44
CHILLED FOODS
Principle
Chilled foods are perishable foods that, in order to extend the time during which they remain wholesome and safe to eat, are kept within controlled and specified conditions, normally below 8 °C. Chilled foods may be divided into those that potentially offer a low risk of the growth of pathogenic organisms and those where the risk is theoretically high.
General
44.1 The factors that invoke the need for additional care in the manufacture of chilled foods are:
- the perishability of raw materials;
- minimal processing to maximise sensory quality;
- the potential for spoilage and/or pathogen growth;
- the rapid despatch of finished products; and
- chilled food chain requirements.
44.2 Given the short shelf life of chilled prepared foods, the emphasis must be on the use of hazard analysis critical control point (HACCP) to develop a system to control processes, premises, personnel and the hygienic status of ingredients/raw materials used, rather than end‐product testing (see Chapter 3). Product safety must be determined by proper consideration of the following:
- ingredient hygienic quality;
- product formulation/characteristics;
- processing parameters;
- allergen control;
- intended use of product;
- storage and distribution conditions;
- manufacturing hygiene; and
- shelf life.
Ingredients
44.3 Points to consider:
- Which pathogen(s) and level of contamination might be present?
- Is there a possibility of preformed toxins?
- What are reasonable specification levels to apply to minimise risk?
- What further processing is to be applied?
- Does the shelf life of the ingredients exceed the shelf life of the finished product?
44.4 Specifications for ingredients/raw materials should include microbiological standards. Ideally, on‐site facilities for microbiological testing should be available. Where this is not available, provision should be made for adequate microbiological testing at a laboratory that has suitable third‐party accreditation (see Chapter 38). The UK Health Protection Agency (now Public Health England (PHE)) in England in 2009 produced guidelines for assessing the microbiological safety of ready‐to‐eat foods placed on the market.1
44.5 Perishable ingredients/raw materials should be purchased only from approved suppliers, who should furnish regular test results and agree to warn the purchaser of any problems in maintaining the standards agreed (see Chapter 23).
44.6 Deliveries of highly perishable raw materials should not be accepted if their temperatures fall outside agreed specified ranges. Guidance on recommended storage conditions should be given on outer packaging. Temperature checks undertaken must be recorded and the equipment used must be calibrated at designated intervals.
44.7 Inspection of perishable raw materials should be based on risk assessment and may include rapid indicative testing methods. However, since testing in itself is not a control measure, as recognised by HACCP, the emphasis is on proactive controls.
Product Formulation/Characteristics
44.8 The growth of pathogenic micro‐organisms can be controlled by product formulation/characteristics. This might include:
- adjustment of pH; and/or
- adjustment of water activity (aw); and/or
- addition of chemical preservative.
An individual factor, such as pH, may be used to reduce microbiological growth. Introducing an additional factor, such as water activity, may produce a synergistic effect, that is, the combination of the two factors as hurdles reduces microbiological growth to a greater than expected or calculated extent. Relatively small changes to both hurdles together (e.g. pH + aw) may be as effective as large changes to either hurdle in isolation. Chemical preservatives are rarely added direct to chilled prepared foods, but may be used in the preservation of chilled cooked meats, for example. However, where they are used, this must be in compliance with the regulatory requirements of the country concerned, and should be at the minimum effective level to ensure product safety, while not themselves presenting a food safety hazard.
Processing Parameters
44.9 This aspect should be considered under the following subdivisions and outcomes:
- Heat Treatments
- None or less than 70 °C for 2 minutes. Possibility that all pathogens present will survive.
- Heated to 70 °C for 2 minutes (or equivalent). All vegetative pathogens present will be reduced to an acceptable level (6 log reduction), for example Listeria monocytogenes, Staphylococcus aureus, Salmonellae and verocytotoxigenic Escherichia coli. However, spores and preformed toxins may persist. The potential for recontamination by vegetative pathogens must be limited by the use of a high‐risk area (HRA) (see 44.17).
- Heated to 90 °C for 10 minutes (or equivalent). In addition to vegetative pathogens, spores of psychrotrophic (non‐proteolytic) Clostridium botulinum will be reduced to an acceptable level (e.g. 6 log reduction in this case). However, more heat‐resistant spores, for example strains of Bacillus cereus and some preformed toxins, may persist. Measures to prevent the outgrowth of psychrotrophic C. botulinum must be in place where chilled products have a shelf life of more than 10 days.
