Chapter 5
Graphene and Graphene Oxide Polymer Composite for Biosensors Applications
Aftab Aslam Parwaz Khan1,2, Anish Khan1,2 and Abdullah M. Asiri1,2
1Center of Excellence for Advanced Materials Research, King Abdulaziz University, Jeddah, 21589, Saudi Arabia
2Chemistry Department, Faculty of Science, King Abdulaziz University, Jeddah, 21589, Saudi Arabia
5.1 Introduction
Polymer nanocomposites have turned into an essential zone in present-day science and technology [1]. They are generally characterized as the combination of a polymer matrix and fillers having a measurement on the nanometer scale. They have uses in numerous areas, for example, energy storage, structural materials, computing and sensors, biomedical, and so on [2, 3]. Graphene, the most recent sensation in current science, has brought about an extraordinary revolution in the field of nanoscience and innovation. Since its discovery in 2004, this “thinnest material” has been receiving huge scientific attention owing to its wonderful chemical and physical properties [4]. Graphene and graphene-based materials have shown tremendous application potential in different fields, for example, in supercapacitors, electronic devices, functional electrodes, rechargeable batteries, biomedical, sensors, and in numerous others [5]. Graphene, a layered carbon material that comprises sp2-hybridized carbon atoms organized in a two-dimensional honeycomb lattice, has drawn a great deal of consideration of late and has been considered as a promising filler for polymer matrices as a result of its extraordinarily large specific surface area, room-temperature Hall effect, a tunable bandgap, high mechanical strength, high electron mobility, high transparency, and electrical conductivity [6, 7]. Due to all these, as described by Novoselov, it is considered as a supernatural occurrence material [8]. The graphene-sheet-incorporated polymer matrix composites have poor attachment between the graphene layers and the polymer matrix. Thus, many methodologies have been completed to enhance the adhesion of graphene sheets by modifying the structure. There are three modified graphitic structures which are named graphene oxide (GO), extended graphite, and graphite intercalated compounds.
The principal form, graphene oxide, is prepared by a simple and modest technique for acid treatment of graphite. During this treatment, graphite is oxidized and delivers GO sheets. The functional groups, for example, carboxyl, epoxy, and hydroxyl groups present on the graphene layers keep the agglomeration of the graphene sheets. This enhances the interaction between the GO and polymer matrix in polymer composite fabrication [9, 10]. The detailed strategies yield 99% of multilayer graphene, named stacked graphene platelets, and just 1% of monolayer graphene sheets [11]. Full depictions of creating graphene through chemical and mechanical, bottom-up, and top-down procedures is beyond the extent of this review; however, they exist in the literature [7, 12, 13]. Different methodologies have been developed to enhance the dispersion, and oxidizing graphene is one of the straightforward and well-known techniques.
GO is thought to be a highly oxidative form of graphene, which has various different types of oxygen functionalities at the edges [14, 15]. The synthesis of GO is dependeent on three preparation methods [16–18]:
- 1. Brodie’s method
- 2. Staudenmaier’s method
- 3. Hummers’ method
The major part of the considerable number of strategies is the chemical exfoliation of graphite utilizing an oxidizing agent within the mineral acid. The Brodie’s and Staudenmaier’s methods concern a mixture of KClO4 and HNO3 so as to oxidize graphite. The Hummers’ method utilizes the expansion of graphite to KMnO4 and H2SO4. At the point when GO is dispersed in water, it forms a stable light-brown colloid GO because of the electrostatic repulsion of adversely charged GO sheets. Figure 5.1 demonstrates the images of GO that are brown in color and the graphene sheets after reduction by hydrazine. Prominently, GO is regularly used toward getting another graphene variation, reduced graphene oxide (rGO), which looks like pristine graphene. Ordinarily used chemical reducing agents incorporate hydrazine monohydrate/sodium borohydride. Thermally reduced graphene oxide (TRGO), then again, can be acquired by quick heating of dry GO in a static environment at 1000 °C till 30 s, delivering sheets of rGO and exfoliated GO [19–21].
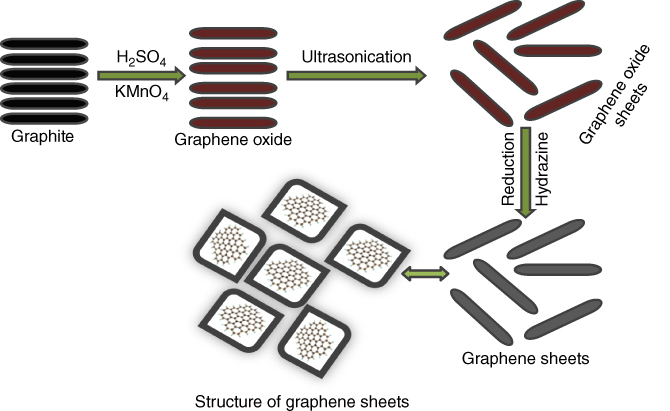
Figure 5.1 Synthesis of graphene and graphene oxide by Hummers’ method.
Exfoliation happens after the pressure produced through the gas CO2 that has evolved because the decay of the hydroxyl and epoxy sites of GO go beyond the van der Waals forces, grasping the GO sheets jointly. Around 30% weight loss is related to the deterioration of the oxygen groups and vanishing of H2O. The exfoliation prompts a volume increase of 100–300 times, producing very low-mass-thickness TRGO. On account of the structural change brought about by the loss of CO2, these sheets are much wrinkled, as shown in Figure 5.2. Eighty percent of the TRGO sheets are single layers with a normal size of around 500 nm, free of the beginning GO size [22].
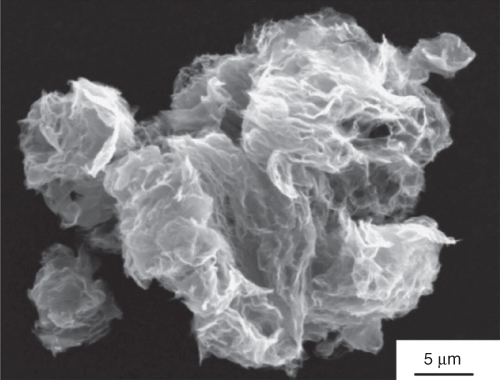
Figure 5.2 SEM of dry, as-produced TRGO powder. The sheets are very agglomerated, and the particles have a fleecy morphology [22].
