Chapter 6
Chemical and Biological Sensors at Component and Device Level
Chapter Contents
6.1 Application Field
6.2 Sensor Principles for the Collection of (Bio)Chemical Information
6.2.1 Optical Techniques
6.2.2 Electrochemical Techniques
6.2.3 Methodology of Sensor Development
6.3 Integrated chemFET Device: Case Study of a Semiconductor-Based pH Sensor Development
6.4 Integrated Clinical Diagnostics: A Medical Application for Electrochemical Sensor Arrays
6.4.1 From Microarray to Biochip Technology
6.4.2 Cell-Based Biosensor
6.5 Conclusions
Developments in microchip-based chemical and biological sensors are closely driven by the requirements of products in the medical and safety devices industry. Specifically, the use of miniaturized devices at the so-called point-of-care, or point-of-action, is a strong driver for innovation in this field. Central laboratories have for many years offered a guarantee of access to state-of-the-art equipment and the highest quality of results for analytical (bio/medical) chemistry. However, logistic constraints and the large investments that are needed in infrastructural housing, as well as maintenance personnel, are extremely expensive in the diversified age of the 21st century.
In addition, decentralized sensor networks can offer novel opportunities. Some problems cannot be solved well by central laboratories, because temporal and spatial information is an essential component of the solution. The development of decentralized sensor networks can tackle such problems, and have stimulated a new innovative wave in the measurement equipment sector. Microfabrication technology presents a solution, not just for decentralization, but also for the simplification of measurement protocols – to the extent that specialized operators are no longer required. One may summarize this trend as an instant measure approach. The pessimists amongst us may suggest that Big Brother is now not only watching us (enabled by a network of cheaply available and distributed video cameras accessible through the cost-efficiency of integrated electro-optical microchip manufacturing), but also punishing us immediately (for example, riding a bike through the warning signal of a traffic light, which may make you liable for an instant fine). The optimists on the other hand, consider the feeling of enhanced safety and fast response to act as quickly as possible to save a life. The same requirement for scanning open space has stimulated developments in the imaging of chemical and biological processes. This chapter gives an introduction to miniaturized chemical and biological sensors at the component and devices level, and also a sketch of a possible methodology for sensor development and testing.
6.1 Application Field
A great many chemical and biochemical sensor products already exist. They were first presented as part of the microelectronic miniaturization era of the early 1980s, when the research and development of electrochemical sensors gained much attention. In 1983, Japan organized the first conference dedicated to chemical microsensors. Micro-integrated chemical sensors are a niche market within the total sensor market, currently counting for approximately 10% of total sales. A large variety of different optical and electrical sensors have been explored in the context of microchip applications for chemical transduction in microreactor devices, due to scalability, in which a measurement can be obtained from very small quantities of gas or liquid.
We will discuss in more detail in the next sections how the need for monitoring is one of the keys for the successful roll-out of miniaturization in industry. For example, a microchip can be implemented to measure the composition of analytes in a chemical or biological sample during down-stream production processes. Such integrated analysis techniques allow product quality to be monitored early in the production line, e.g., using microchip chromatography. Microchip capillary electrophoresis also serves as a new chemical analysis method. Measurement of blood, plasma, serum, urine, droplets from the eye, saliva and spinal-cord fluid samples have already been demonstrated by on-chip measuring. Example applications include proteins, anti-epileptic drugs such as valproate in serum, and also measurements in whole blood, e.g., of anti-depressant pharmaceuticals such as lithium [1–4] Chapter 7 will give details of the lithium measurement method, as part of a business case which illustrates the use of an integrated electroanalytical measurement system, in the point-of-care monitoring of lithium in patients with bipolar disorder. In the current chapter our discussion will concentrate on the component and device level of the sensor itself.
In many cases microchip techniques can be performed with a faster turn-around time than conventional analytical chemistry methods, but the larger scale methods are often more accurate. For point-of-care analysis, the user friendliness of the packaged device, and the robustness of the sensor design are as important as the analysis speed and accuracy.
To illustrate the environment in which such commercial sensors would operate, Figure 6.1 depicts a typical analytical chemistry laboratory environment (left) and the manual loading of a sample into a microplate for optical readout (right), as is often utilized for critical care patients. In the pharmaceutical sciences these samples are mostly loaded automatically by dedicated robotic systems. In the operational environments of the chemical industry, flow-injection analysis (FIA) systems are often utilized, which decouple the harsh conditions of the product process line from the point-of-measurement by a bypass line housing the miniaturized chemical sensors. Timmer et al. present the μTAS approach for integrated microfabricated sensors, which can be utilized in a variety of industries. An example analyte is gaseous ammonia, which needs to be measured in environmental gas analysis as well as automotive, chemical and medical industrial applications [5]. Persaud et al. have presented an overview of “Solid state chemical sensors: Technologies and applications” in which they specifically addressed the requirements for sensor technology in the agricultural industry. The agricultural (bio)industry (intensive farming) needs reliable and affordable sensor technology for monitoring potential risks of environmental pollution, such as ammonia (NH3), carbon dioxide (CO2), hydrogen sulfide (H2S) and methane (CH4) [6].

