Osmolyte Dynamics
New Strategies for Crop Tolerance to Abiotic Stress Signals
Resham Sharma, Renu Bhardwaj, A.K. Thukral, Neha Handa, Ravdeep Kaur and Vinod Kumar
Osmolytes and osmoprotectants have long been identified as pivotal abiotic stress busters because they help plants overcome extremely harsh environmental conditions by constant cellular homeostatic monitoring. This group mainly consists of sugars, polyols, amino acids, and betaines. Together, they shield plants by exercising a number of physiological responses such as membrane integrity strengthening, enzymatic/antioxidant activity balancing, and water adjustments under various abiotic stresses (e.g., temperature fluctuations, water deficit, salinity, heavy metals) and, more recently, pesticide exposure. Crop plant cultivation across the globe needs such comprehensive protection strategies against a vast array of ever-changing environmental conditions by developing tolerance at genetic and molecular levels. Currently, this is being achieved via osmoprotectant genetic engineering of plant genomes and exogenous application of antistress agents to crop plants to obtain high-yield, stress-resistant varieties. A newer modification to this two-dimensional approach is osmoprotectant activation via “omics”—an integral approach comprised of genomics, metabolomics, and transcriptomics. This chapter gives a cumulative account of their biosynthesis, classification, and functioning with respect to transgene induction, exogenous application, and omic approaches for an improved agricultural dimension.
Keywords
abiotic stress responses; environmental stresses; exogenous application; gene expression; osmoprotectants; resistant and transgenic crop varieties
17.1 Introduction
Constantly fluctuating climate and abiotic stresses, such as water deficit, temperature variations, extreme soil salinity, and heavy metal and pesticide toxicity, are serious threats to normal plant growth and development; they are the cause of major crop productivity losses across various geographic zones worldwide (Osakabe et al., 2013, Chamoli and Verma, 2014). Adding to this, modern agricultural practices including monoculturing and excessive use of chemical fertilizers, herbicides, and pesticides end up as perils rather than assets; this is because they persist as xenobiotics that harm delicate ecological components, resulting in more damage than protection (Damalas et al., 2011; Abro et al., 2013; Costa et al., 2014). Understandably, agrarian economies need to develop crop plants tolerant to such environmental stresses for normal growth and balanced functioning. For this reason, genes responsible for active expression of antioxidants, enzymes, osmoprotectants, transporters, and so on have been identified and expressed in the past decade with a very high frequency (Krasensky and Jonak, 2012). By adopting this commendable method, development of transgenic plants through target genetic engineering and exogenous application of osmoprotectants is being achieved (Chen et al., 2014).
Another strategy is the application of chemicals and phytohormones (e.g., nitrous/nitric oxide, salicylic acid, polyamines, brassinosteroids, cytokinins) to increase osmoprotectant expression in crop plants (Duque et al., 2013; Farooq et al., 2013). The novelty of osmoprotectants is their ability to maintain ionic and turgor balances in plant cells, and their heightened accumulation under stress followed by degradation when optimum conditions are achieved (Pinto-Marijuan and Munné-Bosch, 2013). This characteristic feature needs to be introduced into plant species that do not naturally possess them when exposed to abiotic stress. As depicted in Figure 17.1, recent studies indicate that it is better to lump all transgenic and RNA-sensing classes of crop development strategies into what is called “omics”; these include fields such as genomics, transcriptomics, metabolomics, and others. This allows researchers to get a better grasp of gene expression, protein modification, and metabolite composition technologies for osmoprotectants that highlight abiotic stress responses in crops (Urano et al., 2010; Silva et al., 2011). This chapter is an effort to cover the different roles played by osmoprotectants in various plant stress adaptations and the possibility of crop improvement, as well as the degree of effectiveness achieved by promoting stress tolerance using these methods.
17.2 Osmoprotectants in plants
Osmoprotectants or compatible solutes are tiny molecules that make osmotic adjustments between the cell’s surroundings and its cytoplasm; they are selectively released from the protein interface as a result of their tendency to form hydrophilic complexes that in turn stabilize stress inducers (Lang, 2007; Krasensky and Jonak, 2012). To enhance the stress-remediating potential of osmolytes, their engineering into plant genomes has long been considered as one successful approach to apply for normal plant physiological functioning and crop improvement under extreme conditions (Rathinasabapathi, 2000; Rontein et al., 2002)., The three major classes of osmoprotectants in plants that have been reported are briefly discussed in the following subsections.
17.2.1 Sugars and polyols
Sugars, mainly composed of complex saccharides and sugar alcohols or polyols (highly soluble, six-carbon, nonreducing sugars with −OH groups), are the major classes of essential biomolecules that act as structural components and signaling and transport molecules (Noiraud et al., 2001). They are widely discussed as compatible solutes or osmoprotectants and antioxidants under various abiotic stresses and are known to provide innate immunity to plants as well (Moghaddam and Ende, 2012; Kido et al., 2013). Polyols (e.g., mannitol, inositol, glycerol, ribitol) play similar roles due to the presence of hydroxyl groups that form hydration spheres around macromolecules, prohibiting their metabolic inactivation under osmotic stress (Williamson et al., 2002; Janska et al., 2010). Their protective roles in plants are described next.