(Copyright 2006 Reproduced with permission from American Chemical Society.)
The historical backdrop of biosensors started in 1962 with the improvement of the first enzyme-based glucose-sensing device by Clark and Lyons [23]. This novel biosensing-device-dependent happening was a thin layer of glucose oxidase (GOx) entangled in an excess of oxygen electrode by means of a semi-permeable dialysis film. Estimations depended on the observing of the O2 devoured through the enzyme-catalyzed reaction as follows:
A few types of graphene can be utilized as part of biosensor applications, as a mechanism of polymer materials. These have additionally been widely investigated elsewhere [7], so just a few are incorporated here.
5.2 Polymer–Graphene Nanocomposites and Their Applications
One method of utilizing the plenty of appealing properties offered by graphene is to use it in a composite [24], for instance, by including it as a nanofiller in a polymer matrix, forming a polymer–graphene nanocomposite [3, 25, 26]. This approach can be utilized to change/improve existing properties of the polymer and nanofiller, yielding materials with new properties, for example, conductivity or functional groups, along these lines and encouraging new applications. Especially significant to the field of biosensing are characteristically conducting polymers (CPs) [27, 28]. Although various difficulties still remain in developing graphene-based polymer composites, these materials as of now have been investigated for the extent of their use in various fields, as shown in Figure 5.3.

Figure 5.3 Applications of graphene-based polymer composites.
Unlimited exhibits of conceivable polymer–graphene nanocomposites have been generally reviewed [3, 25, 26]. Here, we concentrate on those polymer matrices that present the potential for improvement of polymer–graphene nanocomposite stages that could be used for different biosensing applications. However, there is extraordinary and developing enthusiasm for using the scope of various polymer matrixes to deliver graphene nanocomposite materials for biosensor applications with an extensive variety of properties.
Biosensors are drawing in expanded consideration because of their potential applications in clinical science, food industry, and ecological fields [29]. They are becoming critical to present-day life from analysis of life-undermining ailments to detection of biological agents in warfare. Of late, CPs have drawn much consideration in the advancement of biosensors [30]. An electrochemical biosensor can be made by immobilizing the biological part onto the CP. Utilization of CPs, for example, polyaniline (PANI), polypyrrole (PPy), and poly-3,4-ethylenedioxythiophene, in the preparation of amperometric biosensors has been reported [31–33]. Be that as it may, there is developing enthusiasm for using the scope of various polymer matrixes to create graphene nanocomposite materials for biosensor applications with an extensive variety of properties. In this, we show recent research endeavors that have announced significant sensing execution utilizing nanobiosensors and talk about the possibilities of scientific and innovative difficulties for getting ready cutting-edge biosensors. We concentrate on two CP nanomaterials PANI, and PPy, in light of the fact that they are regular CPs that can be effectively arranged. Thus, the section chose CPs (Figure 5.4a,b) from chemical structures, including the intrinsic CPs PANI and PPy. These chosen polymers have pulled in impressive consideration in fabricating biosensors, as obvious from the developing number of research publications.

Figure 5.4 (a) Polyaniline and (b) polypyrrole.
5.2.1 Polyaniline
PANI is a standout among the most characteristic CPs, with improved conductivity, great environmental stability, and assorted shading changes relating to various redox states. PANI nanomaterials can connect in different gadgets and sensor applications. Therefore, various reviews have concentrated on the synthesis and application of PANI nanomaterials [34]. Unfortunately, the utilization of PANI nanomaterials in biological applications is restricted by their low process ability, the absence of flexibility, and non-biodegradability.
Likewise, these materials have been noted to bring about chronic inflammation once embedded [35, 36]. PANI has been examined for use in biosensors and a few other applications [37]. These different graphene-based PANI nanocomposites have been used for biosensing glucose with the expansion of polyvinylpyrrolidone (PVP), AuNPs, interleukin-6, cholesterol, ammonia, oxalates, serotonin, and medications, for example, artesunate [38–43]. Ruecha et al. used PVP to help balance out the dispersion of graphene and create graphene/PVP/PANI-nanocomposite-based biosensors by means of electrospraying [39]. GN/PVP/PANI-nanocomposite-modified paper-based cholesterol biosensor, H2O2 was created from the enzymatic reaction among cholesterol and cholesterol oxidase, as shown in Figure 5.5.

Figure 5.5 The enzymatic reaction among cholesterol and ChOx on G/PVP/PANI-modified-paper-based biosensor [39].
(Copyright 2013. Reproduced with permission from Elsevier.)
The GN/PVP/PANI paper-based biosensor was likewise work accomplished for the recognition of cholesterol in human serum and acquired percentages of recoveries in the range of 100.0–102.0%, and the RSD was under 5.0%, checking that this sensing system is exceedingly exact. The glucose biosensing by Xu et al. [40] is a recognized graphene/PANI/AuNP nanocomposite for the immediate electron transfer of glucose oxidase. The exceptionally encouraging work of Das and Yoon on urea biosensing [44] includes the utilization of a sulfonated graphene/PANI nanocomposite as an electrochemical signal is produced by the SG-PANI film because of the relationship between graphene SO3− and NH4+ to form graphene–SO3−NH4+. This biosensor showed great sensitivity (0.85 μA cm−2 mM−1), quick response, and better stability, while other related reviews utilized polymer electrolytes. Polyaniline–graphene composites have likewise been used for DNA biosensing. Yang et al. [45] reported a basic and temperate way to deal with and build an immediate DNA-sensing stage in view of the self-redox signal change of very conductive sulfonated polyaniline (SPAN) improved by GO. In view of plentiful sulfonic acid groups, the subsequent nanocomposite showed the self-redox signal even at physiological pH. At the point when the adaptable DNA was effectively united through being covalently connected to the modified electrode, as shown in Figure 5.6, the electron transfer between electrode and buffer was limited. Thus, the inward impedance estimation of SPAN expanded fundamentally. After hybridization, the rigid helix opened the electron channel, which incited the impedance value to diminish drastically. As an underlying utilization of this system, fusion gene sequence formed from promyelocytic leukemia and retinoic acid receptor alpha was effectively recognized.
