Chemistry
Tables
Table 17.1
Physical properties of gases (at 14.7 psia)
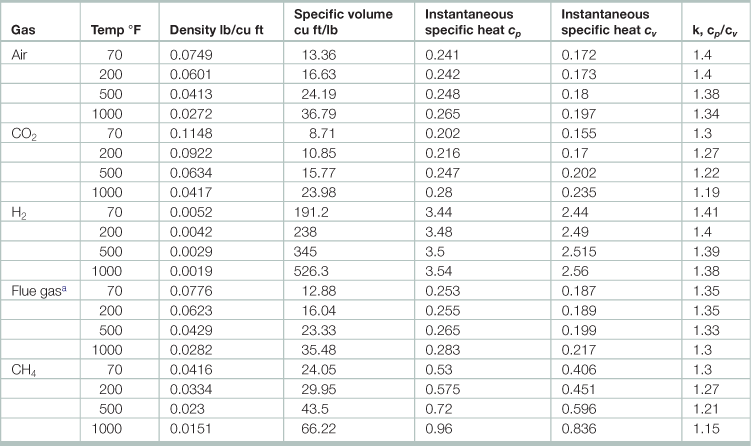
aFrom coal; 120% total air; flue gas molecular weight 30.
Source: Adapted from Babcock and Wilcox. 1978. Steam: Its Generation and Use, (Charlotte, Babcock and Wilcox).
Table 17.2
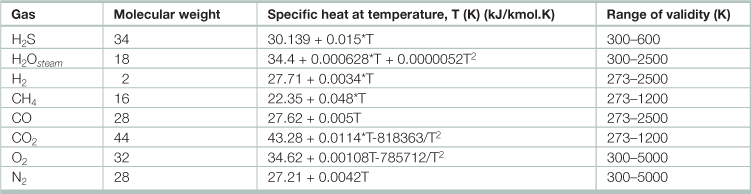
Source: Adapted from Basu, Prabir. 2010. Biomass Gasification and Pyrolysis, (Boston, Academic Press).
Table 17.3
Thermal conductivity, k, of gases (Btu/ sq ft, hr, °F/in thickness)
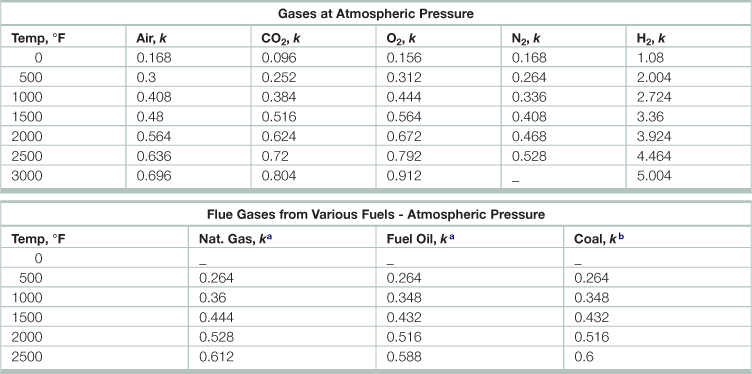
Source: Adapted from Babcock and Wilcox. 1978. Steam: Its Generation and Use, (Charlotte, Babcock and Wilcox).
