Plant Growth-Promoting Bacteria Elicited Induced Systemic Resistance and Tolerance in Plants
Shekhar Jain, Anookul Vaishnav, Amrita Kasotia, Sarita Kumari and Devendra Kumar Choudhary
Plant growth-promoting bacteria (PGPB) are microorganisms that exert benign effects on plants and regulate plant growth. They are the denizen of rhizosphere and along with it form epiphytes (on plant surface) and endophytes (inside plant tissues) that define all the regulatory processes of plants including resistance and tolerance against biotic and abiotic stresses. Several PGPB induce resistance against pathogens by eliciting physiological changes in plants together with tolerance to drought, salt, and other environmental factors. Bacteria elicited induced systemic resistance and tolerance provides plants with stress-responsive mechanisms whereby they mitigate stresses to practical agriculture. Plants acquire an enhanced level of resistance/tolerance after exposure to biotic/abiotic stimuli provided by many different PGPB. The present chapter focuses on mechanisms implicated for bacteria-induced plant growth under biotic and abiotic stresses.
Keywords
abiotic stress; biotic stress; induced resistance; induced tolerance; plant growth-promoting bacteria (PGPB); systemic acquired resistance
5.1 Introduction
In the present agroworld scenario, the first priority of the cultivator is to produce a healthy plant (i.e., a plant without any infectious disease) and to gain high yield in any adverse conditions. There are many microorganisms that affect a plant’s health by causing damage in different ways, ultimately leading to low yield and subsequently low economic value. On the other hand, some environmental factors, such as drought, temperature, salinity, alkalinity, and nutrients, contribute to low production at their extremities. For sustainable agriculture, plants must develop a defensive capacity against various pathogens and show tolerance for adverse environmental conditions. It is difficult to find a place that is exempt from any disease-causing agent, but only natural suppressive soil is the habitat that provides this type of environment (Weller et al., 2002; Choudhary et al., 2007). The wholesome protection of plants against biotic and abiotic stresses is provided by the belowground functioning (microbial activities) of the soil, which works as a protective shield for plants. In this region, plant roots release a substantial amount of elementary molecules, such as C- and N-containing compounds, which are utilized by microbes for growth and functional activities (Ryan and Delhaize, 2001; Choudhary and Johri, 2009).
The benign role of microbes in belowground plant functioning is carried out by so-called plant growth-promoting bacteria (PGPB), and the overall effect on plant growth promotion and development, including resistance against pathogens, is accomplished by mechanism-induced systemic resistance (ISR) (Kloepper et al., 1980; Haynes and Swift, 1990; Jain et al., 2013). These bacteria also help in tolerance of abiotic stress by inducing the production of different osmoprotectants through a mechanism known as induced systemic tolerance (IST) (Choudhary, 2012). PGPB-mediated ISR is accomplished through competition for an ecotype/biotope, production of allelopathic compounds in the rhizosphere, and induced resistance in plants (Jain et al., 2013).
PGPBs are characterized by their colonization with the root and root surface and their ability to promote plant growth. Among PGPB, the predominant genera include Acinetobacter, Agrobacterium, Arthrobacter, Azospirillum, Bacillus, Bradyrhizobium, Frankia, Pseudomonas, Rhizobium, Serratia, Thiobacillus, and others (Lugtenberg et al., 2001; Rothballer et al., 2003; Espinosa-Urgel, 2004; Gamalero et al., 2004; Nivedhitha et al., 2008). Recently researchers used PGPB to describe mechanisms of ISR and IST in various plant species that alleviate biotic and abiotic stresses and promote plant growth (Alvarez et al., 2012; Colebrook et al., 2012; Filippou et al., 2012; Krasensky and Jonak, 2012; Makandar et al., 2012; Morais Neto et al., 2012; Nishiyama et al., 2012; Nouri et al., 2012; Stearns et al., 2012; Tanou et al., 2012a,b; Wang et al., 2012; Weston et al., 2012; Yang et al., 2012; Zamioudis and Pieterse, 2012; Ayliffe et al., 2013; Balmer et al., 2013; Bellin et al., 2013; Bulgarelli et al., 2013; Christou et al., 2013; Jiang et al., 2013; Jogaiah et al., 2013; Miranda et al., 2013; Mitter et al., 2013; Oka et al., 2013; Olivares et al., 2013; Zolla et al., 2013; Zúñiga et al., 2013). In keeping with views of plant growth promotion under biotic and abiotic stresses, the present chapter will unravel the perplexity of ISR and IST mechanisms involved in sustainable development of plants.
5.2 PGPB-elicited response of plants against biotic stress
PGPB-mediated resistance in plants completely overcomes the effect of a pathogen and/or related damaging factors (Agrios, 1988; van Loon, 1997). Plants possess a powerful immune system as a protective guard against microbial pathogens and parasites; this system is coordinated by a complex signaling network. According to the types of molecules they recognize as indicators of a pathogen attack, plants have two types of immune system: PAMP-triggered immunity (PTI) and effector-triggered immunity (ETI) (Jones and Dangl, 2006; Eulgem and Somssich, 2007; Vleesschauwer and Höfte, 2009). In spite of having such a strong immune system, sometimes plants are affected by some infectious microbes. These microbes have some ability to escape a plant’s immune system, which is how they can infect plants and possibly lead to reduced quality and quantity of the product. For these types of microbes, plants require a somewhat enhanced level of resistance and this resistance is provided by PGPB (Choudhary and Johri, 2009).