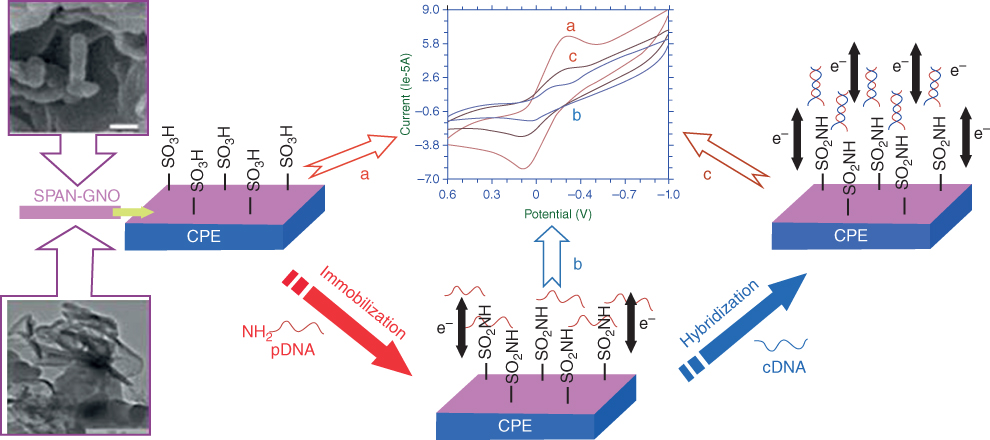
Figure 5.6 Schematic outline of the DNA recognition on various electrode modifications [45].
(Copyright 2013. Reproduced with permission from American Chemical Society.)
Bo et al. [46] have built up a DNA biosensor in view of oxidized graphene–PANI nanowire-modified glassy carbon electrode (GCE). The biosensor showed a quick amperometric response, high sensitivity, and good storage stability for the DNA detection. In this work, daunomycin was utilized as an indicator by means of intercalating in the DNA duplex during hybridization, as shown in Figure 5.7, and delineates the schematic of the preparation technique of the ssDNA/PANI/graphene/GCE electrode.

Figure 5.7 The assembly process of the electrode [46].
(Copyright 2010. Reproduced with permission from Elsevier.)
Feng and coworkers [47] portrayed a DNA electrochemical biosensor for the identification of the BCR/ABL fusion gene in chronic myelogenous leukemia (CML) by electrochemical graphene sheets (GS) arranged on chitosan (CS), PANI layer, and Au nanoparticle (AuNP)-modified GCE to immobilize the capture probes. The catch probe utilized a hairpin structure and was dually named with a 5′-SH and a 3′-biotin. The biotin terminal acted as an affinity tag for the enzyme binding, which was shielded by the atretic hairpin structure from being approached by streptavidin–alkaline phosphatase. Hybridization of target DNA constrained the probe to open and the biotin to be far from the electrode. Consequently, the biotin name ended up noticeably open by streptavidin–alkaline phosphatase, and the catalytic signal was seen by utilizing the 1-naphthyl phosphate as the enzymatic substrate. The detection methodology is shown in Figure 5.8. Taking points of interest from AuNPs/PANI/CS–GS, biotinavidin signaling intensification and alkaline phosphatase, the DNA sensor demonstrated high detection sensitivity, and it had been connected for examining longer DNA chains at around 600 bp in polymerase chain reaction (PCR) genuine specimens with great outcomes.
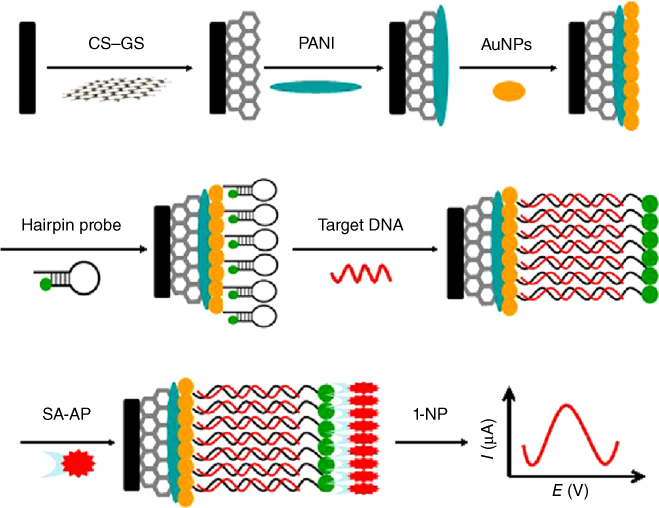
Figure 5.8 Schematic illustration of the electrochemical DNA sensor development process [45]. (
Copyright 2013. Reproduced with permission from Elsevier.
Du et al. [48] depicted a DNA electrochemical biosensor for the detection of specific gene sequences by electrochemically reduced graphene oxide (ERGO) prepared on PANI nanofiber-modified GCE. The change in the interaction between DNA and ERGO can be probed if there should be an occurrence of ssDNA and dsDNA by the redox current changes of [Ru(NH3)6]3+. The active range of the DNA biosensor for detecting the sequence-specific DNA of cauliflower mosaic virus (CaMV35S) gene is from 1.0 × 10−13 to 1.0 × 10−7 M, with a detection limit of 3.2 × 10−14 M. See the schematic outline of the DNA biosensor in Figure 5.9.

Figure 5.9 Schematic outline of the DNA biosensor [48].
(Copyright 2011. Reproduced with permission from Elsevier.)
Jiao and coworkers have created DNA sensors in light of PANI/ERGO-modified electrodes using the voltammetric strategy [49].
The biosensor showed superb execution with a wide linear range (1.0 × 10−15–1.0 × 10−8 M) and a low detection limit of 2.5 × 10−16 M, for the determination of promyelocytic leukemia/retinoic acid receptor alpha sequence with a probe of [Fe(CN)6]3−/4−. See the schematic portrayal of the immobilization and hybridization of DNA on this modified electrode in Figure 5.10, all with extraordinary potential for biosensing applications. Be that as it may, it shows potential work might be offered by the approach of Xue et al. on serotonin biosensing [50] who arranged rGO/PANI nanocomposites film as a base layer for the electropolymerization of an AuNP and p-aminothiophenol MIP biorecognition interface in Figure 5.11.
