Chapter 9
Kinetics of Batch Biodesulfurization Process
This chapter is co-authored by Samiha F. Deriase, Prof. of Chemical Engineering, Egyptian Petroleum Research Institute, Nasr City, Cairo, Egypt
List of Abbreviations and Nomenclature
| CDBTo | Initial DBT concentration |
| C2HBP | Concentration of produced 2-HBP |
| Maximum Biomass Concentration (g/L) | |
| Adjusted Correlation Coefficient | |
| qO2 | Specific Oxygen Uptake Rate (h-1) |
| qp | Specific HBP production rate (h-1) |
| qs | Specific DBT Consumption rate (h-1) |
| µ | Specific Growth Rate (h-1) |
| µa | Apparent viscosity |
| µmax | Maximum Specific Growth Rate (h-1) |
| 2,2’-BHBP | 2,2’-Bihydroxybiphenyl |
| 2-HBP | 2-Hydroxybiphenyl |
| 2-MBP | 2-Methoxybiphenyl |
| 3-MBT | 3-Methylbenzothiophene |
| 4,6-DMDBT | 4,6-Dimethyl Dibenzothiophene |
| 4-MDBT | 4-Methyldibezothiophene |
| ADS | Adsorptive Desulfurization |
| ANN | Artificial Neural Network |
| ATP | Adenosine Triphosphate |
| BBD | Box-Behnken Design |
| BDS | Biodesulfurization |
| BNT | Benzonaphthothiophene |
| BT | Benzothiophene |
| C* | Saturation Concentration of Dissolved Oxygen |
| CCD | Central Composite Design |
| CFU | Colony Forming Unit |
| Co | Initial Concentration of the Dissolved Oxygen |
| Cx-DBTs | Alkylated dibenzothiophenes |
| D | Stirrer diameter |
| DBT | Dibenzothiophene |
| DBTO | Dibenzothiophene Sulfoxide |
| DBTO2 | Dibenzothiophene Sulfone |
| DCW | Dry Cell Weight |
| DDBT | Desulfurizing Capability Index |
| De | Effective diffusivity |
| Di | Impeller diameter |
| DMF | Dimethylformamide |
| DMSO | Dimethylsulfoxide |
| DO | Dissolved Oxygen |
| DOE | Design of Experiment |
| DPS | Diphenylsulphide |
| DSZA | Dibenzothiophene monooxygenase enzyme |
| DSZB | HBPS desulfinase enzyme |
| DSZC | Flavin reductase enzyme |
| E | Fractional Approach to Equilibrium |
| Ea | Activation Energy |
| ED | Entner–Doudoroff |
| EDS | Extractive Desulfurization |
| FWHM | Full Width at Half Maximum |
| GC-AED | Gas Chromatography-Atomic Emission Detector |
| Gr | Growth |
| H/A | Hydrocarbon/Aqueous |
| HB | Higher is Better |
| HBPS | 2′-Hydroxybiphenyl-2-sulfinic acid |
| HDS | Hydrodesulfurization |
| HGO | Heavy Gas Oil |
| HPLC | High Performance Liquid Chromatography |
| IC | Ion Chromatography |
| JA | Jerusalem Artichoke |
| JAJ | Jerusalem Artichoke Juice |
| JAJt | Treated Jerusalem Artichoke Juice |
| kcat | turnover number (min-1) |
| kLa | Gas Liquid Mass Transfer Coefficient (s-1) |
| Km | Michaelis constant (g/L) |
| LB | Lower is Better |
| LGO | Light Gas Oil |
| LSERs | Linear Solvation Energy Relationships |
| m | Maintenance coefficient (gglucose/gcell/h) |
| MD | Middle Distillate |
| MDUF | Middle Distillate Unit Feed |
| MRE | Mean Relative Error |
| n | Number of substitutions |
| NB | Nominal is Better |
| O/W | Oil/Water |
| OA | Orthogonal Arrays |
| ODEs | Ordinary Differential Equations |
| ODS | Oxidative Desulfurization |
| OFP | Oil Fraction Phase |
| OSCs | Organosulfur Compounds |
| OTR | Oxygen Transfer Rate |
| OUR | Oxygen Uptake Rate |
| PASHs | Polyaromatic Sulfur Heterocyclic Compounds |
| PC/O | Partition Coefficients between the Biocatalyst and the Oil Phases |
| PC/W | Partition Coefficients between the Biocatalyst and the Aqueous Phases |
| Qmax | Maximum specific desulfurization rate |
| R | Gas Constant |
| R2 | Correlation Coefficient |
| RMSE | Root Mean Square Errors |
| RPS | Recycled Paper Sludge |
| RSM | Response Surface Methodology |
| S | Substrate concentration (g/L) |
| S/N | Signal to Noise Ratio |
| SBM | Sugar Beet Molasses |
| SKIP | Sum Kinetics Interaction Parameters |
| SSE | Sum of Squares Errors |
| SSF | Solid State Fermentation |
| STBR | Stirred Tank Bioreactor |
| T | Temperature |
| TCA | Tricarboxylic Acid |
| tG | Growth time |
| Th | Thiophene |
| Ug | Superficial Gas Velocity (L/min) |
| Vtip | Impeller tip speed |
| Vtotal | Volumetric desulfurization rate |
| VVM | Vessel Volume per Minute |
| WCW | Wet Cell Weight |
| WOR | Water-to-Oil Ratio |
| X | Biomass concentration (g/L) |
| Ycal | Values calculated by the model |
| YE | Yeast Extract |
| Yexp | Experimental data |
| YG | Growth yield (gglucose/gcell/h) |
| η | Optimization extent value |
| ν | Working Volume (L) |
| ρ | Density |
9.1 Introduction
The main drawback of the BDS process is its low rate compared to the other conventional applied methods. Thus, it is important to know more about the kinetics of the biodesulfurization (BDS) mechanism to enhance the process rate. A representative kinetic evaluation would enhance the commercialization of BDS technology since the output assists in the establishment of an upscale process (Calzada et al., 2012). Serval kinetic studies have been performed for BDS throughout the 4S-pathway. This mainly depends on the determination of the DBT-removal rate and 2-HBP production rate and proposes an empiric kinetic model (Folsom et al., 1999; Galán et al., 2000; Kobayashi et al, 2001; Luo et al., 2003; Martin et al., 2004; 2005; Rashtchi et al., 2006; Alcon et al., 2008; Calzada et al., 2012; Nassar et al., 2017).
In order to scale-up a BDS process, it is important to study the enzymatic activity of the biodesulfurizing microorganisms with time. Kinetic studies on BDS in literature do not frequently focus on the 4S route as a whole four serial reaction network. However, further analysis on kinetic knowledge about intermediate reactions of this route comes down to in vitro works studying the activities of both monooxygenases, DszC (Lei and Tu, 1996) and DszA (Ohshiro and Izumi, 1999; Konishi et al., 2000). Particularly, the last step of this route presents high interest because it has been proven to act as the controlling step of the overall process (Gray et al., 1996; Santos et al., 2007). That is because the final product of the 4S route, 2-hydroxybiphenyl (2-HBP), is known to cause a competitive inhibition on the desulfinase enzyme, DszB, the enzyme of the last reaction (Nakayama et al., 2002; Watkins et al., 2003).
Moreover, in order to design BDS-reactors, the kinetics of the process must be thoroughly understood. Kinetic equations, which describe the activity of an enzyme or the growth of microorganisms on a particular substrate, are crucial in understanding many phenomena in biotechnological processes.
This chapter gives a simple background about the microbial growth and enzyme kinetics involved in the BDS-process with a summary of the published work concerning the kinetics of the biodesulfurization process. Furthermore, to gain a better understanding and full description of the process governed by the microorganisms, this chapter also covers the published kinetics on the BDS of model compounds and real oil feed.
9.2 General Background
9.2.1 Phases of Microbial Growth
The microbial growth curve in any cultural media means the changes of microbial cell numbers are plotted as a function of incubation time in cultural media. It passes through the main four district phases: lag, log, stationary, and decline phases (Figure 9.1). The graph represents the four main phase patterns of population growth when microorganisms are raised in a batch culture, i.e. closed system, where there is no additional space, no additional nutrients are added, and no waste or dead cells are removed. The mathematical description of the four phases is also summarized in Table 9.1. It should be noted that, usually, there are some physiological changes associated with the shifting from one phase to the next.

Figure 9.1 Four Main Phases of Microbial Growth.
9.2.1.1 The Lag Phase
This phase is sometimes known as the adaptation or induction phase. During the lag phase, there is little or no change in the number of cells, but the metabolic activity is high. In a laboratory, microbial cultures are placed in new medium, usually beginning to grow after a “lag phase” due to the physiological adaptation of cells to new physicochemical conditions. The time taken in the lag phase is usually the time to make new enzymes or proteins for the new media.
9.2.1.2 The Log Phase
This phase is also known as the exponential growth or logarithmic phase. During the log phase, the microorganism multiplies at the fastest possible rate (the high rate of cell division and the rate of increase in cells is proportional to the number present at any particular point in time) under the provided conditions (that is a constant supply of fresh media). The log phase represents the period of optimal population growth. Eventually, however, the microorganisms approach the upper limit to their continued growth called the “carrying capacity”. Moreover, it should be known that cells in this phase are typically in the healthiest state.
Table 9.1 Mathematical Description of Microbial Growth Phases.
| Phase | Mathematical description |
| Lag Phase | dx/dt ≅ zero (1) |
| Exponential Phase | dx/dt = µx (2) |
| Stationery Phase | dx/dt = zero (3) |
| Decline Phase | dx/dt ≅ –kdx (4) |
where µ is the specific growth rate (1/time h-1), t is the incubation time (h), kd is the specific death rate (h-1), and x is the number of viable microbial cells (CFU/mL).
9.2.1.3 The Stationary Phase
During the stationary phase, there is an equilibrium between cell division and death (i.e. the n ° of new cells = the n ° of dying cells). Thus, no net growth occurs; within this phase, the available nutrients are exhausted, the physical competition for space and nutrients occurs, and, consequently, metabolically active cells stop dividing. Sometimes, production of some toxic substances occurs and waste products are built up. Furthermore, an increase or decrease in pH sometimes occurs; this is because either an essential nutrient is used up or waste products of the organism accumulate in the medium.
9.2.1.4 The Decline Phase
This phase is also known as the death or logarithmic decline phase. During the decline phase, the number of dead cells exceeds the number of produced new cells. In this phase, the cells stop multiplying and die because of nutrient depletion, toxic effects of some products, and/or intermediates and cell aging. This phase is usually a mirror image of the growth phase. During this phase, there is a net loss of viable cells [though some are still growing and dividing.]. If incubation continues after cells reach the stationary phase, the cells will eventually die.
9.2.2 Modeling of Population Growth as a Function of Incubation Time
A number of mathematical models and equations have been developed for the expression of the microbial growth in food and cultural media (Table 9.2). Most of the models are based on some basic mathematical models, such as the exponential, logistic, and Gompertez models. Figure 9.2 illustrates some of the graphical representations of microbial growth model equations.
Table 9.2 Different Microbial Growth Model Equations.
| Population growth function | Mathematical expression | |
| Differential form | Integrated form | |
| Exponential |  |
N(t) = Noexp(rt) (6) |
| Logistic |  |
 |
| Ordinary Gompertz |  |
 |
| Richards |  |
 |
| Generalized Logistic |  |
 |
| Hyper Logistic |  |
 |
where r is the intrinsic growth rate, No (i.e. N(0)) and Nt (i.e. N(t)) are the initial population size and the population size at time (t), respectively (dN/dt) is the rate of population growth at time (t), k=Nmax is the carrying capacity of the environment (i.e. maximum population), (1/N)(dN / dt) is the relative growth rate, Ninf is the population size at the inflection point, where growth rate is maximum, and α, β, and γ are positive real numbers.

Figure 9.2 Graphical Representation of Microbial Growth (a) Exponential and (b) Logistic Model Equations.
9.3 Microbial Growth Kinetics
9.3.1 Exponential Growth Model
One of the most famous model for microbial growth is the exponential growth function, where there are plenty available nutrients and growth substrates with reasonable space to grow and no threat from predators. It is applicable only for the subset of growth curve when exponential growth occurs, where the growth rate tends to increase at a rate that is proportional to the microbial population:
(19)

where dCx / dt is the growth rate, Cx is the cell concentration (g DCW/L) or change in population, as a function of time t (h), and µ (the proportionality constant) is the specific exponential growth rate (h-1). By integrating equation (20) with initial conditions at t = 0 and Cx = Cxo:
Thus, the basic exponential growth model, eq. 21, is good for modeling microbial growth populations that have unlimited resources over a relatively short span of time.
9.3.2 Logistic Growth Model
In the study of population dynamics, one of the most famous models for a growing, but bounded population is the logistic equation.
By including in the model eq. (20), a factor or a term

which suppresses the rate of growth at a high population, the resulting model is the differential form of the logistic growth kinetic model or Verhulst model (reliant to the Belgian statistician, Pierre Francois Verhulst (1804–1849) who worked on population growth in the 19th Century) (Verhulst, 1845; Verhulst, 1847; Blanch and Clark, 1997). In 1847, Pierre F. Verhulst proposed that the population growth depends not only on the population size, but also on the effect of a “carrying capacity” that would limit growth. That formula is called the “logistic model or the Verhulst model”:
where ![]() is the maximum biomass concentration reached at the late exponential phase when Cx is much smaller than
is the maximum biomass concentration reached at the late exponential phase when Cx is much smaller than ![]() , i.e. Cx <<<
, i.e. Cx <<< ![]() , and the value of that term is almost 1 and does not affect the growth rate, but as Cx increases to approach
, and the value of that term is almost 1 and does not affect the growth rate, but as Cx increases to approach ![]() , i.e. Cx ≈
, i.e. Cx ≈ ![]() , the value of that term approaches zero, thus making the rate of growth, dCx / dt, almost equal zero, which is the case during the stationary phase. However, if Cx >
, the value of that term approaches zero, thus making the rate of growth, dCx / dt, almost equal zero, which is the case during the stationary phase. However, if Cx > ![]() , so
, so

and

then (dCx / dt) < 0 and the population will decrease back towards the carrying capacity, i.e. the saturation level forming a numerical upper bound of growth size, in another word, carrying capacity is the amount that when exceeded in value will result in population decline. Thus, the logistic equation is based on exponential growth, but then adds a “braking force” as numbers increase toward the “carrying capacity,” ![]() .
.
Since the obtained experimental data are integral (i.e. biomass concentration varies with time), these data must be differentiated. The integration of the model introduces much less error than data differentiation. Therefore, eq. (22) has been integrated with initial conditions at t = 0 and Cx = Cxo, yielding the logistic population growth model (or Verhulst model) equation (eq.23), which is frequently used to model biological populations (Verhulst, 1845; Verhulst, 1847; Blanch and Clark, 1997; Martin et al., 2004) and the growth curves of the biological population are often described well with the logistic model (Nassar et al., 2017).
The logistic function can be obtained as a solution to the logistic differential equation (eq.22):
(23)

where Cxo is the initial biomass concentration (g DCW/L) at time, t = 0, Cx is the biomass concentration (g DCW/L) at time, t, ![]() is the maximum biomass concentation (g DCW/L) (i.e. the carrying capacity), t is the incubation period (h), and µ is the specific growth rate (h-1).
is the maximum biomass concentation (g DCW/L) (i.e. the carrying capacity), t is the incubation period (h), and µ is the specific growth rate (h-1).
Del Olmo et al. (2005) studied the influence of different carbon sources (glucose, glutamic acid, and citrate), nitrogen sources (ammonium and/or glutamic acid), and different DMSO concentrations on the growth of Rhodococcus erythropolis IGTS8. The authors employed the logistic kinetic growth model and concluded that the model is able to predict all experiments carried out with a very good fitting of the experimental results and the values of the kinetic parameters (µ and ![]() ) were in agreement with the experimental observations. Del Olmo et al. (2007) studied the influence of operational conditions (such as temperature, pH, and dissolved oxygen concentration) on the growth of Rhodococcus erythropolis IGTS8. A kinetic model based on the logistic equation was also applied to describe biomass concentration during the growth of Rhodococcus erythropolis IGTS8 growth. Kinetic model parameters (µ and
) were in agreement with the experimental observations. Del Olmo et al. (2007) studied the influence of operational conditions (such as temperature, pH, and dissolved oxygen concentration) on the growth of Rhodococcus erythropolis IGTS8. A kinetic model based on the logistic equation was also applied to describe biomass concentration during the growth of Rhodococcus erythropolis IGTS8 growth. Kinetic model parameters (µ and ![]() ) were obtained under several operating conditions and the predicted values of biomass concentration were very close values to those found experimentally. Caro et al. (2008) analyzed the values of biomass concentration by applying logistic model equations and determined the kinetic parameters (µ and
) were obtained under several operating conditions and the predicted values of biomass concentration were very close values to those found experimentally. Caro et al. (2008) analyzed the values of biomass concentration by applying logistic model equations and determined the kinetic parameters (µ and ![]() ) for evaluating the biodesulfurization of dibenzothiophene by growing cells of Pseudomonas putida CECT 5279. Chen et al. (2008) investigated the effect of 2-hydroxybiphenyl, the end product of biodesulfurization of DBT via 4S pathway during cell growth of Microbacterium sp. Strain ZD-M2, using a logistic equation for expressing the cell growth.
) for evaluating the biodesulfurization of dibenzothiophene by growing cells of Pseudomonas putida CECT 5279. Chen et al. (2008) investigated the effect of 2-hydroxybiphenyl, the end product of biodesulfurization of DBT via 4S pathway during cell growth of Microbacterium sp. Strain ZD-M2, using a logistic equation for expressing the cell growth.
9.4 Some of the Classical Kinetic Models Applied in BDS-Studies
For a description of a bioprocess, experimental data can be fitted to several kinetic models to calculate the kinetic parameters for better understanding of the microbial growth, substrate consumption, and product formation.
The relation between the specific growth rate (µ, h-1) of microorganisms and the concentration of substrate (Cs) usually described by the classical empirical growth kinetic models are listed in Table 9.3. Specific growth rates of the culture are fitted to kinetic models, listed above, in order to determine the values of batch growth kinetic parameters like maximum specific growth rate (µmax), half saturation coefficient (Ks), and substrate inhibition constant (Ki). The Monod model (eq.24) has been widely used to describe the growth-linked substrate utilization (Okpokwasili and Nweke, 2005). The Monod kinetic model relates the specific growth rate of microorganisms to substrate concentration via two bio-kinetic parameters (Ks and µmax) (Figure 9.3), where Ks is the half-saturation constant and is defined as the substrate concentration at which µ equals half µmax. The smaller it is, the lower the substrate concentration at which µ approaches µmax Also, the value of Ks shows the affinity of the microorganism to the substrate (Nuhoglu and Yalcin, 2005) and Ks is inversely proportional to the affinity of the microbial system for the substrate (Deriase et al, 2013).
Table 9.3 Classical Empirical Growth Kinetic Models.
| Growth kinetic model | Equation | Reference |
| Monod |  |
Monod (1949) |
| Haldane |  |
Haldane (1930) |
| Moser |  |
Layokun et al. (1987) |
| Aiba |  |
Aiba et al. (1968) |
| Tessier |  |
Edwards (1970) |
| Webb |  |
Edwards (1970) |
| Yano and Koga |  |
Yano and Koga (1969) |

Figure 9.3 Graphical Representation of Monod Kinetic Equation.
9.5 Factors Affecting the Rate of Microbial Growth
The main factors affecting the microbial growth are temperature, pH, oxygen requirements, nutrient levels and, finally, radiation and osmotic pressure.
9.5.1 Effect of Temperature
Temperature is the most important environmental factor that determines the rate of growth, multiplication, survival, and death of all living organisms. High temperature damages microbes by denaturing enzymes, transport carriers, and other proteins. Microbial membranes are known to be disrupted by temperature extremes. At very low temperatures, membranes also solidify and enzymes do not function properly. Cardinal temperatures are the minimum, optimum, and maximum temperatures at which an organism grows, as illustrated in Figure 9.4. Minimum growth temperature is the lowest temperature at which organisms grow. Optimum growth temperature is the temperature at which the most rapid rate of multiplication occurs. Maximum growth temperature is the highest temperature at which growth occurs. A temperature only slightly above this point frequently kills the microorganisms by inactivating the critical enzymes.

Figure 9.4 The Cardinal Temperatures: Minimum, Optimum, and Maximum.
Microorganisms can be classified into groups according to their growth temperature optima, as illustrated in Figure 9.5. A psychrophile is an organism that can live and thrive at temperatures much lower than normal (< 15 °C). A mesophile is an organism that can live and thrive at moderate temperatures (25–35 °C). A thermophile is an organism that can live and thrive at relatively high temperatures (between 45 ° C and 80 °C). A hyperthermophile is an organism that can live and thrive at very high temperatures (i.e. > 80 °C) (Le Borgne and Quintero, 2003).

Figure 9.5 Temperature and Growth Rate Response in Different Temperature Classes of Micro organisms.
The effect of temperature on the specific growth rate is represented mathematically by the Arrhenius model equation, as illustrated in Figure 9.6. The Arrhenius equation relates the specific growth rate to the inverse of absolute temperature (1/T), where A is the proportionality factor, Ea is the activation energy for the process, and R is the gas constant.

Figure 9.6 Effect of Temperature on Specific Growth Rate of Microorganisms.
9.5.2 Effect of pH
pH refers to the negative logarithm of hydrogen ion concentration, that is pH = - log10 [H+]. Microbial growth is strongly affected by the pH of the medium. Drastic variations in cytoplasmic pH disrupt the plasma membrane or inhibit the activity of enzymes and membrane transport proteins. Some organisms have evolved to grow best at low or high pH, but most organisms grow best between a pH of 6 and 8 (neutrophiles). Organisms that grow best at a low pH (<6) are known as acidophiles, while those which grow best at a high pH (>9) are known as alkaliphiles, as illustrated in Figure 9.7.

Figure 9.7 Effect of Acidity and Alkalinity on Growth of Microorganism.
9.5.3 Effect of Oxygen
Oxygen is an important substrate for aerobic organisms. Since metabolic energy production by cells is directly related to the oxygen uptake rate (i.e. the respiration rate), oxygen concentration is very strongly coupled to the growth rate as illustrated in Figure 9.8. The critical dissolved oxygen (DO) concentration refers to the value of DO below which the growth rate is lower than the maximum value. The growth rate sharply rises to its maximum value with DO concentration. The concentration at which the maximum growth rate is attained is often referred to as the critical oxygen concentration ![]() . This value is typically less than 0.5 mg/L for bacteria and yeast.
. This value is typically less than 0.5 mg/L for bacteria and yeast.

Figure 9.8 Effect of Dissolved Oxygen Concentration on Growth of Microorganisms.
9.6 Enzyme Kinetics
The living cell is the site of tremendous biochemical activity called metabolism. This is the process of chemical and physical change which goes on continually in the living organism, including building-up of new tissue, replacement of old tissue, conversion of food to energy, disposal of waste materials, reproduction, etc., briefly, all the activities of “life”.
The phenomenon of catalysis makes biochemical reactions necessary for all life processes possible. Catalysis is defined as the acceleration of a chemical reaction by some substance which itself undergoes no permanent change. The catalysts of biochemical reactions are enzymes and are responsible for bringing about almost all of the chemical reactions in living organisms. Without enzymes, these reactions take place at a rate which is too slow for the pace of metabolism. All known enzymes are high molecular weight proteins made up principally of chains of amino acids (Worthington Biochemical Corporation, 2017).
9.6.1 Basic Enzyme Reactions
The basic Enzymatic reaction mechanism can be represented as follows:
(9.1)
where E represents the enzyme catalyzing the reaction, S represents the substrate (substance being changed), and P represents product of the reaction.
A theory to explain the catalytic action of enzymes was proposed by the Swedish chemist Savante Arrhenius in 1888, where the substrate and enzyme formed some intermediate substance known as the enzyme substrate complex and the reaction can be represented as:
(9.2)
where ES represents enzyme substrate complex.
Combining the aforementioned reactions results in the following reaction:
(9.3)
The study of a large number of chemical reactions revealed that most of these reactions do not go to the true completion, which is likewise true for enzymatically-catalyzed reactions. This is due to the reversibility of most the reactions. Applying this fact to enzymatic reactions results in the following reaction mechanism equation, which is the basic equation upon which most studies related to enzyme activity are based.
(9.4)

where k+1 and k+2 are the forward reaction rate constants and k-1 is the backward reaction rate constant.
Equilibrium (that is the steady state condition) is reached when the forward reaction rates equal the backward rates. Figure 9.9 shows the relatively low and constant concentration of the enzyme – substrate complex (ES).

Figure 9.9 Change in Concentrations of substrate (S), product (P), enzyme (E), and enzyme complex (ES).
9.6.2 Factors Affecting the Enzyme Activity
Several factors affect the rate at which enzymatic reactions proceed, such as enzyme and substrate concentrations, temperature, pH, and the presence of inhibitors or activators (Worthington Biochemical Corporation, 2017).
9.6.2.1 Enzyme Concentration
In order to study the effect of increasing the enzyme concentration upon the reaction rate, the substrate must be present in an excess amount, that is, the reaction must be independent of the substrate concentration and any change in the amount of the product formed over a specific period of time will be dependent upon the level of enzyme present (Figure 9.10).

Figure 9.10 Zero Order Reaction Rate is Independent on the Substrate Concentration.
These reactions are said to be “zero order” because the rates are independent of substrate concentration and are equal to the reaction rate constant (k). The formation of products proceeds at a rate which is linear with time. The amount of enzyme present in a reaction is measured by its activity. Table 9.4 represents the reaction order with respect to substrate concentration.
Table 9.4 Reaction Order with Respect to Substrate Concentration.
| Order | Rate equation |
| Zero | Rate = k (31) rate is independent of substrate concentration |
| First | Rate = k[Cs] (32) rate is proportional to 1st power of concentration |
| Second | Rate = k[Cs]2 (33) rate is proportional to the square of substrate concentration |
| Second | Rate = k[Cs1][Cs2] (34) rate is proportional to 1st power of each substrate concentration |
9.6.2.2 Substrate Concentration
Experimentally, it has been shown that if the amount of the enzyme is kept constant and substrate concentration is then gradually increased, the reaction velocity will increase until it reaches a maximum. After this point, the increase in substrate concentration will not increase the velocity (Figure 9.11). When this maximum velocity has been reached, all of the available enzymes would be converted to ES.

Figure 9.11 Effect of Substrate Concentration on Reaction Velocity.
9.6.2.3 Effect of Inhibitors on Enzyme Activity
Enzyme inhibitors are substances which alter the catalytic action of the enzyme and, consequently, slow down, or in some cases, stop catalysis. There are three types of enzyme inhibition: competitive, non-competitive, and substrate inhibition. Most theories concerning inhibition mechanisms are based on the existence of the enzyme substrate complex (ES). Competitive inhibition occurs when the substrate and a substance resembling the substrate are both added to the enzyme.
A theory called “lock-key theory” of enzyme catalysts can be used to explain why inhibition occurs. This theory utilizes the concept of an “active site”. It holds that one particular portion of the enzyme surface has a strong affinity for the substrate. Figure 9.12 represents the “lock-key” theory considering the enzyme as the lock and the substrate as the key. The key is inserted in the lock, turned, the door is opened, and the reaction proceeds. However, when an inhibitor which resembles the substrate is present, it will compete with the substrate for the position in the enzyme lock. When the inhibitor wins, it gains the lock position, but is unable to open the lock. Hence, the reaction is slowed down because some of the available enzyme sites are occupied by the inhibitor. If a dissimilar substance, which does not fit the site is present, the enzyme rejects it, accepts the substrate, and the reaction proceeds normally.

Figure 9.12 Lock – Key Theory.
Non-competitive inhibitors are substances which when added to the enzyme, alter the enzyme in a way where it cannot accept the substrate (Figure 9.13a) When a non-competitive inhibitor is added, the Vmax is reduced, but the Km remains the same (Figure 9.13b).

Figure 9.13 Non-Competitive Inhibition.
Substrate inhibition will occur when excessive amounts of substrate are present, as according to the Michaelis-Menten equation. Figure 9.14 shows the decrease in reaction velocity after the maximum velocity has been reached, that is excess amounts of substrate added to the reaction mixture decreases the reaction rate. This is thought to be due to the fact that there are many substrate molecules competing for the active sites on the enzyme surfaces that blocked the sites and prevent any other new substrate molecules from occupying them (Figure 9.15).

