Decision Makers’ Summary
This summary is especially addressed to the decision makers within food and drink company chairmen, presidents, chief executives, directors and general managers, who are not normally directly involved in detailed design and implementation of good manufacturing practice (GMP) systems, but whose responsibility it is to establish GMP policies and strategies for their companies, and to provide the necessary authority, facilities and resources to the functional managers and staff to implement the requirements effectively.
In this Guide, GMP is considered as that part of a food and drink control operation that is aimed at ensuring that products are safe, legal, meet integrity criteria and are of the required quality. Effective GMP ensures that products are consistently manufactured to a quality appropriate to their intended use. It is thus concerned with manufacturing practices, food safety, legality, quality and integrity management systems.
The ever‐increasing interest among consumers, retailers, enforcement authorities and other stakeholders such as insurers or shareholders in the conditions and practices employed in food manufacture and distribution heightens the need for the food manufacturer to operate with a clearly defined strategy with high‐level policies together with associated operational procedures and protocols. The ability to demonstrate that the principles and measures identified in this Guide have been fully and effectively implemented could, in the event of a product incident, consumer complaint or formal prosecution, assist the manufacturer in demonstrating that all reasonable steps had been taken to prevent the cause of the incident from occurring, or indeed avoid an offence being committed. Enlightened self‐interest alone should persuade food manufacturers to follow these guidelines.
The manufacturer of a food product must comply with the relevant legal requirements of the country for which the food is intended, for example those of composition, safety, hygiene and labelling. While fulfilling these, however, she/he has a concept of the market at which she/he is aiming and its requirements (e.g. in the case of a food or drink product, its appearance, flavour, texture, presence or absence or amount of particular nutritional components, inbuilt convenience, shelf life, presentation and price). These factors determine the formulation, processing and packaging of the product. Product quality is defined in a product specification that should encompass all of these requirements and express them in a clear, unambiguous manner.
The retailer may approach a manufacturer with a new product concept and request that a manufacturer design a product or process to meet the specific criteria. Of course, the manufacturer’s assessment of what the market wants may be correct or incorrect. While the concept effectively meets all of the law’s requirements, it may, or may not, effectively meet purchasers’ expectations, but unless and until the manufacturer or retailer changes it, the product specification remains the standard with which the product should conform, and GMP is designed to achieve this.
Uniform conformance with product specifications is difficult with food and drink products. The main raw materials for food and drink manufacture derive from nature and are subject to natural variations. In primary production, wide variations may occur among cultivars and also because of seasonal, weather and cultivation differences. In animals, apart from differences between individuals, variance between breeds and rearing systems leads to the potential for inconsistency. Therefore the additional task of the food or drink manufacturer, aided by the knowledge and skills of food science and technology, is to make a reasonably uniform product from variable raw materials by an appropriate combination of raw material selection, raw material pretreatment, formulation adjustment and processing out variation which is outside the boundaries of the product specification.
The Basis for GMP
GMP has two complementary and interacting components: the manufacturing operations and wider management systems [which, for the purposes of this Guide, the Institute of Food Science & Technology (IFST) has designated ‘food control’] (see Figure 1). Both these components must be well designed and effectively implemented. The same complementary nature and interaction must apply to the respective management of these two functions, with the authority and responsibilities of each clearly defined, agreed and mutually recognised. This is not to disregard the importance of other key functions essential to the effective functioning of a company, or indeed of those functions contributing direct services or advice to the manufacturing operation (e.g. purchasing, cost accounting, work study, production planning and engineering maintenance). These terms are explored in more detail in Chapter 2.