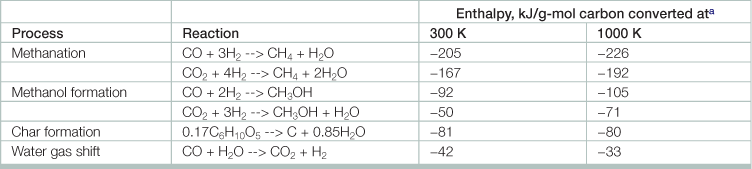
Table 17.4
Heat of formation of various elements and compounds at standard condition, 25 °C, and 1 bar pressurea
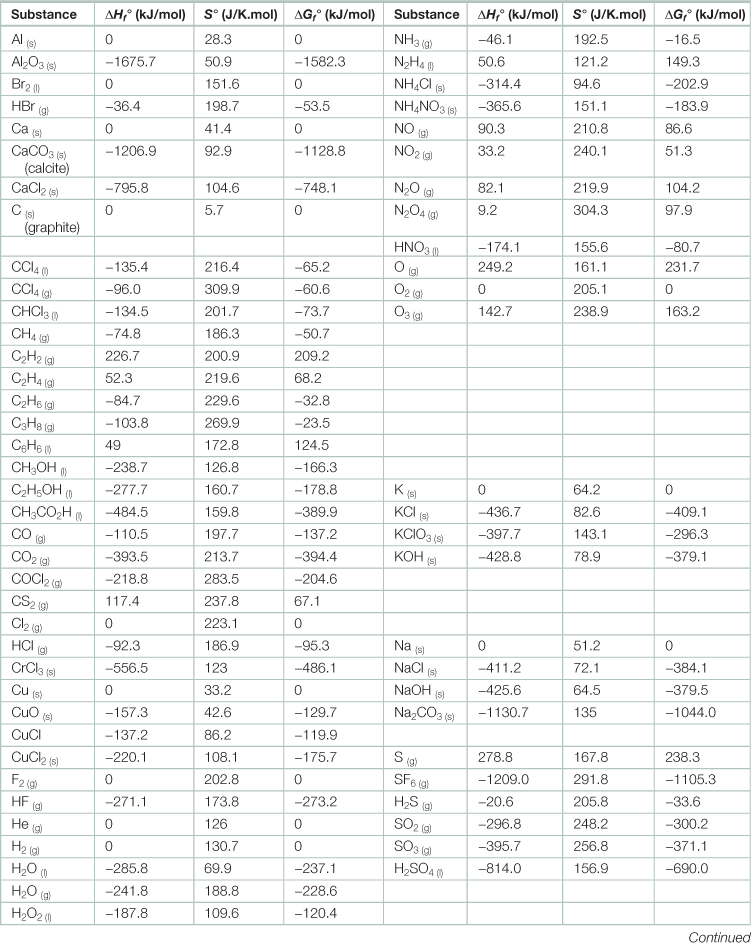
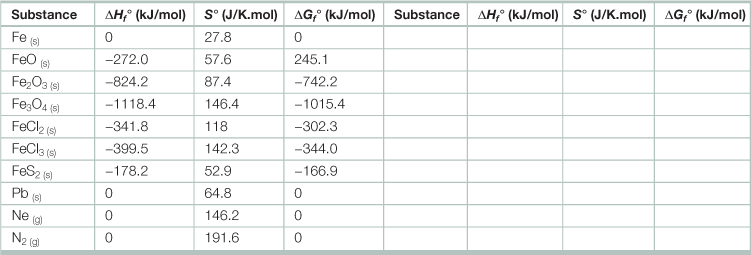
aSubscripts in parentheses indicate the state: solid (s), liquid (l), or gaseous (g).
Source: Adapted from Basu, Prabir. 2010. Biomass Gasification and Pyrolysis, (Boston, Academic Press).
Table 17.5
Physical properties of air at atmospheric pressure
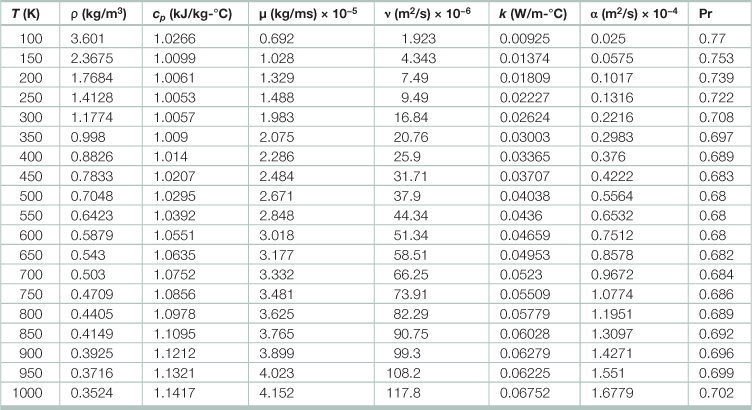
T = temperature, ρ = density, cp = specific heat capacity, µ = viscosity, ν = µ/ρ = kinetic viscosity, k = thermal conductivity, α = cpρ/k = heat (thermal) diffusivity, Pr = Prandtl number.