- Cooling
- Heated product should be cooled as quickly as possible through the so‐called danger zone that is the temperature range of 5–63 °C. This will minimise risk of spore germination and outgrowth. The time taken for cooling will vary from product to product but, as a guideline, should be no more than 2 hours. Blast chillers are often used to achieve this temperature profile.
- Packaging This should be considered under the following subdivisions and outcomes:
- Product cooked in‐pack. Pathogens will be eliminated to the extent indicated in 44.9a and there will be no opportunity for recontamination that may present a food safety hazard (assuming complete integrity of the pack/seal).
- Product cooked, cooled and assembled. Pathogens will be eliminated to the extent indicated in 44.9a, but there is risk of recontamination during assembly that may present a food safety hazard.
Modified atmosphere packaging (MAP) or vacuum packaging may be used to reduce microbiological growth in conjunction with chilled storage, but will not necessarily inhibit the growth of pathogens. There may be particular concerns with respect to C. botulinum, which is anerobic. It is important that the effectiveness of MAP or vacuum packaging is assessed in each case, with reference as necessary to specialist advice given that most pathogens are facultative anaerobes.
Intended Use of Product
44.10 The key point to consider is whether the product is to be eaten:
- without further heating (i.e. is ready to eat);
- following domestic reheating (i.e. requires reheating only prior to consumption); or
- following domestic cooking (i.e. requires a heat process equivalent to at least 70 °C for 2 minutes prior to consumption).
On‐pack instructions are an important control measure to ensure that correct procedures are followed and food safety risk is reduced. Any instructions provided by the manufacturer must be validated to demonstrate their efficacy with records retained, and in the event of a change to the product the instructions must be revalidated, for example increasing the size of chicken pieces in a ready meal or pie.
The Chill Chain
44.11 Temperature and time control are the principal controlling factors for the safety of chilled foods. Effective temperature control throughout the chill chain is particularly important to slow or inhibit the growth of pathogenic bacteria. Chilled foods, for reasons of safety or quality, are designed to be stored at refrigeration temperatures (at or below 8 °C, targeting 5 °C) throughout their entire life. The performance of the proposed distribution chain should be validated and monitored by the responsible party and taken into account when specifying shelf life. The number and location of temperature probes in storage should be such as to ensure effective monitoring. The manufacturer must consider whether automatic failure alert systems, such as visual or audible alarms, or processing fail‐safe systems should be in place. If such systems are used, then they must be monitored at a designated frequency by either quality control or production personnel who have been adequately trained and understand the significance of such failures and the appropriate action to take.
Records should be maintained of the monitoring and verification activity and the corrective action taken in the event of failure. If these points in the process are deemed within the HACCP plan to be critical control points (CCPs), there must be an appropriate level of awareness among all personnel of the consequence of a target level and tolerance and/or a critical limit being exceeded. Visual or audible alarms can be designed into the process to be activated if a critical limit or target level is exceeded, for example at the metal detection stage and diversion of product at a pasteuriser. The automatic failure alert systems must be tested on a routine basis and records maintained of the tests, the results and any remedial action taken. Calibration procedures must be in place for all automatic failure alert systems (see Chapter 34).
Manufacturing Hygiene
44.12 The purpose of establishing specified standards of personnel and premises hygiene is to control the hazard of microbiological contamination and cross‐contamination. The level of hygiene required will depend on the risk of and/or the consequences of cross‐contamination (see Chapters 19 to 22).
44.13 Chilled foods are manufactured using a wide variety of raw materials, processes and packaging systems, and therefore must be expected to have both differing microbiological profiles after manufacture or storage and differing shelf lives, but must be microbiologically safe at the point of consumption. Thus, products made entirely from ingredients that are heated in a container or are assembled from heated ingredient(s) under the special hygiene conditions defined as ‘high risk’ are designed to be free from vegetative pathogens, but not all sporeformers. Those containing raw ingredients will from time to time contain vegetative pathogens such as Listeria as well as toxin producers and/or spore formers. This difference must be taken into account when specifying shelf life in terms of time and temperature and consumer instructions. Since Listeria is able to grow under chilled temperature conditions, and is responsible for the greatest number of deaths from foodborne illness in the UK, EU and USA, this is an important organism to control. In doing so, the risk of other vegetative pathogens being present is also minimised.