Figure 9.14 Substrate Becomes Rate Inhibition.

Figure 9.15 Substrate Inhibition.
9.6.2.4 Effect of Temperature
Like most chemical reactions, the rate of an enzyme-catalyzed reaction increases as the temperature is raised, but, in fact, many enzymes are adversely affected by high temperatures and, as a result, the reaction rate increases with temperature to a maximum level, then declines with a further increase of temperature, as shown in Figure 9.16, because most cell enzymes rapidly become denatured at temperatures above 40 °C.

Figure 9.16 Effect of Temperature on Reaction Rate.
9.6.2.5 Effect of pH
Enzymes are affected by changes in pH. The most favorable pH value, that is, the point where the enzyme is most active, is known as the optimum pH (Figure 9.17). The optimum pH value will vary greatly from one enzyme to another. Extremely high or low pH values generally result in complete loss of activity for most enzymes.

Figure 9.17 Effect of pH on Reaction Rate.
9.7 Michaelis-Menten Equation
In 1903, French physical chemist Victor Henri found that enzyme reactions were initiated by a bond between the enzyme and the substrate. His work was taken up by German biochemist Leonor Michaelis and Canadian physician Maud Menten, who investigated the kinetics of an enzymatic reaction mechanism. In 1913, they proposed a mathematical model of the reaction. Referring to enzymatic reaction mechanism (9.4), it involves the reversible reaction between enzyme [E] and substrate [S], binding together to form the enzyme – substrate complex [ES], which irreversibly yields product [P], regenerating the original enzyme. The enzymatic reaction mechanism has been extensively studied and resulted in the Michaelis–Menten equation (Michaelis and Meten, 1913). The characterization of the enzyme or microbe – substrate interactions involve the estimation of several parameters in the kinetic models from experimental data. In order to describe the true behavior of the system, the estimated and measured kinetic parameters used in developing the models are acceptable and can be used in reactor design for biodesulfurization (Kareem et al., 2014). Theoretically, when the maximum velocity, Vmax, had been reached all of the available enzyme has been converted to ES, the enzyme substrate complex.
Michaelis-Menten developed the following mathematical expression for the reaction velocity in terms of this constant and the substrate concentration-to calculate the enzyme activity in terms of reaction speed from measurable experimental data,
(35)

where Vt is the velocity at any time t, Vmax is the limiting maximum velocity (maximum reaction velocity), Cs is the substrate concentration at time, t, and Km is the Michaelis constant, which is defined as the substrate concentration at half of the maximum velocity, as shown in Figure 9.11_and given by equation (36). A small Km indicates that the enzyme requires only a small amount of substrate to become saturated to approach Vmax. Hence, the maximum velocity is reached at a relatively low substrate concentration. A large Km indicates the need for high substrate concentration to achieve maximum reaction velocity,
where K+1, K+2, and K-1 are rate constants.
When resting cells are employed, reaction kinetics could be governed by the Michaelis-Menten mechanism to explain the desulfurization of DBT and alkyl dibenzothiophenes Cx-DBT and calculate the biokinetic constant parameters, Km and Vmax (Folsom et al., 1999; Mingfeng et al., 2003a, b; Zhang et al. 2003). Nazari et al. (2017) reported that for the commercialization of BDS, the removal of sulfur from petroleum compounds needs enzymes with a high specificity and low Km in a dsz system.
9.7.1 Direct Integration Procedure
To explain the desulfurization pattern, the Michaelis-Menten equation which was originally developed for enzymatic kinetics can be employed.
The direct integration of the Michaelis-Menten equation yielded:
(37)
(38)

(39)

(40)

(41)

Further manipulation of equation (42) gives equation:
(43)

Equation (43) represents the final explicit form of the Michaelis-Menten equation from which the bio-kinetic constants Km and Vmax are estimated (Mingfang et al., 2003a).
It is important to note that most kinetic models and their integrated forms are nonlinear. This makes parameter estimation relatively difficult. However, some of these models can be linearized. Various linearized forms of the integrated expressions have been used for parameter estimation. However, the use of a linearized expression is limited because it transforms the error associated with the dependent variable, making it unable to be normally distributed, resulting in inaccurate parameter estimations. Therefore, non-linear least-squares regression is often used to estimate kinetic parameters from nonlinear expressions. However, the application of nonlinear least- squares regression to the integrated forms of the kinetic expressions is complicated. The parameter estimates obtained from the linearized kinetic expressions can be used as initial estimates in the iterative nonlinear least-squares regression (Kareem et al., 2014).
9.7.2 Lineweaver-Burk Plot Method
(44)

(45)

(46)

(47)

Plotting this relationship over X-Y axes (Figure 9.18.a), the result represents a straight line of slop equal to (Km / Vmax), where the Y axis intercept is equal to (1 / Vmax).

Figure 9.18 Lineweaver-Burk (a) and Eadie-Hofstee (b) Plots Methods.
Abolfazl et al. (2006) applied a Lineweaver-Burk plot method (Lineweaver and Burk, 1934) to calculate the values of maximal velocity, Vmax, and Michaelis constant, Km, for the biodesulfurization of DBT by a moderately thermophilic bacterium.
9.7.3 Eadie-Hofstee
This is another way to represent the enzyme Michaelis-Menten kinetic model equation from which the bio-kinetic constants Km and Vmax can be calculated.
(48)
(49)
(50)
(51)
Figure (9.18b) represents the plot of the above equation which is the Eadie-Hofstee plot, where the plotting of Vt versus Vt/Cs produces a linear plot, the intercept with the X-axis gives the Vmax/Km value, the intercept with the Y- axis gives the value of Vmax and the slope of the line gives (-Km).
9.8 Kinetics of a Multi-Substrates System
In a multi–substrates system, the biomass growth is due to the utilization of all available hydrocarbons in the system to the microorganism, accordingly, the specific growth rate will be the summation of all individual ones for each substrate, as represented by the following equation,
where, µT is the total specific growth rate (h–1) and ![]() is the specific growth rate on substrate i, in the multi-substrate system (h–1), which can be calculated from the multi-substrate form of the Monod kinetic equation, represented by eq. (53), where n is the number of substrates in the system,
is the specific growth rate on substrate i, in the multi-substrate system (h–1), which can be calculated from the multi-substrate form of the Monod kinetic equation, represented by eq. (53), where n is the number of substrates in the system,
where µmax,i is the maximum specific growth rate on substrate i, Ksi and Ksj are the half saturation coefficients for substrates i and j, respectively, and Csi and Csj are the concentrations of substrates i and j in the multisubstrate system, respectively. The value of the interaction term [Ksi /Ksj], in the multi-substrate Monod kinetic model (eq. 53), represents the effect expressed by substrate j on substrate i (Guha et al., 1999; Knightes and Peters, 2006).
9.9 Traditional 4S-Pathway
In 1990, the specific oxidative desulfurization pathway was elucidated for DBT desulfurization. In this pathway, bacteria are exclusively able to remove sulfur atoms from DBT by breaking the carbon-sulfur bond during the oxidation reactions and producing the final 2-HBP product as a result (Chapter 6). The bacteria are also capable of maintaining the heating value of fuel in this pathway, without decomposition of a carbon skeleton (Caro et al 2007; Davoodi-Dehaghani et al., 2010; Nasab et al. 2015). The traditional 4S-route is a multi-enzymatic oxidative system (Chapter 7) of four consecutive reactions which convert DBT into dibenzothiophene sulfoxide (DBTO), then into dibenzothiophene sulfone (DBTO2). The sulfone is transformed into 2′-hydroxybiphenyl 2-sulfinic acid (HBPS) and, finally, 2-hydroxybiphenyl (2-HBP) and sulfate are produced as the final end products.
Current knowledge about the 4S route includes information about both genes and enzymes needed for this transformation (i.e. the transformation of DBT into a sulfur free molecule, 2-HBP). Two monooxygenases, dszC and dszA, and a desulfinase, dszB, participate in the conversion of DBT into 2-HBP, Flavin-dependent monooxygenase, dszC, catalyses the first two steps of DBT oxidation into DBTO and, consecutively, into DBTO2. Monooxygenase dszA catalyzes the transformation of DBTO2 into HBPS. The third enzyme involved in the 4S route, desulfinase dszB, catalyzes the last step of 4S route, which involves the conversion of HBPS into the final product 2-HBP (Calzada et al., 2011). Accordingly, the four genes involved in the multi-enzymatic biodegradation process, which is called 4S metabolic mechanism (Chapter 7) (Mosseini et al., 2006; Calzada et al., 2011), are:
- dszC, encoding a DBT-monooxygenase responsible for the first two oxidations of DBT to sulfoxide and then to sulfone,
- dszA, encoding DBT-sulfone monooxygenase oxidizing DBT-sulfone to 2-HBP-2-sulfinic acid,
- dszB, encoding a 2-HBP- sulfonate desulfinase that converts HBP-sulfinate to 2-HBP and sulfite, and
- dszD, encoding a NADH-flavin mononucleotide oxidoreductase supplying FMNH2 needed for the first three oxidations.
9.9.1 Formulation of a Kinetic Model for DBT Desulfurization According to 4S-Pathway
According to the 4S route, four reactions must be considered as follows (Alcon et al., 2008):
(9.5)
(9.6)
(9.7)
(9.8)
The four reactions involved in 4S route have been studied separately. The DBT, DBTO, DBTO2, and HBP have been chosen as key compounds for each of the four reactions of the route.
The first step is the stoichiometric study of the reaction. It is necessary to simplify the reaction scheme as follows:
(9.9)
(9.10)
(9.11)
The kinetic equations assumed for each reaction are mainly those given in literature, simple Michaelis-Menten kinetics for reactions (9.9–9.11), and product competitive inhibition for reactions (9.12) according to:
(56)

(57)

(58)

(59)

The overall kinetic model for the reaction network is formed by the following set of differential equations, corresponding to the production rates of the four key compounds assumed (Alcon et al., 2008; Calzada et al., 2012; Martinez et al., 2017).
(60)

(61)

(62)

(63)

ki is the kinetic parameter of reaction i (i = 1,2,3,4) µM/min, Ki is the substrate affinity of the reaction substrate i µM, and KI is the inhibition constant µM.
Employing the production rate method, in order to determine reaction rate in the reaction network, this method is expressed as
(64)
R is a vector containing the production rates of the key compounds of the network, γ′ is a matrix containing the stoichiometric coefficients involved in the reaction network, andd r′ is a vector containing the reaction rates of all reactions in the network.
Accordingly, the above equation is expressed as follows:
(65)

Using the inversion matrix of stoichiometric coefficients γ′–1
(66)
This can be expressed as follows:
(67)

According to the above matrix expression, the following set of are equations obtained:
(68)

(69)

(70)

(71)

The integrated form of the above set of equations takes into account t = 0 and ![]() , thus the concentrations of key compounds involved in the 4S-route can be expressed according to the following equations; these equations are allowed to obtain values of
, thus the concentrations of key compounds involved in the 4S-route can be expressed according to the following equations; these equations are allowed to obtain values of

which means calculating values of ri at every time directly from experimental data.
(72)

(73)

(74)

(75)

According to the above equations, each reaction can be studied separately from integral data and the concentration of each compound versus time.
Calzada et al. (2012) proposed a new extended kinetic model by dividing both the numerator and denominator by the affinity of each reaction (Ki) in order to avoid convergence problems during fitting. The concentrations of compounds involved in a 4S-route can be expressed according to the following equations:
(76)

(77)

(78)

(79)

(80)

(81)

where ![]() = ki /Ki and
= ki /Ki and ![]() = 1/Ki.
= 1/Ki.
9.10 Different Kinetic Studies on the Parameters Affecting the BDS Process
Wang et al. (1996) predicted a model for R. erythropolis N1–36, which was based on the Monod growth kinetic equation employing a continuous experimental system. The kinetic parameters (the specific growth rate of cells, the affinity constant for substrate, and the cell yield coefficient) were determined when the sulfur source (dibenzothiophene-sulfone, DBTO2) is growth limiting.
In an attempt to apply biocatalytic oxidation using enzymes as an alternative to BDS using whole cells, Ayala et al. (1998) studied the kinetic properties of chloroperoxidase from Caldariomyces fumago with different organosulfur compounds and the chemical nature of products. Enzymatic oxidations were performed in media containing pure substrates, such as thiophenes and organosulfides, in the presence of 0.25 mM hydrogen peroxide. The specific activity of the oxidation of pure organosulfur compounds with chloroperoxidase from Caldariomyces fumago were ranked in the following decreasing order: ethyl phenyl sulfide (1725 min-1), thianthrene (1310 min-1), bithiophene (840 min-1), phenyl sulfide (831 min-1), benzothiophene (557 min-1), phenyl disulfide (352 h-1), and DBT (126 min-1), and are transformed to their corresponding sulfones. The specific activity is defined as the number of mol of substrate transformed per 1 mol of enzyme per minute (i.e. min-1). The kinetic constants for the oxidation of thianthrene with chloroperoxidase were also estimated in a 1 mL reaction mixture containing 15% acetonitrile and 20 mM KCl in a 60 mM acetate buffer with a pH 3.0 and 1.5 nM of enzyme. The results revealed that the kcat for the oxidation reaction was 64 s-1 and the Km for thianthrene was 90-times lower than for hydrogen peroxide, recording 1.45 and 133 µM, respectively. Moreover, the inactivation constants (kin min-1) for chloroperoxidase determined from a first-order equation, in the presence of different concentrations of hydrogen peroxide, were also calculated and recorded as 0.203, 0.248, and 0.287 min-1 with 0.25, 0.5, and 1 mM H2O2, respectively. So far, the inactivation mechanism has not been clearly elucidated.
DBT biodesulfurization data of two batch cultures inoculated by Rhodococcus erythropolis Ac-1514D and Rhodococcus ruber Ac-1513D were processed using the Monod equation (Zakharyant et al., 2004). The specific growth rates (µ) were obtained by the analysis of the linear ranges of the growth curves that were measured as cell protein vs. time in the semi-logarithmic coordinates. The results revealed that the two µ were nearly the same, recording 0.08 and 0.086 h-1, respectively. The obtained values for Rodococcus sp. are 2.5 times less than those known so far in literature for R. erythropolis (Kaufman et al, 1998; Chang et al., 2000). The yield coefficients for those cultures were 0.86 and 5.85 g protein/mmol DBT for R. erythropolis and R. ruber, respectively, while the specific desulfurization activity was measured by the DBT removal and recorded 185 and 30 mmol DBT/kg DCW/h, respectively. 2-HBP production for R. erythropolis was also reported and recorded 80 mmol 2-HBP /kg DCW/h. This was in accordance with those determined for the non-mutant Rhodococcus sp. (Honda et al., 1998; Kaufman et al, 1998; Kobayashi et al., 2000).
Rhodococcus sp. NCIM2891 has been applied for a batch BDS of hydrodesulfurized diesel oil with varied initial S-concentrations of 200–540 ppm at a different O/W phase ratio (0–100% diesel). NCIM2891 was found to follow the classical Monod type of growth kinetics and the maximum specific growth rate, µmax, was found to be 0.096 h-1 and yielded a coefficient of 0.2 g biomass consumed/g of substrate consumed and the half saturation constant Ks was 71 ppm (Mukhopadhyaya et al., 2006).
Del Olmo et al. (2005a) studied the effects of media composition on the BDS process using Rhodococcus erythropolis IGTS8, which proved great variations in biomass growth and, consequently, the BDS efficiency with the changes in media compositions. First, a kinetic model representing the growth curves, taking into account the composition of the media, was proposed to compare the growth rate in different media compositions throughout the values of the usual parameters employed to describe biomass growth, such as the specific rate (µ) and the maximum biomass concentration reached (![]() ). To evaluate the possibilities of different sources, the biomass yields of the different substrates (that is C, N and S-sources, YCX, YNX, and YSX) have been calculated. Moreover, the desulfurization capability of the cells (XBDS) at different growth times has been evaluated using a desulfurization test of DBT, taking into account only the concentration of the produced 2-HBP. Del Olmo et al. (2005a) also proposed a non-structured kinetic model, which is able to describe growth and desulfurizing capability with good statistical parameters and was applied by a non-linear simple response. A parameter considering the maximum desulfurizing capability of cells obtained during growth cycle is defined that involved both the biomass concentration attaining their BDS capability and the time needed to reach this concentration (DBDS). The advantage of this parameter is its ability to show the best medium composition to obtain biodesulfurizing cells of R. erythropolis IGTS8 and its ability to be applied to compare the results of different microorganisms produced under different operational media and conditions.
). To evaluate the possibilities of different sources, the biomass yields of the different substrates (that is C, N and S-sources, YCX, YNX, and YSX) have been calculated. Moreover, the desulfurization capability of the cells (XBDS) at different growth times has been evaluated using a desulfurization test of DBT, taking into account only the concentration of the produced 2-HBP. Del Olmo et al. (2005a) also proposed a non-structured kinetic model, which is able to describe growth and desulfurizing capability with good statistical parameters and was applied by a non-linear simple response. A parameter considering the maximum desulfurizing capability of cells obtained during growth cycle is defined that involved both the biomass concentration attaining their BDS capability and the time needed to reach this concentration (DBDS). The advantage of this parameter is its ability to show the best medium composition to obtain biodesulfurizing cells of R. erythropolis IGTS8 and its ability to be applied to compare the results of different microorganisms produced under different operational media and conditions.
For all the experiments carried out, the yields of substrates (carbon, nitrogen, and sulfur source) into biomass have been calculated, as follows:
(82)

Two parameters have been defined in order to calculate the desulfurizing capability of the cells.
The percentage of desulfurization, measured as an HBP conversion, XBDS;
(83)

To quantify the desulfurizing capability, taking into account all the variables influencing it (desulfurization percentage obtained at each time by resting cells, together with the biomass concentration reached), the desulfurizing development grade during growth (DBDS) was calculated as follows:
(84)

where CDBT,0 is the initial DBT concentration employed to perform the standard BDS resting cell assay, C2HBP is the concentration of produced 2HBP obtained at the end of the experiment (in that study, 3 h was chosen) of resting cell assay, tG is the time of growth employed to reach a biomass concentration, CX, and XBDS is the percentage of biodesulfurization reached by these cells in the standard test of resting cell assay.
The experiments revealed that the parameter YCX, obtained with citrate was significantly lower than those obtained for the other two C-sources (glucose and glutamic acid). The values obtained for YNX with glucose and citrate were very similar and lower than those obtained using glutamic acid. The highest value for YSX was obtained when citrate was employed as a C-source and the values obtained with glucose and glutamic acid were nearly the same. The XBDS was nearly the same with the three studied C-sources, reaching approximately 75%, but the time to reach that maximum value varied, recording 22 h upon the usage of glucose, but 30 h when using glutamic acid or citrate. The ![]() recorded 5.16, 3.76, and 2.72 using glucose, glutamic acid, and citrate, respectively. The presence of ammonium-nitrogen was found to increase the biomass concentration and expressed higher influence on growth than BDS-activity. However, upon the addition of NH4Cl, the XBDS reached 80% and decreased to 20% in its absence, recording
recorded 5.16, 3.76, and 2.72 using glucose, glutamic acid, and citrate, respectively. The presence of ammonium-nitrogen was found to increase the biomass concentration and expressed higher influence on growth than BDS-activity. However, upon the addition of NH4Cl, the XBDS reached 80% and decreased to 20% in its absence, recording ![]() of 3.76 and 0.93, respectively. The growth was faster with DMSO and sulfate, relative to that with DBT. However, the highest value for YCX was obtained in the experiment carried out using magnesium sulfate while the lowest value was obtained with DMSO as a sulfur source, while the highest values of YNX and YSX occurred when employing DBT. Moreover, upon the calculation of XBDS, it was observed that upon the usage of sulfate, the expression of 4S-route genes and desulfurizing capability throughout the growth were suppressed. A maximum value of XBDS (12%) was reached with the cells grown around 25 h when DMSO was used, but upon the usage of DBT, cells with the same growth time yield had an XBDS value of 9%, but the desulfurizing capability stayed at a constant value for approximately 25 h. The values of YSX increased with the increase in initial DMSO-concentration. However, the change in initial DMSO concentration expressed no significant effect on YNX, but the highest YCX, 9.72 and lowest one occurred at 50 µM and 250 µM, initial DMSO, respectively.
of 3.76 and 0.93, respectively. The growth was faster with DMSO and sulfate, relative to that with DBT. However, the highest value for YCX was obtained in the experiment carried out using magnesium sulfate while the lowest value was obtained with DMSO as a sulfur source, while the highest values of YNX and YSX occurred when employing DBT. Moreover, upon the calculation of XBDS, it was observed that upon the usage of sulfate, the expression of 4S-route genes and desulfurizing capability throughout the growth were suppressed. A maximum value of XBDS (12%) was reached with the cells grown around 25 h when DMSO was used, but upon the usage of DBT, cells with the same growth time yield had an XBDS value of 9%, but the desulfurizing capability stayed at a constant value for approximately 25 h. The values of YSX increased with the increase in initial DMSO-concentration. However, the change in initial DMSO concentration expressed no significant effect on YNX, but the highest YCX, 9.72 and lowest one occurred at 50 µM and 250 µM, initial DMSO, respectively.
Furthermore, experimental data of biomass concentration with time were fitted to the following kinetic model:
As the integration of the model introduces much less error than the data differentiation (Garcia-Ochoa et al., 1992), thus, upon the integration of the above model, with the initial condition t = 0 and Cx = Cxo, the following logistic equation was obtained:
(86)
The fitting has been carried out by non-linear regression using the Fischer and Student’s tests to evaluate the quality of fitting. Moreover, the sum of square residuals has been used as representative of the fitting:
(87)
The BDS-model is a modified one for that proposed by Luedeking-Piret (Luedeking and Piret, 1959):
(88)
with the boundary conditions: t = 10 h and XBDS = 0, which means to take into account a delay time in the development of the desulfurization capability of the cell. Experimental data has been fitted using a fourth order Runge-Kutta algorithm, coupled to a simple-response non-linear algorithm (Marquardt, 1963). The model was able to fit all the runs carried out, showing good results for statistical parameters (Student’s t-test and Fischer’s F-tests). The value of the fitting parameter “α”, considering the production of desulfurizing capability as growth associated, is the highest when glucose was employed. The tendency to decrease this ability during a stationary growth phase (indicated by parameter β) is slightly higher when glucose was employed than that obtained using glutamic acid. The presence of ammonium when glutamic acid was employed also has a clear influence and the value of parameter α presents a value three times higher when ammonium was employed and the decreasing of the desulfurization capability (parameter β) was higher when ammonium was added to the media. When DBT was used as a sulfur source, the value of parameter “α” was lower than that obtained using DMSO. The change in initial DMSO concentration also expressed a clear effect on the values of this parameter; when the higher concentration of DMSO was employed, the higher parameter values were obtained. Thus, the development of the desulfurizing capability of the cells, described as associated to growth (α value), presented the highest value using an initial concentration of 1300 M of DMSO. Although the parameter “β” also had the highest value for an initial concentration of 1300 M of DMSO, the DBDS values always remained higher than that obtained using other concentrations of DMSO.
In a similar study, del Olmo et al. (2005b) studied the effect of operational temperature, pH, and dissolved oxygen concentration on the microbial growth of Rhodococcus erythropolis IGTS8 and its BDS capability (i.e. the biodesulfurizing degree DBDS). The yields of different substrates into biomass (YiX), the percentage of desulfurizing capability of the cells (XBDS), and the desulfurization development degree during growth (DBDS) were calculated. The stationary growth phase was attained faster at temperature values from 30 to 32 °C. The highest and lowest cell concentrations occurred at the stationary phase were recorded at 26 °C and 36 °C, respectively. Moreover, the lowest YCX, YNX, and YSX were recorded at 28 °C. Moreover, the highest BDS percentage of 70% of initial DBT concentration 250 M was recorded at a temperature interval from 28 to 32 °C. This was reached within 24 and 30 h from the beginning of the growth. At the late exponential phase, the BDS percentage recorded a maximum of 20% at 26 °C and 36 °C. The maximum biodesulfurization degree (![]() ) of 5.16 was observed after 24 h of growth at 30 °C. The growth was faster and the biomass concentration was higher when pH was controlled with diluted NaOH at a pH value of 6.5. However, when the pH was not controlled, the lowest YCX, YNX, and YSX were recorded. Also, the highest BDS efficiency was recorded when pH was controlled to 6.5 either by using diluted NaOH or tris-HCl, recording
) of 5.16 was observed after 24 h of growth at 30 °C. The growth was faster and the biomass concentration was higher when pH was controlled with diluted NaOH at a pH value of 6.5. However, when the pH was not controlled, the lowest YCX, YNX, and YSX were recorded. Also, the highest BDS efficiency was recorded when pH was controlled to 6.5 either by using diluted NaOH or tris-HCl, recording ![]() 5.15 and 5.16, within 29 or 24 h of growth time, respectively. Both the growth rate and obtained maximum cell concentration increased with the increase of dissolved oxygen concentrations, recording its maximum at 20% of saturation which indicated oxygen limitation during growth. A maximum desulfurization capacity of ≈ 75% occurred within 24 h of growth at 20% dissolved oxygen concentration. Based on a logistic equation, the highest specific growth rate (µ = 0.202 h–1) was obtained at 30 °C and the
5.15 and 5.16, within 29 or 24 h of growth time, respectively. Both the growth rate and obtained maximum cell concentration increased with the increase of dissolved oxygen concentrations, recording its maximum at 20% of saturation which indicated oxygen limitation during growth. A maximum desulfurization capacity of ≈ 75% occurred within 24 h of growth at 20% dissolved oxygen concentration. Based on a logistic equation, the highest specific growth rate (µ = 0.202 h–1) was obtained at 30 °C and the ![]() parameter decreased with the increase in temperature, recording 2.27 and 0.81 g X/L at 26 °C and 36 °C, respectively.
parameter decreased with the increase in temperature, recording 2.27 and 0.81 g X/L at 26 °C and 36 °C, respectively.
Both kinetic parameters µ and ![]() are functions of temperature and can be described as follows:
are functions of temperature and can be described as follows:
(89)
(90)
where C1 and C2 are parameters of Ratkowsky (Ratkowsky et al., 1983) and a and b are parameters of linear equations. The biomass evolution with time was predicted by the six parameters listed above.
The specific growth rate µ h-1 was significantly affected by pH, however the highest ![]() 2.25 g X/L was obtained at pH 6.5 and that was controlled by diluted NaOH, while the lowest 1.43 g X/L was obtained with an uncontrolled pH of 5.5. Furthermore, the specific growth rate of µ h-1 and
2.25 g X/L was obtained at pH 6.5 and that was controlled by diluted NaOH, while the lowest 1.43 g X/L was obtained with an uncontrolled pH of 5.5. Furthermore, the specific growth rate of µ h-1 and ![]() were found to be increased with the increase of dissolved oxygen concertation, recording their highest values of 0.409 h-1 and 4.27 g X/L at a 20% saturation value and its lowest value, 0.202 h-1, was recorded at a 10% saturation value.
were found to be increased with the increase of dissolved oxygen concertation, recording their highest values of 0.409 h-1 and 4.27 g X/L at a 20% saturation value and its lowest value, 0.202 h-1, was recorded at a 10% saturation value.
The model describing the development of desulfurizing capability during growth that considers XBDS as an associated product to growth and had a production time delay, presented a decreasing tendency as growth finish was used.
(91)