17.2.1.1 Fructans
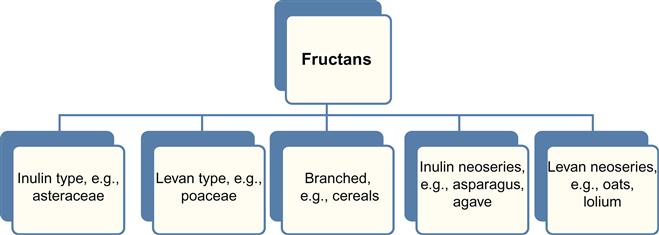
Fructans are water-soluble storage oligosaccharides primarily consisting of fructose units synthesized in plant vacuoles that act as compatible solutes (Valluru and Van den Ende, 2008). Depending on the linkage between fructosyl residues and the spatial arrangement of glucose residues, the fructans are divided into five major categories as shown in Figure 17.2. Fructosyl transferases (FTs) catalyze synthesis of fructans by transferring fructose units from sucrose to a growing fructan chain, followed by incorporation of FTs back into fructans (Krasensky and Jonak, 2012). Fructans accumulate under abiotic stresses, stabilizing cell and organelle membranes and preventing electrolytic leakage of water molecules across them (Peshev et al., 2013; Ahanger et al., 2014). They achieve this by inserting a part of the polysaccharide chain in between the phospholipid mono- and bilayers, thereby fortifying the membranes and acting as effective cryoprotectants (Livingston et al., 2009; Bhandari and Nayyar, 2014; Swati Megha and Basu, 2014).
17.2.1.2 Raffinose family oligosaccharides
Raffinose family oligosaccharides (RFOs) are a group of soluble, nonreducing trisaccharide sugars (e.g., raffinose, stachylose, verbascose, and ajugose) that are actively accumulated under drought and dehydration in plants (Palma et al., 2014; Pirzadah et al., 2014). Raffinose exists ubiquitously while stachylose and other highly polymerized RFOs (e.g., verbascose and ajugose) exist in the vacuoles of certain plants (El Sayed et al., 2013). The first step toward their synthesis is formation of galactinol from myo-inositol and uridine diphosphate galactose in the presence of galactinol synthetase.
This is followed by donation of galactosyl moiety to sucrose and raffinose in the case of raffinose and stachylose synthesis, respectively (Van den Ende, 2013). The same as fructans, RFOs generally act as storage and transport carbohydrates in plants. Expression of galactinol and RFO-synthesizing enzymes elevates during the oxidative burst in plants due to various environmental stresses, which in turn raises endogenous RFOs and galactinol levels (Nishizawa-Yokoi et al., 2008). Numerous reports have been cited for RFO accumulation during salinity, drought, and temperature stress in plants (Kempa et al., 2007; Peters et al., 2007; Usadel et al., 2008; Kim et al., 2010; Gangola et al., 2013). Like fructans, RFOs function as osmoprotectants by acting as phloem-mobile signaling compounds, stabilizing membranes and scavenging reactive oxygen species (ROS) (Nishizawa et al., 2008; Conde et al., 2011; Van den Ende, 2013).
17.2.1.3 Trehalose
Trehalose is a nonreducing disaccharide that accumulates in bacteria, algae, fungi, yeast, invertebrates, and some resurrection plants in response to drought, salinity, temperature variations, and heavy metal stresses (Jang et al., 2003; Cortina and Culianez-Macia, 2005). Trehalose contains two glucose units that are linked by α, α-1, and 1-glycosidic bonds (Redillas et al., 2012). It is synthesized from glucose-6-phosphate and uridine diphosphoglucose in a two-step process. The first step is catalyzed by the enzyme trehalose phosphate synthetase (TPS), resulting in the formation of trehalose-6-phosphate that is further catalyzed by trehalose-6-phosphate phosphatase to trehalose (Penna, 2003). It protects proteins and membranes against denaturation and membrane fusion in stressed plants (Iturriaga et al., 2009). Incorporation of trehalose biosynthetic genes (TPP and TPS) from microbes into transgenic plants has been shown to increase accumulation of trehalose and other soluble sugars, providing increased resistance to several abiotic stresses (Jang et al., 2003; Karim et al., 2007; Miranda et al., 2007; Iordachescu and Imai, 2008; Garg et al., 2012; Redillas et al., 2012).
17.2.1.4 Mannitol
Mannitol is a reduced form of mannose that acts as a chemical chaperone under stress and is the most abundant polyol in plants. It is synthesized in vascular plants from mannose-6-phosphate by simultaneous action of nicotinamide adenine dinucleotide phosphate (NADPH)-dependent mannose-6-phosphate reductase (M6PR) and mannose-6-phosphate phosphatase (Conde et al., 2011). The NAD-dependent enzyme mannitol-1-phosphate dehydrogenase (mtlD) oxidizes mannitol back to mannose (Reis et al., 2012).
17.2.1.5 Myo-inositol
Myo-inositol is a cyclic polyol containing cyclohexanehexol as its main component. It acts as a substrate for synthesis for the RFOs. Myo-inositols are synthesized in a two-step process, as follows:
Myo-inositol and its methylated derivatives (e.g., D-pintol and D-ononitol) accumulate in halophytes under salt stress (Sengupta et al., 2008). More recently, it was observed that myo-inositol derivatives regulate stress responses by serving as compatible solutes and signaling molecules (Kido et al., 2013).
17.2.1.6 Sorbitol
Sorbitol is one of the main photosynthetic end products and serves as a storage and transport sugar in most plant families (Li et al., 2012). Synthesis of sorbitol takes place by catalysis of glucose via NADP-dependent sorbitol-6-phosphate dehydrogenase (S6PDH). Sorbitol is further degraded to fructose by NAD+ sorbitol dehydrogenase in sink tissues (Liang et al., 2012). It plays an important role in osmotic adjustment in cell cytoplasm under various abiotic stresses such as salinity, chilling, and drought (Reis et al., 2012).
