Chapter 7
Towards Direct Electrochemical Control of Gene Expression Level
Abstract
This chapter introduces the design of a strategy towards the implementation of direct electrochemical control of the gene expression level in the paradigmatic case of photosynthetic bacteria, as seen in the previous chapter. The chosen approach will be to dissect the problem in its key steps, identifying a path to the achievement of the stated goals consisting of possible operative solutions to each single problem. As will be clearly evident, a number of solutions are indeed already at hand deriving from diverse scientific and technological contexts. The aim of this chapter is to gather them in a logical sequence in order to make plausible the technological control over such a complex and critical biological phenomenon.
Keywords
redox mediators; liposoluble redox shuttles; giant unilamellar vesicles; large unilamellar vesicles; photosynthetic bacteria; gyrases; thioredoxins7.0. Direct electrochemical control of gene expression level: where to start from?
In the previous chapter we have considered a number of cases, involving different bacteria and subcellular organelles in eukaryotes, of redox-dependent modulation of gene expression pathways. Albeit the general picture of the modulation of gene expression is very complex and mostly not fully elucidated yet, the case of photosynthetic bacteria appears sufficiently clear to select it as a possible candidate for technological exploitation. We are, indeed, interested in developing approaches to the modulation of gene expression by an external stimulus, in order to gain high-level control of the phenomenon. The ultimate goal of this activity deals, of course, with the possibility of making a biosystem amenable to our needs, following a track resembling the one that has led us to exploit bacteria, and cells in general, as protein factories for biotechnological purposes. Approaches that tend toward a similar goal are, for example, those developed by optogenetics, which is a neuromodulation technique employed in neuroscience; it uses a combination of techniques from optics and genetics to control the activity of individual neurons in living tissue – even within freely-moving animals – and to precisely measure the effects of those manipulations in real time (Deisseroth et al., 2006).
The interest in redox-regulated gene expression stems from the notions and the expertise we have gained in all the previous chapters, where we have learnt how to control a working electrode potential, to drive the redox state of immobilized and freely diffusing molecules, etc.
Now we are in a position to try to design a possible electrical approach to the control of gene expression, but, to achieve such an ambitious goal, we first have to select a suitable biological system. A plausible choice appears to be to focus on photosynthetic bacteria of the genus Rhodobacter. The reasons for this choice are connected with a number of interesting and suitable features that one can find in these bacteria, apart from the prerequisite of featuring redox-dependent gene expression molecular machinery. Indeed, as we have seen in section 6.2, variation in oxygen tension is responsible for modulating the oxidative state of the inner bacterial compartment that activates the change of metabolism in the microorganism and makes it a facultative photosynthetic bacterium.
The ability that these bacteria have to switch from photosynthesis to respiration is at the core of a straightforward way to monitor the products of their genes. Indeed, the photosynthetic state (low oxygen tension) tends to enhance expression of those genes that encode for proteins involved in light harvesting and in light-induced charge separation (i.e., LHI, LHII, reaction centers), along with the connected pigments, whereas high oxygen tension tends to depress their expression. Therefore, both R. sphaeroides and R. capsulatus (see section 6.2) are convenient objects to monitor in their metabolic variation, simply by following the variation of the optical absorption bands related to their pigments (bacteriochlorophyll monomers and dimers, bacteriopheophytins) brought about by the proteins of photosynthesis.
Other positive aspects of the genus at issue are its robustness and relative ease of growth, which make them good candidates for repeated experimentation in non-conventional environments.
Affecting the inner redox state of a bacterium by direct electrochemistry implies a “hot-wiring” of the bacterial cytoplasm with an external voltage source, e.g., a potentiostat. A direct physical connection is, however, difficult to implement and doable only one cell at a time; as such it appears of limited technological interest.
We seek, indeed, an approach suitable to affect, contemporarily, the inner redox state of a large bacterial population. A plausible possibility to achieve this goal is to use redox molecular relays or mediators whose oxidation state can be conditioned by the interaction with an electrode in the culture medium, whose potential is driven by a potentiostat. These mediators have to be able to freely diffuse across the bacterial membrane, penetrating the cytoplasm and affecting its redox state. By doing so, they play the role of a metal wire piercing the bacterial membrane and directly affecting the redox state inside the cell.