The experimental data was fitted by using a fourth order Runge–Kutta algorithm to integrate it, with the following boundary condition: XBDS = 0 for t = 10 h, coupled to a simple-response non-linear algorithm (Marquardt, 1963). Model parameter values, α and β, that represent rates of biodesulfurization capability development and losing, respectively, were calculated.
It was found also that α recorded its maximum value at 127.79 at 30 °C and parameter β recorded zero at 26 °C, which was attributed to the maximum BDS capability that could not be reached during the growth time employed. The lowest value of α was obtained at an uncontrolled pH of 5.5 and recorded β, since a maximum BDS capability has not been reached during growth time. However, high α values were recorded over the studied range of dissolving oxygen. However, β recorded the lowest value when dissolved oxygen concentration was kept at 10% saturation, which suggested that 10% saturation was a better choice than 20% saturation since a high BDS capability was kept during more growth time.
A similar study was performed by Martin et al. (2005) to study the influence of different working conditions on the growth and BDS capacity of the genetically modified Pseudomonas putida CECT5979 desulfurizing DBT to 2-HBP. The studied parameters were pH conditions (buffered and non-buffered media) using different carbon sources (glucose, citrate, and glutamic acid), operating temperatures (26–32 °C), and different dissolved oxygen concentrations due to different aeration conditions (different air flows 1 and 3 L/min, using enriched air of 75% in oxygen). For achieving the optimum operating conditions, that is to obtain desulfurizing cells, a parameter (DBDS) that incorporates both biomass concentration and time to reach a particular percentage of desulfurizing capability (XBDS) has been used. This was done by applying the previously mentioned model equations (eqs. 82–84). The effect of different C-sources and changes in pH were performed together and revealed that the growth was better when the microorganism was grown in a non-buffered medium, attaining a value of 4.5 g/L. The maximum biomass concentration reached at the stationary phase was higher when the growth was conducted with glutamic acid as a carbon source in a non-buffered medium. On the other hand, the lowest final concentration of biomass was obtained when citrate was used as a carbon source in a buffered medium, reaching a value of 2.5 g/L. Moreover, the pH increased with time when citrate and glutamic acid were used as carbon sources, but decreased to 4.90 and 5.92 with glucose in non-buffered and buffered BSM, respectively, where the highest YCX, 3.26, was obtained in the run performed with citrate as the carbon source in a buffered medium. The parameter YNX, 0.63, obtained with glutamic acid in a buffered medium, was higher than that obtained for other experiments, while the highest XBDS, 64%, was obtained when glutamic acid is used as the carbon source in a non-buffered medium which also recorded the highest DBDS of 21.65 at 30 °C. The lowest value of XBDS, 5%, was obtained with glucose in a non-buffered medium, however it increased to 20% in a buffered medium. Upon studying the effect of temperature, it was depicted that the maximum cell concentration was nearly the same in all temperatures, but lowered at 32 °C. The highest value of the parameter XBDS was obtained when the microorganism grew at 30 °C in the exponential phase of growth (tG of 9 h) and conserved up to 15 h of growth; after that point, the desulfurizing capability decreased to 40%. The growth and BDS activity increased with the increase of the concentration dissolved oxygen in the growth medium. The highest biomass concentration was obtained when enriched air was used. Nevertheless, although the highest YCX occurred with enriched air, the lowest YNX occurred with enriched air. With an air flow of 1 L/min, the XBDS reached approximately 80%. The growth was fitted with a logistic model equation (eq.86) and the highest ![]() of 4.70 g/L was also obtained when glutamic acid was used as the carbon source in a non-buffered medium which gave the highest DBDS of 21.65 at 30 °C. Moreover, the growth parameters, specific growth rate µ (h-1) and maximum biomass concentration
of 4.70 g/L was also obtained when glutamic acid was used as the carbon source in a non-buffered medium which gave the highest DBDS of 21.65 at 30 °C. Moreover, the growth parameters, specific growth rate µ (h-1) and maximum biomass concentration ![]() (g/L), increased with the increment of dissolved oxygen concentration recorded at 0.73 h-1 and 6.23 g/L, respectively, in an experiment that was performed with enriched air and a flow rate of 3 L/min. Furthermore, upon applying the desulfurization model (eq. 91), the following observations were recorded: α, which considers the production of desulfurizing capability to be growth-associated, was found to be similar when glucose and citrate were used as carbon sources in buffered and non-buffered media, however, when glutamic acid was used, α decreased from 33.72 (in a non-buffered medium) to 18.77 (in a buffered medium). The tendency to decrease the desulfurizing capability during the stationary growth phase (indicated by the parameter β) was found to be higher when glucose was used as the carbon source in a non-buffered medium. The temperature highly influenced the BDS capacity of P. putida CECT5279. The α recorded its highest value, 33.72 at 30 °C and its lowest value, 10.52 at 26 °C. The highest value of β, 1.77, was also recorded at 30 °C. This would give an indication that the tendency to decrease the desulfurizing capability of P. putida CECT5279 is higher when the cells grow at 30 °C. Further, α and β values were higher at an air flow rate of 1 L/min than those recorded applying other air flow rates or upon applying enriched air.
(g/L), increased with the increment of dissolved oxygen concentration recorded at 0.73 h-1 and 6.23 g/L, respectively, in an experiment that was performed with enriched air and a flow rate of 3 L/min. Furthermore, upon applying the desulfurization model (eq. 91), the following observations were recorded: α, which considers the production of desulfurizing capability to be growth-associated, was found to be similar when glucose and citrate were used as carbon sources in buffered and non-buffered media, however, when glutamic acid was used, α decreased from 33.72 (in a non-buffered medium) to 18.77 (in a buffered medium). The tendency to decrease the desulfurizing capability during the stationary growth phase (indicated by the parameter β) was found to be higher when glucose was used as the carbon source in a non-buffered medium. The temperature highly influenced the BDS capacity of P. putida CECT5279. The α recorded its highest value, 33.72 at 30 °C and its lowest value, 10.52 at 26 °C. The highest value of β, 1.77, was also recorded at 30 °C. This would give an indication that the tendency to decrease the desulfurizing capability of P. putida CECT5279 is higher when the cells grow at 30 °C. Further, α and β values were higher at an air flow rate of 1 L/min than those recorded applying other air flow rates or upon applying enriched air.
Upon modeling and simulation of both, the growth of R. erythropolis HN2 and consumption of different concentrations of DBT as a function of incubation time, the logistic model equation was found to accurately describe the change in biomass concentration with time using different initial DBT concentrations. Also, the kinetic model equations relating to the rate of growth of microorganisms and that of DBT consumption with the yield coefficient calculated from the Monod equation allowed the prediction of the DBT–time profile along with microbial growth under the predicted optimal operating conditions, although a slight decrease in the specific growth rate, µ h-1, with an increase of initial DBT concentrations was observed. However, the specific desulfurization rate was found to increase with the increase of initial DBT concentration, recording its maximum at 1000 ppm, where regardless of the initial DBT-concentration, the maximum desulfurization rate occurred at a 72 h incubation period. The recorded maximum specific growth and degradation rates were 0.06667 and 0.8818 h-1, respectively. Thus, that study proved the strong influence of S-concentration on the rate of BDS, the well adaptation of R. erythropolis HN2 towards high DBT concentrations, and its ability to overcome the feedback inhibition of sulfate and 2-HBP (Nassar et al., 2017). This promotes the application of R. erythropolis HN2 in the petroleum desulfurization process.
In another study performed by Dejaloud et al. (2017), a logistic growth model (eq.86) combined with a Pirt model were applied to predict the importance of the maintenance energy during a batch BDS of different initial concentrations of DBT (0.03, 0.05, 0.08, and 0.11 mM) at two levels of glucose-concentrations: energy-limited medium 55 mM and energy-sufficient medium 111 mM using growing cells of Ralstonia eutropha (PTCC1615). Fitting the experimental data to the logistic model was performed using SigmaPlot 12.3 software considering Levenberg–Marquardt as a nonlinear regression algorithm. The lowest values of the sum squares of the differences between the values calculated by the model (Ycal) and the experimental data (Yexp) were determined {SSR = Σ(Yexp – Ycal)2}. The standard error of the estimate, ![]() , was described where df equals the number of data points minus the number of fitted parameters. The coefficient of determination (R2 = 1 – (SSR/SStotal)) which gives the ratio of the sum squares of the residuals to sum squares of the total and shows the extent of closeness of the experimental data to the test model showing the goodness of fit was also obtained. The confidence limits for the estimated parameters were expressed in terms of a 95% confidence interval and determined as follows: estimated parameter ±t0.975,df × the approximated standard error, where t0.975,df is the 0.975th quantile of t-statistic distribution with df degrees of freedom (Lapin, 1997). Additionally, the quality of the data fitting process was quantitatively explained using a t-test which has been defined as the ratio of the coefficient to the relevant standard error. The F-test statistic was also performed to evaluate the contribution of the independent variables in predicting the dependent variable (Lapin, 1997). If F is a large number, it is possible to deduce that the independent variables contribute to the prediction of a dependent variable. All the data revealed good fitting of the model. Moreover, it was found that the increase in the initial glucose concentration from 55 to 111 mM had no significant effect on the maximum cell growth,
, was described where df equals the number of data points minus the number of fitted parameters. The coefficient of determination (R2 = 1 – (SSR/SStotal)) which gives the ratio of the sum squares of the residuals to sum squares of the total and shows the extent of closeness of the experimental data to the test model showing the goodness of fit was also obtained. The confidence limits for the estimated parameters were expressed in terms of a 95% confidence interval and determined as follows: estimated parameter ±t0.975,df × the approximated standard error, where t0.975,df is the 0.975th quantile of t-statistic distribution with df degrees of freedom (Lapin, 1997). Additionally, the quality of the data fitting process was quantitatively explained using a t-test which has been defined as the ratio of the coefficient to the relevant standard error. The F-test statistic was also performed to evaluate the contribution of the independent variables in predicting the dependent variable (Lapin, 1997). If F is a large number, it is possible to deduce that the independent variables contribute to the prediction of a dependent variable. All the data revealed good fitting of the model. Moreover, it was found that the increase in the initial glucose concentration from 55 to 111 mM had no significant effect on the maximum cell growth, ![]() , and minor variations were observed for the maximum levels of the cell content (2.32 to 2.87 g/L). The maximum carrying capacity (
, and minor variations were observed for the maximum levels of the cell content (2.32 to 2.87 g/L). The maximum carrying capacity (![]() ) of the A and B media appears to be similar, but the specific growth rate for the energy-sufficient media was lower than those for the energy-limited cultures of DBT, while the opposite occurred in the control media prepared with ammonium sulfate. The reported high values of µ (0.32–0.63 h-1) indicated the cells’ efforts to be directed toward reproduction and the cells, apparently, are fully capable of using their biotic potential. Moreover, there was an observed difference in the efficiency of substrate consumption and growth and it was described in terms of the maintenance energy by which energy is used for non-growth functions (Pirt, 1965; Russell and Cook, 1995; van Bodegom, 2007). To quantitatively explain the Pirt concept with reference to the overall rate of glucose concentration (ds / dt)T, two terms are important: that for growth (ds / dt)G and that for cell maintenance (ds / dt)M (Pirt, 1965):
) of the A and B media appears to be similar, but the specific growth rate for the energy-sufficient media was lower than those for the energy-limited cultures of DBT, while the opposite occurred in the control media prepared with ammonium sulfate. The reported high values of µ (0.32–0.63 h-1) indicated the cells’ efforts to be directed toward reproduction and the cells, apparently, are fully capable of using their biotic potential. Moreover, there was an observed difference in the efficiency of substrate consumption and growth and it was described in terms of the maintenance energy by which energy is used for non-growth functions (Pirt, 1965; Russell and Cook, 1995; van Bodegom, 2007). To quantitatively explain the Pirt concept with reference to the overall rate of glucose concentration (ds / dt)T, two terms are important: that for growth (ds / dt)G and that for cell maintenance (ds / dt)M (Pirt, 1965):
(92)

By dividing eq. (93) by eq. (85), the following expression is obtained:
Integration of eq. (94) between Cxo and Cx yields:
(95)

The values of the bioenergetic constants, maintenance coefficient (m) and growth yield (YG), for different cultures were estimated and could properly describe the bioprocess. For example, in a control system in the presence of glucose and NH4SO4, the value of m recorded 0.13 and 0.34 gglucose/gcell/h for case A and B, respectively. This indicated that the maintenance coefficient (m) is higher for the energy-sufficient culture than for the energy-limited one, but they recorded the same growth yield 0.26 gcell/gglucose. The results also indicated that 2-HBP production was higher for energy-sufficient cultures, while the values of the specific growth rate and the maintenance coefficient for these media were lower than those of the energy-limited cultures. m and YG values, in limited energy case A, in the presence of DBT were in the range 0.45–0.62 gglucose/gcell/h and 0.58–0.88 gcell/gglucose, respectively, and were higher than those recorded in the case of sufficient energy, case B, ranged between 0.31 and 0.35 gglucose/gcell/h and from 0.21 to 0.28 gcell/gglucose, respectively. Thus, the YG values for the “A” cultures were higher than those for the “B” cultures (YG,As > YG,Bs) and the contrary occurred for the “m” coefficient, which was lower for the “B” than for the “A” cultures (mAs>mBs). This was explained as the excessive energy in cells controlled by the efficient regulation on substrate (glucose) uptake rather than energy spilling activities (Tsai and Lee, 1990) and the cellular pools of the produced NADH and FMNH2 coenzymes in “B” cultures should be directed toward the biodesulfurization process and glucose uptake regulation could be dominant. Not only this, but it was very obvious from the obtained m and YG in sufficient-energy cultures with DBT (case-B) that the increase of initial DBT concentrations did not affect the m or YG values. This indicated no inhibition effect within the studied initial DBT-concentrations. Furthermore, the maximum 2-HBP production in “A” and “B” treatments was about 0.05 mM and inhibition effect of products on the cell growth was not noticeable. The Haldane equation (96) was employed to explain and represent the inhibitory effect of high initial concentrations of DBT, (96)
where the Qmax is the maximum value of the specific desulfurization rate and KS and KI are the half-saturation constant and self-inhibition constant, respectively. The results revealed that with increasing the energy-supplying substrate, the Qmax and KI values kept approximately constant, recording 0.002 mmol/gcell/h and 0.076 and 0.076 mM for “A” and “B” cultures, respectively, but the KS value decreased from 0.1594 mM to 0.0782 mM. Thus, the kinetic studies showed that the half-saturation constant KS for the energy-limited cultures was 2 times higher than the energy-sufficient ones. The data also indicated that the increase in the carbon and energy source resulted in modifying the affinity of the enzymes for the BDS-process. The maximum specific desulfurization activities of the growing cells in these cultures were predicted at 0.002 mmol/gcell/h. Further tests on resting cells showed that the cells harvested from the mid exponential growth phase had the highest desulfurization activity where the specific desulfurization rate was 20 times higher than the growing cells at the same conditions. The desulfurizing capability index (DDBT %gcell/L/h) was calculated using the following equation:
(97)

where both the biomass concentration (Cx) and time (t) to reach a particular percentage of desulfurizing capability are included in the DDBT parameter and recorded 23% gcell/L/h at an initial DBT concentration of 0.5 mM.
The initial biomass concentration has also had an important effect on the BDS process. Caro et al. (2007a) studied the effect of the addition of β-cyclodextrins and different initial biocatalyst concentrations over the BDS process yielded for model oil (50 ppm DBT in –n-hexadecane) in a bi-phase system (1:1 oil:water), using growing cells of R. erythropolis IGTS8 and a 2 L STBR. The BDS-yield (XBDS) was measured as the percentage ratio between the concentration of produced 2-HBP and the initial DBT concentration. This study proved that the addition of 15 ppm β-cyclodextrin in the BDS reaction increased the rate of production of 2-HBP and XBDS, while the DBT removal rate was not affected. The BDS yield increased with the increase of the initial biomass concentration, but up to a certain limit; 2 g DCW/L and the produced 2-HBP decreased at a higher initial biomass concentration.
Caro et al. (2007a) applied another form of the Haldane equation to describe the BDS process and the following equations successfully simulated the removal of DBT and production of 2-HBP:
(99)

(100)

where X is the biomass concentration g/L and S is the substrate concentration g/L and the maximum specific growth rate µmax recorded 70 h-1 and the specific maximum desulfurization rate recorded 0.025 gDBT/gDCW/h with an inhibition rate constant of KI and ![]() of 2 and 2.5 g/L, respectively. The value of the saturation constants, Ks, decreased as the biocatalyst initial concentrations went up, reaching stability from 2 gDCW/L.
of 2 and 2.5 g/L, respectively. The value of the saturation constants, Ks, decreased as the biocatalyst initial concentrations went up, reaching stability from 2 gDCW/L.
Deriase et al. (2013) evaluated the biodegradation kinetics of the toxic 2-hydroxybiphenyl (2-HBP) and 2,2′-bihydroxybiphenyl (2,2′-BHBP) with a different initial concentrations range of S0 (5–50) ppm using suspended cultures of Corynebacterium variabilis Sh42 with a constant initial biomass concentration, Xo 315.8 ppm, in a series of batch experiments. The growth kinetics of C. variabilis Sh42 does not follow simple Monod’s kinetics. The cultures followed substrate inhibition kinetics. By fitting specific growth rates µ (h-1) on suitable substrate inhibition models, evaluating the bio-kinetic constants that are necessary to understand the kinetics of biodegradation process were evaluated, which confirmed good tolerance, growth, and degradation capabilities of Sh42 on the studied concentration ranges of 2-HBP and 2,2′-BHBP. Although Haldane and Yano and Koga bio-kinetic equations for substrate inhibition seemed to be the best adequate expressions for specific growth rates on 2-HBP and 2,2′-BHBP, respectively, with the highest R2 at 1.0 and 0.997, respectively and the least RMSE values at 6 x 10–5 and 9 x 10–5, respectively, an evident disagreement was observed between experimental and simulated profiles for bacterial growth, X (mg/L), and substrate concentration, S (mg/L). A new proposed model based on a modified Haldane equation gave better results.
For a batch biodegradation process, the following differential equations (derived from mass balance considerations) are often used for describing both biomass growth and substrate consumption.
(101)

The change in substrate concentration can be defined by:
where q (h-1) is the specific substrate consumption rate. The relation between q (h-1) and µ (h-1) can be represented by:
The relationship between biomass formation and substrate consumption can be approximately determined by the yield coefficient, Yx/s (dry weight of biomass / weight of utilized substrate), indicating the maximum conversion of unit substrate to cell mass (Okpokwasili and Nweke, 2005).
By introducing the decay rate coefficient [b]:
(105)

By introducing a constant cell decay rate coefficient b (0.001 h-1), there is still an evident disagreement between measured and simulated profiles observed with an average maximum percentage deviation of 22 and 44% for cell growth and 63 and 67% for substrate degradation, respectively.
However, by dividing the time span of the biodegradation process into subintervals, according to the assumption of the production and accumulation of metabolic intermediates during the process, the decay rate coefficient b’ (h-1) is changed for these subintervals according to logical IF statements included in the implemented computer program.
µ (h-1) was represented by a modified Haldane equation given by:
(106)

where f(i) is analogous to substrate inhibition term (S2/Ki) in the classical Haldane equation. f(i) represents the functional relationship of effect of metabolite intermediates on the hydroxybiphenyls (HBPs) biodegradation process. It is expressed as follows:
(107)

These equations were coupled with equation (102), describing the change in HBPs concentration with time. Since the kinetic model of Yano and Koga (eq.30) has no substrate inhibition term (S2 /Ki), the new proposed model was applied for both 2-HBP and 2,2′-BHBP.
To estimate the degree of toxicity of 2-HBP and 2,2′-BHBP on Corynebacterium variabilis Sh42, the Haldane model, which is the most widely used model, was applied. The two bio-kinetic constants are Ki, the inhibition constant which is a measure of sensitivity by inhibitory substances, and Ks, the half saturation constant which is defined as the substrate concentration at which µ equals half of µmax. Since the studied HBPs expressed inhibitory effects on Sh42, according to Nuhoglu and Yalcin, 2005, if the substrate is inhibitory, it is not possible to observe an actual µmax. Thus, Ks could be taken on a hypothetical meaning. It has been shown that the Haldane equation would go through a maximum value of dµ/ds = 0 at substrate concentrations of S* ppm for inhibitory substrates and it is the concentration at which the microorganisms exhibited their maximum utilization rate, S* = (Ks.Ki)1/2 and the corresponding µ value is

This reflects that the degree of inhibition is determined by the [Ks / Ki] ratio and not just by Ki alone. The larger the ratio of [Ks/Ki] is, the smaller the µ* (h-1) relative to umax and the lower the degree of inhibition. From that work, it can be concluded that the maximum specific growth rate on 2,2-′BHBP (0.053 h-1) was greater than that on 2-HBP (0.045 h-1). Considering the fact that Ks ppm is inversely proportional to the affinity of the microbial system for the substrate, Sh42 showed higher affinity to 2-HBP (Ks =0.894 ppm) than that of 2,2′-BHBP (Ks = 1.88 ppm). The S* for 2-HBP was smaller than that of 2,2′-BHBP (8.21 and 11.45 ppm, respectively). The [Ks/Ki] ratio of 2-HBP is smaller than that of 2,2′-BHBP (0.01 and 0.027, respectively) and the corresponding µ* (2.58 x 10–3 and 2.22 x 10–3, respectively). These data indicated that the toxicity and inhibition effects of 2-HBP on Sh42 are higher than those of 2,2′–BHBP (Deriase et al., 2013).
From the quantitative discussion of modeling a relationship between cell growth rate and substrate consumption rate, it was found that the direct coupling of the substrate consumption rate, with the cell growth model is warranted only under certain conditions (e.g. constant cell yield). However, for HBPs degradation over a wide range of initial substrate concentrations, the cell mass yield was found to vary. Variations of the biomass decay rate coefficient during the time course of batch experiments while adjusting the interactive changeable biomass decay rate coefficient b’, it was found to be in the range 0.0001–0.007 h-1 and were found to exert a great influence on the biodegradation process, with the assumption of the existence of some metabolic intermediates that would exert some inhibition on HBPs degradation. Correlation and simulation studies using a new proposed model based on modified the Haldane equation were established and these factors were taken into consideration in the proposed HBPs degradation model. The proposed model is capable of describing growth and HBP degradation profiles very well over the studied initial HBP concentrations (5–50 mg/L). The average maximum percentage deviation reached 6.2 and 7.2% for cell growth and 11 and 10.2% for substrate degradation in the case of 2-HBP and 2,2′-BHBP, respectively (Deriase et al., 2013).
In another study, biodegradation kinetics of different polyaromatic sulfur heterocyclic compounds (PASHs), including thiophene (Th), benzothiophene (BT), dibenzothiophene (DBT), 4- methydibenzothiophene (4-MDBT), and 4,6-dimethyldibenzothiophene (4,6-DMDBT), with different initial concentration ranges of So of (100–1000 mg/L), employing suspended cultures of Bacillus sphaericus HN1 with initial concentrations of Xo ranging from 291.90–362.01 mg/L dry weight, in a series of batch experiments were investigated (Deriase and El-Gendy, 2010). A mathematical model was predicted to describe the biodegradation kinetics of the studied PASHs using HN1. The predicted model is based on the Haldane bio-kinetic equation (eq. 98) for substrate inhibition which is applied to describe the dependence of the specific growth rate µ (h-1) on the intial substrate concentration, So. The Haldane equation seems to be an adequate expression for the cell growth data and the bio-kinetic constants obtained were maximum specific growth rates of µmax = 0.165, 0.231, 2.461, 0.207 h-1, and 0.202 for Th, BT, DBT, 4-MDBT, and 4,6-DMDBT, respectively, a saturation constant of Ks = 3.007, 18.425, 2004.25, 42.25, and 103.43 mg/L for Th, BT, DBT, 4-MDBT, and 4,6-DMDBT, respectively, while the inhibition constant, Ki = 2110.42, 1752.42, 46.849, 2242, and 360.61 mg/L for Th, BT, DBT, 4-MDBT, and 4,6-DMDBT, respectively. This data indicated that the toxicity and inhibition effects of these PASHs on HN1 can be ranked in the following order: Th > BT > DBT. This was attributed to the water solubility of these PASHs, as with the increase of molecular weight and aromatic rings, water solubility decreases. Inhibition phenomenon is often observed when the inhibitory compound is more soluble than other substrates (Nadalig et al., 2002). Similar observations of a higher toxicity of Th than DBT were reported for Agrobacterium MC 501 and the mixed culture XACO (Constanti et al., 1996). Inhibitory effects of BT at higher concentrations were also reported by Van Hamme et al. (2004) and Kirkwood et al. (2007). Moreover, the toxicity and inhibition effects of these three PASHs on HN1 can be ranked in the following order: 4-MDBT > 4,6-DMDBT > DBT. Kirkwood et al. (2007) reported that methylation decreases aqueous solubility, which might explain the lower toxicity effect of 4,6-DMDBT relative to 4-MDBT, but the lowest toxicity effect occurred by DBT, which might be explained by the adaptation of HN1. Regardless of the type of PASHs, growth decreased with an increase in initial substrate concentration. Experimental results have also made it clear that biocatalyst growth was always stopped before the complete removal of the PASHs. This could be attributed to the biodegradation process itself, which might produce toxic metabolites to the cell, but the time profile of each PASH, regardless of the PASHs type and So, expresses the degradation trait at increasing levels during the bacterial growth cycle even after reaching its maximum growth. This indicated that the stationary phase cells have the ability to continue degrading PASHs.
The proposed mathematical model can be described by the following equations:
(108)

(109)

where b (h-1) is the decay rate coefficient found to be in the range 0.0001-0.009 and c is the power of microbial concentration found to be in the range of 0.4–0.6.
The predicted model simulation curves for bacterial growth, X (mg/L), and substrate concentration, S (mg/L), are derived by simultaneously solving the resulting ordinary differential equations (ODEs), 108 and 109 together, with the explicit equations, 103 and 104. These differential equations were numerically solved using POLYMATH 6.10 (professional version). The program used the Felhberg fourth-fifth order Runge-Kutta (FRK45) numerical integration method. The predicted model was adequate in reflecting the PASHs concentration profile. The maximum specific degradation rate (qmax, h-1) recorded 0.042, 0.063, 1.53, 0.088, and 0.053 h-1 for Th, Bt, DBT, 4-MDBT, and 4,6-DMDBT, respectively. The degradation rates ranked in the following increasing order: Th < BT < DBT and 4,6-DMDBT < 4- MDBT < DBT. The highest degradation efficiency recorded in DBT cultures indicated the adaptation of HN1 biodegrading enzymes towards DBT, as it was previously isolated and enriched on DBT.
Nadalig et al. (2002) reported that the presence and position of the methyl group on the PAH molecules govern degradation rates of the compounds. Also, Kirkwood et al. (2007) suggested that steric hindrance influences enzymatic activity. This could explain the lower biodegradation that occurred in 4,6-DMDBT cultures compared to those occurring in 4-MDBT cultures, although the toxicity and inhibition effect of 4-MDBT on HN1 was higher than that of 4,6-DMDBT. Fedorak and Westlake (1983 and 1984) reported that, in general, the more the number of alkyl carbons, the more recalcitrant the compounds. Lu et al. (1999) reported the preference of Shingomonas paucimobilis strain TZS-7 for DBT rather than 4,6-DMDBT. Results from the study performed by Deriase and El-Gendy (2010) are consistent with the results reported by Kropp et al. (1997); C1-DBTs are more susceptible to biodegradation than C2-DBTs. Thus, in environments contaminated with crude oil, DBT and methyl-DBTs will be depleted before the isomers of dimethyl-DBTs.
Guchhait et al. (2005a and b) also described the evolution of BDS by growing cells of Rhodococcus sp. JUBT1 in biphasic media by a typical Haldane equation, as the substrate inhibits both biocatalyst growth and the 4S-pathway. For each OSC and diesel oil with a sulfur content of 500 ppm, having a C1 –DBT of 40 wt.%, C2-DBT of 30 wt.%, C3-DBT of 20 wt.%, and others of 10 wt.%, and an aromatic content of 27.16 wt.%, the value of maximum substrate concentration, CSmax, corresponding to the maximum substrate consumption rate has been determined using the following theoretical concept:

or
(110)

where rs (mg/dm3/h) is the consumption rate of the substrate, t is the time (h), ![]() (mg/dm3) is the modified half saturation constant for a substrate inhibited system, µmax is the maximum specific growth rate (h-1), and i is any compound.
(mg/dm3) is the modified half saturation constant for a substrate inhibited system, µmax is the maximum specific growth rate (h-1), and i is any compound.
The values of the half saturation constant (KS, mg/dm3), maximum substrate concentration (CSmax, mg/dm3), and inhibition constant (KSi, mg/dm3) have been correlated to the number of alkylation in DBTs.
The rate of removal of OSCs was calculated as follows:
(111)