Figure 6.1 Typical chemical analytical laboratory facility with conventional apparatus (left) and example of manual sample loading to a microplate for optical read-out measurements in a biochemical-analysis laboratory (right), University of Twente.
Image courtesy: S. Schlautmann, University of Twente, 2011.
Sensing, sampling and measurement technology as applied to real-time monitoring may require a totally different product configuration for each of the applications to which it is applied.
In the following sections we introduce working principles of sensors for chemical and biological measurands. Here, a biosensor is defined by the fact that it transducts the signal from the analyte by a biological recognition mechanism. We will briefly illustrate examples of sensing applications for measurands of both a chemical and biological nature, including low-molecular weight metabolites, metal ions, gases, anions and biologically active substances, for example, toxins, antibiotics and hormones. To observe and detect changes biologically, cells may be cultured and used to transduct, for example, the effects of pharmaceutics on living systems. The living cell is in this context a complex signal transduction system (“sensor”) developing a positive or negative reaction upon exposure. As far as sensor integration technology is concerned, we are interested in a quantifiable change of the measurand, and a means to present these numbers to the user in an electronic format rather than an analog signal. The detailed evaluation of the technical context of such novel, microfabricated, sensor designs is, of course, very important, but so is the impact of widespread utilization of sensor technology throughout living environments and therefore on society.
6.2 Sensor Principles for the Collection of (Bio)Chemical Information
6.2.1 Optical Techniques
The increasing quality of signal response in analytical chemistry has benefitted from the development of ever better and more sophisticated photometric and fluorometric sensors. The miniaturization of such sensors has interesting applications in microreaction systems, portable systems for in-field and bedside analysis applications, and in fermentation processes. The combination of different, discrete microsensors with fiber-optics or integrated microoptic components is an exciting research area. Optical set-ups that are sensitive to one specific, or multiple wavelengths may be used. The latter will produce light spectra, and thus can be utilized to deliver fingerprint results even of complex fluids, such as blood. For the determination of concentration changes of a specific substance in a background medium, single wavelength absorbance measurements (following the Lambert–Beer law) can be applied, where the intensity of the signal is proportional to the optical density of the medium. Specific markers, which change the optical properties of the measurand so as to produce a signal stronger or more selective. A marker can be introduced to interact with the incident light and produce either a fluorescent signal, or to enhance the optical absorbance of the compound at the selected wavelength. Figure 6.2 depicts a schematic and an example of a microscopic work place for the optical detection of signals as well as an image collected from a cell prepared with specific markers (also called staining of a cell or tissue material).
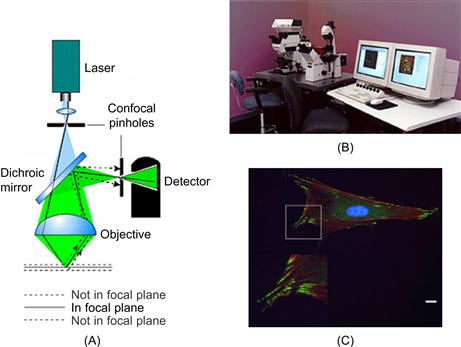
Figure 6.2 Optical microscopy: (A) Schematic of fluorescence imaging microscope and (B) inspection work place (original source: LEICA). (C) Example of immunofluorescence micrograph of osteoblast-like cells.
Image courtesy: E. Lamers, Radboud University Medical Center, 2009.
Biology laboratories would like to utilize the best optical microscope available, to study biological response curves in tissue and cells. So far, there has been little reason to reduce costs in the observation of bioanalytical processes, since we are just about to discover the potential of such a field of analysis to fulfill needs other than fundamental learning about cellular mechanisms. Once such analysis becomes important industrially, more automation will be required, and miniaturizing the optical imaging systems will offer additional advantages (see, for example, also new developments for compact optical detection in analytical systems-on-chip applications in Chapter 7, Section 7.7). Sensor systems that integrate optical components for (bio)chemical analysis are so far relatively rare, although hand-held photospectrometers delivering an electric readout are already available for certain puposes such as the color determination of teeth (shade detection) by a dentist. Another integrated optical measurement system is a miniaturized Mach–Zehnder interferometer, of which the fabrication process has been briefly described in Chapter 3.