The role of these mediators has to be that of mimicking the effect of oxygen tension variations in modulating gene expression. Therefore, as we have seen in section 6.2, they have to be able to affect the redox state of thioredoxins that in turn differentially bind gyrases, resulting in a different supercoiling of the bacterial chromosome, hence in a modulation of the transcription of the photosynthesis genes.
The overall project appears quite ambitious and its various steps are best faced if we try to dissect the process into its basic constituents. For each step or phase, a specific approach will be sought and solutions will be suggested often using a crucial interplay between top-down and bottom-up approaches. In the next sections we will analyze in detail the principal main phases of a process whose completion can lead to implementing the sought electrical control.
7.1. The choice of redox mediators
Which are the features that a redox mediator should have in order to accomplish the task of affecting the redox state of thioredoxins in the desired way? There are, indeed, several aspects to take into account in order to come up with a satisfying choice.
Low-molecular-weight redox species can assist the shuttling of electrons between the intracellular bacterial space and an electrode. However, there are many important requirements that such a mediator should satisfy in order to provide efficient electron transport from/to the bacterial thioredoxins to an external electrode: (a) The mediator should easily penetrate through the bacterial membrane to reach the thioredoxins inside the bacteria. (b) The redox-potential of the mediator should fit the potential of the thioredoxins (e.g., the mediator potential should be positive enough to provide fast electron transfer from the enzymes, but it should not be too positive as to prevent significant loss of potential). (c) Neither oxidation state of the mediator should interfere with other metabolic processes (should not inhibit them or be decomposed by them). (d) The mediator should easily escape from the cell through the bacterial membrane. (e) The oxidation states of the mediator should be chemically stable in the electrolyte solution, they should be well soluble, and they should not adsorb onto the bacterial cells or electrode surface. (f) The electrochemical kinetics of the redox process of the mediator at the electrode should be fast (electrochemically reversible) (Wilkinson, 2000).
Luckily enough, one can take advantage of the extended screening activity on these kinds of redox mediators, conducted in studies dedicated to design and implementation of bacterial fuel cells (Katz et al., 2003).
Many different organic and organometallic compounds have been tested in combination with bacteria to evaluate the efficiency of mediated electron transport from the internal bacterial metabolites to the anode of a biofuel cell. Thionine has been used extensively as a mediator of the electron transport from Proteus vulgaris (Bennetto et al., 1985; Thurston et al., 1985; Delaney et al., 1984; Kim et al., 2000) and from E. coli (Roller et al., 1984; Bennetto et al., 1983). Other organic dyes that have been used include benzylviologen, 2,6-dichlorophenolindophenol, 2-hydroxy-1,4-naphthoquinone, phenazines (phenazine ethosulfate, safranine), phenothiazines (alizarine brilliant blue, N,N-dimethyl disulfonated thionine, methylene blue, phenothiazine, toluidine blue) and phenoxazines (brilliant cresyl blue, gallocyanine, resorufin) (Delaney et al., 1984; Roller et al., 1984; Bennetto et al., 1983; Park & Zeikus, 2000; Ardeleanu et al., 1983; Davis & Yarbrough, 1962; Patchett et al., 1988; Kreysa et al., 1990). These organic dyes were tested with Alcaligenes eutrophus, Anacystis nidulans, Azotobacter chroococcum, Bacillus subtilis, Clostridium butyricum, E. coli, Proteus vulgaris, Pseudomonas aeruginosa, Pseudomonas putida and Staphylococcus aureus bacteria, usually using glucose and succinate as substrates. Among the evaluated dyes, phenoxazine, phenothiazine, phenazine, indophenol, bipyridilium derivatives, thionine and 2-hydroxy-1,4-naphthoquinone were found to be very efficient in maintaining relatively high cell voltage output when current was drawn from the biofuel cell (Delaney et al. 1984). Some other dyes do not function as effective mediators because they are not rapidly reduced by the microorganisms, or they lack sufficient negative potential. Ferric chelate complexes (e.g., Fe(III)EDTA) were successfully used with Lactobacillus plantarum, Streptococcus lactis and Erwinia dissolvens, oxidizing glucose (Vega & Fernández, 1987).