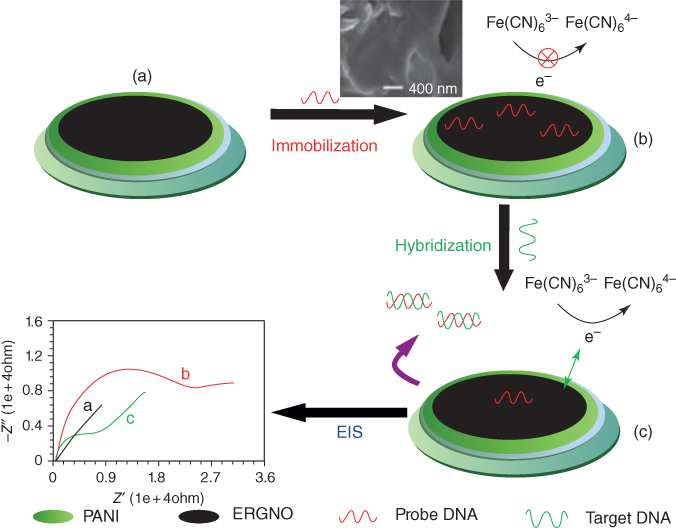
Figure 5.10 Schematic portrayal of the immobilization and hybridization of DNA on the rGO/PANI/GCE [49].
(Copyright 2012. Reproduced with permission from Elsevier.)
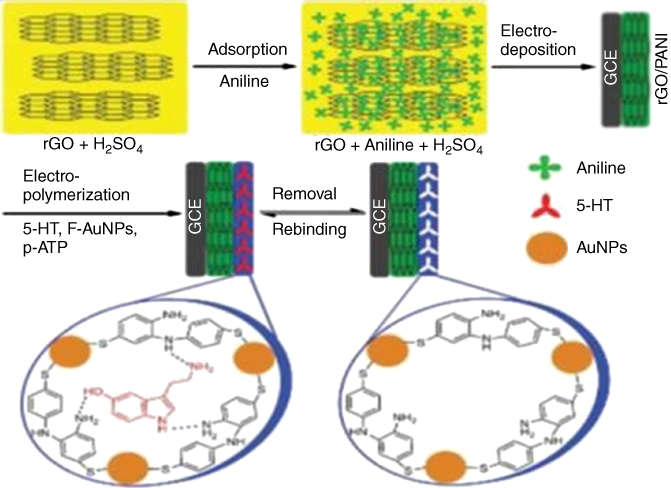
Figure 5.11 Serotonin (5-HT) biosensor preparation scheme [50].
(Copyright 2014. Reproduced with permission from Elsevier.)
This sensor yielded a noteworthy nanomolar detection limit, while resisting interference from different species, for example, dopamine and ascorbic acid.
5.2.2 Polypyrrole
PPy nanomaterials, for example, nanotubes (NTs), nanoparticles, core–shell nanomaterials, and hollow nanospheres, have been utilized as a potential system in electronic gadgets and sensors. In any case, of late, PPy nanomaterials have likewise been utilized for biosensors in view of their facile functionalization and environmental stability. In this area, we talk about a few nanobiosensors utilizing PPy nanomaterials with graphene and GO.
On the premise of the high electrocatalytic movement of graphene toward H2O2 and glucose oxidase, PPy/GR combining with GOx could be an incredible electrode material for oxidase biosensors. Its electrochemical detection can be acknowledged by utilizing GOx as the mediator. Accordingly, an extensive variety of PPy composites, including many using graphene, were accomplished. For example, sulfonated graphene has been used as a major aspect of electrochemical co-deposition [51] and in situ polymerization of pyrrole [52]. Contingent upon the SG/PPy composition and composite synthesis method, it is conceivable to get materials with electrical conductivities of up to 50 S cm−1. Vasantha et al. [53] revealed the rGO-based dopamine sensor with sodium dodecyl benzene sulfonate-doped nano-PPy film-modified electrode which displayed a correlation coefficient of 0.9884, while good sensitivity and LOD were observed to be 13.07 mA mM−1 and 20 nM at S/N = 3, individually. Song et al. [54] utilized enhanced sensor hydrophilic polymer/PPy/GO nanosheets for electrocatalytic applications, all the while determining DA and AA. It was found that the distinctive exhibitions of the derived electrochemical biosensors PAM/PPy/GO, PAA/PPy/GO, and PVP/PPy/GO-modified GCEs in electrocatalytic applications and PAM/PPy/GO indicated excellent sensitivity and selectivity for detection of DA and AA. The synthesis utilizing the technique is illustrated in Figure 5.12, for PAM/PPy/GO, PAA/PPy/GO, and PVP/PPy/GO.
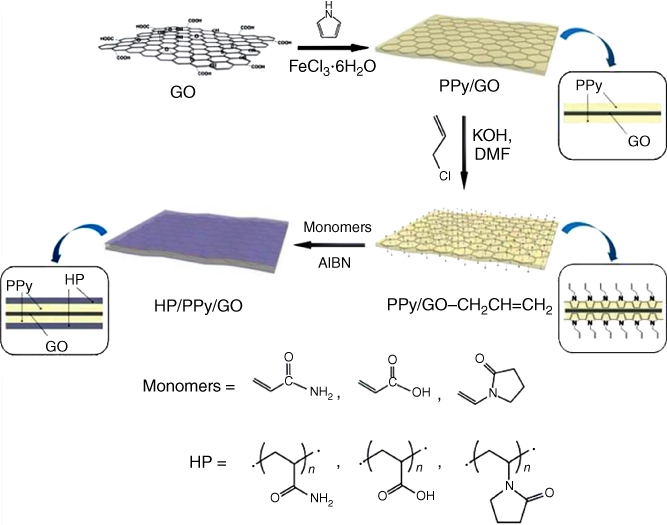
Figure 5.12 The reaction procedure used to set up the HP/PPy/GO nanosheets [54].
(Copyright 2016. Reproduced with permission from Royal Society of Chemistry.)