(112)

where the CB is the biomass concentration mg/dm3, Cs is the substrate concentration, µ is the specific growth rate (h-1), i is any compound, and YX/si is the yield coefficient equal to the mass of biomass produced/mass of substrate consumed.
The specific growth rate, µ, has been calculated using Monod type and Haldane type kinetics during simulation using a 4th order Runga Kutta technique. The data analysis revealed that up to 100 mg/dm3 of each of the studied OSCs’, DBT, C1-DBT, C2-DBT, and C3-DBT, Monod type kinetics were able to explain their transient behavior, while Haldane type kinetics were more suitable to predict the higher removal of higher concentrations, up to 1000 mg/dm3, where the substrate inhibition was validated above 100 mg/dm3 of initial substrate concentration. The value of CSmax increased with the number of substitution, n, in DBT. The value of µmax, however, showed the reverse trend, while CSmax and KS have increased with the number of alkylation and KSi, µmax, and Yx/S have decreased with it. The validity of the Haldane model was also established in the case of diesel. Moreover, the applicability of the linear correlations of the number of alkylation, n, with different kinetic parameters in the lower initial concentration range of diesel, up to 200 mg/dm3, was also confirmed (Guchhait, et al., 2005a).
Caro et al. (2008a) studied the effect of organic solvents and initial S-concentrations on the BDS efficiency of P. putida CECT 5279, using model oil (DBT/n-hexadecane). CECT 5279 was able to tolerate high concentrations of organic solvent up to 1:1 v/v. A concentration of 400 ppm of DBT was converted at a specific rate of generation of the desulfurized final product, 2-HBP, of 2.3 and 1.5 mg HBP/g DCW/h for 27% and 50% (v/v) of hexadecane, respectively. It was found that upon the usage of a no-selective S-source, such as MgSO4, the growth was not affected even at high concentrations of n-hexadecane (i.e. up to 50% v/v of OFP). Nevertheless, when using a selective S-source, such as DBT, the biomass growth was twice lower than that achieved with MgSO4 and the biomass decreased with increase of the OFP. This was attributed to the hydrophobicity of DBT and, consequently, there would be a lower amount of DBT in an aqueous phase with the increase of OFP, which might also depend on the hydrophobicity of the microbial cell itself (Xu et al., 2006; Caro et al., 2007a; Mohebali et al., 2007). It may be also due to the lower oxygen supply in a bi-phase system, especially with the increase of the oil phase. Strain CECT 5279 was probably not capable of being joined at the oil-to-water interface for the uptake of DBT there and, hence, the lower final optical densities were achieved. It is known that the transfer of OSCs from the oil to the aqueous phase is one of the most determinant parameters in the BDS process, especially when biocatalysts without the capacity to be adhered at the interface are used (Maxwell and Yu, 2000; Monticello, 2000; Abbad-Andaloussi et al., 2003). Usually, in the aqueous phase system, there is a lag phase for the BDS of the organosulfur compounds (e.g. DBT). This is the time during which DBT concentration overcomes its solubility in water (Monticello, 2000) and a nucleation process could be produced, as it has been described before (Jia et al., 2006). This time is not always considered in the posterior kinetic analysis because DBT molecules are only assembled by cells when they are completely dissolved into the broth (Wodzinski and Coyle, 1974).
The values of biomass concentration were analyzed by applying the logistic equation to determine the kinetic parameters, µ and ![]() , while the evolution of the BDS process was analyzed applying the Haldane equation, taking into account that the pathway’s final product is stoichiometrically generated. Moreover, the evaluation of the process was done by determining the desulfurization percentage (XBDS). Furthermore, the HBP production rates (rp) were measured as the average cellular activity into a reaction interval. The saturation and inhibition constants, Ks and KI, were found to be increased with the increment of the OFP. It is normally assumed that the increment of KS is related to mass transfer limitations as this effect is similar to a decrease of enzymatic affinity. Moreover, the growth inhibition constant was higher than the desulfurization inhibition constant, indicating that the sensitivity of cell growth towards DBT-concentration was higher than that of desulfurization. It is important to note that the calculated amount of 2-HBP was always higher than the real experimental results since the applied model did not consider the accumulation of intermediates, as they were usually detected only at negligible concentrations in the extracellular media. In another study by Caro et al. (2008b), it was proven that there was accumulation of several products inside the cells. Not only this, but Abbad-Andaloussi et al. (2003) reported that 2-(2′-hydroxyphenyl)benzenesulfinate (HBPSi) was not detected either by gas chromatography (GC) or high performance liquid chromatography (HPLC) due to an analytical selectivity problem since the retention time and spectrum of both HBPSi and HBP are quite similar and the concentrations produced are near the detection limit as well. Upon studying the effect of initial DBT concentration in the model oil (200 to 3200 ppm), the growing cells of P. putida CECT 5279 proved good BDS capabilities. However, the biomass growth stopped before the complete conversion of DBT. This was attributed to the feed-back inhibition effects produced by 2-HBP (Setti et al., 1999; Maxwell and Yu, 2000) and sulfate accumulation (Li et al., 1996). However, due to the hydrophobic nature of 2-HBP, it migrates to the oil phase so its inhibition effect on the cell at the aqueous phase will be decreased. Thus, the growth might be stopped for other reasons, such as several other limiting substrates related with 4S-pathway development, such as the co-factors regeneration and oxygen contribution.
, while the evolution of the BDS process was analyzed applying the Haldane equation, taking into account that the pathway’s final product is stoichiometrically generated. Moreover, the evaluation of the process was done by determining the desulfurization percentage (XBDS). Furthermore, the HBP production rates (rp) were measured as the average cellular activity into a reaction interval. The saturation and inhibition constants, Ks and KI, were found to be increased with the increment of the OFP. It is normally assumed that the increment of KS is related to mass transfer limitations as this effect is similar to a decrease of enzymatic affinity. Moreover, the growth inhibition constant was higher than the desulfurization inhibition constant, indicating that the sensitivity of cell growth towards DBT-concentration was higher than that of desulfurization. It is important to note that the calculated amount of 2-HBP was always higher than the real experimental results since the applied model did not consider the accumulation of intermediates, as they were usually detected only at negligible concentrations in the extracellular media. In another study by Caro et al. (2008b), it was proven that there was accumulation of several products inside the cells. Not only this, but Abbad-Andaloussi et al. (2003) reported that 2-(2′-hydroxyphenyl)benzenesulfinate (HBPSi) was not detected either by gas chromatography (GC) or high performance liquid chromatography (HPLC) due to an analytical selectivity problem since the retention time and spectrum of both HBPSi and HBP are quite similar and the concentrations produced are near the detection limit as well. Upon studying the effect of initial DBT concentration in the model oil (200 to 3200 ppm), the growing cells of P. putida CECT 5279 proved good BDS capabilities. However, the biomass growth stopped before the complete conversion of DBT. This was attributed to the feed-back inhibition effects produced by 2-HBP (Setti et al., 1999; Maxwell and Yu, 2000) and sulfate accumulation (Li et al., 1996). However, due to the hydrophobic nature of 2-HBP, it migrates to the oil phase so its inhibition effect on the cell at the aqueous phase will be decreased. Thus, the growth might be stopped for other reasons, such as several other limiting substrates related with 4S-pathway development, such as the co-factors regeneration and oxygen contribution.
Irani et al. (2011) determined the batch growth kinetic parameters of the aerobic Gram +ve Gordonia alkanivorans RIPI90A for diesel and hydrotreated diesel in biphasic media, with the initial varied range of S-content at 2–5 g/L and 0.007–0.028 g/L, respectively. The experimental results have shown acceptable agreement with the Haldane kinetic for diesel and the Monod kinetic for hyrotreated diesel. The maximum specific growth rate (µmax h-1) on diesel oil and hydrotreated diesel oil recorded 0.459 and 0.095 h-1, with a half saturation coefficient (KS) of 3.55 and 0.02 g/L, respectively, and a substrate inhibition constant (KSi) of 19.24 g/L for diesel oil. This was attributed to the inhibitory high S-concentration in diesel oil. This study revealed that the strong influence of sulfur concentration on its desulfurization rate. Moreover, the inhibition effect of the produced 2-hydroxybiphenyl throughout the 4S-pathway would decrease the desulfurization rate. However, with a low sulfur concentration range, as the availability of sulfur compound increases, better growth of biomass leads to a higher rate of substrate consumption. However, at a high sulfur concentration range, the substrate inhibition effect occurs and, consequently, the rate of BDS decreases.
To determine the kinetics of desulfurization in a batch BDS of model oil (DBT in dodecane) using resting cells of Nocardia globerula R-9, the initial desulfurization rate in a biphasic system (1:1 O/W) was studied with various DBT concentrations in dodecane, at pH7 and 30 °C within 1 h. The obtained results were best represented by the Michaelis–Menten equation:
(113)

where So is the initial DBT-concentration in mmol/L, Vmax is the maximum reaction velocity in mmol S/kg/h, and Km is the Michaelis constant.
The values of Vmax and Km for the model oil BDS were found to be 11.0 mmol S/kg/h and 0.70 mmol/L, respectively (Mingfang et al., 2003b).
To analyze the desulfurization pattern of model oil (DBT in dodecane) using lyophilized cells of Pseudomonas delafieldii R-8, the initial desulfurization rate, under various DBT concentrations in the dodecane with the ratio of oil-to-water and cell density of 1.0 and 20 mg DCW/L of the aqueous phase, was tested. The obtained results were best represented by the Michaelis–Menten equation. The values of the rate constants, the limiting maximal velocity (Vmax), and Michaelis constant (Km), for the desulfurization of DBT were fitted as 0.32 mM/h (13.0 mmol/kg DCW/h) and 1.3 mM, respectively. This indicated that the high concentration of DBT, up to 14 mM (corresponding to 448 ppm), did not inhibit the desulfurization activity of R-8 (Luo et al., 2003).
The time course of desulfurization of the mixture of DBT and 4,6-DMDBT by resting cells of Pseudomonas delafieldii R-8 in a biphasic system was described by Mingfang et al. (2003b). The concentration of each in the dodecane phase was about 0.5 mmol/L. It indicated that the desulfurization process of DBT and 4,6-DMDBT proceeded simultaneously without showing any preference to either one. The desulfurization pattern of DBT was described by the Michaelis–Menten equation. The BDS-initial rate of DBT and 4,6-DMDBT was 1.7 and 0.75 mmol S/kg cell/h, respectively, while the initial desulfurization rate in a single substrate system recorded 6.5 and 2.5 mmol S/kg cell/h for DBT and 4,6-DMDBT.
The initial desulfurization rate in a biphasic system (30 % O/W) of model oil (DBT in hexadecane) was tested under various levels of DBT concentrations, a pH of 6, 30 °C, and 175 rpm, using 11 g DCW/L of resting cells of RIPI-22 bacteria harvested at the end of the logarithmic phase (30 h) (Rashtchi et al., 2006). Considering the fact that BDS is a kind of enzymatic reaction, the Michaelis–Menten equation (eq.113), which is equivalent to Monod, was applied, where dS/dt is the initial specific reaction rate for the production of 2-HBP, S, and the concentration of 2-HBP (mM). The parameter fitting was done using the Curve-Expert 1.3 and the values of Vmax, Km, and the correlation coefficient (R2) were obtained and recorded as 0.21779 mM/h, 0.04099 mM 2-HBP, and 0.9724.
However, in a study performed by Boltes et al. (2013), the Michaelis–Menten kinetics used for data simulation in aqueous media were not valid under biphasic conditions due to the low bioavailability of DBT and its alkylated derivatives (Cx-DBTs) in an aqueous phase where microorganisms exist.
Calzada et al. (2012) developed a kinetic model that fits the experimental results for resting cell operation with cells of different ages in different concentrations, taking into account the enzyme deactivation. The genetically modified Pseudomonas putida CECT 5279 was employed in that study. It is a genetically modified bacterium that successfully performs the 4S-pathway, as it carries the genes dszABC from Rhodococcus erythtropolis IGTS8 and a flavin-oxydo-reductase from Escherichia coli (hpaC) (Galán et al., 2000).
Aggarwal et al. (2013) applied in silico modeling to study the BDS efficiency of BT and DBT, using G. alkanivorans in batch BDS processes with glucose a carbon source. Based on the assumption of a fixed uptake of glucose (20 mg/g DCW/h), with unlimited supplies of BT and DBT and maximized biomass growth, the model consumed BT with a growth rate of 0.021 h-1 and a desulfurization rate of 2.55 mmol/g DCW/h. This corresponds to the minimum in silico sulfur requirement of the cell for maximum growth at a glucose uptake of 20 mg/g DCW/h. In subsequent simulations, they gradually reduced the uptake of BT from 2.55 mmol/g DCW/h to zero. As expected, the DBT uptake gradually increased to meet the cellular demand of sulfur. Since the model has no regulatory mechanics and its uptake system has no bias for DBT or BT, Aggarwal et al. (2013) ruled out the non-specific uptake factors for explaining the preference of BT over DBT. Instead, energy usage offers a plausible explanation. 1 mole BT requires 2 moles NADH, while 1 mole DBT requires 4 moles NADH (Figure 9.19). Thus, for the cell, BT is a better sulfur source than DBT energetically. For investigating the effect of BT and DBT on growth, two simulations were performed. In the first, BT was provided and DBT was provided in the second. For both cases, their uptake rates were fixed at 20 mg/g DCW/h with an unlimited supply of glucose and other nutrients. The obtained maximum growth rate was 1.24 h-1 and 0.90 h-1 with BT and DBT, respectively. Thus, BT promotes higher growth than DBT for G. alkanivorans. This was also attributed to the lower energy requirements of BT. Also, Aggarwal et al. (2013) applied in silico modeling to compare between the R. erythropolis and G. alkanivorans BDS activities. Based on this assumption, maximized cell growth for a fixed glucose uptake of 1 mmol/g DCW/h with unlimited supplies of DBT/BT and other minimal nutrients occurred. Both R. erythropolis and G. alkanivorans can utilize DBT as the sole sulfur source producing 2-HBP, but R. erythropolis cannot utilize BT. Upon simulation for BDS of DBT, based on this assumption, maximized cell growth for a fixed glucose uptake of 1 mmol/g DCW/h, with unlimited supplies of DBT and other minimal nutrients, the growth rate and the corresponding desulfurizing activity were found to be higher for G. alkanivorans (0.15 h-1, 18.13 mmol HBP/g DCW/h) than R. erythropolis (0.14 h-1, 13.54 mmol HBP/g DCW/h). Furthermore, the desulfurization activity exhibited by the two strains increased with the increase in glucose uptake rates. Based on the predicted two models, the minimum sulfur requirements (in terms of DBT) of the two strains’ unit growth rate were calculated to be 120 mmol/g DCW/h and 93.9 mmol/g DCW/h for G. alkanivorans and R. erythropolis, respectively, based on the assumption of supplying all the nutrients in excess and minimized the DBT uptake for a fixed biomass growth rate of 1 h-1. Thus, these analyses showed that G. alkanivorans has higher desulfurization activity than R. erythropolis under the same medium conditions, promoting it as better catalyst for biodesulfurization.

Figure 9.19 Selective Desulfurization of Benzothiophene (BT) and Dibenzothiophene (DBT).
Mathematical modeling and evaluation of bio-kinetic constants are mandatory in the designing and scaling up of bioprocesses for industrial scales. Consequently, it is essential to relate the kinetics of BDS in the petroleum feed with that of the culture growth. The most widely used method for the measurement of bacterial growth is optical density, but this is difficult in the presence of a real oil feed. However, it is more applicable to perform this measuring direct cell counts or dry cell weight, based on the experimental conditions. Nassar et al. (2016) attempted to isolate and identify a new bacterial strain capable of desulfurization without altering the hydrocarbon skeleton. It also aimed to predict a relationship between different bacterial population units, optical density (OD), total viable count (i.e. colony forming unit (CFU/mL)), and biomass dry cell weight (DCW, mg/mL) for further application in the petroleum industry.
Numerical investigation was performed to find out the relationship between CFU/mL and OD600, DCW mg/mL and OD600, and finally, between CFU/mL and DCW mg/mL. The relationship between the DCW and OD600 of DBT-biodesulfurizing bacterium Brevibacillus brevis strain HN1 (accession no. KF018281) was found to be represented by a linear polynomial equation:
where X represents the OD600 and the dependent variable Y represents the corresponding DCW in mg/mL. The coefficients of equation (114) with 95% confidence bounds were P1 = 0.1375 (0.124, 0.151) and P2 = 0.01056 (-0.01481, 0.03593).
The relationship between the total viable count (CFU to cells/mL) of HN1 and OD600 was found to be described by the general Gaussian model for fitting the peak (Giraud, 2008), which can be given by the following equation:
(115)

The converged values of the parameters, a, b, and c (with 95% confidence bounds), were as follows: a = 9.083e+9 (8.6e+9, 9.566e+9), b = 3.066 (2.992, 3.141), and c = 1.272 (1.156, 1.387), where parameter a presents the height of the curve’s peak, b represents the position of the center of the peak, and c controls the width of the curve and is related to the full width at half maximum (FWHM) of the peak.
Finally, based on the previously predicted two model equations, a new relationship was derived that correlated CFU (cells/ml) and DCW, which was found to be represented by the following Gaussian equation:
(116)

Thus, depending on how HN1 would be applied, these predicted correlations would facilitate the monitoring of bacterial growth. This would, consequently, facilitate modeling, simulation, and design of different BDS processes using the obtained bacterial isolate Brevibacillus brevis strain HN1.
Peng and Zhou (2016) studied the desulfurization activity of Rhodococcus sp. MP12 and the specific desulfurization activity in a DBT system was expressed as the amount of 2-HBP produced per gram dry cells weight per hour (µmole HBP/g DCW/h), but in practice, it is difficult to measure 2-HBP production in a biphasic system and a crude oil-aqueous system since 2-HBP production can be divided into oil and water phases. The total HBP buildup in a BDS process of crude oil would include HBP in a buffer phase (i.e. aqueous phase), HBP in a crude oil phase, and HBP absorbed by cells. For the first time, the specific desulfurization activity in a crude oil system was expressed as the amount of sulfate produced per gram dry cells weight per hour (µmole sulfate/g DCW/h) (Peng and Zhou, 2016). This can improve the simplicity and convenience of conventional measurement methods based on the assumption that 1 mol of sulfate and 1 mol of 2-HBP are formed per mole of DBT desulfurized (Gallagher et al., 1993; Oldfield et al., 1997; Rashtchi et al., 2006). Sulfate measurement can be easily fulfilled by a commercially available sulfate kit or ion chromatography (IC). However, Peng and Zhou (2016) used a MOPS-Na buffer instead of a phosphate buffer in crude oil BDS experiments in order to avoid interference of phosphate buffer on sulfate measurement by a sulfate kit. Upon the performance of an experiment on 0.3 (O/W), the sulfur content in desulfurized crude oil, which was measured by an X-ray fluorescent analyzer, was reduced by 8% from 3.42% to 3.13% (w/w) after 168 h of incubation. The analysis of total 2-HBP and total sulfate molar balance showed that the total HBP buildup was found to have a very good match with the total sulfate buildup with no significant difference between total sulfate and 2-HBP buildup by t-test (p > 0.05).
Boltes et al. (2013) studied the effect of mass transfer on BDS-kinetics of alkylated forms of dibenzothiophene (Cx-DBTs) by Pseudomonas putida CECT5279 under aqueous and biphasic resting cell conditions in a 2 L stirred tank reactor. For producing an effective oxygen transfer rate in the broth and volumetric mass transfer coefficient (kLa), the stirring rate was controlled. That study proved that the BDS-process is strongly affected by the mass transfer between liquids, where complete conversion of 10 µmol/L DBT, 4-MDBT, and 4,6-DMDBT was achieved in aqueous reaction media (i.e. one phase system), while the conversions in the presence of an organic liquid (1:1 aqueous:n-hexadecane v:v) were decreased, recording 38%, 19.5%, and 16.5% for DBT, 4MDBT, and 4,6DMDBT, in a mixture with an initial concentration of 271 µmol/L, respectively. The conversion times for DBT, 4-MDBT, and 4,6-DMDBT increased 356, 441, and 498 fold when the oil phase was used. Thus, in a biphasic system, mass transfer between liquids controls the process, increasing reaction time and decreasing the process yield. The 4-MDBT and 4,6-DMDBT conversion times were 4 times slower than DBT in aqueous reactions conducted in a stirred tank reactor, whereas they presented a 5 fold delay compared with DBT conversion times in biphasic reaction media, using the same reactor. This indicates the effect of alkylation and hydrophobicity on the BDS-efficiency.
The desulfurization yield (XBDS) was defined as the ratio of 2HBP accumulated over an established reaction to initial DBT times:
(117)

where C2HBP,t is the concentration of produced 2-HBP at time t (h) and CDBT,to is the intial DBT concentration at time zero.
However, the conversion yield YBDS is calculated as follows:
(118)

where Ccx-DBT,t is the remaining DBT concentration at time t (h) and Cxc-DBT,0 is the intial DBT concentration at time zero.
The XBDS and YBDS give an indication about the microbial BDS efficiency, while the degree to which desulfurization occurred during the growing process (DBDS) was used to correlate the BDS capacity of the cells and the biomass obtained over a given growth time provides information about the optimum biocatalyst production process.
(119)

where tG is the growth time required to attain a biomass concentration, Cx, and XBDS is BDS-yield.
In aqueous media, the kinetic analysis of DBT and Cx-DBT resting cell biodesulfurization under aqueous conditions was based on the Michaelis–Menten equation. Thus, the kinetic equations for 4S-pathway compounds, DBTO and DBTO2, were fitted to a simple Michaelis–Menten equation and to a by-product competitive inhibition for HBP, as follows:
(120)

(121)

(122)

(123)

where S is the 4S compound concentration (µmol/L), vmax is the maximum velocity (µmol/gx/min), and KS is the saturation constant (µmol/L).
For Cx-DBT elimination, only the evolution of the initial compounds measured since the intermediates were unknown.
(124)

(125)

To minimize miscalculation and to calculate the kinetic parameters and volumetric mass transfer, the experimental data was fitted to a well-known exponential concentration–time function (that is, a first-order kinetic equation) for DBT and Cx-DBTs, as the mass transfer controls the process and the transfer rate depends solely on substrate concentration in the oil phase.
(126)
The kinetic parameters (vmax, KS) were obtained from this data using the Lineweaver–Burk method for DBT and Cx-DBT reactions.
In a double phase system, to determine the effect of mass transfer, Boltes et al. (2013) used the concept of characteristic time to compare the BDS process in aqueous media and biphasic liquid reaction media.
(127)

(128)

This study revealed that the maximum specific desulfurization rate of DBT in an aqueous phase was almost four times higher than those of alkylated compound concentrations. Although the saturation constant, Ks (µmol/L), values were similar for all the studied compounds, the calculated values of the maximum velocity (vmax µmol/g/min) varied according to the degree of alkylation and the hydrophobicity of the compounds in aqueous reaction media.
In a biphasic system, the volumetric mass coefficient value for HBP (KHBP) was two times higher than those for KDBT, indicating that HBP accumulation in the oil phase is favored over DBT elimination. The initial elimination rate for DBT and initial accumulation rate for HBP were very similar, as expected, demonstrating that no significant amount of 4S intermediates were accumulated either in the organic or aqueous phases.
The volumetric mass transfer coefficient was calculated for DBT and for HBP from the oil to water phase and also for Cx-DBTs, according to the double-film model. The mass transfer coefficients were determined from the decrease in DBT concentration (KDBT) and the accumulation of HBP (KHBP) in the oil phase, using the integral method for kinetic analyses in both cases. If the reaction takes place very close to the interfacial layer under the water side of the double film where the microorganism exists, the change in watery phase concentrations cannot be measured in the presence of the oil layer. However, the net conversion rate of each compound in the model oil phase could be described according to the following first-order equations:
(129)

(130)

(131)

(132)

The initial Cx-DBT-BDS rates in the oil phase decreased with the presence of substituent groups in the aromatic ring, but were unaffected by the number of methyl substituents. The higher first-order constant K = 2.67 × 10–3 min-1 was recorded for DBT, which has the lowest degree of alkylation and hydrophobicity. This value was two times higher than those obtained for 4-MDBT and 4,6-DMDBT. Furthermore, the DBT desulfurization yield XBDS% recorded 38.80% and its conversion yield (YBDS%) was the highest of all the tested compounds, while 4-MDBT and 4,6DMDBT recorded 19.5% and 16.61%, respectively.
In a similar study performed by Caro et al. (2007a), P. putida CECT5279, using an orbital shaker instead of mechanical stirring, using a biomass cell age of 5.5 h and pH, was controlled at 8.0; the HBP accumulation was favored over DBT elimination and the KHBP value was also two times higher than that for KDBT, but the values of the kinetic coefficients were two orders of magnitude lower than the corresponding results reported in Boltes et al. (2013) using mechanical stirring and Tween 80 as a surfactant, which improved the mass transfer process and aeration.
Kobayashi et al. (2001) studied the BDS rate of different alkyl-DBTs in model oil and light gas oil (LGO) using Rhodococcus erythropolis KA2–5-1 (FERM P-16277) and its genetically improved recombinant, strain rKA2–5-1, as the biocatalysts. The DBS-pattern was represented by the Michaelis–Menten equation and the value of rate contents, the limiting maximum velocity (vmax), and the Michaelis rate constant (Km) were calculated for a biphasic system with 1:1 v/v of different individual DBTs in model oil (DBT, 4-MDBT, 4,6-DMDBT, or 3,4,6-trimethyldibnozothiophene (3,4,6-TMDBT) dissolved in n-tetradecane).
(133)

where S is the concentration of Cx-DBTs (mM), vmax is the limiting maximum velocity (mmol/kg dry cell weight/h), and Km is the Michaelis rate constant (mM).
The DBT-desulfurization activity was 196 and 120 mmol/kg dry cell weight/h for rKA2–5-1 and KA2–5-1, respectively, thus the rKA2–5-1 is 63% higher in DBT-BDS efficiency than the original KA2–5-1. Moreover, the DBT-BDS rate is 2 and 2.5 times higher than those for 4-MDBT and 4,6-DMDBT.
This study revealed that as alkylation increases, the desulfurization activities decrease, where alkyl-DBTs with six carbons of alkyl substituent groups were not desulfurized and the hydrophobicity and size increase, which decreases the transfer of the compound from the solvent phase to inside the cell. Since the hydrophobic compounds are expelled from the aqueous phase, it is hard to be in contact with the bacterial cell surface. However, the type or position of alkyl substituent groups has little effect on BDS activity. Moreover, the BDS activity decreases in the presence of mixtures of alky-DBTs due to the competitive inhibition effect. The inhibition effect of phenolic products was also investigated: 2-hydroxt-biphenyl (2-HBP) for DBT, 2-methy-6-(3-methylphenyl)phenol that is 4,6-dimethylhydroxybiphenyl (4,6-DMHBP) for 4,6DMDBT, and 2,3-dimethyl-6-(3-methylphenyl)phenol that is 3,4,6-trimethylhydroxybi-phenyl (3,4,6-TMHBP) for 3,4,6-TMDBT, within the concentration range of 0–10 mM. It was surprising that no inhibitory effects occurred except for 2-HBP. However, 80% BDS activity occurred even in the presence of 10 mM 2-HBP. This low inhibition effect was attributed to the enzymatic activity of the whole cell system and solubility of 2-HBP in a bi-phasic system, where, in the case of a whole cell system, 2-HBP is excreted from the reaction environment which is the water phase inside the cell to the organic phase that is n-tetradecane. The distribution coefficient of 2-HBP to n-tetradecane and water was found to be 30.
The apparent competitive inhibition model was applied to study the desulfurization pattern of a system of multiple substrates, but in order to apply this model, the kinetic constants of BDS of individual compounds (C0, C1, C2, and C3-DBT, that is DBT, 4-MDBT, 4,6-DMDBT, and 3,4,6-TMDBT) calculated from the Michaelis–Menten equation were used. Based on the assumption that in the BDS of a multiple component system the total concentration of cells was the sum of each concentration of substrate-cell complexes and the concentration of free cells, the model of BDS was extended. Scheme 9.1 summarizes the mutual competitive reaction scheme as suggested by Kobayashi et al. (2001), where one alkyl-DBT acts as the substrate and the other alkyl-DBTs act as inhibitors. That is, when k-1 > k+2, km = k-1/k+1, the dissociation constants for inhibitors are assumed to be K1 = k-3 /k+3, KJ = k-4/k+4, KK= k-5/k+5.