Dasgupta et al. reviewed the use of light-emitting diode-based detectors within a planar flow-through cell, including a variety of application examples for integrated optics on chip [7]. One example of their application is the monitoring of water quality in the drinking water supply network. In these decentralized applications, optical systems are preferred over electrochemical sensors, since they can both generate and transmit the relevant data (an optical fiber telecommunication network is already available with a large area of coverage). In addition, they are insensitive to electromagnetic radiation, and thus are less prone to signal failure. Figure 6.3 illustrates how the signals of an on-chip fluidic network could be read, utilizing the method of absorbance measurement across the depth or the width of a microfluidic channel. This technique could be carried out on-chip using a microscope set-up, similar to the one shown in Figure 6.2B, fiber optics or the integration of planar waveguides into the chip. Methods to increase the optical path inside the chip by means of specially etched sidewalls of the flow channel that act as mirrors have been also described [8].
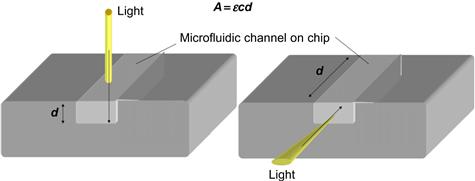
Figure 6.3 Principle of optical absorbance measurements, here d is the optical light path changing the reference signal by the concentration c in the microchannel.
6.2.2 Electrochemical Techniques
Detection and quantification of chemical compounds is often carried out by electrical means, to reduce operator to operator variablity. In a hospital laboratory many samples must be measured rapidly for multiple parameters, and yet decisions have to be made with great care. The integration of electrochemical sensors is a suitable route for automation in this field. Chemical production plants are another potential example, where reading process parameters in real-time allows the utilization of such measurands for process loop control. Electrochemical microsensors can be used for process line monitoring across a range of simultaneous measurement points, bundling different levels of information into the decision-making process. Validating the quality of the signal, remains an on-going topic of debate if both the optical and the electrochemical principle of detection is possible. It may be more important to create sensors which produce an output that can be read directly than to make them robust. The signal validation problem can be solved, for example, by the installation of multi-sensor arrangements. The choice of sensor working principle and design of a sensor package depends very much on the specific field of application. There are many examples of such application fields, including pharmaceutics, medicine, analytical sciences, synthetic chemistry, biotechnology, material sciences and (bio)molecular engineering. The requirements of each for sensitivity, selectivity, portability, multivariant or single-use detection will all influence the choice of a suitable sensor principle, as well as its layout and constituent materials [9]. We will now give a brief introduction to two intensively investigated sensor principles, and then discuss some of their chemical and biological applications at component and device level.
Conductometry
If free ions are available in a solution, we call this solution an electrolyte. The positively and negatively charged ions are called cations and anions, repectively. An electrolytic solution behaves similarly to an electrical conductor, and its electrical properties follow Ohm’s law: I = U/R, where I is the current, U is a voltage and R is the electrical resistance. R depends on the intrinsic properties of the conductor and its shape. This relationship is given as: R = ρ · l/A, in which l is the length of the conductor, A is the cross-sectional area and ρ is the specific resitance. The resistance is also expressed as the conductance of the medium G, hence the name of the measurement principle. ρ is given in units of Ohm · m. The conductance G is given in units of S (Siemens), where 1S=1/Ohm.
We can imagine that an electrolyte could be sealed in a tube with a given length, and cross-sectional diameter A, and thus we can apply the same set of equations as for metallic conductors. To learn about the conductance of an electrolyte, measurements are made in a conductometric cell (container). The ratio l/A is an intrinsic parameter of the design of the conductometric cell and is called its resistive capacity C. This value may be calculated for a straight tube, but for complex geometries C is determined experimentally by using an electrolyte solution with a known specific conductance, for example, KCl solution. Once C is determined, solutions with unknown conductivity can be measured. The specific conductance is a function of temperature and so this must be measured carefully in applications of this sensor working principle. The optimization of planar conductivity sensors suitable for lab-on-a-chip has been presented by Jacobs et al. [10].