Park et al. [55] proposed a fast-response and high-sensitivity H2O2 biosensor in view of a PPy-embedded rGO transducer, which was presented for a FET framework. The rGO/PPyNTs indicated p-type qualities in a liquid-ion gated FET geometry. Their hole transport conduct and conductivity are better than those of rGO sheets or PPy NTs on account of the formation of PPy NT bridges between the rGO sheets. The FET H2O2 biosensor had specific selectivity and rapid sensitivity toward H2O2 in a blend comprising compounds found in biological fluids. Qian et al. depicted extremely encouraging PPy/rGO composites, decorated with AuNPs [56] and created by means of in situ chemical oxidative polymerization on the surface of the GO, using HAuCl4, and took after diminishment with hydrazine is shown in Figure 5.13.
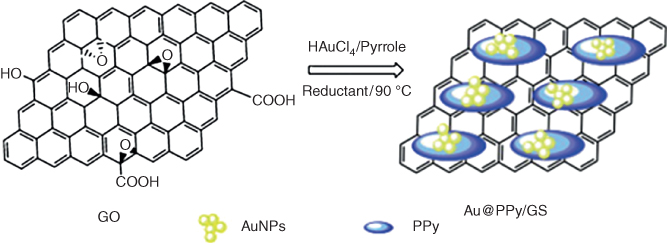
Figure 5.13 Preparation plan of rGO/PPy/AuNP biosensor for dopamine biosensing [56].
(Copyright 2013. Reproduced with permission from Elsevier.)
Lin and coworkers [57] built up a biosensor in light of a gold nanoparticle–PPy–rGO nanocomposite on an acetyl cholinesterase (AChE) biosensor. To stay away from the desquamation and denaturation of the enzymes, AChE was stacked by co-deposition with (NH4)2SiF6. Outcomes demonstrated that the fabricated biosensor had extraordinary bioactivity and stability in a wide pH range. It has an identification limit as low as 0.5 nM and its illustration of the preparation of the AChE biosensor and electrochemical detection of organophosphorus pesticides is shown in Figure 5.14.
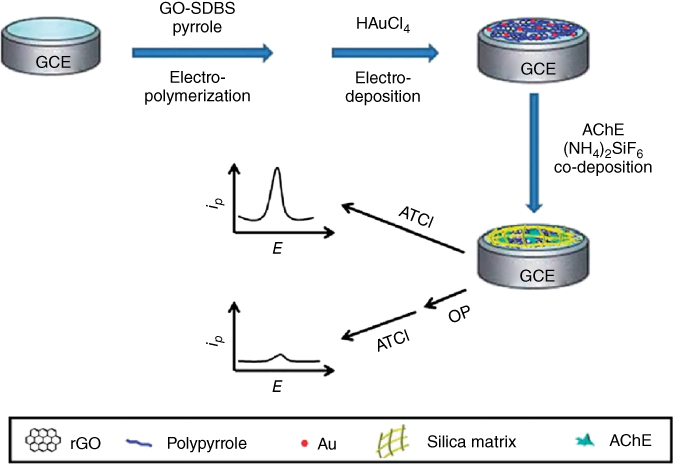
Figure 5.14 Illustration of the preparation of the Au–PPy–rGO-nanocomposite-based AChE biosensor [57].
(Copyright 2014. Reproduced with permission from Royal Society of Chemistry.)
In any case, what may be most encouraging is starting reviews using electropolymerization of pyrrole to create MIP biorecognition components. For example, Sun et al. modified GCEs with PPy MIP/GO films for distinguishing quercetin [58]. A comparable approach was utilized by da Silva et al. to distinguish trimethoprim [59]. Cai et al. then readied a bisphenol biosensor [60] through decorated rGO with silver by electrodeposition of PPy/MIP and it was found to be a selective and sensitive biosensor. Wang et al. then utilized molecularly imprinted PPy–graphene nanocomposites advanced with CdS QDs [61] to deliver a visible-light photoelectrochemical biosensor for 4-aminophenol, a transition in the synthesis of the drug paracetamol.
Likewise, Zheng and coworkers [62] detailed the utilization of PPy to enhance the stacking of alloy nanomaterials on rGO, which has been accounted for a facile, two-step strategy to set up the NiCo alloy altered by PPy/rGO nanohybrids, as outlined in Figure 5.15.

Figure 5.15 Schematic portrayal of the development procedures of NiCo/PPy/rGO nanocomposites [62].
(Copyright 2016. Reproduced with permission from Royal Society of Chemistry.)
Polypyrrole was produced on the surface of GO from beginning to end in in situ chemical polymerization, and afterward, when NiCo was straightforwardly reduced on the supporting substrate, PPy/GO was additionally decreased to form PPy/rGO composition, all the while using hydrazine hydrate. Filling in as a non-enzymatic glucose sensor, the NiCo/PPy/rGO nanocomposite-modified electrode displayed noteworthy electrocatalytic activity toward glucose oxidation. As is normal, the as-prepared NiCo/PPy/rGO electrode demonstrated amazing electrocatalytic activity for glucose oxidation with a wider linear range, a lower detection limit, and excellent anti-interference performances because of promising material candidates for the fabrication of enzyme-free glucose sensors. Recent research has distinguished woven glucose sensing sensors in light of polydopamine PPy nanowires and rGO [63]. Which of these displayed exceptional sensitivity, as high as 0.773 NCR/decade, with a response time as quick as 0.5 s, a linear range of 1 nM to 5 μM, a low detection concentration as well as great repeatability? Importantly, the glucose detected in the presence of ascorbic acid and uric acid interferences and the reliability of the proposed glucose sensor were evaluated in genuine samples of rabbit blood. The schematic graph of a FECT-based glucose sensor with the active layer of PPy/rGO composites is shown in Figure 5.16. The source/drain and the gate electrode filament were made of 133.3D PPy/rGO/PA6 and PPy/rGO/PA6/GOx/Nafion individually. It additionally recommended that the proposed glucose sensor was relevant for practical applications. All the outcomes demonstrated the novel fiber transistors paved the way for high-performance sensors that are anything but difficult to incorporate with convenient/wearable gadgets for real applications.