Plants develop an enhanced defensive capacity when they are appropriately stimulated by specific environmental stimuli, whereby they can acquire resistance against biotic stress. There are two main forms of induced resistance, systemic acquired resistance (SAR) and ISR (previously mentioned), wherein plant defenses are preconditioned by biotic stimuli through prior infection and/or treatment that results in resistance when challenged. Induction and expression of the genes involved in SAR and ISR are discriminated according to the nature of the elicitor and the regulatory pathways involved. These pathways are induced by a specific signaling molecule or elicitor, which activates different intermediate molecules in a cascading manner and forms a network of interconnected signaling pathways that regulate the plant’s induced defense against pathogens, as shown in Figure 5.1 (Choudhary et al., 2007; Jain et al., 2013).
Induction of SAR involved exposure of the plant to virulent, avirulent, and nonpathogenic microbes. A specific time period is required for the establishment of SAR, which depends on type of plant and elicitor. Accumulation of pathogenesis-related (PR) proteins and salicylic acid (SA) is induced in SAR, whereas ISR is triggered by PGPB and does not involve accumulation of PR proteins and/or SA; rather, it relies on pathways regulated by jasmonate (JA) and ethylene (ET) (Pieterse et al., 2001; Yan Z et al., 2002; Choudhary et al., 2007).
A study on plant–microbe interactions found PGPB-elicited ISR against various pathogens to reduce susceptibility to the relevant disease—for example, the carnation (Dianthus caryophillus), with its reduced susceptibility to wilt caused by the pathogenic fungus Fussarium sp., and cucumber (Cucumis sativus), with its reduced susceptibility to foliar disease caused by Colletotrichum orbiculare, respectively (Van Peer et al., 1991; Wei et al., 1991; Compant et al., 2005) (Table 5.1).
Table 5.1
PGPB-Mediated Biocontrol of Different Plant Diseases, Pathogens, and Insects
| PGPBs | Crops | Disease/Pathogen/Insect | References |
| Bacillus amyloliquefaciens | Tomato | Tomato mottle virus | Murphy et al. (2000) |
| Pseudomonas fluorescens | Tobacco | Tobacco necrosis virus | Park and Kloepper, (2000) |
| Bacillus pumilus SE 34 | Tobacco | Blue mold | Zhang et al. (2003) |
| Pseudomonas sp | Groundnut | Rhizoctonia bataticola | Gupta et al. (2002) |
| Streptomyces marcescens 90–116 | Tobacco | Blue mold | Zhang et al. (2003) |
| Bacillus sp. | Cucumber | Cotton aphids | Stout et al. (2002) |
| Bacillus licheniformis | Pepper | Myzus persicae | Lucas et al. (2004) |
| Bacillus cereus MJ-1 | Red pepper | Myzus persicae | Joo et al. (2005) |
| Pseudomonas sp. | White clover Medicago |
Acyrthosiphon kondoi | Kempster et al. (2002) |
| Paenibacillus polymyxa E681 | Sesame | Fungal disease | Ryu et al. (2006) |
| Enterobacter sp | Chickpea | Fusarium avenaceum | Hynes et al. (2008) |
| Azospirillum brasilense | Prunus cerasifera L. | Rhizosphere fungi | Russo et al. (2008) |
| Pseudomonas aeruginosa | Mung bean | Root rot | Siddiqui et al. (2001) |
| Bacillus subtilis G803 | Pepper | Myzus persicae | Kokalis-Burelle et al. (2002) |
| Bacillus amyloliquefaciens | Bell pepper | Myzus persicae Sluzer |
Herman et al. (2008) |

ISR and SAR, both of which are induced-resistance processes, take place in plants by activating a different set of genes, the product of which makes plants resistant to any further pathogen attack. Arabidopsis, a model plant, has been widely used for the plant–microbe interaction. Expression of a specific set of pathogen-inducible defense-related genes was reported in the study of Arabidopsis after induction of SA, JA, and ET pathways. As previously described, whenever plants are affected by any pathogen, accumulation of SA takes place in the infected region and formations of phloem mobile signals are induced. Subsequently, in the distal part of the plant, SA concentration increases and volatile methyl salicylate (MeSA) is released.
The accumulation of SA in SAR was proven in the Arabidopsis SA-nonaccumulating mutant plant NahG. NahG expresses the bacterial salicylate hydroxylase (nahG) gene responsible for conversion of SA into catechol, making it incapable of expressing SAR (Pieterse et al., 1998). SA is the primary molecule for SAR, inducing a further signaling cascade to activate the gene responsible for pathogen resistance; it is called the pathogenesis-related (PR) gene because it encodes different PR proteins in the families PR-2, PR-5, and PR-1.