Scheme 9.1 Mutual Competitive Reaction as Suggested by Kobayashi et al. (2001).
Thus, the reaction velocity of each alkyl-DBT was expressed as follows:
(134)
where r is the reaction velocity (mmol/kg drycell weight/h), Vmax is the limiting maximum velocity (mmol/kg dry cell weight/h), and Km is the Michaelis constant (mM).
Upon the application of a lumping model to describe the multiple BDS of different alkyl-DBTs in the LGO of initial S-content 360 ppm, first, Kobayashi et al. (2001) divided the alkyl-DBTs in LGO to four groups: C1, C2, C3, and Cx, for mono-, di-, tri-, and more than 4- alkyl-substituents. Then, they chose 4-MDBT, 4,6-DMDBt, and 3,4,6-TMDBT as representatives for C1-, C2-, and C3- DBTs, respectively. The GC-AED analysis was done and the total S-concentration of each alkyl-group was calculated by summing the concentrations of compounds belonging to the same group, while the concentration of Cx-DBT was calculated by subtraction of the summed amount of C1-, C2-, and C3- DBTs from the total S-content in LGO.
Similarly, Chen et al. (2008) described the desulfurization efficiency and specific rate of DBT and 4,6- DMDBT elimination using resting cells of Mycobacterium sp. ZD-19 as the biocatalyst, where both values were almost 2 times higher for DBT than for 4,6DMDBT. The rate of BDS of different studied OSCs were reported to be: 12.8, 11.5, 8.58, 3.71, and 1.36 mM/kg DCW/hfor thiophene (Th), benzothiophene (BT), diphenylsulfide (DPS), dibenzothiophene (DBT), and 4,6-dimethyldibenzothiophene (4,6-DMDBT), respectively.
Li et al. (2003) reported the specific desulfurization rate of diesel oil by Mycobacterium sp. X7B to be 0.14 mg sulfur/g DCW/h, while Li et al. (2009) reported the specific desulfurization rates of middle distillate unit feeds (MDUF) and light gas oil (LGO) by resting cells of Gordonia sp. CYKS1 to be 0.17 and 0.15 mg sulfur/g DCW/h, respectively. Bordoloi et al. (2014) reported the specific desulfurization rate of a hydrodesulfurized diesel oil by Achromobacter sp. to be 1.20 mg sulfur/g DCW/h.
According to Soneyink and Lenkins (1980), kinetics of the biodegradation reaction can be described in terms of its order. Goindi et al. (2002) reported that the batch BDS in a hydrocarbon aqueous biphasic system of DBT/n-hexadecane by Staphylococcus sp. strain S3/C follows the first order kinetic model. The resting cells decreased the sulfur content of the hydrocarbon phase by 57% at 2.2 mg S/L/h in the absence of any additional carbon and sulfur source at an optimum hydrocarbon to aqueous phase ratio (H/A) of 2:1, whereas the specific sulfur removal rate was maximized (0.8 mg S/h/g DCW) at H/A 3:1.
The frequently used first- and second-order kinetic models were employed to investigate the biodegradation rate of different PASHs, including Th, BT, and DBT, in single- or multi-substrate systems using growing cells of Bacillus sphaericus HN1 (El-Gendy et al., 2015). It was found that the biodegradation of the three studied PASHs follows the first order kinetic reaction in the single-substrate batch cultures with the regression coefficient R2 ≥ 0.9497, with rate constants of kTh ≈ 0.0095 h-1, kBT 0.0448 h-1, and kDBT 0.0187 h–1 and a half life time of t1/2 of ≈ 72.96, 15.47, and 37.07 h for Th, BT, and DBT, respectively. Biodegradation of BT (R2 ≥ 0.9538), DBT (R2 ≥ 0.9566) follows the first order kinetic reaction in the multi-substrate batch systems, while biodegradation of Th follows the second-order kinetic reaction (R2 ≥ 0.9049). The biodegradation rate of DBT recorded kDBT values of ≈ 0.0117, 0.0411, and 0.0126 h–1 with t1/2 of ≈ 59.24, 16.86, and 55.01 h in a binary mixture with Th or BT and tertiary mixture, respectively. This indicates that the biodegradation of DBT was enhanced in the presence of BT, but depleted in the presence of Th and tertiary multi-substrate batch cultures. The same trend was also observed in case of the biodegradation of BT, where kBT recorded ≈ 0.026 and 0.0571 h–1 with a t1/2 of ≈ 26.66 and 12.14 h in binary mixture with Th or DBT, respectively, while kBT recorded ≈ 0.0405 h–1 with a t1/2 of ≈ 17.11 h in the case of tertiary mixture. This indicates the synergetic effect between BT and DBT and the antagonistic effect of Th on the biodegradation of BT and DBT. For the biodegradation rate of Th, kTh recorded values of ≈ 2×10–4, 1×10–4, and 1×10–4 L/mg/h with a t1/2 of ≈ 50, 100, and 100 h in a binary mixture with Th or BT and a tertiary mixture, respectively. This indicates that the rate of biodegradation of Th is relatively lower than that of BT and DBT, whether in single- or multisubstrate batch cultures. This also shows that the presence of BT enhanced the rate of biodegradation of Th, but there was a strong antagonistic effect expressed on the Th biodegradation rate in a binary mixture with DBT or tertiary-mixture-substrate batch cultures. Briefly, in binary-substrate-batch cultures, BT enhanced the biodegradation of Th and DBT. Although DBT enhanced the biodegradation of BT, it depleted the biodegradation of Th. However, Th depleted the biodegradation of both BT and DBT, but in tertiary-substrate-batch cultures, the biodegradation efficiency of the three PASHs were depleted. Thus, this study proved the competitive inhibition effects of a multi-substrate system.
In another study, the biodegradation kinetics of different polyaromatic sulfur heterocyclic compounds (PASHs), including thiophene (Th), benzothiophene (BT), and dibenzothiophene (DBT), in a series of batch experiments were investigated as mono-, binary-, and tertiary-substrate systems to study the substrate interaction effects and capabilities of Bacillus sphaericus HN1 to utilize PASHs as sole carbon and energy sources in mono-and multi-substrate systems (Deriase et al., 2015). Based on a full factorial design, 23, by varying the studied three PASHs concentrations between 0 and 100 mg/L, eight batch experiments were conducted out in duplicate for a period of 7 days in an orbital shaking incubator set at 200 rpm and 30 °C. A multiple comparison test was performed for the growth of microorganism and substrate biodegradation to obtain pairwise comparisons between the three studied systems. Both tests of ANOVA1 and Kruskal-Wallis showed that there was a highly statistically significant difference in the growth of HN1 between the negative control (free of PASHs) and all other treatments (p = 2.2105e-7 and 9.303e-4, respectively). Although there was a statistically significant difference for biodegradation of DBT in mono– and binary-substrate systems within the time interval 96–168 h (p < 0.05), there was a non-statistically significant difference in the biodegradation of both Th and BT in the three studied systems (p > 0.05). A multi-substrate form of the Monod kinetic model was applied to predict the substrate interactions in binary- and tertiary-substrate systems using the Monod parameters derived from the mono-substrate systems.
Modeling the relationship between cell growth and substrate consumption rates was done by direct coupling of the substrate consumption rate with the cell growth model applying constant cell yield and biomass decay rate coefficients during the time course of batch experiments.
(135)

where the biodegradation rate of a substrate (i) is proportional to both the specific growth rate (µi) on that substrate and the biomass concentration (X) in the system.
The change in microorganism concentration with time can be expressed mathematically as follows:
(136)

where the change in biomass concentration is modeled as if the substrate (i) is the only growth substrate present. The dependent variables were Ci, the concentration of the PASHs, that is the substrate, i (mg/L), and X, the biomass concentration (mg/L), while the independent variable was time, t (h). Yi is the stoichiometric biomass yield coefficient for substrate i (mg biomass/mg substrate) and b is the endogenous decay rate coefficient (h–1) which was estimated using the independent substrate free experiment (–ve control) with a known starting biomass concentration. The endogenous rate of microbial decay, which was characterized by rate coefficient b (h–1), was subtracted from the growth rate of the biomass to account for the energy lost for cell maintenance and b was assumed to be constant for all the studied biodegradation systems. The Monod kinetic model relates growth rate to substrate concentration [µi = f (Ci)] via the two bio-kinetic Monod parameters KSi and µmax,i;
(137)

where µmax,i is the maximum specific growth rate on substrate i (h–1) and KSi is the half saturation coefficient for substrate i (mg/L) and the parameter estimation is challenging for the Monod equation. The relationship between biomass formation and substrate consumption can be determined by the yield coefficient Yi (dry weight of biomass/weight of utilized substrate) indicating the maximal conversion of unit substrate to cell mass.
(138)

where X>Xo and Ci < Cio.
In a multi-substrate system, the biomass growth is due to the utilization of all available hydrocarbons in the system to the microorganism, accordingly and the specific growth rate will be the summation of all individual ones for each substrate, as represented in eq. (52). In that study, correlation and simulation studies using the Monod kinetic model for a mono-substrate system were established and kinetic model parameters were taken into consideration in the multi-substrate Monod kinetic model (eq.53) used to describe the biodegradation process of the studied PASHs in the binary and tertiary substrate batch cultures. Mathematical expressions for modeling and simulating substrate (C mg/L) and biomass (X mg/L) concentration profiles throughout the time span of the batch experiments were also proposed and successfully applied (model equations 54 and 55). Thus, considering the fact that KSi (mg/L) is inversely proportional to the affinity of the microbial system for the substrate (Agarry et al., 2008; Deriase et al., 2013), according to the obtained data from mono-substrate system, the affinity of HN1 towards the studied PASHs could be ranked in the following decreasing order, BT > DBT > Th, indicating the high toxicity of Th compared to BT and DBT. This was confirmed by the lowest µmax,i, 0.01434 h–1, obtained with Th.
In the experimental design of that study, the primary concern was to determine the substrate removal kinetics rather than biomass growth kinetics, where the batch experiments conducted in this study provided a deep understanding of the degradation kinetics and the positive and negative interactions of different PASHs, including Th, BT, and DBT in mono-, binary, and tertiary substrate aqueous systems. Experimentally, the biodegradation rates for the individual substrates in the multi-substrate systems may be enhanced or reduced relevant to the mono-substrate system. The effect of one substrate on the degradation of another is given by Ksi/Ksj terms, where KSi and KSj are the half saturation coefficients for substrates i and j (mg/L) (Guha et al., 1999; Knightes and Peters, 2006). The experimental observations and modeling predictions, i.e. the simulation results for the biodegradation of the three studied PASHs in three studied systems, revealed the following: relative to the biodegradation of the three studied PASHs in mono-substrate-batch cultures, BT enhanced the biodegradation of DBT in the binary substrate-batch cultures of DBT-BT, especially within the time interval of 96–168 h, while, in Th-BT binary substrate-cultures, the BT expressed a negative impact on the biodegradation of Th up to 96 h, but it enhanced the Th-biodegradation within a relatively short time interval of 96–144 h, then the antagonistic effect occurred again with a longer incubation period. Although DBT enhanced the biodegradation of BT in the binary substrate batch cultures of BT-DBT, especially within the time interval 24–168 h, it depleted the biodegradation of Th in the binary substrate batch cultures of Th-DBT. However, Th depleted the biodegradation of both BT and DBT in the binary substrate-batch cultures of BT-Th and DBT-Th, respectively, where the negative impact of Th was very obvious within the time intervals of 12–120 h and 48–168 h, respectively. In the tertiary substrate-batch cultures, Th-BT-DBT, the biodegradation efficiency of the three PASHs were depleted relevant to their biodegradation efficiencies in the mono-substrate batch cultures, where the Σ(Ksi/Ksj) recorded as 5.785, 0.975, and 1.887 for the biodegradation of Th, BT, and DBT, respectively, which indicated that the antagonistic effect between the three studied PASHs on the rate of their biodegradation can be ranked in the following decreasing order: Th > DBT > BT (Deriase et al., 2015).
Simulation of the applied models to describe and estimate the biodegradation kinetics of the three studied PASHs in the different investigated systems was performed and the validity of the models was confirmed. The simulated effectiveness factor of the kinetic models was depicted by using a mean relative error (MRE), eq. (139), to show the goodness of fit between the obtained experimental and simulated data of PASH depletion with time.
where Ci,cal and Ci,exp are the simulated substrate concentration determined from the model and its observed experimental value at time, t, respectively, while m is the number of experimental points. The results of MRE ranged between 0.096 and 0.282 and percentage MRE ranged from 9.6% to 28.2%, which confirmed the adequacy of the mono- and multi-substrate Monod kinetic models for estimating the biodegradation of the studied PASHs. It was also very obvious in the agreement between the measured and simulated profiles for the substrates’ depletion with time. Thus, the approaches of modeling and simulation for the experimental results obtained in that work are considered to be essential in order to facilitate the understanding of the interactive effect of PASH contaminants and their biodegradation processes for better achievement of biotreatment of contaminated sites and industrial effluents.
The total cell-specific desulfurization rate (Rtotal) in a three-component (BDS) experiment, using oil, water, and cells, can be determined according to Abin-Fuentes et al. (2014) by the following relationship:
(140)

where tf is the one-hour length of the experiments, ![]() is the HBP concentration in water at tf,
is the HBP concentration in water at tf, ![]() is the HBP concentration in hexadecane at tf, Ø is the oil fraction, and X is the cell density. Within the three-component BDS experiments, three different biocatalyst populations exist: free cells in an aqueous phase, cells adhered to oil-droplets, and cells that form aggregates. Thus, the Rtotal could be expressed by the following relationship:
is the HBP concentration in hexadecane at tf, Ø is the oil fraction, and X is the cell density. Within the three-component BDS experiments, three different biocatalyst populations exist: free cells in an aqueous phase, cells adhered to oil-droplets, and cells that form aggregates. Thus, the Rtotal could be expressed by the following relationship:
(141)
where Rfree, Roil, and Ragg are the cell-specific desulfurization rates of free cells in an aqueous phase, cells adhered to oil-droplets, and cells that form aggregates, respectively. The ffree, foil, and fagg are the fractions of free cells in an aqueous phase, cells adhered to oil-droplets, and cells that form aggregates, respectively. The values of Roil and Rfree were found to be equal to Rmax as explained in the results obtained by Abin-Fuentes et al. (2014). The specific desulfurization rate of cells in aggregates (Ragg) was determined by the following relationship:
(142)
where η is the effectiveness factor which is defined as the ratio of the actual rate of desulfurization within the aggregates to the rate of desulfurization if there was no intraaggregate diffusion limitation. Rearranging the previous equation allows one to solve for η:
(143)

The effectiveness factor for spherical aggregates and zero-order kinetics (CDBT,water >> Michaelis constant Km in all experiments) is determined from the following relationship (Shuler and Kargi, 2002):
where De is the effective diffusivity within the aggregates and d32,agg is the Sauter mean aggregate diameter. Three-component BDS experiments provided a data pair of (d32,agg, η) values for each mixing speed studied. Then, equation 144 is fit to the (d32,agg, η) data set using the non-linear fitting function nlinfit in MATLAB® to obtain the least-square best-fit value of the parameter De.
The volumetric desulfurization rate (Vtotal) was determined according to the following relationship:
(145)
For each mixing speed tested in the small-scale system, the corresponding power input per unit volume (P/V) and impeller tip speed (vtip) were calculated from the following relationships (Garcia-Ochoa and Gomez, 2009):
(146)
(147)

where ρ is the density of the media, NP is the power number, N is the mixing speed, Di is the impeller diameter, and vtip is proportional to the maximum shear rate, (dV/dy)max.
Arabian et al. (2014) studied the effect of process temperature on the kinetics of BDS of a model oil DBT in dodecane using an inoculum size of 3.6×107 (cell/mL) Bacillus cereus HN, collected at the stationary phase, oil phase fraction of 0.2, initial DBT concentration of 1086 ppmw, and mixing rate of 180 rpm. The general nth order power-law reaction rate expression was chosen to correlate the experimental data. It was found that the best value of n for this process could be 1. Therefore, the rate expression for the BDS of DBT was expressed as follows:
(148)

(149)

where CDBT, t, and k are DBT concentration (ppmw), time (h), and the reaction rate constant (h-1) respectively. R and T represent the universal gas constant (kJ/mol/K) and temperature (K), respectively.
Adlakha et al. (2016) studied the kinetics of BDS of hydrodesulfurized diesel oil with an initial S-content of 70 ppm in a shaken flask batch BDS process of a 1/3 O/W phase ratio at 250 rpm at 30 °C using growing cells of Gordonia sp. IITR100 over a 12 day incubation period. It was found that after four days almost all of the OSCs, including C2-DBT and Cx- BTs, in HDS diesel were used up. The BDS-rates of C2-DBT, C3-BT, and C2-BT were found to be 0.34, 0.76, and 0.038 mg/kg/h (i.e. ppm/h), respectively, while the BDS rate for Cx-thiophenes was found to be the lowest, recording 0.08 mg/kg/h and a minor fraction of thiophene and its derivatives were remaining, with a total S-decrease of approximately 98% and after 6 days of incubation, the S-compounds were below the detectable levels.
In a study performed by Maass et al. (2015) for the evaluation of different kinetic models in the batch BDS of model oil (DBT/n-dodecane) by Rhodococcus erythropolis ATCC 4277, several models were applied, including simple ones, such as Monod and Andrews, and more complex ones, such as competitive, non-competitive, uncompetitive inhibition, sum kinetics interaction parameters (SKIP), and kinetic models to inhibition, and/or limitation effects by multiple-substrate concentrations, to predict the one that best describes the BDS process. This was tested using a parameters minimization method in the MATLAB program and their validation was made by the Fisher’s test. Monod and competitive inhibition models were found to be the best at describing the studied BDS process and exhibited kinetic parameters with a real physical significance. They were able to describe the microbial growth, consumption of DBT and glucose, and production of 2-HBP. One of the important observations reported by Maass et al. (2015) in that study is that the increase in the concentration of dodecane from 20 to 80 % v/v in the studied system did not reflect any change in the kinetic behavior of BDS by R. erythropolis ATCC 4277 and the Monod was the model that presented a fit statistically better for both cases. Simulation of kinetic models to thte BDS process using just an organic phase (100 % v/v) has been also performed. Furthermore, that study revealed that Rhodococcus erythropolis ATCC 4277 is tolerant to high concentrations of organic phasing with a great affinity towards DBT, which is important to real field applications. The obtained yield coefficient, Yx/s (glucose), in that study indicated that the maintenance coefficient, R. erythropolis ATCC4277, is lower than those reported for other R. erythropolis IGTS8, which results in lower costs with a fermentation medium (del Olmo et al., 2005a; Guchhait et al., 2005). The higher Yx/s (DBT) indicated higher utilization of DBT as a co-substrate than R. erythropolis IGTS8, which means a higher BDS efficiency (del Olmo et al., 2005a; Guchhait et al., 2005).
Martinez et al. (2015) studied the effects of the supplementation of BDS-media with some of the metabolites of the Krebs cycle, such as citric acid, succinic acid, and acetic acid, on the BDS efficiency and the intracellular concentrations of both ATP and NADH, using resting cells of Pseudomonas putida CECT5279. The study was performed by a mixture of resting cells of two ages: 67% of cells grown for 23 h and 33% of cells grown for 5 h in batch and fed-batch operations.
The effect of the different co-substrates on the BDS capacity was evaluated using the following parameters:
The maximum BDS-capacity:
(150)

where ![]() is the initial DBT concentration (µM) used in the resting cell assay and
is the initial DBT concentration (µM) used in the resting cell assay and ![]() is the maximum concentration of the 2-HBP concentration (µM) produced during the time employed in the reaction.
is the maximum concentration of the 2-HBP concentration (µM) produced during the time employed in the reaction.
Variation of the BDS capacity (XBDS):
This parameter is used to compare the BDS capacity when different co-substrates are employed with respect to a control experiment.
(151)

A positive value means an improvement in the BDS capacity, while a negative value means a decrease, where BDS is the maximum percentage of BDS.
Variation of the BDS capacity estimated (XBDS|M):
This parameter is employed to estimate the variation of the BDS capacity when the biocatalyst consists of the optimal mixture of cells, taking into account the contribution of each cellular age.
(152)
HBP productivity (PHBP):
This parameter relates to the HBP production in each batch when the BDS is carried out in a fed-batch process.
(153)

where CHBP is the concentration of the produced 2-HBP (µM) in each batch and ∆t is the time duration of each batch (min).
Initial substrate removal rate (![]() ):
):
This parameter shows the initial rate of transformation (µmol/L/min) of each sulfur substrate, j (DBT, DBTO, DBTO2 and HBPS), which can be considered as the initial rate of each 4S route reaction (![]() ).
).
(154)

where ri corresponds to each 4S route reaction (i) as follows:
(9.13)
To obtain this parameter, the evolution with time of each 4S route compound was analyzed and derived.
This study showed that it is possible to improve the yield and BDS-rate by adding co-substrates, since the three examined compounds, acetic, citric, and succinic acids, provided positive results; nevertheless, the best improvement in the BDS capacity was achieved using 1.5% acetic acid. The yield of the process increased up to 140% when using single-aged (5h) cells in a batch process and to 122% using an optimized mixture of cells in a fed-batch process. The consumption of both cofactors, ATP and NADH, in a fed-batch process was very similar with and without a co-substrate (0.50 µmolNADH/gX and 30 nmolATP/gX, respectively), using a consortium of different resting cells’ ages, but the initial intracellular concentrations of ATP and NADH also increased to 58 and 42%, respectively. The differences in the concentrations of the cofactors at the end of the process were much higher when acetic acid was supplied (74% for NADH and 181% for ATP). To determine the influence of acetic acid (1.5%) on each reaction of the 4S route, the initial substrate removal rate (R0j ≈ ri0) was also studied using the consortium of different cells’ age in a batch process. The results showed that the initial rate of all of the reactions was higher upon the usage of 1.5% acetic acid. The improvements were 42, 30, 62, and 117% for r1, r2, r3 and r4, respectively. The increases in the rates of the first three reactions were expected due to the higher concentration of NADH. Moreover, a significant improvement has been observed in the fourth reaction rate, which is the rate-determining step, but is not NADH dependent. Briefly, the addition of co-substrate enhanced the reaction rates of the 4S route and, consequently, the overall rates of all of the reactions were enhanced (Martinez et al., 2015).
Gomez et al. (2015) applied the logistic kinetic model equation to study the effects of hydrodynamic conditions on bacterial cultures of R. erythropolis IGTS8 under different operating conditions in a STBR. Hydrodynamic conditions were changed varying agitation and aeration. Gomez et al. (2015) performed a regime analysis based on characteristic time to provide information about which one is the controlling step by comparing the rates of the different steps. The characteristic or critical time constant is defined as the ratio between capacity and flow; therefore, a small time constant represents a fast process and vice versa. From the regime time analysis, the need for the investigation and optimization at a laboratory scale of certain mechanisms and features can be decided.
The characteristic time for oxygen consumption during growth, tOUR, provides a critical oxygen depletion time and can be calculated from the following equation:
(155)

where C* is the saturation concentration in the liquid phase or, in another word, concentration in equilibrium (mol/m3).
Upon the assumption that the growth rate of microbial cells can be modeled according to the logistic model equation, the maximum oxygen uptake rate (OURmax, mol O2/m3/s), which is reached at the middle of the exponential growth stage, can be calculated as follows (Garcia-Ochoa et al., 2010):
(156)
where µ is the specific growth rate, Yxo presents the overall yield of cells on oxygen, and mO2 is the oxygen consumption coefficient for maintenance (mol O2 kg/X/s).
The characteristic time for oxygen transfer from gas to liquid, tOTR, can be determined by the reciprocal of the volumetric oxygen transfer coefficient according to:
(157)

where kLa is the volumetric oxygen mass transfer coefficient (s–1). The characteristic time for mixing is the time required for complete mixing of the content of the bioreactor, that is the time necessary to pass from an initial non-homogeneous mixture to a certain degree of homogeneity, while a variable with power input and tank geometry is independent of the impeller type (Ma et al., 2006; Nienow, 1997; 2010). For baffled tanks, the mixing time can be related to the design and operation parameters by the following equation (Godoy-Silva et al., 2010):
(158)

This equation indicates that at constant values of vessel diameter (T, m) and Stirrer Diameter (D, m) (fixed for any given equipment), the mixing time decreases only if the energy dissipation rate is increased.
Although the latter equation has been obtained for one single impeller in non-aerated conditions, it is claimed that it can be used for aerated systems by correcting the power input value (Martin et al., 2008). The average energy dissipation rate, e, i.e. total energy dissipation rate per unit mass (W/kg), can be calculated as:
(159)

where P is power input (W) due to agitation, V is the volume of the tank (m3), and ρ is the density (kg/m3).
The power input by the agitation, P, is mainly due to the stirrer speed and, depending on the tank geometry, can be calculated from the power number using the following equation:
(160)

where N is stirrer speed (rpm or rps), Np is the stirrer power number, and P0 (for an un-aerated tank) is calculated; the equation of Michel and Miller (1962) can be selected to calculate the actual power applied to the gas–liquid dispersion according to:
(161)

where the values of the constants α and β depend on the stirrer type and the configuration of the agitation system. For a dual impeller turbine, like the one used in Gomez et al.’s (2015) work, values of α and β were of 1.224 and 0.432, respectively (Abrardi et al., 1988).
Gomez et al. (2015), upon studying the effect of different stirring rates on the biomass growth and BDS activity of R. erythropolis IGTS8, found that the effectiveness factor for growth, η, changed with respect to specific growth rate and the stirrer speed (N) or the impeller Reynolds number:
(162)

where µa is the apparent viscosity (Pa s), D is the stirrer diameter (m), and ρ is the density (kg/m3).
It was observed that oxygen limitation occurs at agitation conditions from approximately 100 to 250 rpm, with an optimization extent value (η) less than 1. This revealed the strong limitations of mass transfer rates that could occur at lower stirring speeds. However, with the increase of agitation speed from 250 to 450 rpm, the η is independent of the agitation conditions, values close to 1.0 are found (the same value as that is in shaken flasks at 210 rpm), mass transport limitations are overcome, and the growth rate is kinetically limited. This behavior is due to the combined effect of improved mixing and a decreased resistance to the transportation of nutrients resulting in an increment of cell growth rate to a point at which the transfer rate is fast enough and does not affect the growth rate any more. However, at a higher agitation speed (> 450 rpm) a sharp decrease in η occurs as the high stirrer speeds change the turbulence inside the stirred tank reactor, which in turn affects the cells, probably through shear effects. Thus, it was concluded that the cultures carried out runs at stirrer speeds higher than 450 rpm are harmful for growth. The impeller Reynolds number under these conditions is higher than 9375 and the value of the average power drawn (e) is 0.6 W/kg (Gomez et al., 2015). Garcia-Ochoa et al. (2013) reported the same observation for the culture of Xanthomonas campestris, where the growth rate was related to the oxygen limitation conditions or damage produced by hydrodynamic stress also using an optimization extent (η).
A bioprocess consists of a series of several successive steps like that of the 4S-pathway BDS process. The overall rate that is generally observed depends on the slower among those. These steps (oxygen transfer, biochemical reaction, etc.) take place at rates dictated by different phenomena (turbulence, heat transfer, and kinetics). The evaluation of each of these particular rates is a key revealing which of them is the one controlling the overall rate. One of the most instinctive methods of doing this is the comparison of the characteristic times of each step. Average values of the calculated characteristic times for oxygen consumption, oxygen transfer, and mixing in runs conducted at different stirrer speeds in batch BDS processes, using R. erythropolis IGTS8, have been reported (Gomez et al., 2015), where the average values of mO2, YOX, and C* of 2.3 × 10–4 mol O2 kg/X/s, 18.5 mol O2 kg/X, and 0.245 mol/m3, respectively, have been assumed considering the operational conditions at which the runs have been conducted. Mass transfer characteristic times over the range of the operating conditions studied were generally in the interval from 1578.5 to 16.1 s and mixing characteristic times were in the range from 43.3 to 6.2 s. Both characteristic times decrease when the stirrer speed is increased (from 100 to 700 rpm). The values estimated for mixing characteristic periods are within the range reported in the literature (Lu et al., 1997; Jahoda et al., 2007). A characteristic time for mixing much longer than that for oxygen uptake suggests that significant spatial differences in oxygen concentration could occur and the opposite situation indicates that dissolved oxygen (DO) concentration is homogeneous in the liquid phase. A characteristic time for oxygen transfer greater than that for oxygen uptake indicates potential oxygen transfer limitations. According to the obtained results, the characteristic times for oxygen uptake, tOUR, are above 263.5 s. These values suggest that for agitation between 100 and 350 rpm, oxygen transfer limitation will occur in the STBR because the oxygen transfer time is much longer than the oxygen consumption time. However, mixing time is short compared to oxygen transfer time, indicating homogeneous DO concentration.
The regime analysis performed by Gomez et al. (2015) showed that the mixing characteristic time is much lower than transfer time and oxygen consumption time, indicating that the mixing time for the different agitation regimes is acceptable and that there are no mixing limitations. The minimum stirrer speed necessary for a satisfactory performance of the bioreactor from the point of view of the IGTS8 cells oxygen demand was found to be 350 rpm, while at stirrer speeds over 450 rpm, growth rate and desulfurization capacity are both negatively affected.
In a similar study, Escobar et al. (2016) applied the logistic kinetic model equation to evaluate the effects of hydrodynamic conditions on growth by changing the stirrer speed in a STBR using the genetically engineered Pseudomonas putida KTH2. This parameter was calculated as the effectiveness factor for growth and the ratio between the growth rates, in the presence of external limitations (bad mixing conditions, oxygen transfer limitations, or cell damage), was calculated under the best conditions, that is when the growth rate is the controlling step of the overall process rate and is not affected by any other physical phenomena, according to the following equation:
(163)