Gao and coworkers (2001) found that transgenic Diospyros kaki Thunb. trees overexpressing sorbitol-6-phosphate dehydrogenase had photosystem II that was affected less under salinity stress. Exogenous sorbitol application had positive effects on growth of salt-stressed plants and also reduced stress-induced H2O2 and MDA content in salt-sensitive rice seedlings (Theerakulpisut and Gunnula, 2012).
17.2.1.7 Glycerol
Glycerol is a simple polyol with three hydroxyl groups. Its backbone is focal to all triglycerides. Many marine algae are known to accumulate glycerol under abiotic stresses (Rathinasabapathi, 2000). These are known to offer osmotic buffering and membrane protection (Ashraf and Foolad, 2007). Glycerol-stimulating genes have been incorporated into tobacco plants to counter abiotic stresses (Chamoli and Verma, 2014).
17.2.2 Amino acids, peptides, and amines
Plants respond to environmental fluctuations via accumulation of various amino acids (e.g., asparagine, glycine, histidine, serine, peptides), glutathione and phytochelatins, and amines (e.g., spermine, spermidine, putrescine, nicotianamine, mugineic acid) (Less and Galili, 2008). These osmolytes are directly linked to metabolic pathways and help in regulation of cytosolic pH, ionic homeostasis, free radical removal and stabilization of organelles, and macromolecules such as protein complexes and membranes (Bray et al., 2000; Szabados and Savouré, 2010). Cysteine (Cys) is a sulfur-containing amino acid involved in synthesis of methionine, vitamins, and cofactors, and it is directly linked to osmoregulation in plants under stress (Anjum et al., 2012). Histidine (His) in its free form is a well-known metal chelator, especially for nickel because it has high-affinity and detoxification properties. By doing so, it maintains osmotic balance in the cells under stress (Kerkeb and Kramer, 2003). γ-Aminobutyric acid (GABA) is a well-known nonprotein amino acid involved in the carbon–nitrogen metabolism, signaling, and osmotic adjustments (Sharma and Dietz, 2006; Seher et al., 2013).
The foremost amino acid with the maximum reports for osmoprotectants under abiotic stress is proline (Mattioli et al., 2008). As the first osmoprotectant that accumulates in the cytoplasm in response to any abiotic stress and in addition to being an osmolyte, proline plays other important roles too: a signaling molecule, a metal chelator, an antioxidant defense molecule, and a buffering agent to control the redox potential across stressed cells (Ashraf and Foolad, 2007; Urano et al., 2010; Celik and Atak, 2012; Hayat et al., 2013). In plants, proline is synthesized by two pathways that involve glutamate and ornithine as precursor molecules. The glutamate pathway, as shown in Figure 17.3, is the major pathway for proline production during osmotic stress (Hayat et al., 2013; Rai and Penna, 2013).
The ornithine pathway is activated mostly during seedling development (Figure 17.4) in some species of plants during stress-induced proline accumulation (Armengaud et al., 2004; Xue et al., 2009). Proline is synthesized from glutamate, which is converted to glutamate-1-semialdehyde by the action of the enzyme pyrroline-5-carboxylate synthetase (P5CS); in turn it is spontaneously converted to pyrroline-5-carboxylate (P5C), which is further converted to proline by the action of the enzyme P5C reductase (P5CR) (Turchetto-Zolet et al., 2009; Sharma and Vaslues, 2010; Liang et al., 2012).
Proline levels were found to increase in many angiosperms under abiotic stress as reported by Mohammadkhani and Heidari (2008) and Sharma and coworkers (2011); the authors noted a positive relationship between proline accumulation and stress tolerance. This specific tendency of certain plants to accumulate proline was extolled and utilized further for crop plants that are not natural proline accumulators by using the transgenic approach and exogenous application techniques. These reports are discussed further later in the stress sections (Wang et al., 2012; Soshinkova et al., 2013; Xu et al., 2013; Surekha et al., 2014). The second most important class of osmoprotectants contains the polyamines (PA)—small aliphatic molecules involved in maintaining cellular pH—that are activated under drought and salinity stress (Macro et al., 2012). Another amino acid, 5-aminolevulinic acid (ALA), is involved in osmoprotection against cold stress and water deficit in many plants, and it has been used as an exogenous application for the same (Balestrasse et al., 2010; Korkmaz et al., 2010).
17.2.3 Quaternary ammonium compounds
Betaines are a group of osmotic protectants that are derivatives of amino acids containing fully methylated nitrogen atom. Glycine betaine (GB), proline betaine, β-alanine betaine, choline-O-sulfate, and 3-dimethylsulfoniopropionate are the most common types of betaines reported in stressed plants (Wani et al., 2013; Chen et al., 2014). Out of these, GB (N, N, N-trimethylglycine), which is a zwitter ion, is one of the most comprehensively studied compatible solutes. Its distribution is widely reported in bacteria, cyanobacteria, algae, fungi, animals, and many plant families (Chen and Murata, 2011). It plays an important role in maintaining cellular compatibility as well as osmoregulation in plant cells by absorbing water from the surroundings, leading to the maintenance of osmotic pressure (Giri, 2011).
Two different pathways for GB biosynthesis have been recognized in living organisms as illustrated in Figure 17.5. In most microorganisms, plants, and animals, choline acts as a precursor molecule that is oxidized to form betaine aldehyde with the help of choline monooxygenase. Betaine aldehyde is further converted into glycine betaine via catalysis of betaine aldehyde dehydrogenase (Wargo, 2013). Another pathway with glycine as a precursor is reported in halophytic cyanobacteria Actinopolyspora halophila and Ectothiorhodospira halochloris (Nyyssola et al., 2000). In this three-step pathway, glycinesarcosine methyltransferase (GSMT) catalyzes the conversion of glycine to N-methylglycine (sarcosine); this is further converted to N, N-dimethylglycine via dimethylglycine methyltransferase (SDMT). The methylation occurring in the second step can also be catalyzed by GSMT. N, N-dimethylglycine formed at the end of the second step is methylated to glycine betaine only with the help of SDMT (Waditee et al., 2005).