Shelf Life
44.14 Shelf life depends on the control of all the preceding factors, but must be validated by challenge testing and/or modelling for each product and process for defined chill storage conditions. It must be recognised that the integrity of the whole of the chill chain is vital to ensure the safety and quality of chilled foods.
44.15 By developing a product utilising a combination of factors such as raw material quality, hygienic processing, temperature, water activity, acidity and modified atmosphere, microbiological growth can be controlled and thus spoilage and/or food‐borne illness prevented. The choice and combination of hurdles will determine the shelf life and the conditions of use of the products.
44.16 Consult CCFRA Guideline G46 Evaluation of Product Shelf Life for Chilled Foods (2004) and Shelf life of ready to eat food in relation to L. monocytogenes—Guidance for food business operators (CFA/BRC/FSA, 2010) for further information on the determination of shelf life. Further guidance can be found in the guidance section of this publication (Appendix IV).
Application of HACCP
44.17 There are distinct terms that need to be considered when applying HACCP to the safety of chilled foods:
- Ready to eat (RTE): Food intended by the producer or the manufacturer for direct human consumption without the need for cooking or other processing that is effective to reduce to an acceptable level or eliminate microorganisms of concern (i.e. cold eating).
- Ready to cook (RTC): Food designed to be given a heat process by the consumer that will deliver a 6‐log kill with respect to vegetative pathogens (a minimum process equivalent to 70 °C for 2 minutes) throughout all components.
- Ready to reheat (RTRH): Food manufactured in a high‐care area (HCA) or HRA that has been designed to be reheated by the final consumer.
- Low‐risk area (LRA): An area where good manufacturing practice standards are in place as described within this publication, but the area and the practices have not been specifically designed to minimise microbial contamination, for example raw material intake, storage areas of RTC foods and packaged product where the product is fully enclosed (see 43.19).
- High‐care area (HCA): An area designed to a high standard of facility specification and hygienic design where practices relating to personnel, ingredients, equipment and environment are managed to minimise microbial contamination of a RTE or RTRH product containing uncooked ingredients. HCAs include:
- areas where RTE and RTRH food is being produced/assembled; and
- areas where RTE/RTRH ingredients not thermally processed (minimum 70 °C for 2 minutes) but that have been decontaminated and grown/produced to RTE standards are stored and handled.
- High‐risk area (HRA): An area designed to a high standard of facility specification and hygiene design where practices relating to personnel, ingredients, equipment and environment are managed to minimise microbial contamination of a RTE or RTRH product comprising only cooked ingredients:
- areas where RTE and RTRH food is being produced/assembled; and
- areas where only thermally processed foods (minimum 70 °C for 2 minutes for <10‐day shelf life) are stored and handled.
44.18 Figure 44.1 represents decision trees formulated by the Chilled Food Association (CFA) (http://www.chilledfood.org), whose permission to include them and assistance with the preparation of this chapter are gratefully acknowledged. The first decision tree shows the basic decision pathway. Both the heat treatment (if any) applied in manufacturing the food product, any microbiological hazards arising between the heat treatment and final pack sealing and the final status of the food (i.e. ready to eat, ready to reheat or ready to cook) must be considered.