In that batch operation, the following mass balance equation for the dissolved oxygen concentration, assuming a well mixed liquid phase has been applied:
(164)

where the OTR and OUR are the oxygen- transfer and uptake rates (mol O2/L/s), respectively, and the OTR is proportional to the mass transfer coefficient, kL, the specific interfacial area, a, and the difference between the saturation concentration and the dissolved oxygen concentration in the broth.
The OTR was calculated from the following equation:
(165)
The OUR was calculated using the following equation:
(166)

where the CO2 and ![]() are the dissolved oxygen concentrations in the broth and in equilibrium at the gas–liquid interface, respectively. In that study,
are the dissolved oxygen concentrations in the broth and in equilibrium at the gas–liquid interface, respectively. In that study, ![]() was 0.21 mol/m3.
was 0.21 mol/m3.
In another study performed by Martinez et al. (2016) to study the effect of the hydrodynamic conditions of the oxygen transfer rate during the scale-up of a BDS process using mixed culture of resting cells with different ages of Pseudomonas putida CECT5279, the change of dissolved oxygen concentration with time, (dCO2/dt), in the broth was expressed as the difference of OTR and OUR (eq. 164). The OTR is described as being proportional to the oxygen concentration gradient using the volumetric mass transfer coefficient, kLa, as the proportional constant (eq. 165). The OUR was expressed as the product of the specific oxygen consumption, qO2, and the biomass concentration:
(167)
where qO2 is the specific oxygen consumption rate (molO2 /g/s) and Cx is the concentration of biomass (g DCW/L).
The kLa in a shaken flask must be determined using empirical equations, such as eq. 168, which was proposed by Liu et al. (2006) and modified by Garcia-Ochoa et al. (2013):
where N is the stirrer speed (rpm), VF and VL are the volume (mL) of the flask and liquid, respectively, and µL and µW are the viscosity (Pa s) of the medium and water, respectively.
Martinez et al. (2016) evaluated the BDS capacity of XBDS at different stirring speeds of 200–500 rpm, after 180 min, using an airflow of 1 vvm, where the XBDS increased with the stirring speed and recorded its maximum at 400 rpm, where a complete conversion of 25 µmol/L DBT to 2-HBP was obtained at 400 rpm and 1 vvm within 180 min, while upon varying the airflow from 1–3 vvm and decreasing the time to 90 min. The maximum conversion to 2-HBP occurred at 2 vvm within 90 min. Thus, the optimum condition for the BDS of 25 µM DBT in STBR using P. putida CECT5279 were 400 rpm, 90 min, and 2 vvm. Upon the study os different stirring speeds, the evolution of the dissolved oxygen (DO) fell to zero and the time needed to deplete the oxygen increased with the kLa value. This suggests that the potential value of OUR was higher than the actual value of OTR. The kLa recorded its highest value of 1.68 x 10–2 s–1 at 400 rpm and 3 vvm. It is important to keep in mind the fact that the microorganism consumes all the oxygen and that just because it is transported, does not imply an increase in BDS capacity with OTR. Thus, this study proved that the 4S route is highly sensitive to oxygen availability working under oxygen limiting conditions. Moreover, the hydrodynamic conditions highly affect the 4S route, but not the metabolism of cell growth. Upon comparing the results obtained with shaken flasks and STBR, they were found to give the same BDS-capacity. This occurred because both have the same hydrodynamic conditions since the most important principle for the scale-up of aerobic bioprocesses consists of maintaining a constant OTR defined by kLa at different scales (Garcia-Ochoa and Gomez, 2009), so by applying eq. 168, it was possible to calculate the kLa values for the shaken flask operation at different agitation speeds; when BDS is carried out at 200 rpm, the value of kLa was recorded 1.39 × 10–2 s–1 when BDS was conducted in STBR and a predictive method was employed to estimate kLa (Garcia-Ochoa and Gomez, 2009).
The kLa values can also be correlated with the combination of stirrer speed, N, superficial gas velocity, VS, and liquid effective viscosity, µa, obtaining equations such as the following:
(169)
where the constant, C, depends on the geometric parameters of the vessel and the stirrer employed. Other equations proposed substitute the average power input per volume, P/V, by the effect of stirrer speed, N. The exponent (a, b, and c) values, show a wide variation range in the different correlations proposed by different authors: 0.3≤a≤0.7, 0.4≤b≤1, and –0.4≤c≤ –0.7. For non-viscous systems, the most frequently used correlation is that from Van’t Riet (1979).
Upon the best conditions previously determined (400 rpm and 2 vvm of aeration), the value obtained for kLa was 1.37 × 10–2 s–1, which was almost the same as that calculated at a shaken flask scale. Consequently, Martinez et al. (2016) proved with these results the suitability of the constant-kLa criterion for STBR scale-up.
Gomez et al. (2015) divided the change of the effectiveness factor for growth, η, with the operating conditions into a bioreactor of aerobic bioprocesses into three stages: the first stage occurs when the fluid dynamic conditions concomitantly occur with the increase of the OTR, which provides an increase on the growth rate (e.g. by changing stirrer speed) because this is the limiting step controlling the overall rate of the process. The second stage is the plateau stage that corresponds to the situation where the oxygen transfer rate (OTR) is equal to or greater than the maximum oxygen uptake rate (OUR), which is the controlling step of the overall rate. The third stage occurs upon further increment in agitation speed; this stage is undesirable because it occurs concomitantly with the occurrence of some cell damage or some change in the metabolism provoked by a hydrodynamic or oxidative stress.
The BDS capacity of the cells accumulated during the growth was monitored through resting cell desulfurization assays. For a quantitative analysis of the desulfurization capacity under different agitation conditions, the dibenzothiophene (DBT) conversion (XDBT) and the yield of 2-hydroxybiphenyl (X2-HBP) were calculated as follows:
(170)

(171)

where the CtDBT and CtHBP were DBT and 2-HBP concentrations at 120 min of resting cell assay.
The enzymes activities were determined in resting cell assays from cells of different time courses of growth using DBT, DBTO, DBTO2, and HBPS as sulfur substrates, independently, and then were calculated at the specific time-zero reaction rate according to equation:
(172)

where Cj is the concentration of the enzyme substrate, j, and CX is the concentration of the biomass employed in the resting cell assays.
Gomez et al. (2015) found that upon using different R. erythropolis biocatalyst ages, the values of XDBT and XHBP change with agitation speed. For stirrer speeds above 450 rpm, a decrease in the consumption rate of DBT is evident and, therefore, lower HBP concentrations are obtained. Furthermore, an increase of the agitation from 100 to 450 rpm has a positive influence on the development of the desulfurization capacity of the cells during growth, where XDBT recorded 60% and 100% at 100 and 450 rpm, reaching 100%.
Escobar et al. (2016) observed results revealing that at stirrer speeds < 700 rpm, the biochemical reactions were limited by mass transfer and η values increased from 0.53 (400 rpm) to 1.0 (700 rpm). These results proved that limitations of the mass transfer rate could occur when agitation < 700 rpm. The increase of η was attributed to the increase of the oxygen transport rate, OUR, and, consequently, the growth rate. Nevertheless, η recorded a constant value of 1 at an agitation speed of 700 to 2000 rpm, which was attributed to the overcome of the mass transport limitations and the process rate was determined by the cell metabolism reaching the maximum growth rate. That is, the OUR increased with the increase of the stirring rate (N) because OUR is taking the highest possible value under these conditions, being equal to the OTR value. When N is increased, kL and a increase and, therefore, OTR increases and OUR can take higher values until the maximum value is reached. Then, the increase of N will provoke an increase of kLa and OTR, but now OUR value is not affected and the value of the effectiveness factor is constant and equal to 1. The changes of XDBT and XHBP with different agitation speeds and resting cell ages proved that both parameters were constant within the agitation speed of 100 and 300 rpm. However, the cell age at this stirring speed range expressed a significant impact. The XDBT recorded 50% for cells of 5 h and 100% with cells of 15 and 23 h of age, while the XHBP recorded 15% at 5 h and increased to approximately 40 and 60 % for cells of 15 and 23 h, respectively. Then, they were decreased at higher speeds and lost at 800 rpm.
Escobar et al. (2016) followed up the kinetics of each enzyme, DszA, DszB, and DszD, where the recombinant protein was expressed in and purified from E. coli. The kinetic data obtained were appropriately modeled by the Michaelis-Menten model.
(173)

where v is the enzyme activity, kcat is the turnover number, E0 is the enzyme concentration in each assay, S is the substrate concentration, Km is the Michaelis constant, and Vmax is the maximum enzyme activity. The kinetics of DszC could not be modeled accurately with a simple Michaelis-Menten model because it was found that DszC was inhibited by its substrate, DBT. Thus, Haldane (i.e. the inhibition mode) was applied to fit the data obtained with DszC.
(174)

where KSI is the substrate (DBT) inhibition constant. The catalytic efficiency of an enzyme is reported to be best defined by the ratio of the kinetic constants kcat and Km (Copeland, 2000).
The concentrations of DszA, DszB, and DszC in that study were assumed to be all the same and equal to E0, which was obtained by allowing its value to vary until the initial desulfurization rate predicted by the model matched the measured desulfurization rate and was found to be 15 mg/mL, so that the total concentration of desulfurization enzymes in the cytoplasm was 45 mg/mL. This value was found to be of the same order as the total cytoplasmic concentration of protein which has been reported to be approximately 200 mg/mL (Ellis, 2001).
The kcat values of DszB, DszA, and DszD were found to be approximately 1.7 ±0.2, 11 ± 2, and 760±10 min-1, respectively. The Km value of DszB was calculated to be approximately 1.3 ± 0.3 µM. Nevertheless, DszC was inhibited by its substrate (DBT) and its kinetic parameters were found to be approximately 1.6 ± 0.3 min-1, 1.4 ± 0.3 M, and 1.8 ± 0.2 M for kcat, Km, and KSI, respectively, while the activity of DszC was found to be 31.3 nmol DBTO2/mg DszC/min. The catalytic efficiencies of DszA, DszB, and DszC were calculated to be approximately 3.1, 1.3, and 1.1 µM-1min-1, respectively. Furthermore, the catalytic efficiency of DszD on NADH and FMN was calculated to be 6.7 and 100 M-1min-1, respectively. Consequently, the efficiency of the 4S-enzymes was suggested to be listed in the following decreasing order: DszD > DszA > DszB ≈ DszC.
Copeland (2000) reported the dose-response equation describing the effect of the inhibitor concentration on enzyme activity:
(175)

where vi is the enzyme’s activity at an inhibitor concentration of I, v0 is the enzyme’s activity in the absence of inhibitor, and IC50 is the concentration of the inhibitor required to reduce the enzyme’s activity by 50% and it is phenomenological and has no mechanistic implications. The value of IC50 can be obtained by fitting the data for vi/v0 versus I to the dose-response equation using the nonlinear fitting package, nlinfit, in the MATLAB program.
Abin-Fuentes et al. (2013) reported the four major inhibitory interactions of the 4S-pathway in an order of decreasing strength, as follows: DszC inhibition by HBPS (IC50 = 15 ± 2 µM), DszC inhibition by HBP (IC50 50 ± 5 µM), DszA inhibition by HBP (IC50 60 ± 5 µM), and DszB inhibition by HBP (IC50 110 ± 10 µM). It is important to note that the major inhibitory compounds in the pathway are the last two intermediates, HBPS and HBP. Consequently, the 4S-pathway suffers from the pattern of inhibition from the type of feedback inhibition of linear pathways; for example, in the tricarboxylic acid (TCA) cycle, the first enzyme in the pathway is strongly inhibited by the end product, ATP.
Abin-Fuentes et al. (2013) concluded that IC50s of all four inhibitory interactions are significantly less than the estimated cytoplasmic HBP concentration. Even in the best case scenario, when 50 g/L of a highly HBP-selective resin was added to the BDS mixture, the estimated cytoplasmic HBP concentration was still 260 M. This finding suggests that these four inhibitory interactions are likely responsible for the reduction in biocatalyst activity that is observed during a typical BDS process when HBP is generated endogenously from DBT within the biocatalyst.
The model for non-competitive inhibition of an enzyme that obeys Michaelis-Menten kinetics is given by Copeland (2000):
(176)

where I is the inhibitor concentration, Ki is the inhibition constant, and α is a parameter that reflects the effect of the inhibitor on the affinity of the enzyme for its substrate and, likewise, the effect of the substrate on the affinity of the enzyme for the inhibitor. Non-competitive inhibition refers to the case in which an inhibitor displays binding affinity for both the free enzyme and the enzyme-substrate binary complex. This form of inhibition is the most general case. In fact, competitive and uncompetitive inhibition can be viewed as special, restricted cases of non-competitive inhibition in which the values of α are infinity and zero, respectively.
Abin-Fuentes et al. (2013) reported the occurrence of the telltale sign of non-competitive inhibition, which is a reduced Vmax without a change in Km as the inhibitor concentration is increased, in the inhibition of DszC by both HBPS and HBP in R. erythropolis IGTS8 and suggested a modified non-competitive model:
(177)

The Ki and α for DszC of R. erythropolis IGTS8 were 13.5 µM and 0.13, respectively, for HBPS inhibition and 40 µM and 0.4, respectively, for HBP. These parameters were calculated by graphical plots; the construction of the double-reciprocal Lineweaver-Burk plot and the Dixon plot of 1/ Vmax, as a function of I, from which the value of –αKi can be determined as the x intercept, while the slopes of the double-reciprocal lines (from the Lineweaver-Burk plot) are plotted as a function of I. For this plot, the x intercept is equal to Ki. Unfortunately, the mechanism of DszB inhibition by HBP and DszA inhibition by HBPS could not be determined due to the limited resolution of the HPLC detector.
Kareem et al. (2013a) carried out the anaerobic biodesulfurization of diesel using an isolated bacterial strain, Desulfobacterium indolicum.
The mass balance on the OSCs in diesel resulted in the following model equation:
(178)

where Ki is the distribution coefficient of the OSCs between diesel and water. The direct integration of the Michaelis-Menten equation yielded:
(179)

Manipulation of equation 179 gave a more explicit result:
(180)

Different OSCs in diesel oil were monitored. The kinetic parameters of BDS, like the maximum velocity rate constant, Vmax, and Michaelis-Menten constant, Km, were determined for BT and DBT were calculated to be 0.540 and 5.992 mg/L/h and 19.837 and 192.782 mg/L, respectively.
The partition coefficient was estimated through the efficacy of combining linear solvation energy relationships (LSERs) developed for pure systems through the application of the linear solvent strength theory of Arey and Gschwend (2005):
(181)

where R is the universal gas constant, T is the temperature at which the coefficient is measured, Vf is the molar volume of diesel, Pi° is the vapor pressure of the components of the fuels parameters, R2 describes the excess molar refraction of solute i, ![]() describes the polarity/polarizability of solute i,
describes the polarity/polarizability of solute i, ![]() describes the hydrogen-bonding acidity of solute i,
describes the hydrogen-bonding acidity of solute i, ![]() describes the hydrogen bonding basicity of solute i, and Vx describes the group-contributable molecular volume of solute i, while c, r, s, a, b, and v are adjusted coefficients specific to the two-phase system. The Ki,fw recorded 2.312 and 2.038 for BT and DBT, respectively. A mathematical model showing the pattern of diesel biodesulfurization was developed. The developed model was solved numerically by the Finite Difference method. The model was able to describe all the experimental data with good statistical parameters and the simulated data were compared with the experimental findings to validate the model developed; the simulated results showed very good agreement with the experimental findings.
describes the hydrogen bonding basicity of solute i, and Vx describes the group-contributable molecular volume of solute i, while c, r, s, a, b, and v are adjusted coefficients specific to the two-phase system. The Ki,fw recorded 2.312 and 2.038 for BT and DBT, respectively. A mathematical model showing the pattern of diesel biodesulfurization was developed. The developed model was solved numerically by the Finite Difference method. The model was able to describe all the experimental data with good statistical parameters and the simulated data were compared with the experimental findings to validate the model developed; the simulated results showed very good agreement with the experimental findings.
Kareem et al. (2013b) also modelled the BDS of kerosene by taking a mass balance on the substrates, i.e. the OSCs in the kerosene.
The material balance on the solute (substrates in kerosen) over the time period from t to t + Δt over the element of volume of a batch reactor from z to z + Δz was obtained:
(182)

In a batch reactor there is no inflow or outflow of material, thus:
(183)

The mass transfer rate to the solid is:
(184)
The accumulation in the fluid phase is:
(185)
There was no reaction in the fluid phase in the reactor since all the reactions were assumed to take place on the cell surface, hence, the reaction term was considered equal to zero. Thus, by substituting eqs. 184 and 185 in eq.182, the following expression is obtained:
Dividing eq. 186 by AΔz:
where CS is the concentration of the substrate on the surface of the organism which is not easily measurable. This was solved by applying the law of conservation of mass to the absorbable solute contained in the fluid phase and the solid based on the assumption that the adsorption transfers material from the fluid phase and adds to the solid phase, i.e. the microorganism Desulfobacterium anilini, taking into consideration that the solid phase loses material by desulfurization and generates none. Thus, the solid phase mass balance for the sulfur specific reductive BDS pathway is expressed as follows:
Dividing eq. 188 by AΔz:
Substituting eq. 189 into eq. 187:
The solutions of eq. 190 were assumed to be simple when q is a linear function of C, that is if the adsorption was assumed to be linear, then dq/dt can be replaced by -KdC/dt, thus:
(191)
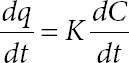
where K is a distribution coefficient:
where eq. 192 represents the mass transfer based kinetic rate expression for the specific reductive BDS pathway and it is a first order differential equation which was solved numerically using the Finite Difference Method. The simulated results were found to have a high correlation with known and reliable experimental data. The maximum velocity rate constant and the Michaelis-Menten constant, Km, were obtained by subjecting the obtained parameters from linear plots to the Macquardt’s non-linear regression analysis. The Vmax and Km were found to be 0.103 mg/L/h and 0.575 mg/L for thiophene (Th) and 1.350 mg/L/h and 54.700 mg/L for 2,5-dimethylthiophene (2,5-DMTh), respectively. The partition (distribution) coefficients (Ki,fw) of the organic sulfur compounds in the kerosene used in the simulation were estimated through the efficacy of combining linear solvation energy relationships (LSERs) developed for pure systems through the application of the linear solvent strength theory (eq.181). The Ki,fw for Th and 2,5-DMTh recorded 1.281 and 1.054, respectively. The quantitative parameters estimated in the course of modelling the anaerobic biodesulfurization of kerosene (Kareem et al., 2013b) would be useful in a bioreactor design of the process that would eventually take the technology to the market.
Moreover, Kareem et al. (2014) investigated the kinetics of the anaerobic biodesulfurization of diesel. This was done by simulating the kinetics of the process alone and then with and without the effect of mass transfer. The kinetic parameters Vmax and Km of the Michaelis-Menten equation were estimated using linear equations of Hanes, Lineweaver-Buck, and Eadie-Hofstee plots (Eadie, 1942; Hofstee, 1959). However, the values obtained for each parameter from the linear equations were close, but not the same. This necessitated the need to carry out a non-linear regression analysis using Marquardt’s algorithm (Marquardt, 1963), where the parameters were estimated and then subjected to Marquardt iterative nonlinear least squares regression and the obtained bio-kinetic parameters were used to model the BDS of diesel by Desulfobacterium anilini in a batch reactor. The Vmax and Michaelis-Menten constant, Km, determined for BT and DBT were calculated to be 0.572 and 6.118 mg/L/h and 18.050 and 182.278 mg/L, respectively. The mass balance was performed by taking the mass balance of the substrates and the sulfur containing hydrocarbons in the fuel and diesel were the same as reported by Kareem et al. (2013b). The distribution coefficient, Ki,fw, was estimated using the model developed by Arey and Gschwend (2005) for sulfur-containing organic substances in the fuel phase (eq. 181). The Ki,fw for BT and DBT recorded 2.312 and 2.038, respectively. The Implicit Finite Difference Method was used to obtain a substrate concentration-time data from eq. 192. The results were presented in terms of a percentage biodesulfurization-time profile. There was good agreement between simulated and experimental data proving that the assumptions made in developing the models were correct. The kinetics of the biodesulfurization of diesel by Desulfobacterium anilini has been found to be mass transfer driven (Kareem et al., 2014).
Similar work has been published by Kareem (2016), where the kinetics of the anaerobic BDS of kerosene using Desulfatiglans anilini comb. nov with and without the influence of mass transfer were investigated. The bio-kinetic parameters were estimated using the various methods of Hanes (eq. 193), Lineweaver-Buck (eq. 194), and Eadie-Hofstee (eq. 195) all from the linear transformation of the Michaelis-Menten equation. The obtained results when compared caused confusion, thus the Michaelis-Menten equation was integrated and the bio-kinetic parameters were estimated. The Vmax and Michaelis-Menten constant, Km, were determined for Th and 2,5-DMTh were calculated to be 0.104 and 1.426 mg/L/h and 0.548 and 6.7 mg/L, respectively. The partition coefficients of sulfur containing hydrocarbons in kerosene and in water were used instead of the mass transfer coefficient; this was done using the Arey and Gschwend model (eq.181). The developed model equations were solved numerically using the Finite Difference Method. Furthermore, the simulated concentration-time profile of the mass transfer plus kinetics has a better agreement with the known experimental values than those with kinetics alone. The good agreement of simulated data and the experimental ones proved that the assumptions made in developing the models were valid. The result showed that mass transfer played a significant role on the kinetics of the process.
The Hanes-Woolf plot, known as the Hanes plot for short, is obtained by rearranging the Michaelis-Menten equation, such that:
(193)

The transformation gives the best applying 1 / vmax instead of vmax, but the main drawback of this transformation is dependent of both abscissa and ordinate on the substrate concentration.
The Lineweaver-Burk plot can be obtained by linearly transforming the Michaelis-Menten equation to:
(194)

The double reciprocal plot is prone to error because it distorts the error structure of the data. In the Eadie-Hofstee plot, the Michaelis-Menten equation can be linearly transformed so that the reaction rate is plotted as a function of the ratio between the rate of reaction and substrate concentration:
(195)

The abscissa and ordinates are independent variables both dependent on the reaction rate, so, like the Hanes-Woolf plot, any experimental error will be present in both axes. Parameters estimated from this plot are more reliable than those of Eadie-Hofstee and Lineweaver-Burk because they give equal weight to data points in any given range of substrate concentration or reaction rate. All these short comings may be attributed to the nonlinearity of the Michaelis-Menten equation itself, thus a non-linear regression method will give a better estimate of the kinetic parameters.
Zhang et al. (2013) studied the interactions among three typical Cx-DBTs, such as dibenzothiophenes (DBT), 4-methyldibenzothiophene (4- MDBT), and 4,6-dimethyldibenzothiophene (4,6-DMDBT), using Mycobacterium sp. ZD-19 in an airlift reactor.
The specific desulfurization rate in the airlift reactor, Vi, according to the multi-substrate competitive inhibition kinetics using the Michaelis–Menten pattern was described as follows:
(196)

where Vi is the specific desulfurization rate of substrate i in the multiple substrate system, Csi is the concentration of substrate i, Ksi is the half-saturation constant of substrate i, Ksj and Csj are the half-saturation constant and concentration of another co-existing substrate, j, in the multi-substrate system. Additionally, a fractional velocity rate equation (Segel, 1975) was used to estimate the inhibition of a multi-substrate on a sole substrate in the competitive inhibition system.
where V0 is the biodesulfurization rate of substrate i in the sole substrate system and Vi is the biodesulfurization rate of substrate i in the multiple substrates system.
The Michaelis–Menten kinetic parameters were obtained from the sole substrate experiments using a Lineweaver–Burk plot method. The values of Vmax exhibited a decrease tendency for each additional alkyl substitution. The Vmax values of DBT, 4-MDBT, and 4,6-DMDBT were 0.328, 0.233, and 0.0.92 mM/h, respectively, indicating that the biodesulfurization rate decreased for each additional alkyl substitution. The Km values of DBT, 4-MDBT, and 4,6-DMDBT were 0.591, 0.747, and 0.590 mM, respectively. The higher Km value was considered as an indication of a weaker affinity of the Mycobacterium sp. with substrate. Though a weaker affinity of the Mycobacterium sp. with 4-MDBT was obtained compared to that with 4,6-DMDBT, a higher desulfurization of 4-MDBT was achieved compared with that of 4,6-DMDBT. These results were attributed to the steric hindrance effect that prevented Mycobacterium sp. ZD-19 from attacking C–S for desulfurization of 4,6-DMDBT. Briefly, the experimental results indicated that the desulfurization rates would decrease in the multiple Cx- DBTs system compared to the single Cx-DBT system. The extent of inhibition depended upon the substrate numbers, concentrations, and affinities of the co-existing substrates. For example, compared to the individual desulfurization rate (100 %), the DBT desulfurization rate decreased to 75.2 % (DBT + 4,6-DMDBT), 64.8 % (DBT + 4-MDBT), and 54.7 % (DBT + 4,6-DMDBT + 4-MDBT), respectively. This phenomenon was caused by an apparent competitive inhibition of substrates, which was well predicted by a Michaelis–Menten competitive inhibition model (eq. 196). The DBT desulfurization rate decreased to 0.093 and 0.108 mM/h in the presence of 4-MDBTand 4,6-DMDBT, respectively. The 4-MDBT desulfurization rate decreased from 0.121 to 0.074 and 0.092 mM/h in the presence of DBT and 4,6-DMDBT, respectively, while the 4,6-DMDBT desulfurization rate decreased from 0.023 to 0.013 and 0.014 mM/h in the presence of DBT and 4-MDBT, respectively. The fractional velocity rate equation (eq. 197) estimated the inhibition of multiple substrates on a single substrate. In both binary and ternary systems, the presence of other Cx-DBTs had a significant inhibition effect on the desulfurization of DBT. Similarly, the initial desulfurization rates of 4-MDBT and 4,6-DMDBT also decreased in the binary and ternary mixtures compared with the individual substrate condition. An indication of the competitive effect of the co-existing substrates on a sole substrate were determined by the term

in the denominator of the fractional velocity rate equation (eq. 197). The obtained results of the single, binary, and ternary systems indicate that the extent of the competitive inhibition in the multiple substrates system depended upon the numbers, concentrations, and affinities of the co-existing substrates (Zhang et al., 2013).
9.11 Evaluation of the Tested Biocatalysts
The evaluation of the tested biocatalysts can be performed by investigation of:
9.11.1 Kinetics of the Overall Biodesulfurization Reaction
Although BDS the via 4S-pathway takes place in several steps, the overall process can be summarized by BDS of DBT to the dead end product 2-HBP. Moreover, the overall rate kinetics are affected by the concentration and distribution of DBT.
where k is the rate coefficient (cm3/sec), t is the incubation period (sec), CDBT is the initial DBT concentration (ppm), CX is the cell concentration (cells/cm) (i.e. the concentration of bacteria), and C2-HBP is the 2-HBP concentration (ppm).
The integrated form of eq. 198:
(199)