Because betaines play an important role in osmoregulation, their presence has been noted in chloroplasts, cytoplasm, and vacuoles (Chen and Murata, 2011). The same as in the case of other osmoprotectant classes, GB has been induced via transgenic technology and the exogenous approach in many nonaccumulating crop species to alleviate abiotic stresses without harming yield and quality (Joseph et al., 2013; Kaya et al., 2013).
17.3 Metabolic expression and exogenous application of osmoprotectants under abiotic stresses
The basic strategies used by most plants in antistress mechanics involves activation of osmoregulatory molecular cascades that in turn trigger the perception and transduction of stress and the expression of specific stress-related genes and metabolites; this is the basic strategy most plants use as an antistress mechanism (Figure 17.6). Recent progress in metabolomic analyses in a model crop, Arabidopsis, indicated the critical understanding of possible metabolic fluctuations in crops under varying environmental conditions (Iwaki et al., 2013). Such a study corroborates previous work and pushes forward the concept of engineering osmoprotectant genes and exogenous application of osmolytes and phytohormones for enhancing tolerance to various abiotic stresses, as discussed in the next subsections.

17.3.1 Temperature stress
Temperature stress manifests itself in crop plants at two diverse levels: injuries due to heat and chilling stresses. Because plants are sessile organisms, they lack the ability to move to optimum environments when faced with a sudden rise or drop in temperature; as a result, growth and development are hindered significantly (Lobell and Field, 2007; Wigge, 2013). All biochemical reactions are temperature sensitive from enzymatic activity to molecular expression, so it is imperative to cover losses caused to plants under temperature stress. High temperature (HT) is a threat to crop productivity, therefore it is necessary to sustain high-yielding yet resistant plants (Wahid and Close, 2007). Cold temperature (CT) stress includes chilling and freezing.
HT and CT prevent the complete genetic expression of plants resulting in inhibition of metabolic reactions and induction of osmotic and oxidative stress (Chinnusamy et al., 2007). Because of this, the geographical occurrence of plants is restricted and huge losses in agricultural productivity are foreseen (Adam and Murthy, 2014). Endogenous osmoprotectants are involved in signal cascading and transcriptional control activation to counter these biochemical and physiological aberrations that are otherwise irreversible in many plants. However, current theories propose inducing them into nonosmoprotectant accumulators via transgenic technology or to counter oxidative stress by exogenous application of osmolytes and phytohormones as foliar sprays into plant tissue culture trials followed by field conditioning (Bita and Gerats, 2013; Hasanuzzaman et al., 2013).
Transgenic induction confirmed the positive effects of proline gene overexpression during HT stress as transformed plants showed a negative leaf osmotic potential and increased xanthophyll accumulation (Dobra et al., 2010). Similarly, high glycine betaine (GB) contents were reported in maize and sugarcane (Quan et al., 2004; Wahid and Close, 2007) under HT. On the other hand, there are plant species (e.g., rice, mustard, tobacco) that are not natural GB–sugar accumulators under HT; however, the transgenic approach allowed induction of the GB–trehalose–fructan-biosynthetic pathways into them (Sakamoto and Murata, 2002; Quan et al., 2004).
The Raffinose family oligosaccharides (RFO) were marked for desiccation tolerance in plant seeds. RFO contents were determined based on analysis of the genes involved in their biosynthesis. Extensive sugar analysis of RFO contents in Arabidopsis thaliana under CT revealed a huge amount of raffinose and galactinol accumulation in transgenic plants. Three stress-responsive galactinol synthase (GolS) genes (AtGolS1, 2, and 3) among seven Arabidopsis GolS genes were identified. Of these, AtGolS3 was found to be CT-induced (Taji et al., 2002). Tobacco cultivars were transformed so they can overaccumulate proline, fructans, and GB by incorporation of transgene constructs from Arabidopsis spp. and Vigna spp. genes (AtP5Cs and VacP5Cs) for D1-pyroline-5-carboxylate synthetase production. The gene coding of Bacillus subtilis SacB for levansucrase and Arthrobacter globiformis codA for choline oxidase were also integrated and expressed in these tobacco plants; then they were subjected to subzero temperatures and osmotic interplays. Their subsequent CT-resistant progenies were established for stable transgenic line selection (Konstantinova et al., 2002). Levels of oxidative stress markers, such as leakage of electrolytes and osmoprotectant accumulation, were recorded for freezing stress. Control tobacco plants showed marked damage; however, transformed tobacco plants showed a lower extent of oxidative damage (Parvanova et al., 2004).
Many recent reports on cold–heat stress have noted a rise in the cellular levels of proline, GB, sugars, and sugar alcohols; these serve as cryoprotectants to protect cellular metabolism (Bhandari and Nayyar, 2014; Pirzadah et al., 2014; Sağlam and Jan, 2014). An interesting study of cold stress responses in two cultivated tomato varieties, Gerry (cold stress sensitive) and T47657 (moderately cold stress tolerant), found that exogenous GB induced the expression of lypoxygenase-13 (TomLOXF) and fatty acid desaturase 7 (FAD7) genes; this led to increased membrane stability and defense mechanisms via the octadecanoid pathway or lipid peroxidation products (Karabudak et al., 2014). This is still a very potential area for further research into temperature stress induction, it needs more review.