In plants all of these PRs have some antimicrobial properties, primarily directed against fungal pathogens (Uknes et al., 1992; Kombrink and Somssich, 1997; Saskia et al., 1999). The NPR-1 protein encoded by the npr-1 gene allows SAR establishment because it activates PRs genes after receiving a signal from SA accumulation (Pieterse et al., 1998). Therefore, the sequence of the signaling events in SAR is such that, after recognition of pathogen, SA accumulation takes place that activates the npr-1 gene followed by PR gene activation. It has been proven that the volatile MeSA can act as a long-distance mobile signal for SAR, whereas MeSA itself appears to be biologically inactive; however, it is in the systemic tissue that MeSA is hydrolyzed to SA by the MeSA-esterase activity of SA-binding protein-2 (Park et al., 2007; Heil and Ton, 2008; Vlot et al., 2008a,b; Vleesschauwer and Höfte, 2009).
ISR takes a more diverse and complex route to establish a higher degree of prior resistance with no infection. In place of the PRs gene, defense-related gene activation takes place in ISR via JA- and ET-mediated signaling. A thionin molecule is expressed as a defense-related protein after induction of JA signaling (Epple et al., 1995; Wasternack and Parthier, 1997; Pieterse et al., 1998), including that of proteinase inhibitors (Farmer et al., 1992), whereas pathogen-inducible genes are induced in ET signaling (Saskia et al., 1999). Unlike SAR, ISR is elicited by nonpathogenic rhizobacteria or PGPB and there is no need for initial infection as is required in SAR.
After induction by PGPB, synthesis of JA and ET takes place in the plant and after challenge inoculation, the JA and ET responses activate npr-1 gene expression, which encodes the NPR-1 protein followed by activation of a defense-related gene. NPR-1 proteins are known as master regulators of both defense pathways because after receiving the preceding signal, this protein activates expression of either the PR gene or a defense-related gene for the establishment of SAR and ISR, respectively. Like MeSA, methyl jasmonate (MeJA) also works as a volatile signal for the distal part of the plant. Expression of a different defense-related gene depends on whether that NPR-1 is getting a signal from JA or ET or from both in concert. Saskia et al. (1999) have elaborately described the different defense-related gene activations by JA and ET (Figure 5.2).
Expression of the pathogen-inducible genes—Hel (encoding a hevein-like protein), ChiB (encoding a basic chitinase), and Pdf1.2 (encoding a plant defensin)—and the proteins encoded by all three, was shown to have antifungal activity through ET signaling (Samac et al., 1990; Potter et al., 1993; Penninckx et al., 1996). Likewise, the activation of the Hel, ChiB, and Pdf1.2 genes were mediated by JA signaling (Penninckx et al., 1996; Thomma et al., 1998). For the expression of plant defense proteins that exhibit antagonistic and proteinase inhibitory activities, ET- and JA-mediated signaling is required in a cohort manner (Penninckx et al., 1998). The pal1 gene, which encodes phenylalanine ammonia-lyase (PAL), plays an important regulatory role in the synthesis of lignin and SA in Arabidopsis; it was found to be induced by JA (Mauch-Mani and Slusarenko, 1996; McConn et al., 1997).
JA is also involved in plant protection from insects and herbivores—for example, in the tomato, JA-induced expression of the Pin gene, which encodes for the proteinase inhibitor proteins (Farmer and Ryan, 1992) and protects the plant against herbivory (Heitz et al., 1999). Expression of the Atvsp gene, which encodes the vegetative storage protein (VSP), is also induced by JA signaling in Arabidopsis. VSP possesses acid phosphate activity and by using this activity it retards development of insects and increases their mortality rate. That is how, by activation of such a wide range of different defense-related genes, PGPB-elicited ISR helps protect plants against a broad range of pathogens, insects, and herbivores (Berger et al., 1995).
5.3 PGPB-produced elicitors of ISR against biotic stress
A number of bioactive natural chemicals, known as allelochemicals, are produced during plant–microbe and microbe–microbe interactions. They are a subset of metabolites that are not required for an organism’s growth, development, and reproduction. Some PGPBs produce different allelochemicals (e.g., siderophores, antibiotics, volatiles), which are used as a weapon against plant pathogens to protect plants from pathogenic diseases. Allelochemicals may work in a competitive manner, such as siderophores, for the acquisition of iron or may directly cause damage by inhibiting the gene machinery of target pathogens such as antibiotics and volatiles (Choudhary et al., 2007).
5.3.1 Siderophore
Iron, a transition metal, is one of the most important and essential micronutrients in animals and plants because it is crucial for some life-holding processes such as respiration, photosynthesis, N2-fixation, and so on. In spite of being the fourth most frequent element on earth, it is not readily available in many environments because of the very low solubility of the Fe3+ ion. In such iron-limiting environments, it is difficult for plants and microbes to survive and be productive. For the survival of the self and the host plant, PGPB secretes an iron-binding ligand called “siderophore” in such an environment, which makes a complex with the Fe3+ ion and thus is available to the host organism (Gupta and Gopal, 2008). Siderophores are low-molecular-weight organic compounds with a very high and specific affinity to chelate iron (Boukhalfa and Crumbliss, 2002).