Amperometry
Amperometric three-electrode measurement systems are state-of-the-art technology for the determination of toxins in the air, in which the gas diffuses into an electrochemical cell (electrolyte container with the electrodes). Planar microfabrication technology made it possible for systems to be batch fabricated at relatively low cost, and thus to be used as personalized meters for health and safety applications. The main advantage of microfabrication is the possibility to deposit different types of electrode materials acting as catalysts within a multi-sensor layout and reducing the electrode distance. Multi-layouts offer the potential for selective measurement of different specific compounds in a mixture and the reduction of the electrode distance enhances the sensor sensitivity [11]. The sensor working principle is based on the reaction of the electrochemical active compound at the working electrode, while new molecules diffuse towards the electrode and thus compensate the current flow. The electrical potential between the working electrode and the reference electrode is set and controlled by a potentiostat, while the current flowing between the working and the counter electrode provides the concentration-dependent signal.
The selection of the offered voltage at the working electrode depends on the measurand and if properly chosen, the measured current will be proportional to the amount of reduced compound. To control the mass transfer at the electrolyte–electrode interface, a diffusion-barrier is used by depositing a permeable material onto the electrode. These membrane-type materials should be deposited in as thin a layer as possible onto the electrode, to allow a fast sensor response and a sufficiently high sensor current. Membranes of several tens of micrometers may be easier to deposit than thin membranes, however the sensors will lose sensitivity. Developing a new microfabrication process is therefore always a trade-off between ease and robustness of manufacture and sensor performance. One of the best established and most investigated cases of amperometry is the electrochemical glucose sensor and its application in diabetes management [12].
Figure 6.4 shows a set up for amperometric sensing in a microfluidic channel structure using a conventional copper wire (left) and a variety of planar microfabricated electrodes (right) as studied by Cai et al. [13].
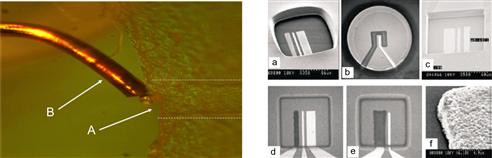
Figure 6.4 Left: principle of amperometric detection using a thin copper wire inserted into the microchip reservoir at the end face of the microchannel. (Image courtesy: E. X. Vrouwe, University of Twente, 2002.) Right: (a–e) different designs for microfabricated electrodes and (f) detail of electrodeposited electrode.
Reproduced with permission from reference [13]
6.2.3 Methodology of Sensor Development
Prior to the introduction of a novel sensor into a real-world analytical process, the components have to be tested at component level and assembled. Often the interfacing of different components can generate additional challenges, one of which is the reduction of dead volumes in the sensor design. However, joining two different materials can also cause an unexpected failure, particularly if electrochemistry is utilized, e.g., eroding the integrated electrodes. Figure 6.5 presents a road map for the selection and design of a sensor principle and particular aspects to consider for electrochemical sensors. The design methodology for electrochemical sensors has been discussed previously, for example by Brett [14].
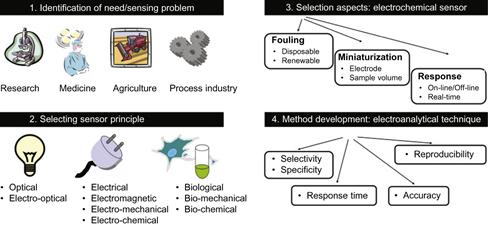
Figure 6.5 Overview of sensor development methodology.
6.3 Integrated chemFET Device: Case Study of a Semiconductor-Based pH Sensor Development
pH is one of the most important parameters affecting chemical and biological reactions. As mentioned previously, it is common practice in process control and quality assurance to collect the process-related parameters downstream and use them to run (bio)chemical production processes safely and efficiently. The accurate measurement of a universal parameter such as pH or temperature often gives a good first order indication if a process is proceeding correctly. A shift in pH potentially indicates that the product quality has shifted, too. For production control, it is not always necessary to know what precise changes are occurring in product properties, although these may be measured during process optimization. Fluctuations in the environmental temperature at the measurement point may lead to error in reading the pH value and we need to look into the magnitude of these errors carefully depending on the customer’s needs. The most established instrument for measuring pH is the pH-glass electrode, the measurement signal of which is a low voltage that is directly proportional to the pH value. For practical applications knowledge of pH sensing by conventional techniques is still useful; in this context we will consider how a change in pH influences the working of a semiconductor device.
Semiconductors can be used as chemical sensors in addition to their more widely-known applications [15, 16]. The so-called ion-sensitive field-effect transistor (ISFET) principle has been explored for the measurement of pH by Bergveld et al. [17–22] amongst others. ISFET design is based on the metal oxide semiconductor field effect transistor, in which the metal is replaced by an aqueous solution and a reference electrode, as depicted in Figure 6.6. The conductance between source and drain is a function of the electrical field perpendicular to the gate oxide surface. To measure pH, the ISFET’s gate oxide is exposed to the aqueous solution. If the pH in the solution changes, then the electronic signal will also change as a function of the state of ionization of the surface bound SiOH groups. For background on the working principles of semiconductor devices further reading is encouraged, e.g., [23]. There is also considerable literature available on the ISFET, and at the University of Twente, The Netherlands, a number of PhD projects have focused on the development of this specific class of electrochemical sensors and the relevant PhD theses may be found through the University’s library system, for example, de Rooij in 1978, Bousse in 1982 and van den Berg in 1988 [36–38].