17.3.2 Water deficit
Water deficit is one of the most common forms of abiotic stress leading to reduced plant productivity (Yadav et al., 2014). Relatively long durations of water scarcity may lead to premature senescence and plant death. Plants being sedentary by nature are subject to strong selection pressures for managing variations in water availability, which includes the increasing content of organic osmolytes such as GB, proline, and pinitol (Miller et al., 2010). Under water deficit, aspargine, proline, and valine levels increase significantly in Bermuda grass, wheat, potatoes, and hot peppers (Sairam et al., 2002; Kerkeb and Kramer, 2003; Knipp and Honermeier, 2006; Anjum et al., 2012). Increased proline content and escalated expression of P5CS1 in transgenic Arabidopsis spp. was also recorded (Mattioli et al., 2008, 2009; Lehmann et al., 2010).
Additionally, reports exist for the involvement of proline in boosting of antioxidant enzyme activity (Khedr et al., 2003; Campos et al., 2011). Plant tolerance to water stress has been determined by various biochemical pathways that cause the acquisition or retention of water, protection of chloroplast function, and maintenance of ion homeostasis (Shinozaki and Yamaguchi-Shinozaki, 2007). Biosynthesis of both polyamines and compatible osmolytes (e.g., betaines, proline, and sugars) increases under water stress (Yang et al., 2007; Kubis, 2003, 2008). In some cases, the suppression of proline biosynthesis in transgenic plants resulted in increased sensitivity to water stress (De Ronde et al., 2000).
Water deficit in transgenic tobacco resulted in overexpression of proline biosynthesis enzymes (Roosens et al., 2002). Similarly, soluble sugars in arbuscular mycorrhizal plants act as osmoprotectants by providing stability to plant organelles under water deficit (Auge, 2001; Porcel and Ruiz-Lozano, 2004). In addition, soluble sugar content in arbuscular mycorrhizal associated Erythrina variegata (Monoharan et al., 2010) and Casuarina equisetifolia (Zhang et al., 2010) seemed to decrease oxidative damage linked to water stress. The enhancement of soluble sugars in the leaves of arbuscular mycorrhizal associated Citrus tangerine plants was reported under water stress by 1.37-fold to that of well-watered control plants (Wu and Xia, 2006).
As discussed earlier, GB is a well-known osmoprotectant involved in the protection of protein–enzymatic activities under water stress and results in the stabilization of membranes during freezing (Subbarao et al., 2001; Xing et al., 2010). Thus, induction of GB biosynthetic genes to overexpress in plants is a commonly practiced adaptive strategy. When transformation of heterologous P5CS into the high proline-producing species of citrus (~100 μmol g−1 of leaf dry weight) was done, Molinari and coworkers (2004) found that the transformed plants contained 8-fold more proline than the nontransformed plants; this resulted in an osmotic adjustment and maintenance of pressure potential under water stress. In sugarcane, GB and other osmolytes and soluble sugars play a major role in osmotic adjustment and act as oxidative damage shields for managing water stress (Wahid, 2004; Gandonou et al., 2006; Patade et al., 2008). Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 in transgenic rice revealed an increased tolerance to abiotic stresses compared to control plants (Li et al., 2011).
Similarly, exogenous application of trehalose in maize plants alleviated drought resistance by up-regulating growth, photosynthetic and water relations, as well as antioxidant defense mechanisms (Ali and Ashraf, 2011; Theerakulpisut and Gunnula, 2012). IMT (myo-inositol methyltransferase) cDNA from Glycine max was isolated and, via RT-PCR analysis, it was revealed that GmIMT transcripts were induced by both drought and salinity stress in soybean seedlings (Ahn et al., 2011). Recently, in response to water deficit stress, transgenic Arabidopsis plants were marked for an increase in galactinol and raffinose concentrations resulting in a better ROS scavenging capacity (El Sayed et al., 2013). Under conditions of normal water supply and water stress, the isoforms of three antioxidant enzymes (i.e., APX, GR, and CAT) were differentially regulated in leaves of transgenic Swingle citrumelo plants with enhanced endogenous proline accumulation. Findings indicated that proline also caused a 2-fold increase in transcription activity of the genes—CAT2, APX1, APXcl, and cu/znSOD2—involved in antioxidant enzyme generation under water stress (Carvalho et al., 2013). Another report indicated a vacuolar H+-pyrophosphatase gene in the wheat genome (TaVP) with an expression that is up-regulated under drought stress and linked to sugar hyperaccumulation as a subsidiary response in targeted plant cells (Li et al., 2014b). Cumulatively, these reports convey that osmolytes are instrumental in protecting plants, especially at times of water deprivation.
17.3.3 Salinity stress
Salinity stands for hyper salt accumulation in soils beyond the tolerance limits for most plants and approximately 20% of the world’s total irrigated agricultural land suffers from poor yield due to high salt content (Selvakumar et al., 2014). Salt stress affects crops under extreme saline conditions by severely impairing plants’ metabolism due to osmotic stress and loss of turgor. One of the mechanisms adopted by plants to tolerate salt stress is the accumulation of compatible solutes that help maintain osmotic homeostasis (Gill et al., 2014). Salinity stress has a marked effect on sugar and polyol accumulation. In different genotypes of seashore paspalum, sugars (e.g., glucose, sucrose, and myo-inositol) increased due to salinity stress while mannitol and sorbitol showed no effect (Lee et al., 2008).