Although a wide range of siderophores are produced by different microorganisms’ pseudobactines, also known as pyoverdin or fluorescein, are the most important that exhibit a distinctive phenotypic trait of the rRNA homology group I species of the genus Pseudomonas (Visca et al., 2007). By sequestering the Fe3+ ion, siderophores produced by different PGPBs do not allow growth of pathogenic fungi in the vicinity and showed heterologous siderophores produced by a coinhabitant (Loper and Henkels, 1999; Whipps, 2001; Compant et al., 2005). Fungi also produce siderophores, but these have a lower affinity for ferric ion (O’Sullivan and O’Gara, 1992; Loper and Henkels, 1999; Compant et al., 2005). In addition to protection of ferric iron against biocontrol bacteria and plant deleterious microorganisms, siderophores also trigger immune response in plants (Höfte and Bakker, 2007).
Much of the research conducted on pseudobactines during the last decade demonstrates their role in triggering plant resistance. For instance, pseudobactines produced by Pseudomonas putida WCS358 have been shown to suppress Ralstonia solanacearum in Eucalyptus urophylla (Ran et al., 2005), Erwinia carotovora in tobacco (Nicotiana tabacumn) (van Loon et al., 2008), and Botrytis cinerea in tomato (Solanum lycopersicum) (Meziane et al., 2005). Pseudobactines are also effective against viral pathogens; for example, those produced by Pseudomonas fluorescens WCS374r make Arabidopsis plants resistant against turnip crinkle virus (TCV) (Djavaheri, 2007), while those produced by Pseudomonas fluorescens CHA0 protect the tobacco plant from tobacco necrosis virus (TNV) (Maurhofer et al., 1994). Arora et al. (2001) isolated two strains of PGPB Rhizobium meliloti, RMP3 and RMP5, from Mucna pruriens, which produce siderophores and show strong antagonism against the pathogen Macrophomina phaseolina.
5.3.2 Antibiotics
The finding that PGPBs produce antibiotics has significantly increased our knowledge of the biocontrol of disease. Fluorescent pseudomonads produce a wide range of antibiotics, including 2, 4-diacetylphloroglucinol (DAPG), pyoluteorin (PLT), pyrrolnitrin (PRN), phenazine-1-carboxylic acid (PCA), 2-hydroxy phenazines, and phenazine-1-carboxamide (PCN), which have different structural configurations. A wide range of other bacteria also produce different types of antibiotics that target different pathogens and protect plants from different pathogenic diseases, as detailed in Table 5.2 (Raaijmakers and Weller, 1998; Weller et al., 2002; Fernando et al., 2005).
Table 5.2
List of Some Antibiotics Produced by Bacteria against Target Pathogen
| Antibiotic | Source | Target Pathogen | Disease | Reference |
| 2,4-diacetyl-phloroglucinol | Pseudomonas fluorescens F113 | Pythium spp. | Damping off | Shanahan et al. (1992) |
| Agrocin 84 | Agrobacterium radiobacter |
Agrobacterium tumefaciens |
Crown gall | Kerr, (1980) |
| Bacillomycin D | Bacillus subtilis AU195 | Aspergillus flavus | Aflatoxin contamination | Moyne et al. (2001) |
| Bacillomycin, Fengycin | Bacillus amyloliquefaciens FZB42 | Fusarium oxysporum | Wilt | Koumoutsi et al. (2004) |
| Xanthobaccin A | Lysobacter sp. strain SB-K88 | Aphanomyces cochlioides |
Damping off | Islam et al. (2005) |
| Gliotoxin | Trichoderma virens | Rhizoctonia solani | Root rots | Wilhite et al. (2001) |
| Herbicolin | Pantoea agglomerans C9-1 | Erwinia amylovora | Fire blight | Sandra et al. (2001) |
| Iturin A | B. subtilis QST713 | Botrytis cinerea and R. solani | Damping off | Paulitz and Belanger, (2001); Kloepper et al. (2004) |
| Mycosubtilin | B. subtilis BBG100 | Pythium aphanidermatum | Damping off | Leclere et al. (2005) |
| Phenazines | P. fluorescens 2-79 and 30-84 | Gaeumannomyces graminis var. tritici | Take-all | Thomashow et al. (1990) |
| Pyoluteorin, Pyrrolnitrin | P. fluorescens Pf-5 | Pythium ultimum and R. solani | Damping off | Howell and Stipanovic, (1980) |
| Pyrrolnitrin, Pseudane | Burkholderia cepacia | R. solani and Pyricularia oryzae | Damping off and rice blast | Homma et al. (1989) |
| Zwittermicin A | Bacillus cereus UW85 | Phytophthora medicaginis and P. aphanidermatum | Damping off | Smith et al. (1993) |

Among the aforementioned antibiotics, DAPG, the most frequently reported in PGPB-mediated disease control, is produced by Pseudomonas fluorescens CHA0, which induces resistance against oomycete Hyaloperonospora arabidopsidis (Iavicoli et al., 2003), and the root knot nematode Meloidogyne javanica (Siddiqui and Shaukat, 2003). Pseudomonas chlororaphis Q2-87 produces DAPG to elicit ISR in Arabidopsis against the leaf pathogen Pseudomonas syringae pv. tomato (Vleesschauwer and Höfte, 2009; Weller et al., 2012). Several bacterial strains have the ability to produce a huge array of antibiotics and help in suppression of diverse microbial competitors—for example, Bacillus cereus strain UW85-produced zwittermycin (Silo-Suh et al., 1994; Pal and Gardener, 2006) and kanosamine (Milner et al., 1996).