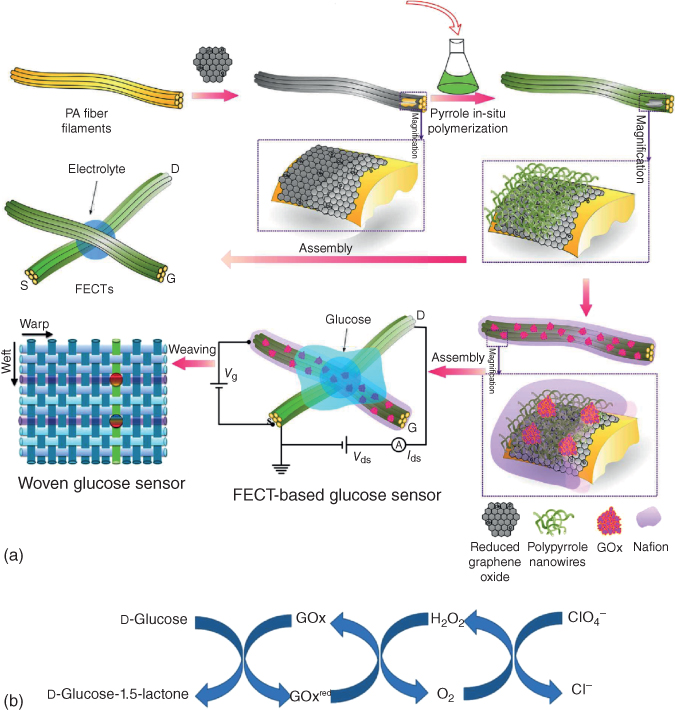
Figure 5.16 (a) The schematic diagram of glucose sensors in view of fiber organic electrochemical transistors with an active layer of PPy nanowires and rGO. (b) Reaction cycle involved in glucose sensing using GOx [63].
(Copyright 2017. Reproduced with permission from Royal Society of Chemistry.)
5.3 Conclusions, Challenges, and Future Scope
The exploration of graphene/GO-based polymer nanocomposites is an energizing field of research these days. These great materials have demonstrated the assorted range of applications in areas such as energy storage, optoelectronics, sensors, solar cells, biomedical, and many others. We have given an understanding of the graphene/GO-based PANI and PPy of the prepared composites and studied their biosensor applications. CP has brought in a lot of enthusiasm because of its good stability, interesting electro activity, high capacitance, and unusual doping or doping chemistry; and especially when combined with graphene/GO, the performance of nanocomposites is enormously improved. These applications are additionally of incredible advantage to the earth and day-by-day life. As it were, more noteworthy endeavors ought to be made to enhance the exhibitions of graphene-based polymer nanocomposites, enlarge their applications, and acknowledge commercialization in the near future. It is hard to get a uniform dispersion of GR in the polymer matrix as it tends to frame agglomeration. Utilization of some newly propelled dispersion methods may take care of this issue. Another issue is the absence of large-scale production methods to synthesize graphene with desirable thickness. It is still exceptionally difficult to prepare single-layer graphene. Therefore, some successful, minimal effort strategies to produce high-quality graphene in large scale are yet to happen. In spite of the fact that works on advancement of graphene-based materials are developing quickly, there is still space for broad essential research.
References
- 1 Stankovich, S. , Dikin, D.A. , Dommett, G.H.B. , Kohlhaas, K.M. , Zimney, E.J. , Stach, E.A. , Piner, R.D. , Nguyen, S.T. , and Ruoff, R.S. (2006) Graphene-based composite materials. Nature, 442, 282–286.
- 2 Kuilla, T. , Bhadra, S. , Yao, D. , Kim, N.H. , Bose, S. , and Lee, J.H. (2010) Recent advances in graphene based polymer composites. Prog. Polym. Sci., 35, 1350–1375.
- 3 Kim, H. , Abdala, A.A. , and Macosko, C.W. (2010) Graphene/polymer nanocomposites. Macromolecules, 43, 6515–6530.
- 4 Rao, C.N.R. , Sood, A.K. , Subrahmanyam, K.S. , and Govindaraj, A. (2009) Graphene: the new two-dimensional nanomaterial. Angew. Chem. Int. Ed., 48, 7752–7777.
- 5 Rao, C.N.R. , Sood, A.K. , Voggu, R. , and Subrahmanyam, K.S. (2010) Some novel attributes of graphene. J. Phys. Chem. Lett., 1, 572–580.
- 6 Geim, A.K. and Novoselov, K.S. (2007) The rise of graphene. Nat. Mater., 6, 183–191.
- 7 Zhu, Y. , Murali, S. , Cai, W. , Li, X. , Suk, J.W. , Potts, J.R. , and Ruoff, R.S. (2010) Graphene and graphene oxide: synthesis, properties, and applications. Adv. Mater., 22, 3906–3924.
- 8 Novoselov, K.S. , FalKo, V.I. , Colombo, L. , Gellert, P.R. , Schwab, M.G. , and Kim, K. (2012) A roadmap for graphene. Nature, 490, 192–200.
- 9 Xiao, H. , Xiaoying, Q. , Freddy, B. , and Hua, Z. (2012) Graphene-based composites. Chem. Soc. Rev., 107, 525–944.
- 10 Rajatendu, S. , Mithun, B. , Bandyopadhyay, S. , and Anil, K.B. (2001) A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites. Prog. Polym. Sci., 36, 638–670.
- 11 Pumera, M. , Ambrosi, A. , Bonanni, A. , Chng, E.L.K. , and Poh, H.L. (2010) Graphene for electrochemical sensing and biosensing. TrAC, Trends Anal. Chem., 29, 954–965.
- 12 Stankovich, S. , Dikin, D. , Piner, R.D. , Kohlhaas, K. , Kleinhammes, A. , Jia, Y. , Wu, Y. , Nguyen, S.T. , and Ruoff, R.S. (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon, 45, 1558–1565.
- 13 Allen, M.J. , Tung, V.C. , and Kaner, R.B. (2010) Honeycomb carbon: a review of graphene. Chem. Rev., 110, 132–145.
- 14 Dreyer, D.R. , Park, S. , Bielawski, C.W. , and Ruoff, R.S. (2010) The chemistry of graphene oxide. Chem. Soc. Rev., 39, 228–240.