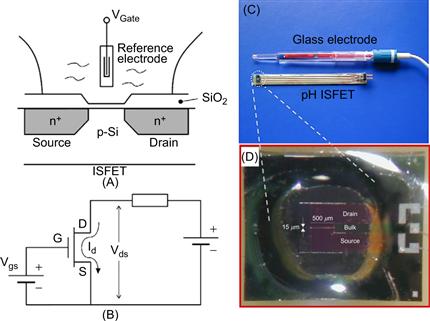
Figure 6.6 Microfabricated pH sensor. (A) Schematic of the working principle of an ion-selective field effect transistor (ISFET) and (B) ISFET electronic diagram. (Reproduced from reference [22].) (C) Realization of the packaged device compared to a standard glass hydrogen electrode and (D) detail of the silicon sensor element.
Images courtesy: P. Bergveld, University of Twente.
6.4 Integrated Clinical Diagnostics: A Medical Application for Electrochemical Sensor Arrays
Clinical diagnostics often relies on the measurement of a variety of blood parameters, e.g., the electrolyte balance or the blood gas analysis. The required analysis methods have often been developed in clinical chemistry. Since the discovery of DNA and its importance to health patterns and prophylactic disease management, many molecular diagnostic assays on chip have been developed. The interaction sites are organized in a standardized x,y-matrix – so-called microarrays. These arrays may be read-out by optical means or by electrochemical sensors. Figure 6.7 depicts a schematic example of the assembly process of a DNA microarray.
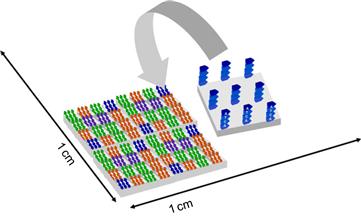
Figure 6.7 Schematic design of a DNA microarray.
6.4.1 From Microarray to Biochip Technology
Assays for method development in drug discovery, biochemistry and chemical sciences can also be performed on-chip. This is more efficient than conventional methods for bio-liquid analysis because of its high throughput and the much reduced amounts of solvents and reagents that are needed. This integrated microfluidic approach has already demonstrated remarkable results in a diveristy of life-science applications. Biolog Inc., for instance, followed this logical miniaturization roadmap with their Phenotype MicroArray™ technology. A BioChip design relies on the symbiosis between biology and physical instrumentation. In many applications biological phenomena of protein–protein and protein–cell interactions are investigated and monitored using microelectronic read-out systems that have been developed by microfabrication technology. DNA optical read-out has gained wide acceptance in applying fluorescence for gene expression profiling within the so-called microplate reader format. This approach has been used for the manipulation and analysis of thousands of different DNA sequences and proteins, either spotted or synthesized in-situ on a few cm2 surface area [24, 25]. One example of a novel diagnostic sensor platform enabled by smart bio-photolithography and thin-film patterning is illustrated in Figure 6.8.
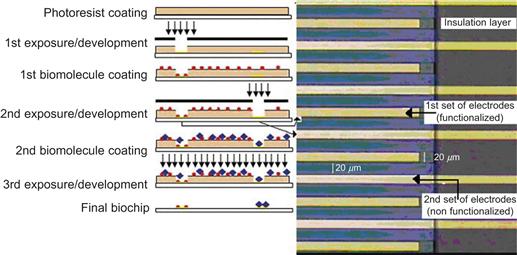
Figure 6.8 Micro-electrochemical biomolecular detection. Bio-photolithographic process for biomolecular patterning on the electrode array. On the right, gold interdigital electrode (IDE) array detail; each set of electrodes consists of 50 microelectrodes with a width of 20 μm and a distance between electrodes of 20 μm
Reproduced from reference [26].
There is already a significant number of industrial players exploiting integrated label-free microarray approaches. Figure 6.9 illustrates an example of a bioelectrochemical integrated sensor array for advanced bio-assays. Such biochip designs may also employ flow-through systems with various types of biophysical detection methods. Infineon, for example, has developed such a type of array, based on their expertise in miniaturization for electronic systems, but in this instance using an optical read-out [27, 28].