A study performed using Arabidopsis mutants and transgenic lines implicated defense-signaling pathways wherein DAPG-induced resistance follows a signaling route different from that into ISR. This pathway does not depend on the master regulator NPR-1 or functional JAR1 protein but is regulated by the ethylene-insensitive root-1 (eir1) gene, which is ET insensitive in the roots only (Roman et al., 1995; Vleesschauwer and Höfte, 2009). The absence of ISR expression after exogenous exposure of DAPG on the eir1 mutant suggested that an intact ET-signaling pathway is required for the establishment of DAPG-inducible resistance (Iavicoli et al., 2003; Vleesschauwer and Höfte, 2009).
PCA, a green-pigmented heterocyclic nitrogenous compound, is produced extracellularly by several PGPBs with antagonistic activity coupled with the accumulation of toxic superoxide radicals in the target cells (Hassett et al., 1992, 1993; Chin-A-Woeng et al., 1998; Fernando et al., 2005). PCA produced by Pseudomonas fluorescens 2-79 and Pseudomonas aureofaciens 30-84 exhibits antagonism against Gaeumannomyces graminis var. tritici (Thomashow et al., 1990). Stem rot disease in canola, which is caused by Sclerotinia, is suppressed by the activity of Pseudomonas chlororaphis strain PA-23 (Zhang and Fernando, 2004). Hu et al. (2005) have isolated strain M-18 from the rhizosphere soil of sweet melon, using 1-aminocyclopropane-1-carboxylate (ACC) as the sole nitrogen source; it was found that this strain has a capability of producting PCA and pyoluteorin antibiotics.
5.3.3 Volatiles
In the context of plant defense, PGPB produces volatile organic compounds (VOCs) that promote plant growth and induce systemic resistance, which provides a new insight into PGPB–plant interaction. Several types of VOCs produced by bacteria have been reported so far; they play a crucial role in plant defense. Some of the most common VOCs include dodecane, 2-undecanone, 2-tridecanone, 2-tridecanol, tetramethyl pyrazine 2,3-butanediol, 3-hydroxy-2-butanone (acetoin), and others. Among these 2,3-butanediol and 3-hydroxy-2-butanone are the most important, and recent research work on bacteria-produced VOCs verified their role in the elicitation of ISR (Ryu et al., 2003).
Two bacterial strains—Bacillus subtilis GB03 and Bacillus amyloliquefaciens IN937a—consistently release 2,3-butanediol and 3-hydroxy-2-butanone, which are not released by Escherichia coli DH5α. Further treatment of A. thaliana plants with these strains has shown that there is significant resistance against challenge inoculation with Erwinia carotovora subsp. carotovora SCC1. Absence of disease protection upon treatment with genetically modified Bacillus strain which is unable to produce 2,3-butanediol confirmed the priming activity of such VOCs to induce resistance against disease (Ryu et al., 2003). Besides Bacillus, several strains of Pseudomonas fluorescens were reported to produce VOCs and have shown more effectiveness in controlling root and seedling diseases (Shanahan et al., 1992; Pierson and Weller, 1994; Schnider et al., 1995; Cronin et al., 1997; Duffy and Défago, 1997; Raaijmakers et al., 1997, 1999; Raaijmakers and Weller, 1998, 2001; Landa et al., 2002).
5.4 PGPB-elicited plant response against abiotic stress
Abiotic stresses include drought, low temperature, salinity, and alkalinity, all of which adversely influence growth and induce senescence, leading to cell death or reduced crop yield. Plants respond to these stresses by producing different compatible solutes that include organic ions or other low-molecular-weight organic solutes (Rhodes et al., 2002). These compatible solutes comprised quaternary amino acid derivatives (proline, glycine betain, β-alaninebetaine, and prolinebetaine); tertiary amines (1,4,5,6-tetrahydro-2-methyl-4-carboxyl pyrimidine); mono-, di-, oligo-, and polysaccharides (glucose, fructose, sucrose, trehalose, raffinose, and fructans); sugar alcohols (mannitol, glycerol, and methylated inositols); and sulfonium compounds (choline-O-sulfate, dimethylsulfoniopropionate) (Vinocur and Altman, 2005; Flowers and Colmer, 2008). Despite producing a range of molecules against abiotic stress, plants struggle for survival under stress conditions and show a lower growth rate and poor yield.