Since thionine has frequently been used as a mediator in microbial fuel cells, mono- and disulfonated derivatives of thionine have been applied to determine the effect of hydrophilic substituents on mediation of electron transfer from E. coli to an anode (Lithgow et al., 1986). Changing from thionine to 2-sulfonated thionine and 2,6-disulfonated thionine results in an increased efficiency of mediated electron transport. The low efficiencies of the biofuel cells operating with thionine and 2-sulfonated thionine were attributed to interference with electron transfer by adsorption of the mediator on the microbial membrane. It should be noted that the overall efficiency of the electron-transfer mediators depends also on many other parameters, and in particular on the electrochemical rate constant of mediator re-oxidation, which depend, in turn, on the electrode material.
Since an electron-transfer mediator needs to meet many requirements, some of which are mutually exclusive, it is not possible to reach perfect conditions for electron transport from a bacterial cell to an electrode. A mixture of two mediators can be useful in optimizing efficiency. A solution containing thionine and Fe(III)EDTA was applied to mediate electron transport from E. coli, oxidizing glucose as a primary substrate to an anode (Tanaka et al., 1983). Although both mediators can be reduced by E. coli, thionine is reduced over 100 times faster than Fe(III)EDTA. The electrochemical oxidation of the reduced thionine is much slower than oxidation of Fe(II)EDTA, however. Therefore, electrons obtained from the oxidation of glucose in the presence of E. coli are transferred mainly to thionine under the operational conditions of the cell. The reduced thionine is rapidly re-oxidized by Fe(III)EDTA, the rate of which has been shown to be very fast, ket = 4.8×104 M−1s−1. Finally, the reduced chelate complex, Fe(II)EDTA, transfers electrons to the anode by the electrode reaction of a Fe(III)EDTA/Fe(II)EDTA couple with a sufficiently large rate constant. One more example of enhanced electron transport in the presence of a mixture of mediators was shown for Bacillus strains oxidizing glucose as a primary substrate. The biofuel cell was operated in the presence of methylviologen (MV2+) and 2-hydroxy-1,4-naphthoquinone or Fe(III)EDTA (Akiba et al., 1985). Methylviologen can efficiently accept electrons from the bacterial cells, but its reduced state (MV•+) is highly toxic for the bacteria and immediately inhibits the fermentation process. In the presence of a secondary mediator that has a more positive potential, MV•+ is efficiently re-oxidized to MV2+. The reduced secondary mediator (quinone or Fe(II)EDTA) then transports the electrons to the anode.
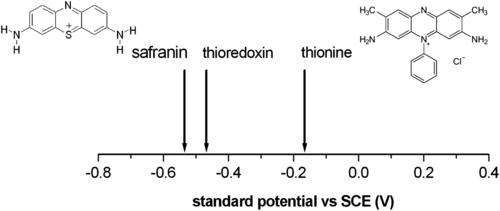
In Figure 7.1 the relative typical redox potentials of thioredoxin (−0.47 V vs SCE) (Watson et al., 2003) along with those of safranin (−0.53 mV) and thionine (0.18 mV) are reported with the structure of both redox mediators. These two dyes appear to be suitable for affecting the redox state of thioredoxin. Particularly, whereas safranin can reduce the enzyme, thionine can oxidize it. Therefore, these two molecules seem to be a suitable choice for controlling the redox state of the disulfides of thioredoxins. We know, from section 6.2, that the redox state of thioredoxin’s S-S bridges determines whether the molecule is able to bind gyrase, determining eventually the fate of the gene expression in Rhodobacter.
7.2. How to go further?
After having selected a suitable pair of redox mediators that could in principle accomplish the task of affecting the redox state of thioredoxin’s disulfides in the desired way, one has to face the question of how to proceed in tackling the overall problem. Indeed, the variables to be controlled are so numerous that it is difficult to proceed immediately towards a real biological system if one aims at understanding what is going on. It is of course possible to try to evaluate whether a serendipitous approach can provide any positive results, but a detailed understanding and control of the various steps that can lead to gene expression control definitely require the implementation of a systematic study.