Source: Kalogirou, Soteris A. 2009. Solar Energy Engineering, (Boston, Academic Press).
Table 17.6
Physical properties of saturated liquid water
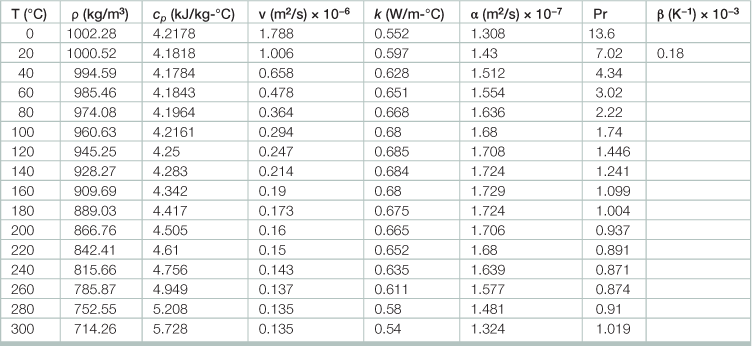
Notes: T = temperature, ρ = density, cp = specific heat capacity, ν = µ/ρ = kinetic viscosity, k = thermal conductivity, α = cpρ/k = heat (thermal) diffusivity, Pr = Prandtl number.
Source: Kalogirou, Soteris A. 2009. Solar Energy Engineering, (Boston, Academic Press).
Table 17.7
Content of different elements in inanimate and living matter
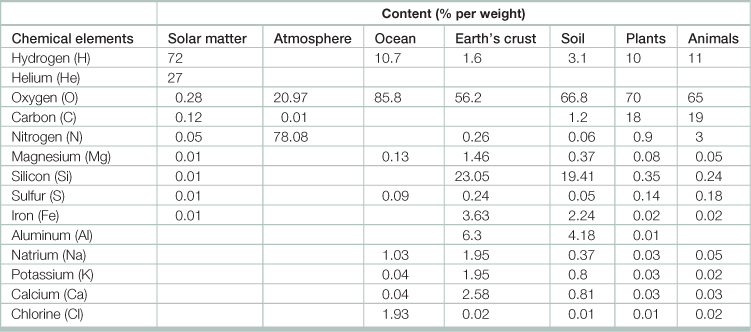
Source: Adapted from Chernyshenko, S.V. 2008. Structure and History of Life, In: Sven Erik Jorgensen and Brian Fath, Editor(s)-in-Chief, Encyclopedia of Ecology,(Oxford, Academic Press), Pages 3403–3416.
Table 17.8
General properties of gaseous hydrocarbons
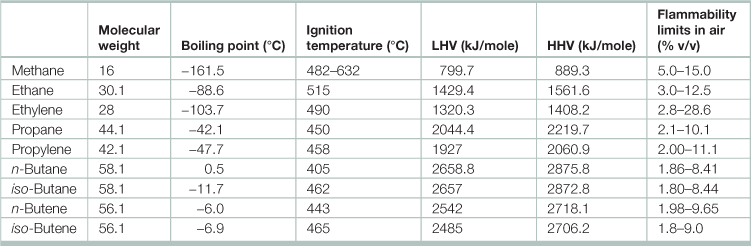
Source: Adapted from Speight, James G. 2011. Chapter 3 - Fuels for Fuel Cells, In: Dushyant Shekhawat, J.J. Spivey And David A. Berry, Editor(s), Fuel Cells: Technologies for Fuel Processing, (Amsterdam, Elsevier), 2011, Pages 29–48.