PGPBs play a crucial role against abiotic stress by enhancing plant tolerance. PGPB-induced tolerance has been proposed, including physical and chemical changes (Figure 5.3). A huge range of PGPBs have been reported to provide tolerance to host plants under different abiotic stress environments (Blanco and Bernard, 1994; Dardanelli et al., 2008; Dimkpa et al., 2009; Egamberdieva and Kucharova, 2009; Yang et al., 2009; Choudhary et al., 2012). To date, many bacteria have been found that involve alleviation of different abiotic stresses (Table 5.3).
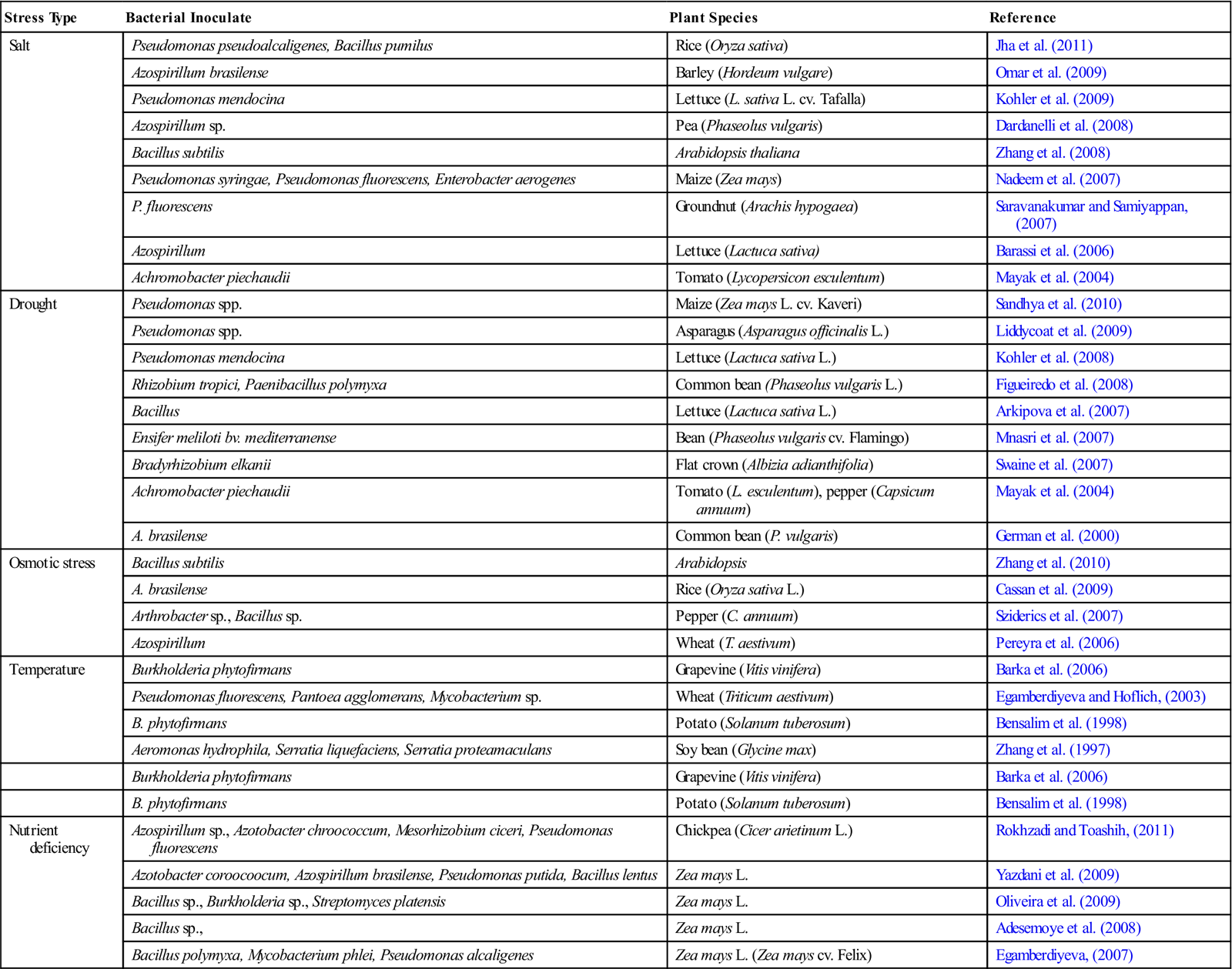
Table 5.3
PGPB Mediated IST against Abiotic Stress
| Stress Type | Bacterial Inoculate | Plant Species | Reference |
| Salt | Pseudomonas pseudoalcaligenes, Bacillus pumilus | Rice (Oryza sativa) | Jha et al. (2011) |
| Azospirillum brasilense | Barley (Hordeum vulgare) | Omar et al. (2009) | |
| Pseudomonas mendocina | Lettuce (L. sativa L. cv. Tafalla) | Kohler et al. (2009) | |
| Azospirillum sp. | Pea (Phaseolus vulgaris) | Dardanelli et al. (2008) | |
| Bacillus subtilis | Arabidopsis thaliana | Zhang et al. (2008) | |
| Pseudomonas syringae, Pseudomonas fluorescens, Enterobacter aerogenes | Maize (Zea mays) | Nadeem et al. (2007) | |
| P. fluorescens | Groundnut (Arachis hypogaea) | Saravanakumar and Samiyappan, (2007) | |
| Azospirillum | Lettuce (Lactuca sativa) | Barassi et al. (2006) | |
| Achromobacter piechaudii | Tomato (Lycopersicon esculentum) | Mayak et al. (2004) | |
| Drought | Pseudomonas spp. | Maize (Zea mays L. cv. Kaveri) | Sandhya et al. (2010) |
| Pseudomonas spp. | Asparagus (Asparagus officinalis L.) | Liddycoat et al. (2009) | |
| Pseudomonas mendocina | Lettuce (Lactuca sativa L.) | Kohler et al. (2008) | |
| Rhizobium tropici, Paenibacillus polymyxa | Common bean (Phaseolus vulgaris L.) | Figueiredo et al. (2008) | |
| Bacillus | Lettuce (Lactuca sativa L.) | Arkipova et al. (2007) | |
| Ensifer meliloti bv. mediterranense | Bean (Phaseolus vulgaris cv. Flamingo) | Mnasri et al. (2007) | |
| Bradyrhizobium elkanii | Flat crown (Albizia adianthifolia) | Swaine et al. (2007) | |
| Achromobacter piechaudii | Tomato (L. esculentum), pepper (Capsicum annuum) | Mayak et al. (2004) | |
| A. brasilense | Common bean (P. vulgaris) | German et al. (2000) | |
| Osmotic stress | Bacillus subtilis | Arabidopsis | Zhang et al. (2010) |
| A. brasilense | Rice (Oryza sativa L.) | Cassan et al. (2009) | |
| Arthrobacter sp., Bacillus sp. | Pepper (C. annuum) | Sziderics et al. (2007) | |
| Azospirillum | Wheat (T. aestivum) | Pereyra et al. (2006) | |