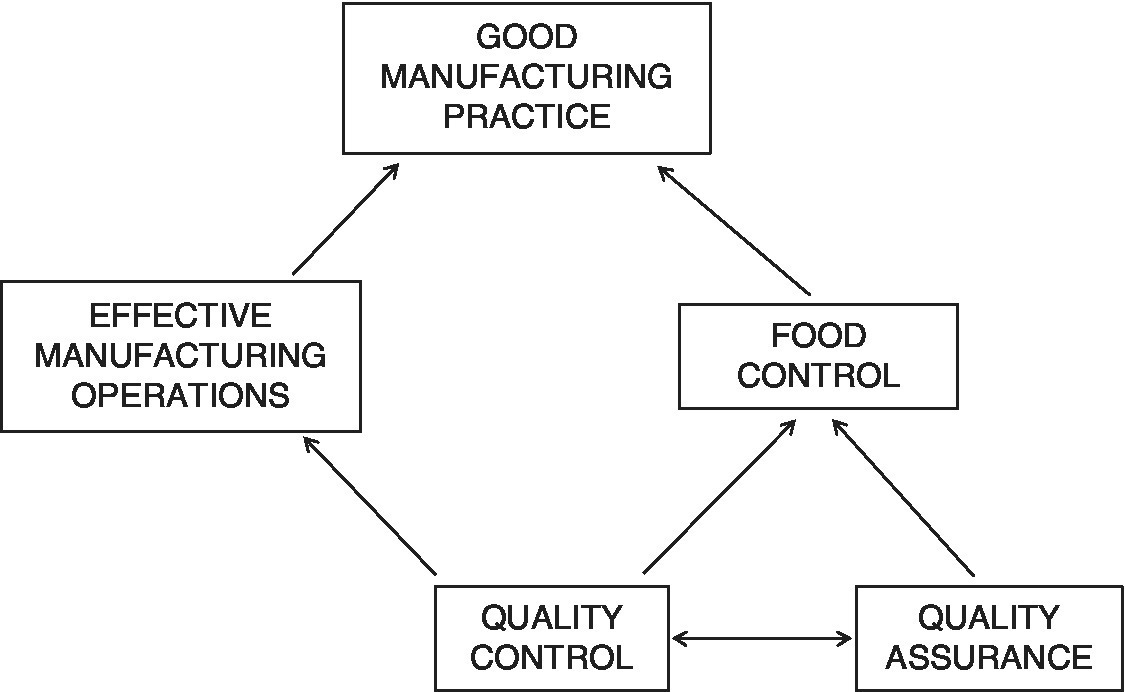
What constitutes ‘well designed’ in these two contexts mentioned above is not just a matter of common sense, or something that would be self‐evident to non‐technical business people. As well as management skills, it also involves extensive and up‐to‐date knowledge of current and emerging quality issues, food safety hazards and best practice in terms of food science and technology relating to the ingredients, processes, packaging and products concerned.
Effective Manufacturing Operations
GMP requires that every aspect of manufacture is fully defined in advance and that all the resources and facilities are specified, namely:
- specific measures undertaken at critical control points (CCPs) based on food safety hazard analysis and food integrity threat analysis, or critical quality points (CQPs) identified in the quality planning process;
- adequate design of premises and suitable manufacturing and storage space;
- suitable process flow with process design to streamline the process and minimise the potential for cross‐contamination;
- correct and adequately maintained equipment;
- appropriately trained people;
- correct raw materials, processing aids and packaging materials;
- appropriate storage and transport facilities;
- documented operational procedures and cleaning schedules;
- appropriate management and supervision; and
- adequate technical, administrative and maintenance services
are provided, in the right quantities, at the right times and places, and are utilised as intended. In order to ensure that operations do proceed according to plan, it is also necessary to:
- provide operators with documented procedures in clear unambiguous instructional language (with due regard to reading, numeracy and language problems);
- train and motivate the operators to carry out the procedures correctly;
- undertake formal review to ensure that training and instruction have been effective;
- avoid, if possible, incentive bonus schemes, but, if unavoidable, to build into any incentive bonus schemes adequate safeguards against unauthorised ‘short cuts’ or trade‐offs;
- provide a food control programme working along the lines indicated below;
- ensure that genuine records are completed during production and that they demonstrate that specified procedures were in fact complied with, and to enable the history of manufacture and distribution of a batch subsequently to be traced should a problem arise or a product withdrawal or recall be necessary;
- establish a well‐planned and effective system to carry out a product withdrawal or recall, should that prove necessary; and
- establish a tried and proved business continuity and crisis management procedure in case of need.
Effective Food Control
The other and complementary major component of GMP is effective food control. Effectiveness requires:
- well‐qualified and appropriately experienced individuals working in food control management participating in the development and validation of process controls and specifications that address the safety, legality, quality and integrity of food;
- competent staff and adequate facilities to undertake all the relevant inspection, sampling and testing of materials, and monitoring of process conditions and relevant aspects of the production environment (including all aspects of hygiene) and management of potential food safety hazards and food integrity threats;
- verification activities that are developed and implemented by appropriately experienced personnel in order to demonstrate that the food products and the process are consistently under the appropriate level of control and identify areas for preventive action where system weaknesses and vulnerabilities are detected; and
- rapid feedback of information (accompanied where necessary by advice) to manufacturing personnel, thereby enabling prompt adjustment or corrective action to be taken and enabling processed material to be approved as fit for either further processing or sale, or to be segregated for decision as to appropriate disposition, for example reject, regrade or reprocessing.
Responsible Management
Of course, the requirements of effective manufacturing operations and of effective food control mentioned above are merely headings and within each there are very many aspects that are considered more fully within the body of this Guide. The Institute hopes that the Guide will prove of help to the management of food and drink companies, to those concerned with private and public verification activities, food law enforcement and consumer protection, to the students who will be the food technologists, engineers and production managers of tomorrow and to those responsible for training them.
The full title of the Guide is Food & Drink – Good Manufacturing Practice: A Guide to its Responsible Management. The reference to responsible management is deliberate. GMP can only stem from policy firmly and uncompromisingly stated and continuously pursued by a company board and general management, which, moreover, provides adequate physical, financial and human resources for the purpose.
