigger a cellular response via binding to the surface receptors. Thus, members of a bacterial population “know” how many others are present.
Finally, many bacteria form dense, structured communities called biofilms, often on surfaces such as teeth, blood vessels, and catheters. Cells in biofilms tend to be less susceptible to antibiotics than the same species growing as a cell suspension. For some bacterial pathogens, quorum sensing appears to be related to biofilm formation. Thus, interrupting quorum sensing might be a way to lower biofilm formation and increase the susceptibility of these bacteria to antibiotics.52
Perspective
Working with pathogens often uses simple technology that is easily adapted for detection of resistance. For example, a resistant bacterium will form a colony on drug-containing agar that prevents growth of susceptible cells. Measuring growth can also be simple. When microbes grow and divide in a liquid culture, they eventually become so concentrated that the medium becomes turbid (cloudy). By measuring the turbidity of a culture, we can obtain a rough estimate of the concentration of microbes.
In the late 1990s, the scientific community mounted a massive effort to determine the nucleotide sequence of the human genome. Rapid sequencing methods emerged that were subsequently applied to many pathogens. As a result, we now have complete nucleotide sequences for most of the medically important microbes. The availability of these sequences encouraged the development of many innovative diagnostic strategies based on nucleic acids. Sequence information also enabled the design of nucleic acid-based antibiotics that were expected to be highly specific. By comparison, our current antibiotics are rather crude agents. So far, few successful nucleic acid antibiotics have been developed, largely due to delivery problems. In the next chapter, we describe the major antibiotic classes to provide a context for considering resistance.