| Temperature | Burkholderia phytofirmans | Grapevine (Vitis vinifera) | Barka et al. (2006) |
| Pseudomonas fluorescens, Pantoea agglomerans, Mycobacterium sp. | Wheat (Triticum aestivum) | Egamberdiyeva and Hoflich, (2003) | |
| B. phytofirmans | Potato (Solanum tuberosum) | Bensalim et al. (1998) | |
| Aeromonas hydrophila, Serratia liquefaciens, Serratia proteamaculans | Soy bean (Glycine max) | Zhang et al. (1997) | |
| Burkholderia phytofirmans | Grapevine (Vitis vinifera) | Barka et al. (2006) | |
| B. phytofirmans | Potato (Solanum tuberosum) | Bensalim et al. (1998) | |
| Nutrient deficiency | Azospirillum sp., Azotobacter chroococcum, Mesorhizobium ciceri, Pseudomonas fluorescens | Chickpea (Cicer arietinum L.) | Rokhzadi and Toashih, (2011) |
| Azotobacter coroocoocum, Azospirillum brasilense, Pseudomonas putida, Bacillus lentus | Zea mays L. | Yazdani et al. (2009) | |
| Bacillus sp., Burkholderia sp., Streptomyces platensis | Zea mays L. | Oliveira et al. (2009) | |
| Bacillus sp., | Zea mays L. | Adesemoye et al. (2008) | |
| Bacillus polymyxa, Mycobacterium phlei, Pseudomonas alcaligenes | Zea mays L. (Zea mays cv. Felix) | Egamberdiyeva, (2007) |
Promotion of root growth that produces a larger root surface area provides uptake of nutrients and water and thus increases their availability to the plant. Inoculation of the Azospirillum and cell-free supernatant of A. brasiliense in the plant has been shown to promote morphological root changes, including the production of phytohormones, auxins, cytokinins, and gibberellins (Spaepen et al., 2008). This is confirmed by exogenous application of IAA to bean roots (Remans et al., 2008a,b). A widespread characteristic of the PGPB is ACC deaminase (ACC-D) activity where bacteria regulate ACC and help abiotically stressed plants survive (Mayak et al., 2004; Saleem et al., 2007; Dimkpa et al., 2009; Lugtenberg and Kamilova, 2009). Under abiotic stress, PGPBs use the immediate ethylene precursor ACC as a source of nitrogen and, using ACC-D, degrade it into 2-oxobutanoate and ammonia, thus indirectly increasing plant growth (Glick et al., 2007; Kang et al., 2010; Bianco and Defez, 2011). Inoculation with bacteria containing ACC-D has been found to induce longer roots that help in the uptake of water from deep soil under drought stress conditions, thereby ameliorating a plant’s water use efficiency under drought conditions (Saleem et al., 2007; Zahir et al., 2008). Researchers performed experiments on ACC-D under abiotic stress conditions and found decreasing levels of ethylene in the rhizosphere (Saravanakumar and Samiyappan, 2007; Bianco and Defez, 2011).