Table 17.9
Hydrocarbon synthesis product distributions (reduced iron catalyst)
| Variable | Fixed bed | Fluid bed |
| Temperature | 220–240 °C | 320–340 °C |
| H2/CO feed ratio | 1.7 | 3.5 |
| Product selectivity (%) | ||
| Gases (C1–C4) | 22 | 52.7 |
| Liquids | 75.7 | 39 |
| Nonacid chemicals | 2.3 | 7.3 |
| Acids | — | 1 |
| Gases (% total product) | ||
| C1 | 7.8 | 13.1 |
| C2 | 3.2 | 10.2 |
| C3 | 6.1 | 16.2 |
| C4 | 4.9 | 13.2 |
| Liquids (as % of liquid products) | ||
| C3–C4 (liquified petroleum gas) | 5.6 | 7.7 |
| C5–C11 (gasoline) | 33.4 | 72.3 |
| C12–C20 (diesel) | 16.6 | 3.4 |
| Waxy oils | 10.3 | 3 |
| Medium wax (mp 60–65 °C) | 11.8 | — |
| Hard wax (mp 95–100 °C) | 18 | — |
| Alcohols and ketones | 4.3 | 12.6 |
| Organic acids | — | 1 |
| Paraffin/olefin ratio | ~1:1 | ~0.25:1 |
Source: Larsen, John W. and Martin L. Gorbaty, Coal Structure and Reactivity. 2003. In: Robert A. Meyers, Editor-in-Chief, Encyclopedia of Physical Science and Technology (Third Edition), (New York, Academic Press), Pages 107–122.
Table 17.10
Properties of gasoline, natural gas, and hydrogen
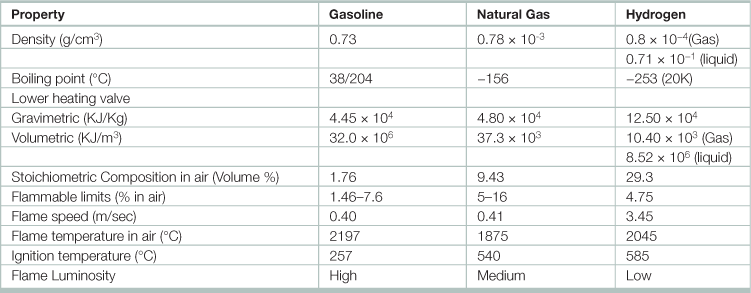
Source: Adapted from Garg, H. P., S. C. Mullick, and A. K. Bhargava. 1985. Solar thermal energy storage, (Dordrecht, D. Reidel Publishing).
Table 17.11
High-temperature, closed-loop chemical C-H-O reactions
| Closed-loop system | EnthalpyaΔH0 (kJ mol−1) | Temperature range (K) |
| CH4 + H2O ↔ CO + 3H2 | 206 (250)b | 700−1200 |
| CH4 + CO2 ↔ 2CO + 2H2 | 247 | 700−1200 |
| CH4 + 2H2O ↔ CO2 + 4H2 | 165 | 500−700 |
| C6H12 ↔ C6H6 + 3H2 | 207 | 500−750 |
| C7H14 ↔ C7H8 + 3H2 | 213 | 450−700 |
| C10H18 ↔ C10H8 + 5H2 | 314 | 450−700 |
aStandard enthalpy for complete reaction.
bIncluding heat of evaporation of water.
Source: Adapted from Sørensen, Bent. 2012. Hydrogen and Fuel Cells-Emerging Technologies and Applications (Second Edition), (Boston, Academic Press).