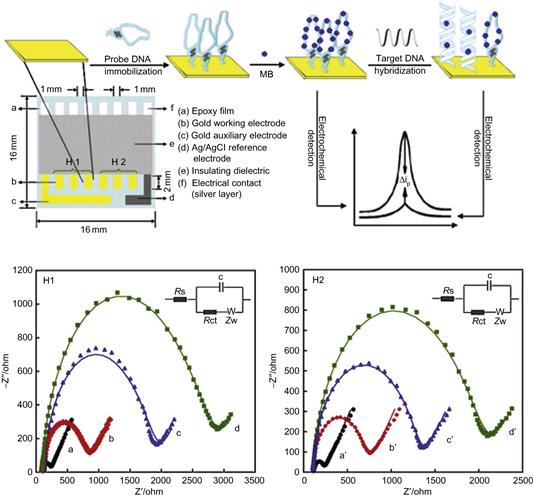
Figure 6.9 Top: schematic diagram of multi-electrode array and representation of biosensor array with fabrication steps and performance. Bottom: Faradic impedance spectra (FIS) of multi-electrode array in each immobilization step and binding step in 100 μL of 0.1 M PBS solution (pH 7.40) containing 10 mM K3Fe(CN)6-10 mM K4Fe(CN)6. H1: (a) Bare Au; (b)H1/Au; (c) H1/MCH/Au; H2: (a’) bare Au; (b’) H2/Au; (c’) H2/MCH/Au; (d’) HIV-2/H2/MCH/Au. Fitted data (solid line). The frequency was from 100 kHz to 0.1 Hz and the amplitude was 5.0 mV. The inset shows the equivalent circuit applied to fit the impedance spectroscopy.
Reproduced from reference [27].
6.4.2 Cell-Based Biosensor
In a new generation of transducers for the detection of biologically active substances, living cells can be utilized as the central unit [29, 30]. Recently, mammalian cell-based sensors have been investigated, as a tool for detecting pathogen and toxins in food, and for security applications with respect to defense strategies against chemical and biological attacks [31, 32]. A major challenge for the development of such systems is to keep the cell alive in a sealed microenvironment. Devices have been demonstrated using cell culture chambers made from polydimethylsiloxane (PDMS) and glass, while the electronic functions were realized in CMOS technology. Often in such cell-based biosensors the interaction between the cell and the electrochemical interface needs to be modified by cell-compatible molecules, such as molecules that enhance the adhesion of the cells to the sensor surfaces [33]. A variety of polymer grafting methods have been developed to coat the sensor’s surface. Some of these polymers are combined with biological membrane proteins into an artificially designed surface layer. The selection of a specific surface modification also impacts the cellular response. Inappropriate mechanochemical transduction between surface and cell may even lead to cell death (apoptosis) or up- or down-regulation of gene expression may influence the observations of the pharmacological or toxicological response studies. Within stem-cell research, cells seeded on different types of surface structures may also differentiate differently, making the investigations even more complex.
Three-dimensional cellular networks have also been investigated. Cells and biomolecules may be deposited by principles similar to ink-jet printing. An example of this approach is given by Zhang et al., who demonstrated an industrial-scale, high-throughput, sandwich cell-based drug screening platform [34]. They applied micromachined Si3N4 sieves to enhance perfusion and maintain the three-dimensional morphology of the cells in culture over the long term. In addition to sensor technology, these techniques are also suitable for exploration in tissue regeneration processes. Figure 6.10 depicts an overview of different types of three-dimensional cell culture scaffolds that have typically been exploited in the artificial growth of tissues such as, for example, bone, cartilage, heart valves or skin [35].
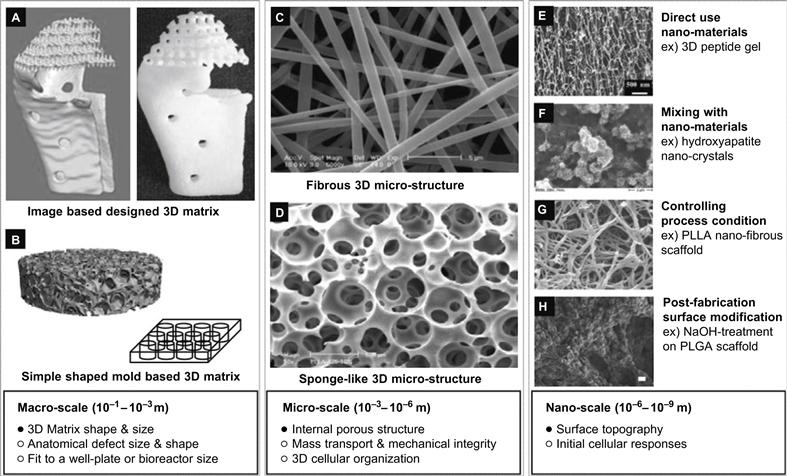
Figure 6.10 Design criteria of three-dimensional, multi-scale cell culture matrix.