Under extreme salt stress, the accumulation of asparagine, arginine, serine, and glycine was reported in Coleus blumei Benth, Spinacia oleracea, and Oryza sativa (Martino et al., 2003; Summart et al., 2010). Sugar and starch accumulation was also reported in Solanum lycopersicum plants (especially fruits) subjected to a 160 mM concentration of NaCl stress (Yin et al., 2010). Mannitol overexpressing the M6PR gene from celery was induced in Arabidopsis, and it elevated the expression of other stress-inducible genes as well. The M6PR gene increased salt tolerance in Arabidopsis by lowering salt injury, minimizing vegetative growth inhibition, and enhancing seed production as compared to wild-type plants (Chan et al., 2011).
Further study substantiated the up-regulation of the expression of a sucrose transporter in leaves and enhanced activity of ADP-glucose pyrophosphorylase (AGPase) in fruits. AGPase is responsible for catalysis of the synthesis of ADP-glucose from glucose-1-phosphate and ATP. Bread wheat cv. Giza 163 was made salt-tolerant by transforming the mtlD gene (encoding mannitol-1-phosphate dehydrogenase) of E. coli (Ramadan et al., 2013). It has been observed that expression of mannitol in the transgenic plant led to enhanced grain yield in plants treated with salt (NaCl and CaCl2). Other reports indicate that the fructose, glucose, and galactronic acid content increased significantly while sorbitol, mannose, and galactose levels decreased. PcINO1 gene coding for L-myo-inositol-1-phosphate synthase (MIPS) from a halophytic variety of wild rice tolerant to salinity was induced into cultivated mustard and tobacco plants. The resulting transgenic plants showed increased salt tolerance and less oxidative damage with inositol accumulation in both roots and shoots. The yield and crop quality of transgenic plants remained uncompromised and the plants were able to grow stably, set seeds, and germinate in saline environments (Das-Chatterjee et al., 2006).
Amino acids are also very sensitive to changes in salt concentrations in the soil. Proline is one of the most sensitive amino acids that accumulates in the cytosol and vacuoles in response to salt stress and offers protection against the damaging effects of 1O2 and −OH by binding to redox-active metal ions (Miller et al., 2010). Its increase was reported in two varieties of rice cultivars subjected to increasing NaCl concentrations (Demiral and Türkan, 2005). A marked increase in the concentration of proline was also observed in Trachyspermum ammi (L.) Sprague in response to NaCl stress (Ashraf and Orooj, 2006).
A study conducted on two cultivers of salt-stressed Sesamum indicum showed an enhanced level of proline accumulation at the highest salt concentration in salt-tolerant cultivars; this suggested its important role in maintaining plant homeostsis (Koca et al., 2007). Similar results have been reported in Paspalum vaginatum where proline content was enhanced significantly in response to stress and was documented to be a major osmolyte for maintaining osmotic homeostasis in a variety of plant genotypes (Lee et al., 2008). Proline metabolism is also affected by salt stress. The activities of the enzymes involved in its metabolic pathways are reported to be altered to significant levels, leading to enhancement of proline accumulation in plants. Metabolic enzymes were analyzed in Oryza sativa subjected to salt stress and the authors found that the activities of the proline synthesizing enzymes (i.e., P5CS, P5CR, and ornithine-δ-aminotransferase) were increased while it decreased in proline hydrolysis enzyme proline dehydrogenase (PDH) (Bagdi and Shaw, 2013).
Similar results have been reported in a study carried out on cell suspension cultures of Cucumis sativus treated with NaCl. Two types of cell cultures (i.e., acclimated and nonacclimated) were maintained. The activities of P5CS and P5CR were enhanced and PDH was reported to decline in both types of cultures. Naliwajski and Skłodowska (2014) also noted that proline content in the cultures increased significantly and acclimated cultures responded to the stress quickly. In another study carried out on Helianthus tuberosus L. by Huang et al. (2013), the effect of salt stress was observed on activities of biosynthetic enzymes and accumulation of proline. The authors found that roots, stems, and leaves of plants accumulated significant amounts of proline and reported that activities of the enzymes involved in the glutamate pathway were high in response to stress. However, those involved in the ornithine pathway and proline catabolism decreased significantly thereby indicating that the glutamate pathway is the major process activated during salt stress tolerance.
Exogenous application of proline also has been reported to have a protective effect on stressed plants. Cucumis melo was subjected to 150 mM NaCl alone and in combination with 10 mM of proline. Kaya et al. (2007) observed that proline was instrumental in ameliorating the adverse effects of salt on growth, fruit yield, and other physiological parameters. A similar investigation on a salt-sensitive variety, “Jinchun No. 2” of Cucumis sativus, was done by Huang and coworkers (2009). Stress was given in the form of NaCl (100 mM) and a significant change in dry weight; leaf relative water; malondialdehyde; content of K+, Na+, Cl−; and enzymes was observed. Foliar application of proline (25 ml per plant) was effective in alleviating stress and enhanced the tolerance of plants to saline conditions.
Glycine betaine content was found to increase in the two cultivars of Beta vulgaris L. when exposed to saline conditions (Subbarao et al., 2001). NaCl treatment was given to plants and GB content was correlated with the amount of Na+ in the leaves, thereby indicating that salinity may be one of the factors prompting synthesis of GB in plants. Similar results have been reported by Kumar and his companions (Kumar et al., 2003) in two cultivars of Morus alba L. where the salt-tolerant variety was able to accumulate more betaines compared to the salt-sensitive variety; this suggests the protective role of this osmolyte. Atriplex nummularia and Atriplex semibaccata are two highly salt-tolerant plant species and were studied for the genetic basis for GB biosynthesis when subjected to high concentrations of salt (Joseph et al., 2013). The authors identified and analyzed choline monooxygenase and betaine aldehyde dehydrogenase, two enzymes involved in GB biosynthesis. They found that the expressions of both the genes encoding these enzymes were significantly up-regulated in leaf tissues and accumulated a high level of GB in both species. Exogenous application of GB to plants also enhances the defense system against salt stress. Maize plants subjected to NaCl stress were treated with different concentrations of GB. The plants showed higher resistance to salt stress by enhancing the activities of antioxidative enzymes and increasing pigment content. Exogenous application of GB also enhanced the endogenous GB levels, thereby highlighting its ameliorative effect (Nawaz and Ashraf, 2010).