Such an all-or-none attempt has indeed been done using Rhodobacter sphaeroides. The experiment compared the absorption spectra of two different bacterial suspensions in the bacteriochlorophyll spectral range. One of these was left to grow for 24 h in normal aerobic conditions, whereas the second was incubated with 1 mM thionine. The flask also contained three electrodes, a Au wire as WE, a reference (SCE) and a counter (Pt). The working electrode was kept at a potential of 0 V for 24 h. Such a potential helped maintain thionine in an oxidized state. Oxidized thionine, once diffusing inside bacteria, could reduce the disulfides of thioredoxins, providing an increase in the supercoiling activity of gyrases on the bacterial chromosome that should cause a decrease in puc and puf operon transcription (see section 6.2), hence a reduced amount of bacteriochlorophyll present in the bacteria. After 24 h, the amount of bacteria in the two flasks was evaluated by optical absorption spectroscopy; subsequently, bacterial concentration was adjusted to a value suitable for absorption spectroscopy in the bacteriochlorophyll spectral range (700–900 nm) and the corresponding spectra were acquired in a comparative fashion. Figure 7.2 shows the spectra obtained, which show a decrease in the normalized bacteriochlorophyll absorption of the sample under potentiostatic control, whereas the control, without the redox mediator, does not show any appreciable difference due to the application of a potential.
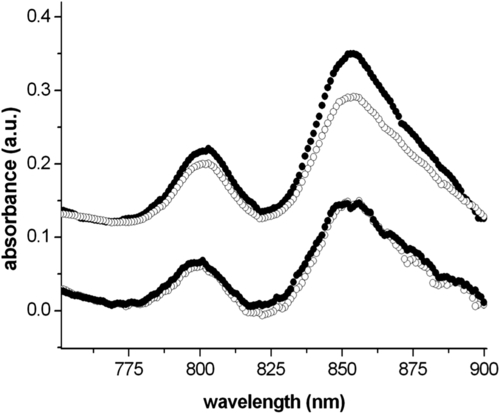
These data are just preliminary and, as such, they do not allow one to draw any robust conclusions on the validity of the approach used. Of course, the questions still to be answered are numerous and even the positive identification of the mechanisms at play can be questioned (e.g., whether the redox mediator operates according to the described mechanism, see section 6.2, or whether it directly affects the bacteriochlorophyll oxidation state).
We believe that to go any further in the mechanistic understanding of the phenomena taking place during redox control of the cytoplasmic space by direct electrochemistry, one needs a more systematic approach probably involving the dissection of the problem into its main constituent parts. At any rate, these preliminary results appear to be promising and deserve further attention.
It is now useful to think of possible ways to analyze the process by subdividing it into a number of steps that allow one to achieve full control of the phenomenon. One can think that the first step to overcome is to demonstrate that the proposed approach is really able to control the oxidation state of the inner space of a model system mimicking a cell envelope. It is conceivable to have an artificial lipid envelope filled with a suitable redox molecule whose oxidation state could be affected by the use of the selected redox mediator. A possibility could be to fill the envelope with potassium ferricyanide, which possesses a redox potential ≈ 436 mV at pH 7, and which could be easily reduced to ferrocyanide by thionine or safranin according to: [Fe(CN)6]4− ⇌ [Fe(CN)6]3− + e−. This reaction can be followed spectroscopically (at 420 nm, molar extinction coefficient = 1040 M−1cm−1) or optically (under a microscope and with suitable concentrations, the reduction reaction makes the dye change its color from brown to pale yellow). Furthermore both ferro- and ferricyanide are known to be impermeable through the plasma membrane, thus ensuring their confinement inside the envelope.
Quite natural candidates for this kind of test appear to be liposomes. They are one of the several possible self-assembling structures that amphiphilic molecules such as phospholipids can form when dispersed in an aqueous solution. For a comprehensive exposition of these and other self-assembling structures the reader can refer to any of the numerous reviews and books present in the literature (for a quantitative description see Israelachvili, 2005). These aggregates can be formed in various sizes in the range from a few nm to several tens of micrometers. According to their typical size, they are called SUVs (for Small Unilamellar Vesicles, from a few to tens of nanometers), LUVs (for Large Unilamellar Vesicles, from tens to hundreds of nanometers), or GUVs (for Giant Unilamellar Vesicles, in the micron to tens of microns range). They consist of a single phospholipid bilayer that, for thermodynamic reasons connected to the minimization of the free energy associated with the unsaturated boundaries, bends and forms a closed envelope that mimics a biological membrane. Considering that it is possible to assemble liposomes from lipid mixtures that are present in bacterial membranes (e.g., PE:PG in 3:1 molar ratio is found in the inner E. coli membrane), these structures appear to be suitable candidates for mimicking their behavior with regard to the main aspect of our interest, which is the possibility of affecting their inner redox state by redox mediators.