Table 17.12
Properties of common heat transfer liquidsa
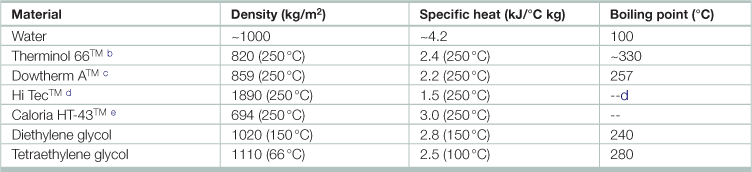
aData collected directly from manufacturers.
bTrademark of Monsanto Chemical Co.
cTrademark of Dow Chemical Co; Dowtherm is a mixture of diphenyl and diphenyl oxide.
dTrademark of E.I. duPont; solidifies below approximately 190°C; does not boil or decompose below 500°; consists of 40% NaNO2, 7% NaNO3, 53% KNO3.
Source: Adapted from Goswami, Yogi D., Frank Kreith, and Jan F. Kreider. 2000. Principles of Solar Engineering, 2nd edition, (Philadelphia, Taylor & Francis).
Table 17.13
Specific heat capacity of selected substances
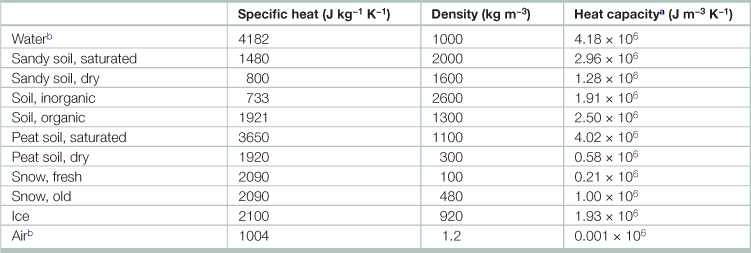
aSubstances change their temperature by differing amounts for a given amount of heat, depending on their specific heat capacity and their density. The last column, the product of the former two quantities, describes the amount of heat that is necessary to raise the temperature of 1 m3 of a given substance by 1 K.
bDensity depends on temperature. Values given are for 293 K.
Source: Adapted from Kleidon, A. 2008. Energy Balance, In: Sven Erik Jorgensen and Brian Fath, Editor(s)-in-Chief, Encyclopedia of Ecology, (Oxford, Academic Press), Pages 1276–1289.
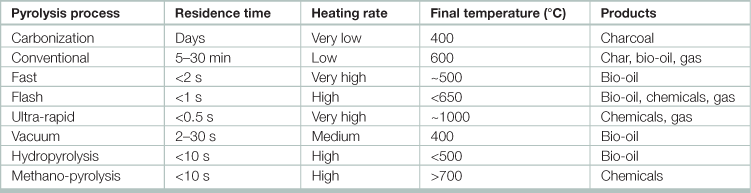
Table 17.14
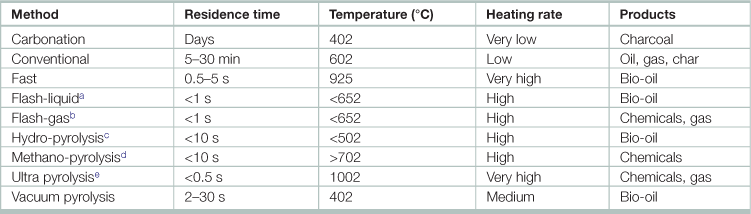
aFlash-liquid: liquid obtained from flash pyrolysis accomplished in a time of <1 s.
bFlash-gas: gaseous material obtained from flash pyrolysis within a time of <1 s.
cHydropyrolysis: pyrolysis with water.
dMethanopyrolysis: pyrolysis with methanol.
eUltra pyrolysis: pyrolysis with very high degradation rate.
Source: Adapted from Panwar, N.L., Richa Kothari, V.V. Tyagi. 2012. Thermo chemical conversion of biomass – Eco friendly energy routes, Renewable and Sustainable Energy Reviews, Volume 16, Issue 4, Pages 1801–1816.