A similar study conducted by Hu et al. (2012) on Lolium perenne also confirmed the role of GB as a stress protectant. The plants were subjected to NaCl stress and application of GB to them significantly enhanced growth, relative water content, transpiration rate, pigment content, and activities of the antioxidative enzymes. The same study confirmed the role of GB in maintaining the cellular homeostasis and K+–Na+ ratio. In another study carried out on salt-stressed maize treated with GB exogenously, Kaya et al. (2013) reported that foliar application fortified the antioxidative defense system of the plants and alleviated the detrimental effects of salinity. Glycine betaine has also been reported to be instrumental in affecting Ca2+ signaling in stressed plants. GB pretreated tobacco plants when subjected to NaCl stress showed Ca2+ efflux in epidermal cells of root; however, after 24 h of treatment, Ca2+ influx was observed. The study concluded that GB induced an increase in free Ca2+ ion concentration and enhanced expression of calmodulin and heat shock transcription factors in the cells, leading to increased levels of heat shock proteins (Li et al., 2014a).
Transgenic studies conducted with plants also confirmed the importance of GB in salt tolerance and the technique has been successfully applied to various crops to obtain stress-resistant varieties. Usually, the genes engineered in the plants are those that encode the enzymes involved in the biosynthetic pathway. Solanum lycopersicum plants, when engineered with genes encoding betaine aldehyde dehydrogenase and choline oxidase, showed tolerance to salt stress in both seedling and mature phases (Zhou et al., 2007; Goel et al., 2011). These two genes were also reported to be introduced in S. tuberosum thereby enhancing the plant’s ability to resist oxidative, salt, and drought stresses (Ahmad et al., 2008; Zhang et al., 2011).
Similarly, He et al. (2010) transformed the choline dehydrogenase endcoding gene betA in Triticum aestivum, an important enzyme in the biosynthetic pathway of GB. The authors observed that transgenic plants had a higher content of GB and showed higher germination, vigorous growth, and more photosynthesis rates under high salt concentrations. In another study, Medicago sativa plants were observed to grow vigorously under salt stress compared to wild types when a gene encoding betaine aldehyde dehydrogenase was transformed (Liu et al., 2011). Thus, GB may be of vital importance not only in normal cellular functioning but also as a stress protectant to aid in enhancing the tolerance of plants to stress.
Additionally, many compounds, including some phytohormones, when applied exogenously alleviate plants’ stress from salt by increasing endogenous osmolyte accumulation (Iqbal et al., 2014). Nitric oxide (NO) helps in the maintenance of osmoregulation and ion homeostasis under salinity stress, as discussed in Farooq et al. (2013). Exogenous application of 24-epibrassinolide (EBL) on a Pusa Basmati-1 variety of rice under NaCl stress showed an improvement in proline content compared to the control plants (Sharma et al., 2013).
17.3.4 Heavy metal stress
Proline, betaines, and soluble sugars are the main osmolytes reported to accumulate in crop plants growing in soils laden with heavy metals. Currently, the significance of multiple transcription factors, protein–enzyme complexes involved in osmolyte production, and osmolyte-induced specific gene expression in plant tolerance is a hotbed for global research (Bhardwaj et al., 2014). Early reports indicated proline to be the first accumulated osmoprotectant in response to Cd, Cu, and Zn stress in crop plants such as sunflower, wheat, and rice (Chen et al., 2001). Similarly, proline levels were found to rise in Anethum graveolens, Brassica juncea, Cynara scolymus, and Plantago psyllium under Cd, Cu, and Pb stress (Karimi et al., 2012; Aghaz et al., 2013; Mohammadi et al., 2013). Co, Cu, and Fe toxicity led to escalated GB accumulation in addition to activated antioxidant enzymes in plants (Chen and Murata, 2011; Dhir et al., 2004, 2012). A significant increase in proline, GB, and total sugar levels was observed in response to Cd, Cu, Cr, and Zn exposure in plants such as Brassica juncea L., Lactuca sativa, Salvinia natans, and Solanum lycopersicum (Aly and Mohamed, 2012; Dhir et al., 2012; Handa et al., 2013; Hashem et al., 2013; Muslu and Ergun, 2013; Sharma et al., 2013c).
However now, osmolyte expressions via genetic engineering, as well as exogenous application formats, clearly symbolize their contribution to water adjustments and antioxidant induction at microcellular levels under heavy metal stress. The osmolyte-centered proteomic and metabolomic approaches are quickly evolving as significant tools to determine plants’ responses to various external stimuli including heavy metal stress (Rodziewicz et al., 2014). Earliest indications revealed metal induced proline accumulation and its protective role in transgenic plants and algae (Sharma and Dietz, 2006). Transgenic Chlamydomonas reinhardtii expressing P5CS isolated from moth bean accumulated 80% more proline than the wild type under Cd toxicity (Siripornadulsil et al., 2002). Transgenic Oryza sativa cultivar overexpressing the betaine synthesis gene BADH showed improved Cd tolerance compared to nontransgenic cultivars (Shao et al., 2008). Exogenous application of proline and GB to mung bean and tomato cultivars under Cd stress fortified the antioxidative defense mechanisms and reduced Cd-associated toxicity (Islam et al., 2009; Duman et al., 2010; Hossain et al., 2010).