7.2.1. LUVs and GUVs
The relative ease of preparation of self-assembling phospholipid structures along with the existence of suitable experimental methods to probe phenomena taking place in their interior make LUVs and GUVs systems of choice for implementing the early steps towards a mechanistic understanding of the phenomena behind the direct electrochemical control of the redox environment inside cells.
LUVs and GUVs have a fundamental difference in that the former are amenable to optical spectroscopic, bulk investigation (e.g., UV-Vis optical absorption spectroscopy), whereas the latter, given their quite large size, can be studied and observed under an optical microscope (e.g., fluorescence microscopy). Therefore we propose both of these systems as suitable bench tests for tracking changes in the oxidation state of their interior.
Preparations of liposomes of different sizes share a number of common steps. When dealing with liposomes of mixed lipid composition, the lipids must first be dissolved and mixed in an organic solvent to ensure a homogeneous mixture is achieved. Usually this process is carried out using chloroform or CHCl3/MetOH mixtures (according to lipid solubility). One has to obtain a clear lipid solution for complete mixing of the lipids. Typically, lipid solutions are prepared at 10–20 mg lipid/ml organic solvent. Once the lipids are thoroughly mixed in the organic solvent, the solvent is removed to yield a lipid film. In the case of small solvent volumes (< 1 ml), the solvent can be evaporated using a dry nitrogen or argon stream in a fume hood. For larger volumes, the organic solvent should be removed by rotary evaporation, yielding a thin lipid film on the sides of a round-bottom flask. The lipid film is thoroughly dried to remove residual organic solvent by placing the vial under vacuum for a variable time (usually several hours). Dry lipid films can then be stored frozen until ready to hydrate.
Hydration of dry lipid films can be achieved by simply adding an aqueous medium to the container of dry lipids and agitating. The temperature of the hydrating medium should be above the gel–liquid crystal transition temperature (Tc or Tm) of the lipid with the highest Tc before adding to the dry lipid. After adding the hydrating medium, the lipid suspension should be kept above the Tc during the whole period. The hydration medium is generally determined by the application of the lipid vesicles. Suitable hydration media typically include distilled water, buffer solutions, saline, and non-electrolytes such as sugar solutions.
Typical hydrating solution compositions that meet these conditions are 0.9% saline, 5% dextrose and 10% sucrose. During hydration some lipids form complexes unique to their structure. The results of the described hydration procedure are large multilamellar vesicles (LMVs) whose structure resembles an onion, with each lipid bilayer separated by a water layer. Once a stable, hydrated LMV suspension has been achieved, the particles can be downsized by a variety of techniques, including sonication or extrusion.
Disruption of LMV suspensions using sonication typically gives rise to small unilamellar vesicles (SUV) with diameters in the range of 15–50 nm that can also be useful in some cases for our aims. The most common techniques for preparation of such particles are bath and probe tip sonicators. Bath sonicators, due to their limited power and higher level of cleanness, are the most widely used instrumentation for preparation of SUV. Sonication of an LMV dispersion is achieved by placing a test tube containing the suspension in a bath for 5–10 minutes above the Tc of the lipid. As a result, the lipid suspension begins to clarify to yield a slightly hazy transparent solution. The achievable mean size and distribution of the SUVs are influenced by composition and concentration, temperature, sonication time and power, and volume. Furthermore, due to the high degree of curvature of these membranes, SUVs are inherently unstable and spontaneously fuse to form larger vesicles when stored below their phase-transition temperature.
Lipid extrusion is the approach of choice to yield LUVs. It consists in forcing a lipid suspension through a polycarbonate filter with a defined pore size to yield particles having a diameter dictated by the pore size of the filter used. This procedure has to be repeated typically around 10 times, to achieve repeatable final results. Prior to extrusion through the final pore size, LMV suspensions are disrupted either by several freeze–thaw cycles or by pre-filtering the suspension through a larger pore size (typically 0.2–1.0 μm). This method helps prevent the membranes from fouling and improves the homogeneity of the size distribution of the final suspension. As with all procedures for downsizing LMV dispersions, the extrusion should be performed at a temperature above the Tc of the lipid. Extrusion through filters with 100 nm pores typically yields large unilamellar vesicles (LUV) with a mean diameter of 120–140 nm. Mean particle size also depends on lipid composition and is quite reproducible from batch to batch. In Figure 7.3 a typical extruding set-up is shown.