(200)

(201)
9.11.2 Maximum Percentage of Desulfurization ( )
)
This parameter indicates the maximum desulfurizing capability of cells obtained during the resting cell assay:
(202)

where ![]() is the maximum 2-HBP concentration obtained during the time employed in the resting cell assay and CDBTO is the initial concentration of DBT used to perform desulfurization with resting cell assay.
is the maximum 2-HBP concentration obtained during the time employed in the resting cell assay and CDBTO is the initial concentration of DBT used to perform desulfurization with resting cell assay.
9.11.3 Time for Maximum Biodesulfurization tBDSmax (min)
This parameter indicates the time at which the maximum percentage of biodesulfurization has been reached in the resting cell assay.
9.11.4 Initial DBT Removal Rate  (µmol/L/min)
(µmol/L/min)
This parameter shows the initial rate of DBT transformation. It is calculated by applying differential method over the evolution of the DBT concentration throughout time in order to estimate the value of the DBT removal rate at time, zero, according to the following expression:
(203)

9.11.5 Maximum Productivity  (%/min)
(%/min)
This parameter relates maximum percentage of desulfurization to the time needed for this conversion:
(204)

9.11.6 Specific Conversion Rate (SE %L/g/min)
This parameter regards the performance of each tested biocatalyst. It relates ![]() , concentration of biomass, CX, and time needed for the maximum achieved conversion, according to:
, concentration of biomass, CX, and time needed for the maximum achieved conversion, according to:
(205)