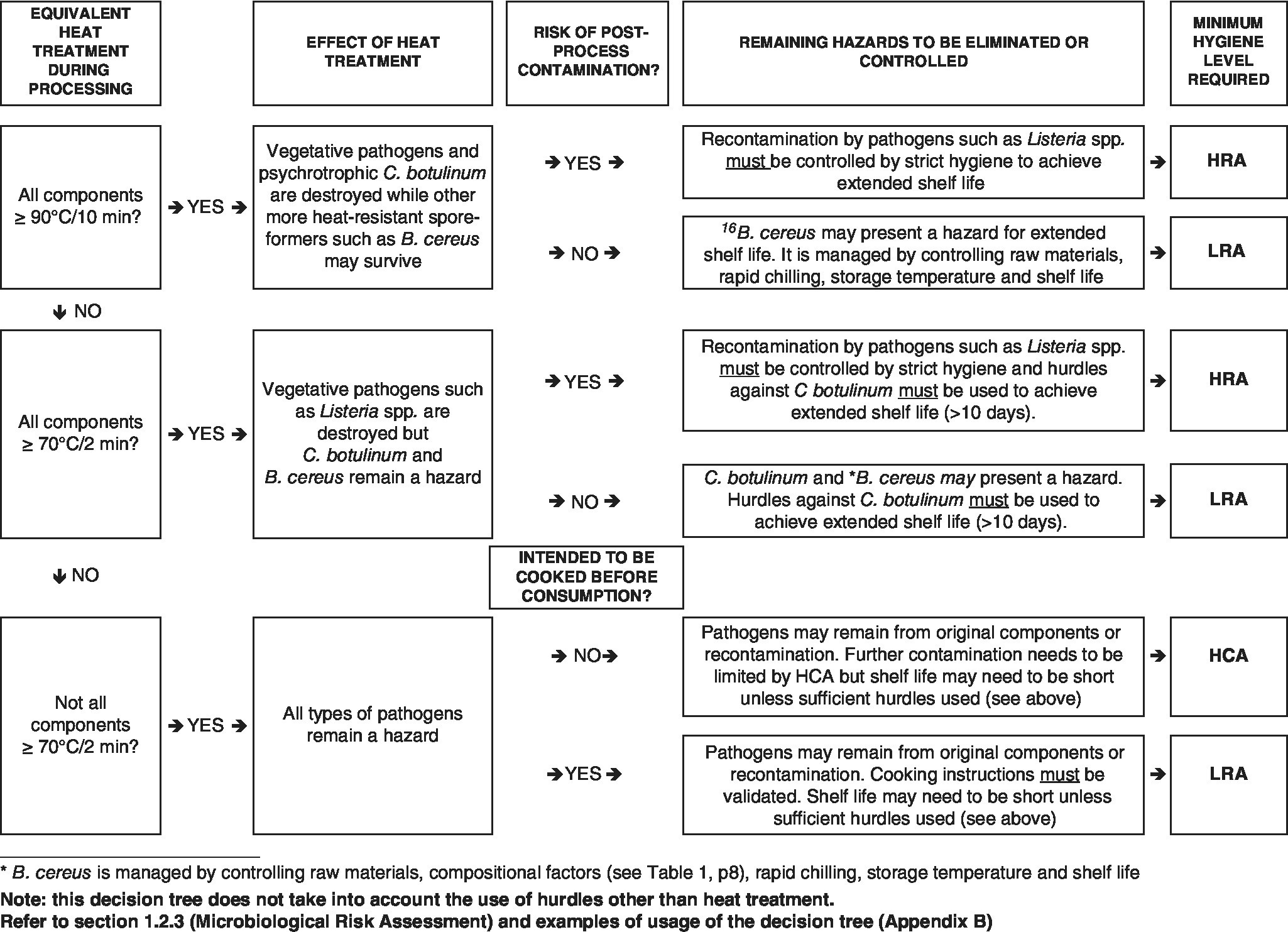
Figure 44.1 Decision tree to determine the minimum hygienic status required for chilled products.
In general, only cooked‐in‐pack products are free of any risk of recontamination between heat treatment and final pack sealing and can therefore be produced in an LRA. The completed pathway through the decision tree shows the minimum hygiene status of the product handling environment between heat treatment and final pack sealing.
44.19 A detailed description of the requirements in each of the above areas is given in the CFA’s Best Practice Guidelines for the Production of Chilled Food (4th edition, 2006). The standards highlighted are minima for each type of product. Higher standards can be used, but due regard should be paid to the potential for cross‐contamination between lower‐status and higher‐status products within a single‐status environment. Other products must not be produced in an HCA unless HACCP shows there are no additional risks to all products. Expert microbiological advice should be sought if necessary.
Quality Control
44.20 Microbiological testing of raw materials, intermediate products and finished products should aim at monitoring and verifying standards and trends, not simply accepting or rejecting on the basis of results. In cases of unsatisfactory results, recall procedures would be necessary; therefore any adverse trend in results calls for immediate investigation of process conditions and controls, including in the supply chain, thus reducing the risk of a recall situation developing (see Chapter 27). It should be determined whether a positive or negative release system is in place with regard to microbiological testing.