Reproduced from reference [35]. The publisher for this copyrighted material is Mary Ann Liebert, Inc.
6.5 Conclusions
Chemical processes and the intensification of (bio)chemical reactions rely on the optimization of dedicated technologies. These optimization studies can be carried out off-line, by measuring a set of feedback process parameters in a centralized laboratory. However, it is more efficient to measure the performance of a chemical reaction as early and as directly as possible during production processes. Appreciation of this fact led to the developments of the total analysis system (TAS). Originally, a TAS was simply a trolley containing all the equipment required for taking a reading from a specific production pipeline in the plant. Once the value of a specific parameter was known, a process optimization could take place immediately. With the ongoing requirement for cost reduction and even faster response times in industrial processes, microelectronic devices were used for the purpose of automation. The need to couple the read-outs into the (bio)chemical sensor systems quickly led to a new trend: microTAS (μTAS). This applied to chemical production plant optimization, as well as information retrieval concerning a variety of body functions that required rapid intervention.
At the component level, the electrochemical working principles can be integrated with widely established electronic functions and data-log systems. However, good design requires minimal dead volume and intelligent choice of materials.
Even more recent developments have seen multi-sampling of micro-arrays, by either optical or electrical sensors. The intensely high throughput demands of such fields as genomics and proteomics research have extended the boundaries of sensor developments to the point where the transduction processes of living cells are put to work in novel sensor designs. These novel platform technologies may be utilized in a variety of applications. The development of microsensors for universal parameters such as pH, conductivity and temperature are considered as milestones in chemical engineering, while the bio-recognition and interaction of molecules within specific biochip or microarray format gain importance for the understanding of biofunctions and their subsequent regulation in case of malfunctioning, e.g., during disease.
Unfortunately, the field of chemical and biochemical detection cannot be approached by a one-fits-all concept, and besides sensors for universal parameters such as temperature and pH, many distinguished sensor working principles and their working range performance are being investigated in the μTAS R&D community.
REFERENCES
1. Colyer CL, Mangru SD, Harrison DJ. Microchip-based capillary electrophoresis of human serum proteins. J Chromatography. 1997;A 781(1–2):271–276.
2. Colyer CL, Tang T, Chiem N, Harrison DJ. Clinical potential of microchip capillary electrophoresis systems. Electrophoresis. 1997;18(10):1733–1741.
3. Ölvecká E, Koníková M, Grobuschek N, Kaniansky D, Stanislawski B. Direct determination of valproate in serum by zone electrophoresis–isotachophoresis on a column-coupling chip. J Sep Sci. 2003;26(8):693–700.
4. Vrouwe EX, Luttge R, van den Berg A. Direct measurement of lithium in whole blood using microchip capillary electrophoresis with integrated conductivity detection. Electrophoresis. 2004;25(10–11):1660–1667.
5. Timmer B, Olthuis W, Van Den Berg A. Ammonia sensors and their applications – a review. Sens Actuators B Chem. 2005;107(2):666–677.
6. Persaud KC, Flint A, Sneath RW. Solid state chemical sensors: Technologies and applications. In: 2007;1–6. EuroSime 2007: International Conference on Thermal, Mechanical and Multi-Physics Simulation Experiments in Microelectronics and Micro-Systems 16–18, April 2007.
7. Dasgupta PK, Eom I-Y, Morris KJ, Li J. Light emitting diode-based detectors: Absorbance, fluorescence and spectroelectrochemical measurements in a planar flow-through cell. Anal Chim Acta. 2003;500(1–2):337–364.
8. Tiggelaar RM, Veenstra TT, Sanders RGP, Gardeniers JGE, Elwenspoek MC, Van Den Berg A. A light detection cell to be used in a micro analysis system for ammonia. Talanta. 2002;56(2):331–339.
9. Thévenot DR, Toth K, Durst RA, Wilson GS. Electrochemical biosensors: Recommended definitions and classification. Biosens Bioelectron. 2001;16(1–2):121–131.
10. Jacobs P, Varlan A, Sansen W. Design optimisation of planar electrolytic conductivity sensors. Med Biol Eng Comput. 1995;33(6):802–810.
11. Kimura J, Murakami T, Kuriyama T, Karube I. An integrated multibiosensor for simultaneous amperometric and potentiometric measurement. Sens Actuators. 1988;15(4):435–443.