- 15 Lerf, A. , He, H. , Forster, M. , and Klinowski, J. (1998) Structure of graphite oxide revisited. J. Phys. Chem. B, 102, 4477–4482.
- 16 Brodie, B.C. (1859) On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. Ser. A, 149, 249–259.
- 17 Staudenmaier, L. (1898) Verfahren zur darstellung der graphitsaure. Ber. Dtsch. Chem. Ges., 31, 1481–1487.
- 18 Hummers, W.S. Jr. and Offeman, R.E. (1958) Preparation of graphitic oxide. J. Am. Chem. Soc., 80, 1339.
- 19 McAllister, M.J. , Li, J.L. , Adamson, D.H. , Schniepp, H.C. , Abdala, A.A. , Liu, J. , Herrera, A.M. , Milius, D.L. , Car, R. , Prud’homme, R.K. , and Aksay, I.A. (2007) Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater., 19, 4396–4404.
- 20 Prudhomme, R.K. , Aksay, I.A. , Adamson, D. , and Abdala, A. (2007) Thermally exfoliated graphite oxide. US Patent 20,070,092,432
- 21 Steurer, P. , Wissert, R. , Thomann, R. , and Mulhaupt, R. (2009) Functionalized graphenes and thermoplastic nanocomposites based upon expanded graphite oxide. Macromol. Rapid Commun., 30, 316–327.
- 22 Schniepp, H.C. , Li, J.L. , McAllister, M.J. , Sai, H. , Herrera, A.M. , Adamson, D.H. , Prudhomme, R.K. , Car, R. , Saville, D.A. , and Aksay, I.A. (2006) Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B, 110, 8535–8539.
- 23 Clark, L.C. and Lyons, C. (1962) Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N.Y. Acad. Sci., 102, 29–45.
- 24 Yu, X. , Zhang, W. , Zhang, P. , and Su, Z. (2017) Fabrication technologies and sensing applications of graphene-based composite films: advances and challenges. Biosens. Bioelectron., 89, 72–84.
- 25 Potts, J. , Dreyer, D. , Bielawski, C. , and Ruoff, R. (2011) Graphene-based polymer nanocomposites. Polymer, 52, 5–25.
- 26 Zhang, M. , Li, Y. , Su, Z. , and Wei, G. (2015) Recent advances in the synthesis and applications of graphene–polymer nanocomposites. Polym. Chem., 6, 6107–6124.
- 27 Gerard, M. (2002) Application of conducting polymers to biosensors. Biosens. Bioelectron., 17, 345–359.
- 28 Lei, W. , Si, W. , Xu, Y. , Gu, Z. , and Hao, Q. (2014) Conducting polymer composites with graphene for use in chemical sensors and biosensors. Microchim. Acta, 181, 707–722.
- 29 O’Regan, B. and Grätzel, M.A. (1991) A low-cost, high-efficiency solar cell based on dye sensitized colloidal TiO2 films. Nature, 353, 737–740.
- 30 Wang, P.S. et al (2003) Gelation of ionic liquid-based electrolytes with silica nanoparticles for quasi-solid-state dye-sensitized solar cells. J. Am. Chem. Soc., 125, 1166–1167.
- 31 Grätzel, M. (2001) Photoelectrochemical cells. Nature, 414, 338–344.
- 32 Grätzel, M. (2005) Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem., 44, 6841–6851.
- 33 Agarwala, S. , Kevin, M. , Wong, A.S.W. , Peh, C.K.N. , Thavasi, V. , and Ho, G.W. (2010) Mesophase ordering of TiO2 film with high surface area and strong light harvesting for dye-sensitized solar cell. ACS Appl. Mater. Interfaces, 2, 1844–1850.
- 34 Jang, J. (2006) Emissive Materials Nanomaterials, Springer, Berlin, Germany, pp. 189–260.
- 35 Huang, L. , Zhuang, X. , Hu, J. , Lang, L. , Zhang, P. , Wang, Y. , Chen, X. , Wei, Y. , and Jing, X. (2008) Synthesis of biodegradable and electroactive multiblock polylactide and aniline pentamer copolymer for tissue engineering applications. Biomacromolecules, 9, 850–858.
- 36 Guo, Y. , Li, M. , Mylonakis, A. , Han, J. , MacDiarmid, A.G. , Chen, X. , Lelkes, P.I. , and Wei, Y. (2007) Electroactive oligoaniline-containing self-assembled monolayers for tissue engineering applications. Biomacromolecules, 8, 3025–3034.
- 37 Humpolicek, P. , Kasparkova, V. , Saha, P. , and Stejskal, J. (2012) Biocompatibility of polyaniline. Synth. Met., 162, 722–727.
- 38 Liu, P.Z. , Hu, X.W. , Mao, C.J. , Niu, H.L. , Song, J.M. , Jin, B.K. , and Zhang, S.Y. (2013) Electrochemiluminescence immunosensor based on graphene oxide nanosheets/polyaniline nanowires/CdSe quantum dots nanocomposites for ultrasensitive determination of human interleukin-6. Electrochim. Acta, 113, 176–180.
- 39 Ruecha, N. , Rangkupan, R. , Rodthongkum, N. , and Chailapakul, O. (2014) Novel paper-based cholesterol biosensor using graphene/polyvinylpyrrolidone/polyaniline nanocomposite. Biosens. Bioelectron., 52, 13–19.
- 40 Xu, Q. , Gu, S.X. , Jin, L. , Zhou, Y. , Yang, Z. , Wang, W. , and Hu, X. (2014) Graphene/polyaniline/gold nanoparticles nanocomposite for the direct electron transfer of glucose oxidase and glucose biosensing. Sens. Actuators, B, 190, 562–569.
- 41 Radhapyari, K. , Kotoky, P. , Das, M.R. , and Khan, R. (2013) Graphene–polyaniline nanocomposite based biosensor for detection of antimalarial drug artesunate in pharmaceutical formulation and biological fluids. Talanta, 111, 47–53.
- 42 Devi, R. , Relhan, S. , and Pundir, C.S. (2013) Construction of a chitosan/polyaniline/graphene oxide nanoparticles/polypyrrole/Au electrode for amperometric determination of urinary/plasma oxalate. Sens. Actuators, B, 186, 17–26.