Similarly, exogenous application of trehalose was found to enhance Cd accumulation and an increase in antioxidative activity in duckweed (Duman et al., 2010). More recently, proline as a foliar spray was reported to alleviate Cd toxicity in Cicer arietinum by boosting its growth, photosynthetic attributes, yield, and the activity of the essential photosynthetic enzyme, carbonic anhydrase (Hayat et al., 2013). Exogenously applied GB effectively countered heavy metal toxicity in Triticum aestivum L. by enhancing root shoot length, biomass, photosynthetic pigments, and Na+ and K+ ion levels (Bhatti et al., 2013). Exogenous abscisic acid (ABA) when applied to leaves of Atractylodes macrocephala under Pb toxicity led to enhanced soluble sugar levels indicating osmolytic oxidative damage control compared to the control plants (Wang et al., 2012).
17.3.5 Pesticide toxicity
It is amply clear that osmoprotectants are well documented for their role in water, salt, and temperature stress, but their importance in pesticide stress still remains to be explored thoroughly. Most studies have indicated the active accumulation of proline under pesticide stress in lower plants, and now this concept is being considered for higher crop plants as well. Cyanobacteria species have been studied extensively in this regard because they are effective “biofertilizers” and are often used for maintenance of fertility in many crop plants (e.g., rice) belonging to the food grain group. Three cyanobacterial species (i.e., Aulosira fertilissima, Anabaena variabilis, and Nostoc muscorum) were subjected to endosulfan toxicity and this led to a considerable rise in total proline content levels (Kumar et al., 2008). Another pertinent study pointed out the effect of pesticide stress on rising proline activity in cells of Anabaena variabilis. Under altered endosulfan treatments, the intracellular proline concentration was found to show a steady linear increase, indicating its free radical scavenging role (Syiem and Nongrum, 2011).
The importance of endogenous and exogenous osmolyte accumulation and application was studied in Anabaena variabilis under the effect of varying concentrations of malathion. A marked increase in the endogenous levels of proline, sucrose, mannitol, trehalose, and glycogen was reported. Next, the effect of these osmolytes as exogenous sprays was recorded for growth and antioxidant enzyme activity in A. variabilis under malathion exposure. Interestingly, these exogenous osmolytes seemed to provide an additional protection, with the order of magnitude being trehalose>glycogen>sucrose>mannitol>proline (Ningthoujam et al., 2013). Similar results were reported by Manikar and coworkers (2013) for the role of osmoprotectants in reducing oxidative stress in combination with antioxidative enzymes, again in A. variabilis exposed to malathion. However, the focus is now shifting toward osmoprotectant interplay in crop plants under pesticide stress and the most pertinent reports for the same follow.
Tomato and brinjal are well known to be highly susceptible to insect attacks so to ensure a good yield and less economic losses, heavy doses of chloropyrifos and malathion are applied to crop plants, leading to xenobiotic pollution and ROS generation; this, ultimately, causes cellular damage and plant death. Both pesticides significantly stimulated antioxidant and osmolyte activity (Mahnaz et al., 2011). A more site-specific study was done for selected osmoprotectant contents (i.e., proline, total amino nitrogen, and soluble sugars) in three crop plants—turnip, tomato, and lettuce—when irrigated with pesticide- and fertilizer-laden industrial wastewater of the El-Amia drain in Egypt. Notably, steady escalation in the activities of antioxidant enzymes, glutathione, proline, soluble sugars, and total amino nitrogen content was seen in response to irrigation with the pesticide-rich wastewater (Hashem et al., 2013). Increased proline accumulation was seen in response to exogenous application of 24-epibrassinolide under the stress induced in rice by neonicotinoid and imidacloprid (Sharma et al., 2013a). As far as the prospects of inducing the expression of osmoprotectant genes in crop plants against pesticide stress is concerned, the area is very nascent and needs pioneering efforts based on its marketing as an effective agricultural practice to reduce yield and economic losses.
17.4 Conclusion and future prospects
Crop plants, the same as other plant classes, exhibit a large variety of protection mechanisms in response to abiotic stresses, often at the expense of their yield and performance. However, numerous reports indicate that osmoprotectant generation and accumulation is a novel, noninterfering mechanism against the deleterious effects of stresses while keeping quality and performance damage at bay. Recent studies show that the molecular and metabolic response of plants to a combination of abiotic stresses is the most unique and can be enhanced by inducing the expression of osmolyte synthetic genes for reducing huge losses to the agricultural sector worldwide. Additionally, exogenous application of foliar sprays rich in osmoprotectants, as well as phytohormones that induce osmoprotectant release in plants, are effective alternatives to breed crop varieties that are less susceptible to abiotic stress. Stress conditions (e.g., drought, salinity, heat, cold, heavy metal, pesticides) are still being researched especially in combination with each other. The current scenario is all about identification and characterization of known as well as new classes of osmoprotectants and to understand their basic working as stress busters in plants using omics. The research branch boasts techniques such as mass spectroscopy (MS), electrophoresis, and chromatographic advances. This is leading to further perspectives on the molecular, physiological, and metabolic aspects of stress remediation via osmoprotectants and is in turn narrowing the relevant knowledge gap between lab and field trials to develop such abiotic stress-resistant crop varieties. Another important aspect is the harnessing of amine-derived osmoregulators (e.g., 5-ALA) in photodynamic therapy and photodiagnoses applicable to cancer therapy. The amine-derived molecules are key metabolites in porphyrin precursor molecule biosynthesis that convert monooxygen into less toxic forms. The development of crop plants for further novel medical applications, such as antiaging cosmetics and disease therapy, can also be explored in the future.