44.21 Production programmes should provide sufficient time for adequate cooling; a deadline for acceptance of late orders should be established to ensure this. Care should be taken to avoid condensation on cold product by exposure to warm product or humid conditions.
44.22 The correct functioning of refrigerated despatch vehicles should be checked before loading, and product temperatures should be checked and marked on delivery notes before loading. At maximum load, despatch vehicles should be able to maintain specified temperatures and ensure that products carried remain within appropriate temperature profiles throughout the load and for the whole journey. A system should be in place to monitor the vehicle’s operating temperature. This could include manual logging of data on the appropriate record during transport of the product, inspections using a calibrated temperature probe at each delivery point (where the temperature is formally recorded) or data logging devices where the data can be electronically downloaded. All equipment must be checked at routine intervals to verify that the results obtained are accurate and the equipment is working within an acceptable tolerance (see Chapter 34). Documented transport breakdown procedures should be in place that define the actions to be taken in the event of vehicle or refrigeration unit breakdown, and the records to be maintained that detail the incident and corrective action taken.
44.23 The quality control function should liaise with distribution management and customers to establish that there is adequate provision for maintenance of the temperature‐controlled chain during transport and storage with the need for this being understood by all personnel. Major control points also include the occasions when products may not be in a temperature‐controlled environment during the loading and unloading of vehicles, chilled stores and retail display cabinets.
44.24 Any frustrated deliveries that are returned should be examined by quality control, who should ensure that only goods in standard condition are accepted for further despatch. Other returns, suitably labelled, should be kept separately until disposal is agreed and actioned.
High‐risk Areas
44.25 Special conditions must be provided for the production of high‐risk products. Detailed requirements are set out in the CFA Best Practice Guidelines. The production area should be completely separated from other areas from floor to ceiling. Positive pressure ventilation with micro‐filtered air at the appropriate temperature and humidity should be provided. However, rather than a specific pressure being recommended, which is technically challenging to measure, a minimum number of air changes per hour is the preferred control parameter. Entry and exit should be through changing rooms, with ‘no‐touch’ washing facilities. Emergency exits from the production area should be alarmed so that access via these exits can be monitored. Personnel must wear, as instructed, designated internal footwear and clothing. Only fully processed food components and packaging materials should be admitted, with entry through hatches/airlocks. Chill rooms for products awaiting prepacking should be part of the designated special area. Construction and finish should provide for easy cleaning and disinfection. Only essential equipment and materials should be permitted in the production area. External packaging and ingredients entering the area may be subject to a sanitising procedure. Production should be interrupted for frequent thorough cleaning and disinfection. The frequency should be determined by risk assessment, which should be formally recorded and retained together with any results of verification activities such as product sampling or swab results. Otherwise cleaning and disinfection or sanitation may be undertaken between product types or varieties in addition to other hygiene activities, for example between the assembly of different sandwich types.
44.26 Only named, authorised and medically cleared individuals should enter the production area. This includes management, technicians, engineers and visitors. It must be impressed on visitors, contractors and staff how critical it is to maintain personal hygiene and minimise the risk of product contamination (see Chapter 17). Medical screening should be undertaken with emphasis on:
- skin health – absence of acne, boils, other infections, wounds and burns;
- ear, nose and throat infections;
- gastrointestinal disorders; and
- contact with known cases of food poisoning.
44.27 Pre‐employment screening should be made by a physician with experience in occupational medicine. The health status of selected employees should be reviewed by the physician at appropriate intervals, and a daily check, before starting work, should be made by an occupational nurse or designated individual.
44.28 Before entering the special area, outer garments should be changed for clean overalls and internal footwear. Effective head covering, coloured distinctively, should be worn. Beards and long hair should be discouraged, but if worn should be fully contained. Hands should be washed with a non‐aromatic antibacterial soap or lotion. Personnel should not use the same catering or toilet facilities as personnel from other areas (see Chapter 17).
44.29 Designated equipment should be provided in each area to prevent cross‐contamination. This includes maintenance equipment that should be designated to specific areas, for example HRA, so that the tools can only be used in that area.