- 43 Wu, Z. , Chen, X. , Zhu, S. , Zhou, Z. , Yao, Y. , Quan, W. , and Liu, B. (2013) Enhanced sensitivity of ammonia sensor using graphene/polyaniline nanocomposite. Sens. Actuators, B, 178, 485–493.
- 44 Das, G. and Yoon, H.H. (2015) Amperometric urea biosensors based on sulfonated graphene/polyaniline nanocomposite. Int. J. Nanomed., 10, 55–66.
- 45 Yang, T. , Meng, L. , Wang, X. , Wang, L. , and Jiao, K. (2013) Direct electrochemical DNA detection originated from the self-redox signal of sulfonated polyaniline enhanced by graphene oxide in neutral solution. ACS Appl. Mater. Interfaces, 5, 10889–10894.
- 46 Bo, Y. , Yang, H. , Hu, Y. , Yao, T. , and Huang, S. (2011) A novel electrochemical DNA biosensor based on graphene and polyaniline nanowires. Electrochim. Acta, 56, 2676–2681.
- 47 Wang, L. , Hua, E. , Liang, M. , Maa, C. , Liu, Z. , Sheng, S. , Liu, M. , Xie, G. , and Feng, W. (2014) Graphene sheets, polyaniline, and AuNPs based DNA sensor for electrochemical determination of BCR/ABL fusion gene with functional hairpin probe. Biosens. Bioelectron., 51, 201–207.
- 48 Du, M. , Yang, T. , Li, X. , and Jiao, K. (2012) Fabrication of DNA/graphene/polyaniline nano complex for label-free voltammetric detection of DNA hybridization. Talanta, 88, 439–444.
- 49 Yang, T. , Li, Q. , Li, X. , Wang, X. , Du, M. , and Jiao, K. (2013) Freely switchable impedimetric detection of target gene sequence based on synergistic effect of ERGNO/PANI nanocomposites. Biosens. Bioelectron., 42, 415–418.
- 50 Xue, C. , Wang, X. , Zhu, W. , Han, Q. , Zhu, C. , Hong, J. , Zhou, X. , and Jiang, H. (2014) Electrochemical serotonin sensing interface based on double-layered membrane of reduced graphene oxide/polyaniline nanocomposites and molecularly imprinted polymers embedded with gold nanoparticles. Sens. Actuators, B, 196, 57–63.
- 51 Liu, A. , Li, C. , Bai, H. , and Shi, G. (2010) Electrochemical deposition of polypyrrole/sulfonated graphene composite films. J. Phys. Chem. C, 114, 22783–22789.
- 52 Wang, X. , Yang, C. , Li, H. , and Liu, P. (2013) Synthesis and electrochemical performance of well-defined flake-shaped sulfonated graphene/polypyrrole composites via facile in situ doping polymerization. Electrochim. Acta, 111, 729–737.
- 53 Arulraj, A.D. , Arunkumar, A. , Vijayan, M. , Viswanath, K.B. , and Vairathevar, S.V. (2016) A simple route to develop highly porous nano polypyrrole/reduced graphene oxide composite film for selective determination of dopamine. Electrochim. Acta, 206, 77–85.
- 54 Mao, H. , Ji, C. , Liu, M. , Sun, Y. , Liu, D. , Wu, S. , Zhang, Y. , and Song, X.M. (2016) Hydrophilic polymer/polypyrrole/graphene oxide nanosheets with different performances in electrocatalytic applications to simultaneously determine dopamine and ascorbic acid. RSC Adv., 6, 111632–111639.
- 55 Park, J.W. , Park, S.J. , Kwon, O.S. , Lee, C. , and Jang, J. (2014) Polypyrrole nanotube embedded reduced graphene oxide transducer for field-effect transistor-type H2O2 biosensor. Anal. Chem., 86, 1822–1828.
- 56 Qian, T. , Yu, C. , Zhou, X. , Wu, S. , and Shen, J. (2014) Au nanoparticles decorated polypyrrole/reduced graphene oxide hybrid sheets for ultrasensitive dopamine detection. Sens. Actuators, B, 193, 759–763.
- 57 Yang, Y. , Asiri, A.M. , Du, D. , and Lin, Y. (2014) Acetyl cholinesterase biosensor based on gold nanoparticle–polypyrrole–reduced graphene oxide nanocomposite modified electrode for the amperometric detection of organophosphorus pesticides. Analyst, 139, 3055–3060.
- 58 Sun, S. , Zhang, M. , Li, Y. , and He, X. (2013) A molecularly imprinted polymer with incorporated graphene oxide for electrochemical determination of quercetin. Sensors, 13, 5493–5506.
- 59 da Silva, H. , Pacheco, J.G. , Magalhaes, J.M.C.S. , Viswanathan, S. , and Delerue-Matos, C. (2014) MIP-graphene-modified glassy carbon electrode for the determination of trimethoprim. Biosens. Bioelectron., 52, 56–61.
- 60 Cai, R. , Rao, W. , Zhang, Z. , Long, F. , and Yin, Y. (2014) An imprinted electrochemical sensor for bisphenol A determination based on electro deposition of a graphene and Ag nanoparticle modified carbon electrode. Anal. Methods, 6, 1590–1597.
- 61 Wang, R. , Yan, K. , Wang, F. , and Zhang, J. (2014) A highly sensitive photo electrochemical sensor for 4-aminophenol based on CdS–graphene nanocomposites and molecularly imprinted polypyrrole. Electrochim. Acta, 121, 102–108.
- 62 Sheng, Q. , Liu, D. , and Zheng, J. (2016) NiCo alloy nanoparticles anchored on polypyrrole/reduced graphene oxide nanocomposites for nonenzymatic glucose sensing. New J. Chem., 40, 6658–6665.
- 63 Wang, Y. , Qing, X. , Zhou, Q. , Zhang, Y. , Liu, Q. , Liu, K. , Wang, W. , Li, M. , Lu, Z. , Chen, Y. , and Wang, D. (2017) The woven fiber organic electrochemical transistors based on polypyrrole nanowires/reduced graphene oxide composites for glucose sensing. Biosens. Bioelectron., 95, 138–145.