For example, for the genetically modified Pseudomonas putida CECT 5279 and the maximum in vivo activities of monooxygenase enzymes (DszA and DszC) are shown when the late exponential growth phase is reached (23 h), while the desulfinase enzyme DszB presents a maximum activity during the early exponential growth phase (5 h) (Calzada et al., 2009b). Also, a consortium of these two cell ages was reported to yield excellent BDS results (Calzada et al., 2009a). Calzada et al. (2011) optimized the ratio and total biomass concentration of both 5 h and 23 h growth time in cells in a complex consortium for desulfurization by performing resting cells of biodesulfurization assays using dibenzothiophene (DBT) as a sulfur model compound. A particular cell mixture containing 66.7% of 23 h growth time cells was found to work as the most effective desulfurization biocatalyst. The authors showed that the complex biocatalyst that combines 5 and 23 h cells of ![]() achieve better behavior for BDS than the 9 h simple catalyst. That led to a higher DBT conversion into 2-HBP, a higher initial DBT removal rate, and maximum productivity. Selection took into account the highest conversion of DBT into 2-HBP in the shortest operation time with the lowest possible amount of biomass for each tested biocatalyst. The mixture composed by a 1:2 ratio of cells collected at 5 and 23 h of growth time of Pseudomonas putida CECT 5279, using a total biomass concentration of 2.1 g DCW/L, showed the best capabilities for desulfurization in resting cells among all the proposed biocatalyst formulations according to the specific conversion rate previously defined. Improving complex biocatalysts can offer better biodesulfurization processes, achieving higher DBT conversions and reducing time needed for biodesulfurization.
achieve better behavior for BDS than the 9 h simple catalyst. That led to a higher DBT conversion into 2-HBP, a higher initial DBT removal rate, and maximum productivity. Selection took into account the highest conversion of DBT into 2-HBP in the shortest operation time with the lowest possible amount of biomass for each tested biocatalyst. The mixture composed by a 1:2 ratio of cells collected at 5 and 23 h of growth time of Pseudomonas putida CECT 5279, using a total biomass concentration of 2.1 g DCW/L, showed the best capabilities for desulfurization in resting cells among all the proposed biocatalyst formulations according to the specific conversion rate previously defined. Improving complex biocatalysts can offer better biodesulfurization processes, achieving higher DBT conversions and reducing time needed for biodesulfurization.
References
Abbad-Andaloussi, S., Lagnel, C., Warzywoda, M., Monot, F. (2003) Multicriteria comparison of resting cell activities of bacterial strains selected for biodesulfurization of petroleum compounds. Enzyme and Microbial Technology. 32(3/4): 446–454.
Abin Fuentes, A., Leung, J.C., Mohamed, M.E.S., Wang, D.I.C., Prather, K.L.J. (2014) Rate limiting step analysis of the microbial desulfurization of dibenzothiophene in a model oil system. Biotechnology and Bioengineering. 111(5): 876–884.
Abin-Fuentes, A., Mohamed, M.E., Wang, D.I.C., Prather, K.L.J. (2013) Exploring the mechanism of biocatalyst inhibition in microbial desulfurization. Applied and Environmental Microbiology. 79(24): 7807–7817.
Abolfazl, S.H., Soheila, Y., Mohammad, M.S. (2006) Biodesulfurization of dibenzothiophene by a newly isolated thermophilic bacteria strain. Iranian Journal of Chemistry and Chemical Engineering. 25:65–71.
Abrardi, V., Rover, G., Sicardi, S., Baldi, G., Conti, R. (1988) Sparged vessels agitated by multiple turbines. Proceeding of 6th European Conference on Mixing. Pavia, Italy, 24–26 May, 1988. Pp. 329–336.
Adlakha, J., Singh, P., Ram, S. K., Kumar, M., Singh, M. P., Singh, D., Srivastava, P. (2016). Optimization of conditions for deep desulfurization of heavy crude oil and hydrodesulfurized diesel by Gordonia sp. IITR100. Fuel.184: 761–769.
Agarry, S.E., Solomon, B.O., Layokun, S.K. (2008) Substrate inhibition kinetics of phenol degradation by binary mixed culture of Pseudomonas aeruginosa and Pseudomonas fluorescence from steady state and wash-out data. African Journal Biotechnology. 7: 3927–3933.
Aggarwall, S., Karimi1 I.A., Ivan, G.R. (2013) In silico modeling and evaluation of Gordonia alkanivorans for biodesulfurization. Molecular BioSyststem. 9: 2530–2540.
Aiba, S., Shoda, M., Nagatami, M. (1968) Kinetics of product inhibition in alcohol fermentation. Biotechnology and Bioengineering journal. 10: 845–864.
Alcon, A., Martin, A.B., Santos, V.E., Gomez, E., Garcia-Ochoa, F. (2008) Kinetic model for DBT biodesulphurization by resting whole cells of Pseudomonas putida CECT5279. Biochemical Engineering Journal. 39:486–495.
Alcon, A., Santos, V.E., Martin, A.B., Yustos, P., Garcia-Ochoa, F. (2005) Biodesulfurisation of DBT with Pseudomonas putida CECT5279 by resting cells: Influence of cell growth time on reducing equivalent concentration and HpaC activity. Biochemical Engineering Journal. 26: 168–175.
Alonso del Aguila, R., Boltes, K., Leton, P., Rodríguez, A., Rosal, R., Perdigón, J., Garcia-Calvo, E. (2007) Biodesulfurization of DBT in model oil by resting cell of Pseudomonas putida CECT5279: Process enhancement. European Congress of Chemical Engineering - 6, (September), 1–6.
Alves, L., Paixao, S.M. (2011) Toxicity evaluation of 2-hydroxybiphenyl and other compounds involved in studies of fossil fuels biodesulphurisation. Bioresource Technology, 102(19), 9162–9166.
Ansari, F., Grigoriev, P., Libor, S., Tothill, I. E., Ramsden, J.J. (2009) DBT degradation enhancement by decorating Rhodococcus erythropolis IGST8 with magnetic Fe3O4 nanoparticles. Biotechnology and Bioengineering, 102(5): 1505–1512.
Arabian, D., Najafi, H., Farhadi, F., Dehkordi, A.M. (2014) Biodesulfurization of simulated light fuel oil by a native isolated bacteria Bacillus cereus HN. Journal of Petroleum Science and Technology. 4(1): 31–40.
Arey, J.S., Gschwend, P.M. (2005) Estimating the partition coefficients for fuel – water systems: developing linear salvation energy relationships using linear solvent strength theory to handle mixtures. Environmental Science and Technology. 39: 2702 – 2710.
Arnaud, J.P., Lacroix, C., Foussereau, C., Choplin, L. (1993) Shear stress effects on growth and activity of Lactobacillus delbrueckii subsp. Bulgaricus. Journal of Biotechnology. 29: 157–175.
Ayala, M., Tinoco, R., Hernandez, V., Bremauntz, P., Vazquez-Duhalt, R. (1998) Biocatalytic oxidation of fuel as an alternative to biodesulfurization. Fuel Processing Technology. 57: 101–11.
Bahuguna, A., Lily, M.K., Munjal, A., Singh, R.N. Dangwal, K. (2011) Desulfurization of dibenzothiophene (DBT) by a novel strain Lysinibacillus sphaericus DMT-7 isolated from diesel contaminated soil. Journal of Environmental Sciences. 23: 975–982.
Bandyopadhyay, S., Chowdhury, R., Bhattacharjee, C. (2013) Steady state performance of a bioreactor for production of near zero sulfur diesel (NZSD) and bio-surfactant. Journal of Clean Energy Technologies. 1(3): 189–193.
Blanch, H.W., Clark, D.S. (1997) Biochemical Engineering. Marcel Dekker Inc., New York.
Boltes, K., Alonso del Aguila, R., García-Calvo, E. (2013) Effect of mass transfer on biodesulfurization kinetics of alkylated forms of dibenzothiophene by Pseudomonas putida CECT5279. Journal of Chemical Technology and Biotechnology. 88: 422–431.
Bordoloi, N.K., Rai, S.K., Chaudhuri, M.K., Mukherjee, A.K. (2014) Deep-desulfurization of dibenzothiophene and its derivatives present in diesel oil by a newly isolated bacterium Achromobacter sp. to reduce the environmental pollution from fossil fuel combustion. Fuel Processing Technology, 119: 236–244.
Bronnenmeier, R., Markl, H. (1982) Hydrodynamic stress capacity of microorganisms. Biotechnology and Bioengineering. 24: 553–578.
Calik, P., Yilgör, P., Ayhan, P., Demir, A. (2004) Oxygen transfer effects on recombinant benzaldehyde lyase production. Chemical Engineering Science. 59: 5075–5083.
Calzada, J., Alcon, A., Santos, V.E., Garcia-Ochoa, F. (2011) Mixtures of Pseudomonas putida CECT 5279 cells of different ages: optimization as biodesulfurization catalyst. Process Biochemistry. 46:1323–1328.
Calzada, J., Alcon, A., Santos, V.E., Garcia-Ochoa, F. (2012) Extended kinetic model for DBT desulfurization using Pseudomonas Putida CECT5279 in resting cells. Biochemical Engineering Journal. 66: 52–60.
Calzada, J., Heras, S., Alcon, A., Santos, V.E., Garcia-Ochoa, F. (2009a) Biodesulfurization of dibenzothiophene (DBT) using Pseudomonas putida CECT 5279: a biocatalyst formulation comparison. Energy and Fuels. 23: 5491–5495.
Calzada, J., Zamarro, M.T., Alcon, A., Santos, V.E., Diaz, E., Garcia, J.L., Garcia-Ochoa, F. (2009b) Analysis of dibenzothiophene desulfurization in a recombinant Pseudomonas putida strain. Applied and Environmental Microbiology. 75: 875–877.
Caro, A, Boltes, K., Leton, P., Garcia-Calvo, E. (2008b) Description of by-product inhibition effects on biodesulfurization of dibenzothiophene in biphasic media. Biodegradation. 19: 599–611.
Caro, A., Boltes, K., Letón, P., García-Calvo, E. (2007b) Dibenzothiophene biodesulfurization in resting cell conditions by aerobic bacteria. Biochemical Engineering Journal. 35:191–197.
Caro, A., Boltes, K., Leton, P., Garcia-Calvo, E. (2008a) Biodesulfurization of dibenzothiophene by growing cells of Pseudomonas putida CECT 5279 in biphasic media. Chemosphere. 73: 663–669.
Caro, A., Letón, P., García-Calvo, E., Setti, L. (2007a) Enhancement of dibenzothiophene biodesulfurization using β-cyclodextrins in oil-to-water media. Fuel. 86: 2632–2636.
Chalmers, J.J. (1994) Cells and bubbles in sparged bioreactors. Cytotechnology. 15: 311–320.
Chang, J.H., Chang, Y.K., Cho, K.-S., Chang, H.N.: (2000) Desulfurization of model and diesel oils by resting cells of Gordona sp. Biotechnology Letters. 22(3): 193.196
Chen H., Cai Y.B., Zhang W.J., Li W. (2009) Methoxylation pathway in biodesulfurization of model organosulfur compounds with Mycobacterium sp. Bioresource Technology. 100:2085–2087.
Chen, H., Cai, Y.B., Zhang, W.J., Li, W. (2009) Methoxylation pathway in biodesulfurization of model organosulfur compounds with Mycobacterium sp. Bioresource Technology. 100:2085–2087.
Chen, H., Zhang, W.J., Cai, Y.B., Zhang, Y., Li, W. (2008) Elucidation of 2-hydroxybiphenyl effect on dibenzothiophene desulfurization by Microbacterium sp. strain ZD-M2. Bioresource Technology. 99(15): 6928–6933.
Chen, H., Zhang, W.J., Cai, Y.B., Zhang, Y., Li, W. (2008a) Elucidation of 2-hydroxybiphenyl effect on dibenzothiophene desulfurization by Microbacterium sp. strain ZD-M2. Bioresource Technology. 99(15): 6928–6933.
Chen, H., Zhang, W.J., Chen, J.M., Cai, Y.B., Li. W. (2008b) Desulfurization of various organic sulfur compounds and the mixture of DBT + 4,6DMDBT by Micobacterium sp. ZD-19. Bioresource Technology. 99: 3630–3634.
Chisty, Y. (2010) In: M.C. Flickinger (ed) Shear Sensitivity in Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology. John Wiley & Sons, New York.
Choi, O.K., Cho, K.S., Ryu, H.W., Chang, Y.K. (2003) Enhancement of phase separation by the addition of de-emulsifiers to three phase (diesel oil/biocatalyst/aqueous phase) emulsion in diesel biodesulfurization. Biotechnology Letters. 25(1): 73–77.
Constanti, M., Giralt, J., Bordons, A. (1996) Degradation and desulfurization of dibenzothiophene sulfone and other sulfur compounds by Agrobacterium NC501 and a mixed culture. Enzyme and Microbial Technology. 19: 214–219.
Copeland, R.A. (2000) Enzymes: a practical introduction to structure, mechanism, and data analysis, 2nd ed. Pp. 397, Wiley-VCH, Weinheim, Germany.
Davoodi-Dehaghani, F., Vosoughi, M., Ziaee, A.A. (2010) Biodesulfurization of dibenzothiophene by a newly isolated Rhodococcus erythropolis strain. Journal of Bioresource Technology. 101: 1102–1105.
Dejaloud, A., Vahabzadeh, F., Habibi, A. (2017) Ralstonia eutropha as a biocatalyst for desulfurization of dibenzothiophene. Bioprocess and Biosystems Engineering. 40: 969–980.
del Olmo, C.H., Alcon, A., Santos, V.E., Garcia-Ochoa, F. (2005a) Modeling the production of a Rhodococcus erythropolis IGTS8 biocatalyst for DBT biodesulfurization: influence of media composition. Enzyme and Microbial Technology. 37: 157–166.
del Olmo, C.H., Santos, V.E., Alcon, A., Garcia-Ochoa, F. (2005b) Production of a Rhodococcus erythropolis IGTS8 biocatalyst for DBT biodesulfurization: influence of operational conditions. Biochemical Engineering Journal. 22: 229–237.
Denome, S.A., Oldfield, C., Nash, L.J., Young, K.D. (1994) Characterization of the desulfurization genes from Rhodococcus sp. strain IGTS8. Journal of Bacteriology. 176: 6707–6716.
Deriase, S.F., Nassar, H.N., El-Gendy, N.Sh. (2015) Modeling and simulation for biodegradation of mono-, binary-, and tertiary-substrate batch systems of different polyaromatic sulfur heterocyclic compounds. Petroleum Science and Technology. 33(10): 1063–1076.
Deriase, S.F., El-Gendy, N.Sh. (2010) Modeling and simulation for degradation of different polyaromatic sulfur heterocyclic compounds by Bacillus sphaericus HN1 in a batch reactor. 4(2): 1–24.
Deriase, S.F., Younis, Sh.A., El-Gendy, N.Sh. (2013) Kinetic evaluation and modeling for batch degradation of 2-hydroxybiphenyl and 2,2’-dihdroxybipheny by Corynebacterium variabilis Sh42. Desalination and Water Treatment. 51: 4719–4728.
Derikvand p., Etemadifar Z. (2014) Improvement of biodesulfurization rate of alginate immobilized Rhodococcus erythropolis R1. Jundishapur Journal of Microbiology. 7:1–7.
Eadie, G. S., (1942) The Inhibition of Cholinesterase by Physostigmine and Prostigmine. Journal of Biological Chemistry.146:85–93.
Edwards, V.H. (1970) The influence of high substrate concentrations on microbial kinetics. Biotechnology and Bioengineering journal. 12: 679 - 712.
El-Gendy, N.Sh., Nassar, H.N., Younis, S.A. (2015) Main and interactive effects of polyaromatic sulfur heterocyclic compounds on growth and biodegradation efficiencies of Bacillus sphaericus HN1: modeling and statistical analysis. Petroleum Science and Technology. 33(11): 1167–1181.
Ellis, R.J. (2001) Macromolecular crowding: obvious but under appreciated. Trends in Biochemical Sciences. 26:5 97–604.
Escobar, S., Rodriguez, A., Gomez, E., Alcon, A., Santos, V.E., Garcia-Ocho, F. (2016) Influence of oxygen transfer on Pseudomonas putida effects on growth rate and biodesulfurization capacity. Bioprocess and Biosystems Engineering. 39: 545–554.
Fedorak, P.M., Westlake, D.W.S. (1983) Microbial degradation of organic sulfur compounds in Prudhoe Bay crude oil. Canadian Journal of Microbiology. 29: 291–296.
Fedorak, P.M., Westlake, D.W.S. (1984) Degradation of sulfur heterocycles in Prudhoe Bay crude oil by soil enrichments. Water Air Soil Pollution. 21: 225–230.
Folsom B.R., Schieche D.R., Digrazia P.M., Werner J. and Palmer S. (1999) Microbial desulfurization of alkylated dibenzothiophenes from a hydrodesulfurized middle distillate by Rhodococcus erythropolis I-19. Applied Environmental Microbiology. 65: 4967–4972.
Galán, B., Díaz, E., García, J.L. (2000). Enhancing desulphurization by engineering a flavin reductase encoding gene cassette in recombinant biocatalysts. Environmental Microbiology. 2: 687–694.
Gallagher, J.R., Olson, E.S., Stanley, D.C. (1993) Microbial desulfurization of dibenzothiophene: a sulfur-specific pathway. FEMS Microbiology Letters. 197: 31–36.
García Camacho, F., Molina Grima, E., Sánchez Mirón, A., González Pascual, V., Chisti, Y. (2001) Carboxymethyl cellulose protects algal cells against hydrodynamic stress. Enzyme and Microbial Technology. 29: 602–610.
Garcia-Ochoa, F., Escobar, S., Gomez, E. (2015) Specific oxygen uptake rate as indicator of cell response of Rhodococcus erythropolis cultures to shear effects. Chemical Engineering Science. 122: 491–499.
Garcia-Ochoa, F., Gomez, E. (2009) Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnology Advances. 27: 153–176.
Garcia-Ochoa, F., Gomez, E., Alcon, A., Santos, V.E. (2013) The effect of hydrodynamic stress on the growth of Xanthomonas campestris cultures in a stirred and sparged tank bioreactor. Bioprocess and Biosystems Engineering. 36: 911–925.
Garcia-Ochoa, F., Gomez, E., Santos. V.E., Merchuk, J.C. (2010) Oxygen uptake rate in microbial processes: an overview. Biochemical Engineering Journal. 49: 289–307.
Garcia-Ochoa, F., Romero, A., Santos, V.E. (1992) Comparison of Methods for determining kinetic parameters in complex reactions. International chemical engineering 32(3): 538–551.
Giraud, C. (2008). Estimation of Gaussian graphs by model selection. Electronic Journal of Statistics. 2:542–563.
Godoy-Silva, C. Berdugo, J.J. Chalmers, Aeration, mixing and hydrodynamics of animal cell bioreactors, in: M.C. Flickinger (Ed.), Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology, John Wiley & Sons, 2010.
Goindi, H.K., Saini, V.S., Verma, P.S., Adhikari, D.K. (2002) Dibenzothiophene desulfurization in hydrocarbon environment by Staphylococcus sp. resting cells. Biotechnology Letters. 24:779–781.
Gomez, E., Alcon, A., Escobar, S., Santos, V.E., Garcia-Ochoa, F. (2015) Effect of fluid dynamic conditions on growth rate and biodesulfurization capacity of Rhodococcus erythropolis IGTS8. Biochemical Engineering Journal. 99: 138–146.
Gomez, E., Santos, V., Alcon, A., Garcia-Ochoa, F. (2006a) Oxygen transport rate on Rhodococcus erythropolis cultures: effect on growth and BDS capability. Chemical Engineering Science. 61: 4595–4604.
Gomez, E., Santos, V., Alcon, A., Martin, A.B., Garcia-Ochoa, F. (2006b) Oxygen-uptake and mass-transfer rates on the growth of Pseudomonas putida CECT5279: influence on biodesulfurization (BDS) capability. Energy and Fuels. 20: 1565–1571.
Gray, K.A., Mrachko, G.T., Squires, C.H. (2003) Biodesulfurization of fossil fuels. Current Opinion in Microbiology. 6: 229–235.
Gray, K.A., Pogrebinsky, O.S., Mrachko, G.T., Xi, L., Monticello, D.J., Squires C.H. (1996) Molecular mechanisms of biocatalytic desulfurization of fossil fuels. Nature Biotechnology. 14: 1705–1709.
Guchhait, S., Biswas, D., Bhattacharya, P., Chowdhury, R. (2005a) Bio-desulfurization of model organo-sulfur compounds and hydrotreated diesel – experiments and modelling. Chemical Engineering Journal. 112: 145–151.
Guha, S., Peters, C.A., Jaffe, P.R. (1999) Multi-substrate biodegradation kinetics of naphthalene, phenanthrene, and pyrene mixtures. Biotechnology Bioenergy. 65: 491–499.
Gunam, I.B.W., Yaku, Y., Hirano, M., Yamamura, K., Tomita, F., Sone, T., Asano, K., (2006) Biodesulfurization of alkylated forms of dibenzothiophene and benzothiophenes by Sphingomonas subarctica T7b. Journal of Bioscience and Bioengineering. 101: 322–327.
Gunam, I.B.W., Yamamura, K., Nengah Sujaya, I., Antara, N.S., Aryanta, W.R., Tanaka, M., Asano, K. (2013) Biodesulfurization of dibenzothiophene and its derivatives using resting and immobilized cells of Sphingomonas subarctica T7b. Journal of Microbiology and Biotechnology,.23(4): 473–482.
Guobin S., Huaiying Z., Weiquan C., Jianmin X., Huizhou L. (2005) Improvement of biodesulfurization rate by assembling nanosorbents on the surfaces of microbial cells. Biophysical Journal. 89(6): 58–60.
Haldane, J.B.S. (1930) Enzymes. Longmans, Green & Company, London, England, UK.
Hewitt, C.J., Boon, L.A., McFarlane, C.M., Nienow, A.W. (1998) The use of flow cytometry to study the impact of fluid mechanical stress on Escherichia coli W3110 during continuous cultivation in an agitated bioreactor. Biotechnology and Bioengineering. 59: 612–620.
Hodaifa, G., Martinez, M.E., Orpez, R., Sanchez, S. (2010) Influence of hydrodynamic stress in the growth of Scenedesmus obliquus using a culture medium based on olive mill waste water. Chemical Engineering Process. 49: 1161–1168.
Hofstee, B.H.J. (1952) Specificity of Esterases: I. Identification of Two Pancreatic Aliesterases, Journal of Biological Chemistry. 199:357–364.
Honda, H., Sugiyama, H., Saito, I., Kobayashi, T. (1998) High cell density culture of Rhodococcus rhodochrous by pH-stat feeding and dibenzothiophene degradation. Journal Fermentation and Bioengineering. 85:334–338. http://chemwiki.ucdavis.edu/Biological_Chemistry/Catalysts/Enzymatickinetics.
Introduction to enzymes (2017) worthington biochemical corporation. http://www.worthington-biochem.com/introbiochem/introEnzymes.html
Irani, Z.A., Yazdian, F., Mohebali, G., Soheili, M., Mehrnia, M.R. (2011) Determination of growth kinetic parameters of a desulfurizing bacterium, Gordonia alkanivorans RIPI90A. Chemical Engineering Transactions. 24: 937–942.
Izumi, Y., Oshiro, T., Ogino, H., Hine, Y., Shimao, M. (1994) Selective desulfurization of dibenzothiophene by Rhodococcus erythropolis D-1. Applied and Environmental Microbiology. 60: 223–236.
Jahoda, M., Mostek, M., Kukukova, A., Machon, V. (2007) CFD modelling of liquid homogenizationin stirred tanks with one and two impellers using large eddy simulation. Chemical Engineering Research and Design. 85: 616–625.
Jia, X., Wen, J., Sun, Z., Caiyin, Q., Xie, S. (2006) Modeling of DBT biodegradation behaviors by resting cells of Gordonia sp WQ-01 and its mutant in oil–water dispersions. Chemical Engineering Science. 61: 1987–2000.
Jorjani, E., Chehreh Chelgani, S., Mesroghli, S. (2007) Prediction of microbial desulfurization of coal using artificial neural networks. Minerals Engineering. 20(14): 1285–1292.
Kao, P.-M., Chen, C.-I., Huang, S.-C., Chang, Y.-C., Tsay, P.-Y., Liu, Y.-C. (2007) Effects of shear stress and mass transfer on chitinase production by Paenibacillus sp. CHE-N1. Biochemical Engineering Journal. 34: 172–178.
Kareem, S.A. (2016) Anaerobic biodesulfurization of kerosene part II: investigating its kinetics. IOSR Journal of Biotechnology and Biochemistry. 2(5): 37–45.
Kareem, S.A., Susu, A.A., Aribike, D.S., Nwachukwu, S.C.U. (2013a) Modelling the biodesulfurization of diesel. International Journal of Engineering Research and Technology. 2(7): 1010–1015.
Kareem, S.A., Susu, A.A., Aribike, D.S., Nwachukwu, S.C.U. (2013b) Modelling the biodesulfurization of kerosene in a batch reactor. International Journal of Engineering Science Invention. 2(4): 61–66.
Kareem, S.A., Susu, A.A., Aribike, D.S., Nwachukwu, S.C.U. (2014) Investigating the kinetics of biodesulfurization of diesel. Assumption University : AU Journal of Technology. 18 (1): 27–35.
Kaufman, E.N., Borole, A.P., Shong, R., Sides, J.L., Juengst, C. (1999) Sulfur specificity in the bench-scale biological desulfurization of crude oil by Rhodococcus IGTS8. Journal of Chemical Technology and Biotechnology. 74: 1000–1004.
Kaufman, E.N., Hankins, J.B., Borole, A.P. (1998) Comparison of batch-stirred and electrospray reactors for biodesulfurization of dibenzothiophene in crude oil and hydrocarbon feedstocks. Applied Biochemistry and Biotechnology. 73: 127–143.
Kayser, Y., Bielagajones, B.A., Jackowski, K., Odusan, O., Kilbane, J.J. (1993) Utilization of organusulphur compounds by axenic and mixed cultures of Rhodococcus rhodochrous IGTS8. Journal of General Microbiology. 139: 3123–3129.
Kilbane, J.J. (1992) Mutant microorganisms useful for cleavage of organic C-S bonds. US patent 5,104,801.
Kilbane, J.J. (2006) Microbial biocatalyst development to upgrade fossil fuels. Current Opinion in Microbiology. 17: 305–314.
Kilbane, J.J., Jackowski, K. (1992) Biodesulfurization of water soluble coal derived material by Rhodococcus rhodochrous IGTS8. Biotechnology and Bioengineering. 40: 1107–1114.
Kim, Y.J., Chang, J.H., Cho, K.-S., Ryu, H.W., Chang, Y.K. (2004) A physiological study on growth and dibenzothiophene (DBT) desulfurization characteristics of Gordonia sp. CYKS1. Korean Journal of Chemical Engineering. 21(2): 436–441.
Kirkwood, K.M., Andersson, J.T., Fedorak, P.M., Foght, J.M., Gray, M.R. (2007): Sulfur from benzothiophene and alkyl benzothiophenes supports growth of Rhodococcus sp. strain J7H1. Biodegradation. 18: 541–549.
Kishimoto, M., Inui, M., Omasa, T., Katakura, Y., Suga, K., Okumura, K. (2000) Efficient production of desulfurizing cells with the aid of expert system. Biochemical Engineering Journal. 5: 143–147.
Knightes, C.D., Peters, C.A. (2006) Multi-Substrate biodegradation kinetics for binary and complex mixtures of polycyclic aromatic hydrocarbons. Environmental Toxicology and Chemistry. 25:1746–1756.
Kobayashi, M., Horiuchi, K., Yoshikama, O., Hirasawa, K., Ishii, Y., Fujino, K., Sugiyama, H., Maruhashi, K. (2001) Kinetic analysis of microbial desulfurization of model and light gas oils containing multiple alkyl dibenzothiophenes. Bioscience, Biotechnology, and Biochemistry. 65: 298–304.
Kobayashi, M., Onaka, T., Ishii, Y., Konishi, J., Takaki, M., Okada, H., Ohta, Y., Koizumi, K., Suzuki, M. (2000) Desulfurization of alkylated forms of both dibenzothiophene and benzothiophene by a single bacterial strain. FEMS Microbiology Letters. 187: 123–126.
Konishi, J., Ishii, Y., Osaka, T., Ohta, Y., Suzuki, M., Maruhashi, K. (2000) Purification and characterization of dibenzothiophene sulfone monooxygenase and FMN-dependent NADH oxidoreductase from the thermophilic bacterium Paenibacillus sp. strain Al l-2. Journal of Bioscience and Bioengineering. 90 (2000) 607–613.
Konishi, J., Ishii, Y., Osaka, T., Okumura, K., Suzuki, M. (1997) Thermophilic carbon-sulfur-bond-targeted biodesulfurization. Applied and Environmental Microbiology. 63(8): 3164–3169.
Kropp, K.G., Andersson, J.T., Fedorak, P.M. (1997) Bacterial transformations of three dimethyl dibenzothiophenes by pure and mixed bacterial cultures. Environmental Science and Technology. 31: 1547–1554.
Lapin, L.L. (1997) Modern engineering statistics. 1st ed. Duxbury Press, Belmon.
Layokun, S.K., Umoh, E.F., Solomon, B.O. (1987) A kinetic model for the degradation of dodecane by P. fluorescens isolated from the oil polluted area, Warri in Nigeria. Journal of Nigerian Society of Chemical Engineers. 16: 48 - 52.
Le Borgne, S., Quintero, R. (2003) Biotechnological processes for the refining of petroleum. Fuel Processing Technology. 81: 155–169.
Lei, B., Tu, S.-Ch. (1996) Gene over-expression, purification and identification of a desulfurization enzyme from Rhodococcus sp. Strain IGTS8 as a sulfide/sulfoxide monooxygenase. Journal of Bacteriology. 178: 5699–5705.
Li, F.L., Xu, P., Ma, C.Q., Luo, L.L., Wang, X.S. (2003) Deep desulfurization of hydrodesulfurization-treated diesel oil by a facultative thermophilic bacterium Mycobacterium sp. X7B, FEMS Microbiology Letters. 223: 301–307.
Li, M.Z., Squires, C.H., Monticello, D.J., Chids, J.D. (1996) Genetic analysis of the dsz promoter and associated regulatory regions of Rhodococcus erythropolis IGTS8. Journal of Bacteriology. 178(22): 6409–6418.
Li, W., Tang, H., Liu, Q., Xing, J., Li, Q., Wang, D., Yang, M., Li, X., Liu, H. (2009) Deep desulfurization of diesel by integrating adsorption and microbial method. Biochemical Engineering Journal. 44: 297–301.
Lichtinger, T., Reiss, G., Benz, R. (2000) Biochemical identification and biophysical characterization of a channel-forming protein from Rhodococcus erythropolis. Journal of Bacteriology. 182: 764–770.
Lineweaver, H., Burk, D. (1934) The Determination of Enzyme Dissociation Constants. Journal of American Chemical Society. 56: 658–666.
Liu, Y.S., Wu, J.Y., Ho. K.P. (2006) Characterization of oxygen transfer conditions and their effects on Phaffia rhodozyma growth and carotenoid production in shake-flask cultures. Biochemical Engineering Journal. 27: 331–335.
Lu, J., Nakajima-Kampe, T., Shigeno, T., Ohbo, A., Nomura, N., Nakahara, T. (1999) Biodegradation of dibenzothiophene and 4,6- dimethyl dibenzothiophene by Sphingomonas paucimobilis strain TZS-7. Bioscience and Bioengineering. 88(3): 293–299.
Lu, W.-M., Wu, H.-Z., Ju, M.-Y. (1997) Effects of baffle design on the liquid mixing in an aerated stirred tank with standard Rushton turbine impellers. Chemical Engineering Science. 21: 3843–3851.
Luedeking, R., Piret, E.L. (1959) A Kinetic study of the lactic acid fermentation batch process at controlled pH. Biotechnology and Bioengineering. 1: 393–412.
Luo, M.F., Xing, J.M., Gou, Z.X., Li, S., Liu, H.Z., Chen, J.Y. (2003) Desulfurization of dibenzothiophene by lyophilized of Pseudomonas delafieldii R-8 in the presence of dodecane. Biochemical Engineering Journal. 13: 1–6.
Ma, N., Mollet, M., Chalmers, J.J. (2006) Aeration, mixing and hydrodynamics in bioreactors, in: W.-S. Hu, S.S. Ozturk (Eds.), Cell Culture Technology for Pharmaceutical and Cell-based Therapies, CRC Press, Boca Raton (FL). Pp. 225–248.
Maass D., Mayer D.A., Moritz D.E., Oliveira D., de Souza A.A.U., Souza S.M.A.G. (2015) An Evaluation of Kinetic Models in the Biodesulfurization of Synthetic Oil by Rhodococcus erythropolis ATCC 4277. Applied Biochemistry and Biotechnology. 177(3): 759–770.
Maghsoudi, S., Vossoughi, M., Kheirolomoom, A., Tanaka, E., Katoh, S. (2001) Biodesulfurization of hydrocarbons and diesel fuels by Rhodococcus sp. strain P32C1. Biochemical Engineering Journal. 8: 151–156.
Marcelis, C.L.M., van Leeuwen, M., Polderman, H.G., Jannsen, A.J.H., Lettinga, G. (2003) Model description of dibenzothiophene mass transfer in oil/water dispersions with respect to biodesulfurization. Biochemical Engineering Journal. 16: 253–264.
Märkl, H., Bronnenmeier, R., Wittek, B. (1991) The resistance of microorganisms to hydrodynamic stress. International Chemical Engineering. 31: 185–197.
Marquardt, A., W. (1963) An algorithm for least-squares estimation of non-linear parameters. Journal of the Society for Industrial and Applied Mathematics. 11: 431–41.
Martin, A.B., Alcon, A., Santos, V.E., Garcia-Ochoa, F. (2004) Production of a biocatalyst of Pseudomonas putida CECT5279 for dibenzothiophene (DBT) biodesulfurization for different media compositions. Energy and Fuel. 18: 851–857.
Martin, A.B., Alcon, A., Santos, V.E., Garcia-Ochoa, F. (2005) Production of a Biocatalyst of Pseudomonas putida CECT5279 for DBT Biodesulfurization: Influence of the Operational Conditions. Energy & Fuels. 19(3): 775–782.
Martin, M., Montes, F.J., Galan, M.A. (2008) On the contribution of the scales of mixing to the oxygen transfer in stirred tanks. Chemical Engineering Journal. 145: 232–241.
Martinez, I., Santos, V.E., Alcon, A., Garcia-Ochoa, F. (2015) Enhancement of the biodesulfurization capacity of Pseudomonas putida CECT5279 by co-substrate addition. Process Biochemistry 50: 119–124.
Martinez, I., Santos, V.E., Garcia-Ochoa, F. (2017) Metabolic kinetic model for dibenzothiophene desulfurization through 4S pathway using intracellular compound concentrations. Biochemical Engineering Journal. 117: 89–96.
Martinez, I., Santos, V.E., Gomez, E., Garcia-Ochoa, F. (2016) Biodesulfurization of dibenzothiophene by resting cells of Pseudomonas putida CECT5279: influence of the oxygen transfer rate in the scale-up from shaken flask to stirred tank reactor. Journal of Chemical Technology and Biotechnology. 91: 184–189.
Marzona, M., Pessione, E., Di Martino, S., Giunta, C. (1997) Benzothiophene and dibenzothiophene as the sole sulfur source in Acinetobacter: growth kinetics and oxidation products. Fuel Processing Technology. 52: 199–205.
Matsui, T., Hirasawa, K., Konishi, J., Tanaka, Y., Maruhashi, K., Kurane, K. (2001) Microbial desulfurisation of alkylated dibenzothiophene and alkylated benzothiophene by recombinant Rhodococcus sp. Strain T09. Applied Microbiology and Biotechnology. 56: 196–200.
Maxwell, S., Yu, J., (2000) Selective desulphurisation of dibenzothiophene by a soil bacterium: microbial DBT desulphurisation. Process Biochemistry. 35: 551–556.
McFarland, B.L. (1999) Biodesulfurization. Current Opinion in Microbiology. 2,257–264.
Meijer, J.J., ten Hoopen, H.J.G., van Gameren Y.M., Luyben, K.Ch.A.M., Libbenga, K.R. (1994) Effects of hydrodynamic stress on the growth of plant cells in batch and continuous culture. Enzyme and Microbial Technology. 16: 467–477.
Michaelis, L., Menten, M.L. (1913) Die Kinetik der Invertinwirkung. Biochemische Zeitschrift 49:333–369.
Michel, B.J. and Miller, S.A. (1962) Power requirements of gas–liquid agitated systems, AIChE Journal. 8: 262–267.
Mingfang, L., Jianmin, X., Zhongxuan, G., Huizhou, L., Jiayong, C. (2003b) Microbial desulfurization of dibenzothiophene and 4,6-dimethyldibenzothiophene in dodecane and straight-run diesel oil. Korean Journal of Chemical Engineering. 20(4): 702–704.
Mingfang, L., Zhongxuan, G., Jianmin, X., Huizhou, L., Jiayong, C. (2003a) Microbial desulfurization of model and straight-run diesel oils. Journal of Chemical Technology and Biotechnology. 78: 873–876.
Mohebali, G., Ball A.S. (2008) Biocatalytic desulfurization (BDS) of petrodiesel fuels. Microbiology. 154: 2169–2183.
Mohebali, G., Ball, A.S., Rasekh, B., Kaytash, A. (2007) Biodesulfurization potential of a newly isolated bacterium, Gordonia alkanivorans RIPI90A. Enzyme and Microbial Technology. 40: 578–584.
Monod, J. (1949) The growth of bacterial cultures. Annual Review of Microbiology. 3:371–94.
Monticello, D.J. (2000) Biodesulfurization and the upgrading of petroleum distillates. Current Opinion in Microbiology. 11: 540–546.
Monticello, D.J., Finnerty, W.R. (1985) Microbial desulfurization of fossil fuels. Annual Review of Microbiology. 39. 371–389.
Mukhopadhyaya, M., Chowdhury, R., Bhattacharya, P. (2006) Biodesulfurization of hydrodesulfurized diesel in a trickle bed reactor - Experiments and modeling. Journal of Scientific and Industrial Research. 65(5): 432–436.
Nadalig, T., Raymond, N., Nimatuzahroh, M., Budzinsk, H., Bertrand, J.C. (2002) Degradation of phenanthrene, methylphenanthrenes and dibenzothiophene by a Sphingomonas strain 2mpII. Applied Microbiology and Biotechnology. 59(1): 79–85.
Naito, M., Kawamoto, T., Fujino, K., Kobayashi, M., Maruhashi, K., Tanaka, A. (2001) Long-term repeated desulfurization by immobilized Rhodococcus erythropolis KA2–5-1 cells. Applied Microbiology and Biotechnology. 55: 374–378.
Nakayama, N., Matsubara, T., Ohshiro, T., Moroto, Y., Kawata, Y., Koizumi, K., Hirakawa, Y., Suzuki, M., Maruhashi, K., Izumi, Y., Kurane, R. (2002) A novel enzyme, 2-hydroxybiphenyl-2-sulfinate desulfinase (DszB), from a dibenzothiphene-desulfurizing bacterium Rhodococcus erythropolis KA2–5-1: gene over-expression and enzyme characterization. Biochimica et Biophysica Acta (BBA)1598(1/2): 122–130.
Nasab, N.A., Kumleh, H.H., Kazemzad, M., Panjeh, F.G., Davoodi-Dehaghani, F. (2015) Improvement of Desulfurization Performance of Rhodococcus erythropolis IGTS8 by Assembling Spherical Mesoporous Silica Nanosorbents on the Surface of the Bacterial Cells. Journal of Applied Chemical Research. 9(2): 81–91.
Nassar, H.N., Deriase, S.F., El-Gendy, N.Sh. (2016) Modeling the relationship between microbial biomass and total viable count for a new bacterial isolate used in biodesulfurization of petroleum and its fractions. Petroleum Science and Technology. 34(11–12): 980–985.
Nassar, H.N., Deriase, S.F., El-Gendy, N.Sh. (2017) Kinetic modeling and simulation of a batch biodesulfurization process by Rhodococcus erythropolis HN2. Petroleum Science and Technology. 35(20): 1995–2002.
Nazari, F., Kefayati, M.E., Raheb, J. (2017) The study of biological technologies for the removal of sulfur compounds. Journal of Sciences, Islamic Republic of Iran. 28(3): 205–219.
Nienow, A.W. (1997) On impeller circulation and mixing effectiveness in the turbulent flow regime. Chemical Engineering Science. 15(52): 2557–2565.
Nienow, A.W. (2010) Scale-up, stirred tank reactors, in: M.C. Flickinger (Ed.), Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology, John Wiley & Sons.
Noda, K., Watanabe, K., Maruhashi, K. (2003) Isolation of the Pseudomonas aeruginosa gene affecting uptake of dibenzothiophene in n-tetradecane. Journal of Bioscience and Bioengineering. 95(5): 504–511.
Nuhoglu, N., Yalcin, B. (2005) Modeling of phenol removal in a batch reactor. Process Biochemistry. 40: 1233–1239.
Ohshiro, T., Hine, Y., Izumi, Y. (1994) Enzymatic desulfurization of dibenzothiophene by a cell-free system of Rhodococcus erythropolis D-1. FEMS Microbiology Letters. 118: 341–344.
Ohshiro, T., Hirata, T., Izumi, Y. (1995a) Microbial desulfurization of dibenzothiophene in the presence of hydrocarbon. Applied Microbiology and Biotechnology. 44: 249–252.
Ohshiro, T., Izumi, Y. (1999) Review microbial desulfurization of organic compounds in petroleum. Bioscience, Biotechnology and Biochemistry. 63: 1–9.
Ohshiro, T., Kobayashi, Y., Hine, Y., Izumi, Y. (1995b) Involvement of flavin coenzyme in dibenzothiophene degrading enzyme system from Rhodococcus erythropolis D-1. Bioscience, Biotechnology and Biochemistry. 59(7): 1349–1351.
Ohshiro, T., Suzuki, K., Izumi, Y. (1996) Regulation of dibenzothiophene degrading enzyme activity of Rhodococcus erythropolis D-1. Journal Fermentation and Bioengineering. 81: 121–124.
Ohshiro, T., Suzuki, K., Izumi, Y. (1997) Dibenzothiophene (DBT) degrading enzyme responsible for the first step of DBT desulfurization by Rhodococcus erythropolis D-1: purification and characterization. Journal of Fermentation and Bioengineering. 83(2): 233–237.
Ohshiro, T., Suzuki, K., Izumi, Y. (1998) Regulation of dibenzothiophene degrading enzyme activity of Rhodococcus erythropolis D-1. Journal of Fermentation and Bioengineering. 81: 121–124.
Okpokwasili G.C., Nweke, C.O. (2005) Microbial growth and substrate utilization kinetics. African Journal of Biotechnology. 5(4): 305–317.
Oldfield, C., Pogrebinsky, O., Simmonds, J., Olson, E.S., Kulpa, C.H. (1997) Elucidation of the metabolic pathway for dibenzothiophene desulfurization by Rhodococcus sp. strain IGTS8 (ATCC 53968). Microbiology. 143: 2961–2973.
Olmos, E., Mehmood, N., Haj-Husein, L., Goergen, J.L., Fick, M., Delaunay, S. (2013) Effects of bioreactor hydrodynamics on the physiology of Streptomyces. Bioprocess and Biosystems Engineering. 36:259–272.
Omori, T., Saiki, Y., Kasuga, K., Kodama, T. (1995) Desulfurization of alkyl and aromatic sulfides and sulfonates by dibenzothiophene desulfurizing Rhodococcussp. strain SY1. Bioscience, Biotechnology and Biochemistry. 59(7): 1195–1198.
Onaka, T., Kobayashi, M., Ishii, Y., Okumura, K., Suzuk,i M. (2000) Application of solid-phase extraction to the analysis of the isomers generated in biodesulfurization against methylated dibenzothiophenes. Journal of Chromatography A 903: 193–202.
Patel, S.B., Kilbane, J.J., Webster, D.A. (1997) Biodesulphurisation of dibenzothiophene in hydrophobic media by Rhodococcus erythropolis sp. strain IGTS8. Journal of Chemical Technology and Biotechnology. 69: 100–106.
Peng, B., Zhou, Z. (2016) Study on growth characteristic and microbial desulfurization activity of the bacterial stain MP12. Biochemical Engineering Journal. 112: 202–207.
Pirt, S.J. (1965) The maintenance energy of bacteria in growing cultures. Proceedings of the Royal Society of London. Series B. 163: 224–231.
Prokop, A., Bajpai, R.K. (1992) The sensitivity of biocatalysts to hydrodynamic shear stress. Advances in Applied Microbiology. 37: 165–232.
Ramirez-Corredores, M.M., Borole, A.P. (2007) Biocatalysis in Oil Refining (Studies in Surface Science and Catalysis). Elsevier Science; 1st edition (September 22, 2011).
Rashtchi, M., Mohebali, G.H., Akbarnejad, M.M., Towfighi, J., Rasekh, B., Keytash, A. (2006) Analysis of biodesulfurization of model oil system by the bacterium, strain RIPI-22. Biochemical Engineering Journal, 29(3): 169–173.
Ratkowsky, D.A., Lowry, R.K., Stokes, T.A., Chandler, R.E. (1983) Model for bacterial culture growth rate throughout the entire bio kinetic temperature range. Journal of Bacteriology. 154: 1222–1226.
Russell, J.B., Cook, G.M. (1995) Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiological Reviews. 59: 48–62.
Sahoo, S., Verma R.K., Suresh, A.K., Rao, K.K., Bellare, J., Suraishkumar, G.K. (2003) Macro-level and genetic-level responses of bacillus subtilis to shear stress. Biotechnology Progress. 19: 1689–1696.
Santos, V.E., Alcon, A., Martin, A.B., Gomez, E., Garcia-Ochoa, F. (2007) Desulfurization of dibenzothiophene using the 4S enzymatic route: influence of operational conditions on initial reaction rates. Biocatalysis and Biotransformation. 25: 286–294.
Schilling, B.M., Alvarez, L.M., Wang, D.I.C., Cooney, C.L. (2002) Continuous desulfurization of dibenzothiophene with R. rhodochrous IGTS8 (ATCC 53968). Biotechnology Progress. 18: 1207–1213.
Segel, I.H. (1975) Enzyme kinetics. Wiley, New York.
Setti, L., Bonoli, S., Badiali, E., Giuliani, S. (2003) Inverse phase transfer biocatalysis for a biodesulfurization process of middle distillates. Вестник Московского университета. Серия 2. 44(1): 80–83.
Setti, L., Farinelli, P., Di Martino, S., Frassinetti, S., Lanzarini, G., Pifferi, P.G. (1999) Developments in destructive and non-destrucitve pathways for selective desulfurizations in oil biorefining processes. Applied Microbiology and Biotechnology. 52: 111–117.
Setti, L., Lanzarini, G., Pifferi, P.G. (1994) Diffusion as a rate controlling step in heavy oil biodesulfurization processes. Fuel Processing Technology. 40, 311–317.
Setti, L., Lanzarini, G., Pifferi, P.G. (1995) Dibenzothiophene biodegradation by a Pseudomonas sp. in model solutions. Process Biochemistry. 30(8): 721–728.
Setti, L., Lanzarini, G., Pifferi, P.G. (1996) Immobilized cells for applications in non-conventional systems. Progress in Biotechnology. 11: 777–784.
Setti, L., Lanzarini, G., Pifferi, P.G. (1997) Whole cell biocatalysis for an oil desulfurization process. Fuel Processing Technology. 52: 145–153.
Shan, G., Xing, J., Zhang, H., Liu, H. (2005) Biodesulphurization of dibenzothiophene by microbial cells coated with magnetite nanoparticles. Applied and Environmental Microbiology. 71: 4497–4502.
Shuler, M.L., Kargi, F. (2002) Bioprocess Engineering: Basic concepts. Second Edition. Prentice Hall, Inc. Pp. 268–273.
Soneyink, V.L., Lenkins, D. (1980) Water Chemistry. John Wiley and Sons, New York, USA.
Sutcliffe, I.C., Brown, A.K., Dover, L.G. (2010) The rhodococcal cell envelope: composition, organization and biosynthesis. Biology of Rhodococcus. Microbiology monographs 16. Springer-Verlag, Berlin, Germany.
Tao, F., Yu, B., Xu, P., Ma, C. (2006) Biodesulfurization in biphasic systems containing organic solvents. Applied and Environmental Microbiology. 72: 4604–4609.
Tsai, S.P., Lee, Y.H. (1990) A model for energy-sufficient culture growth. Biotechnology and Bioengineering. 35: 138–145.
Van Bodegom P (2007) Microbial maintenance: a critical review on its quantification. Microbial Ecology. 53: 513–523.
van Hamme, J.D., Fedorak, P.M., Foght, J.M., Gray, M.R., Dettman, H.D. (2004) Use of novel fluorinated organosulfur compound to isolate bacteria capable of carbon-sulfur bond cleavage. Applied and Environmental Microbiology. 70: 1487–1493
Van’t Riet, K. (1979) Review of measuring methods and nonviscous gas–liquid mass transfer in stirred vessels. Industrial and Engineering Chemistry Process Design and Devlopment. 18: 357–364.
Verhulst, P.F. (1845) Recherches math mematiques sur la loi d’accroissement de la population. Nouv. Mem. Acad. R. Sci. B.-lett. Brux.18: 1–45. gdz.sub.uni-goettingen.de
Verhulst, P.F. (1847) Deuxieme memoire sur la loi d’accroissement de la population. Mem. Acad. R. Sci. Lett. B.-Arts Belg. 20: 142–173. www.digizeit-schriften.de
Wang, J., Butler, R.R., Wu, F., Pombert, J.F., Kilbane, J.J., Stark B.C. (2017) Enhancement of microbial biodesulfurization via genetic engineering and adaptive evolution. PLoS ONE. 12(1): 1–20.
Wang, P., Humphrey, A.E., Krawiec, S. (1996) Kinetic analysis of desulfurization of dibenzothiophene by Rhodococcus erythropolis in continuous cultures. Applied and Environmental Microbiology. 62: 3066–3068
Wang, P., Krawiec, S. (1996) Kinetic analyses of desulfurization of dibenzothiophene by Rhodococcus erythropolis in batch and fed-batch cultures. Applied and Environmental Microbiology. 61: 1670–1675.
Watkins, L.M., Rodriguez, R., Schneider, D., Broderick, R., Cruz, M., Chambers, R., Ruckman, E., Cody, M., Mrachko, G.T. (2003) Purification and characterization of the aromatic desulfinase 2-(2′-hydroxyphenyl) benzenesulfinate desulfinase. Archives of Biochemistry and Biophysics. 415:14–23.
Wodzinski, R.S., Coyle, J.E. (1974) Physical state of phenanthrene for utilization by bacteria. Applied Microbiology. 1091–1094.
Worthington Biochemical Corporation (2017) Introduction to Enzymes http://www.worthington-biochem.com/introbiochem/Enzymes.pdf
Xu X., Cai Y., Song Z., Qiu X., Zhou J., Liu Y., Mu T., Wu D.,Guan Y., Xing, J. (2015). Desulfurization of immobilized sulfur-oxidizing bacteria, Thialkalivibrio versutus, by magnetic nanaoparticles under haloalkaliphilic conditions. Biotechnology Letters. 37(8): 1631–1635.
Xu, P., Yu, B., Li, F.L., Cai, X.F., Ma, C.Q. (2006) Microbial degradation of sulfur, nitrogen and sulfur heterocycles. Trends in Microbiology. 14: 398–405.
Xu, X., Cai, Y., Song, Z., Qiu, X., Zhou, J., Liu, Y., Mu, T., Wu, D., Guan, Y., Xing, J. (2015) Desulfurization of immobilized sulfur-oxidizing bacteria, Thialkalivibrio versutus, by magnetic nanaoparticles under haloalkaliphilic conditions. Biotechnology Letters. 37(8): 1631–1635.
Yan, H., Kishimoto, M., Omasa, T., Katakura, Y., Suga, K., Okumura, K., Yoshikawa, O. (2000) Increase in desulfurization activity of Rhodococcus erythropolis KA2–5-1 using ethanol feeding. Journal of Bioscience and Bioengineering. 89(4): 361–366.
Yang, J., Hu, Y., Zhao, D., Wang, S., Lau, P.C.K., Marison, I.W. (2007) Two-layer continuous-process design for the biodesulfurization of diesel oils under bacterial growth conditions. Biochemical Engineering Journal. 37: 212–218.
Yang, J., Marison I. (2005) Two-stage process design for the biodesulphurisation of a model diesel by a newly isolated Rhodococcus globerulus DAQ3. Biochemical Engineering Journal. 27: 77–82.
Yano, T., Koga, S. (1969) Dynamic behavior of the chemostat subject to substrate inhibition. Biotechnology and Bioengineering journal. 11: 139 - 153.
Yepez, B.O., Maugeri, F. (2005) Agitation, aeration and shear stress as key factors in inulinase production by Kluyveromyces marxianus. Enzyme and Microbial Technology. 36: 717–724.
Yu, B., Ma, C., Zhou, W., Wang, Y., Cai, X., Tao, F., Zhang, Q., Tong, M., Qu, J., Xu, P. (2006a) Microbial desulfurization of gasoline by free whole-cells of Rhodococcus erythropolis XP. FEMS Microbiology Letters. 258: 284–289.
Yu, B., Xu, P., Shi, Q., Ma, C. (2006b) Deep desulfurization of diesel oil and crude oils by a newly isolated Rhodococcus erythropolis strain. Applied and Environmental Microbiology. 72: 54–58.
Zakharyants, A.A., Murygina, P., Kalyuzhnyi, S. (2004) Screening of Rhodococcus species revealing desulfurization activity with regard to dibenzothiophene. In: Biocatalytic technology and nanotechnology. G E. Zaikov. Nova Science Publishers, Inc. Pp. 51–58.
Zeelani, G.G., Ashrafi, A., Dhakad, A., Gupta, G., Pal, S.L. (2016) A Review on Desulfurization Techniques of Liquid Fuels. International Journal of Science and Research (IJSR). 5(5): 331–336.
Zhang, S.H., Chen, H., Li, W. (2013) Kinetic analysis of biodesulfurization of model oil containing multiple alkyl dibenzothiophenes. Applied Microbiology and Biotechnology. 97(5): 2193–2200.


