In the specific case at issue, LUVs have to be extruded in a solution also containing the target molecule that is going to be used as an indicator of the redox state inside the liposome. In the present case 1 mg/ml potassium ferricyanide could be enough. LUVs eventually have to be dialyzed against a suitable (physiological) buffer in order to get rid of external ferricyanide from the solution.
Preparation of GUVs can be performed following different approaches. One of the most reliable is to use a dedicated growth chamber where two Pt wires (typically 0.5 mm in diameter) are electrically biased with a slowly varying sinusoidal potential (a few volts p-p and a frequency in the range 2–10 Hz) after having been painted with phospholipid solution. According to the different types of phospholipids used, solvent composition can also vary. In the case of a PC headgroup, as reported in the following example, 1 mg/ml DOPC in CHCl3/MetOH (50%/50% vol.) has been used. After proper drying, the growth process is to be carried out in a water-based solution with a composition that depends upon the particular lipids used and their foreseen application but that usually contains sucrose (200 mM) and a salt at quite low ionic strength (< 10 mM).
Figure 7.4 shows a typical image of a GUV attached to a suction pipette, while the inset shows a GUV filled with methylene blue.
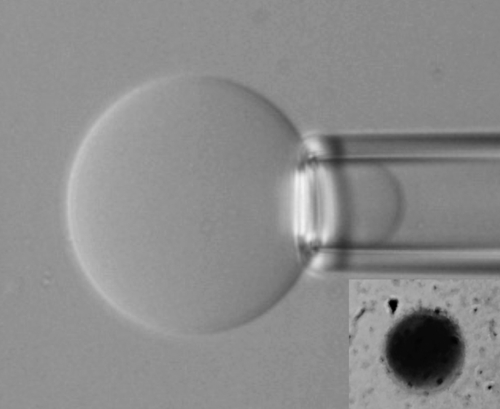
It is therefore suggested that the proposed systems can be valuable to test the effect of redox mediators in influencing the oxidation state of the inner compartment of lipid vesicles. Measurements on LUVs and GUVs will enable one to quantify the kinetics of the phenomenon, as well as the critical concentration and potential values to be used in order to achieve the desired result.
7.2.2. Complicating the system
If one wishes to go further along the steps needed to attain complete control over the electrochemical modulation of the gene expression level in photosynthetic bacteria, one has also to implement a strategy suitable to check whether the electrochemical conditioning of the inner vesicle space can really cause the foreseen cascade of events (see section 6.2).
Therefore, one can continue with the vesicle approach, complicating the system. A possible way of doing that is to make use of recombinant enzymes that can more closely resemble the real, biological system that remains the final goal of the experimentation. The availability of recombinant thioredoxins and DNA gyrases allows one to think of an artificial system where there are present all the main constituents of the foreseen cascade of molecular events that should lead to the modulation of the supercoiling of the bacterial chromosome, hence driving the access of DNA polymerases to different portions of the genome.
The proposal foresees therefore the formation of liposomes in the presence of thioredoxin, gyrase, ATP and circular DNA (e.g., circular λ-DNA or plasmids). Circular DNA supercoiling can be checked by gel-electrophoresis of the pristine DNA. Once all the molecular components are ready, it will be necessary to form liposomes containing all of them in the correct relative concentrations. Submitting the loaded liposomes to redox state control with either safranin or thionine, the modulation of the oxidation state of the S-S bridges of thioredoxin will be achieved, providing as a consequence the cascade of events outlined in section 6.2 and schematically summarized in Figure 6.1.
After a suitable conditioning time, the recovery of DNA and the analysis of its supercoiling (by electrophoresis or even by atomic force microscopy) will provide a quite direct answer to the question about the efficacy of the proposed approach.
In particular, decreased supercoiling of the recovered DNA (Nelson & Cox 2008) with respect to that inserted in the liposomes will be direct evidence of the fact that the control of the SS vs the SH state in thioredoxin has been effectively achieved by direct electrochemistry. The proposed synthetic approach has the obvious advantage of allowing for a detailed screening of the various phases constituting the logical development of the process of DNA-supercoiling modulation. Once achieved, the described result will enable a much safer belief in the results that have been preliminarily shown in Figure 7.2 and that of course will be amenable to optimization in light of the outcome of the “synthetic” experimental approach hereby proposed.
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.