In addition, under abiotic stress conditions, the level of osmoprotectant proline increased in plants in the presence of PGPB (Smirnoff and Cumbes, 1989; Barka et al., 2006; Chen et al., 2007; Sziderics et al., 2007; Bianco and Defez, 2009; Bano and Fatima, 2009; Kohler et al., 2009; Sandhya et al., 2010; Jha et al., 2011; Vardharajula et al., 2011). Proline alters the effect of abiotic stress in a different way, such as by scavenging reactive oxygen species (ROS) using antioxidant activity and by stabilizing the protein structure through molecular chaperones (Kavi Kishor et al., 2005; Verbruggen and Hermans, 2008). Researchers performed experiments on microbial determinants under abiotic stress; these included exopolysaccharides (EPS) (Sandhya et al., 2009), trehalose (Figueiredo et al., 2008; Suarez et al., 2008), glycine betaine (GB) (Murata et al., 1992; Mohanty et al., 1993; Jagendorf and Takabe, 2001), potassium (Blumwald, 2000; Maser et al., 2002; Takahashi et al., 2007), and VOCs (Ryu, 2004). VOCs emitted by PGPB down-regulate and up-regulate hkt1 expression in roots and shoots and maintain lower Na+ levels under salt stress (Zhang et al., 2008; Yang et al., 2009).
Temperature is another crucial parameter affecting plant growth and development. Most of the normal physiological processes in plants range from approximately 0°C to 40°C. Very high and very low temperatures cause injury effects in different ways. The thermotolerant Pseudomonas putida strain NBRI0987 shows a high level of stress sigma (S) (RpoS) in drought-affected chickpea (Cicer arietinum) rhizosphere under high-temperature stress at 40°C (Srivastava et al., 2008). Similar results were reported for various other crops under high temperatures (Zhang et al., 1997; Ali et al., 2009). Several PGPBs deal with cold- and/or low-temperature stress (Bensalim et al., 1998; Compant et al., 2005; Ait Bakra et al., 2006; Selvakumar et al., 2007a,b).
Metabolic processes, such as photosynthesis and respiration, occupy different cellular compartments in living plants. Different ROS, such as superoxide (O2−), hydrogen peroxide (H2O2), hydroxyl radical (•OH), and singlet oxygen (1O2), are continuously produced as by-products of these metabolic pathways (Apel and Hirt, 2004). Major plant ROS-scavenging mechanisms include superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione reductase (GR), and catalase (CAT) enzymes. PGPBs, too, play a significant role in ROS scavenging (Bianco and Defez, 2009; Omar et al., 2009; Kohler et al., 2010; Sandhya et al., 2010).
In addition, nutrient elements, such as phosphorus, potassium, iron, zinc, and copper, possess limited mobility in the soil. In one study, phosphorus accumulation was shown to decrease in plants under salt stress when P-deficiency symptoms were induced (Navarro et al., 2001; Rogers et al., 2003; Parida and Das, 2004). Several PGPB strains solubilize insoluble inorganic phosphate compounds (e.g., tri-calcium phosphate, di-calcium phosphate, hydroxyapatite, and rock phosphate) by producing organic acids (Rodrìguez and Fraga, 1999; Egamberdiyeva, 2007; Richardson et al., 2009; Khan et al., 2009). Different PGPBs with different/matching PGP activity also showed synergistic effects when inoculated in cohorts (Tchebotar et al., 1998; Parmar and Dadarwal, 1999; Itzigsohn et al., 2000; Hamaoui et al., 2001; Sindhu et al., 2002a,b; Remans et al., 2007, 2008a,b; Elkoca et al., 2008; Yang et al., 2009; Figueiredo et al., 2010; Yadegari and Rahmani, 2010).
5.5 Conclusion and future prospects
This chapter focused on the role of PGPBs in the plant protection against biotic stresses—ranging from microorganisms and parasites to nematodes and insects—and in plant tolerance of biotic stresses. They do so by producing different osmoprotectants. PGPB-elicited ISR and IST were elaborately described along with signaling cascades and gene-expression mechanisms. Given a rapidly growing global population, the demand for increased crop yields is ever increasing; that is why it has become more and more important to use agrochemicals in the form of fertilizers and pesticides. Although agrochemicals show an instant effect on growth and disease control, their effects are not long lasting and they reduce soil fertility.
Plant growth-promoting bacteria are now considered the best alternative to these agrochemicals because they have many positive benefits. Besides promoting plant growth, PGPBs defend plants from different disease-causing agents. In this chapter, the role of PGPBs in biotic stress was shown in the form of ISR and allelochemicals; in the abiotic stress tolerance, it was shown in the form of IST. Another role of different PGPB strains alone and synergistically is in enhancing plant tolerance for abiotic stress. Moreover, PGPBs can be used to determine the roles of plant–microbe interaction and rhizoremediation in the degradation of soil pollutants. To more successfully apply PGPB in the agricultural field, a greater understanding of their ecology is needed.
Acknowledgments
The authors gratefully acknowledge DBT grant No. BT/PR1231/AGR/21/340/2011 to DKC. Some of the research in this chapter was supported by SERB grant No. SR/FT/LS-129/2012.