12. Heller A, Feldman B. Electrochemical glucose sensors and their applications in diabetes management. Chem Rev. 2008;108(7):2482–2505.
13. Cai X, Glidle A, Cooper JM. Miniaturized electroanalytical sensor systems in micromachined structures. Electroanalysis. 2000;12(9):631–639.
14. Brett CMA. Electroanalytical techniques for the future: The challenges of miniaturization and of real-time measurements. Electroanalysis. 1999;11(14):1013–1016.
15. Cané C, Grácia I, Merlos A. Microtechnologies for pH ISFET chemical sensors. Microelectronics J. 1997;28(4 SPEC.ISS.):389–405.
16. Bratov A, Abramova N, Ipatov A. Recent trends in potentiometric sensor arrays – a review. Anal Chim Acta. 2010;678(2):149–159.
17. Bergveld P. Ion sensitive field effect transistor. Proc Conf Biocapt’75, International Conference on Biomed Transducers. 1975;1(3–7):299–304 Paris, France, November 1975.
18. Bergveld P. The operation of an ISFET as an electronic device. Sens Actuators. 1981;1(1):17–29.
19. Bergveld P, Schoot BHvd, Onokiewicz JHL. Development of a microprocessor-controlled coulometric system for stable pH control. Anal Chim Acta. 1983;151:143–151.
20. Bergveld P. The development and application of FET-based biosensors. Biosensors. 1986;2(1):15–33.
21. Bergveld P. Future applications of ISFETs. Sens Actuators B Chem. 1991;4(1–2):125–133.
22. Bergveld P. Thirty years of ISFETOLOGY: What happened in the past 30 years and what may happen in the next 30 years. Sens Actuators B Chem. 2003;88(1):1–20.
23. Sze SM. Semiconductor Devices: Physics and Technology second ed. Hoboken, NJ: Wiley; 2001.
24. Lagraulet A. Current clinical and pharmaceutical applications of microarrays: From disease biomarkers discovery to automated diagnostics. JALA. 2010;15(5):405–413.
25. Saaem I, Ma KS, Marchi AN, LaBean TH, Tian J. In situ synthesis of DNA microarray on functionalized cyclic olefin copolymer substrate. ACS Appl Mater Interfaces. 2010;2(2):491–497.
26. Mir M, Dondapati SK, Duarte MV, et al. Electrochemical biosensor microarray functionalized by means of biomolecule friendly photolithography. Biosens Bioelectron. 2010;25(9):2115–2121.
27. Zhang D, Peng Y, Qi H, Gao Q, Zhang C. Label-free electrochemical DNA biosensor array for simultaneous detection of the HIV-1 and HIV-2 oligonucleotides incorporating different hairpin-DNA probes and redox indicator. Biosens Bioelectron. 2010;25(5):1088–1094.
28. Kessler N, Ferraris O, Palmer K, Marsh W, Steel A. Use of the DNA flow-thru chip, a three-dimensional biochip, for typing and subtyping of influenza viruses. J Clin Microbiol. 2004;42(5):2173–2185.
29. Bousse L. Whole cell biosensors. Sens Actuators B Chem. 1996;34(1–3):270–275.
30. O’Shaughnessy TJ, Pancrazio JJ. Broadband detection of environmental neurotoxicants. Anal Chem. 2007;79(23):8838–8845.
31. Bhunia A. Mammalian cell-based sensors: A rapid screening tool for pathogens and toxins in food. Food Eng Ingredients. 2010;35:15–17.
32. Janata J. Role of analytical chemistry in defense strategies against chemical and biological attack. Annu Rev Anal Chem. 2009;2:321–331.
33. Ni M, Tong WH, Choudhury D, Rahim NAA, Iliescu C, Yu H. Cell culture on MEMS platforms: A review. Int J Mol Sci. 2009;10(12):5411–5441.
34. Zhang S, Tong W, Zheng B, et al. A robust high-throughput sandwich cell-based drug screening platform. Biomaterials. 2011;32(4):1229–1241.
35. Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: State of the art. Tissue Eng Part B Rev. 2008;14(1):61–86.
36. N.F. de Rooij, The ISFET in electrochemisty: the influence of ionic compositions of solutions on the response of the ion-sensitive field effect transistor, PhD Thesis, University of Twente, Enschede, The Netherlands, 1978.
37. L.J. Bousse, The chemical sensitivity of electrolyte/insulator/silicon structure: fundamentals of ISFET operation, PhD Thesis, University of Twente, Enschede, The Netherlands, 1982.
38. A. van den Berg, Ion sensors based ISFET’s with synthetic ionophores, PhD Thesis, University of Twente, Enschede, The Netherlands, 1988.
