Chapter 5
Competing Storage Methods
Electrochemical secondary batteries are still the predominant means of energy storage in everyday situations. They take the form of either the ubiquitous dry cells (button cells included) or the familiar lead storage battery. Uses have a very wide range, from small electronic devices to power systems for diesel railway locomotives and emergency standby power sources. Despite their undeniable utility and the fact that they have made possible a vast variety of consumer and industrial products, they each suffer from some debilitating problems.
Unfortunately, the life of most batteries is very limited either in terms of the number of charge/discharge cycles or simply their age. The aging and deterioration of batteries, as compared to mechanical or electronic devices, are caused by many inherent factors over which we have little control. These inherent factors have mostly to do with the very nature of chemical changes and their limited reversibility. Frequently, this irreversibility and cumulative deterioration are not only due to chemical changes but also the physical changes brought about by the chemical processes, such as lowering the mechanical strength of materials resulting from chemical changes.
Why and how do batteries fail? What have we done to circumvent or improve upon the limitations? It is not our intention here to analyze in any detail the many mechanisms that contribute to battery failure. However, a brief review of some of the more familiar electrochemical cells might contribute to our understanding of why the search continues for improved electrochemical processes that might be applied to the purpose of energy storage.
Some typical, common secondary cell reactions include the following:
- NiCad Cd + 2Ni(OH)3 = CdO + H2O + Ni(OH)2 (from Junger)
- Zinc/Iron cell Zn + Fe2O3 = 2FeO + ZnO
- Lead-Acid Pb + PbO2 + H2SO4 = 2PbSO4 + 2H2O (from Plante)
- Nickel-Iron Fe + 2Ni(OH)3 = FeO + 2Ni(OH)2 + H2O (from Edison)
- Ni-MH Charge to the right, discharge to the left:
- Ni(OH)2 + M = NiOOH + MH, where the metal MH is usually a charged hydrogen absorbing alloy such as LaNi4.7Al0.3(H6)
- Lithium Ion (–) anode: C + Li+ + xe– = LixC
- (+) cathode: LiMO2= Li1–xMO2 + xLi+ + xe–
An anode is where oxidation takes place, and cathodes are reduction sites. There are other lithium cells that employ solid and/or polymer electrolytes such as the Li-ion SPE cells that use LiCoO2 cathodes and graphite anodes.
The Li/SOCl2, lithium thionyl chloride, cell has energy producing reactions at the electrodes during discharge – anode is Li = Li+ + e–, and cathode is 2SOCl2 + 4e– = 4Cl– + SO2 + S.
5.1 Problems with Batteries
Despite their convenience, batteries have a number of limitations and serious disadvantages. The cost per unit of stored energy is hardly important when the application calls for very small amounts of energy such as in watches, cell phones, and other small electronic communication devices. However, it becomes a major consideration in motive power or bulk energy storage cases.
When one considers large-sized energy facilities, other matters in addition to initial capital equipment (battery) costs become important. Safety, cycle, standing life, and dependability become issues to address. In virtually all electrochemical batteries, solid materials that are part of the electrode structures participate in the energy storing processes. Electrochemical reactions in which solid materials undergo changes in structure or composition are not completely reversible. Hence, there are inexorable and unwanted changes that occur with each cycle that eventually result in the demise of the battery.
The lead-acid cell is an example of this situation. The principle energy storing reaction is essentially
(5.1)

The inherent failure mechanisms of batteries include the following:
- Shrinkage of the negative plate sponge lead
- Sulfation of plates, exposed plates, or long discharged times
- Changes in crystalline structure
- Spallation of active materials
- Grid corrosion by long overcharging.
These are all consequences of chemical reactions involving solids at or part of the electrode structures. Other batteries, such as the Edison cell, nickel-cadmium, lithium-ion, etc., involve changing the chemical composition of solid materials.
All of the energy cells listed above are fairly common and presently available. They all have solid reagents at both electrodes. The continual cycling of cells directly involves or necessitates the restructuring of these solids as the cell is charged and discharged. Unfortunately, such physical (chemical) processes are not entirely reversible. Each time the electrode materials chemically change, a certain degree of irreversibility is encountered. Materials are redeposited with the same uniformity, some of the solids become physically detached from the electrode, and some loss of continuity or capacity results. The operating life of the electrochemical cells is limited by these mechanisms. In addition to the limitations cited above, there are also concerns with the passivation of electrode materials, or memory effects in which cell capacity or electrical performance is adversely changed as a result of continued partial charge cycling.
Because of these factors and the need to develop energy systems with longer lives and more useable operational characteristics, a search has been continuing for electrochemical cells with energy storing (producing) reagents in either a liquid or gas phase. Such systems are usually referred to as either fuel cells or redox batteries. In the former gas phase, reagents such as oxygen and hydrogen are introduced into the reaction chamber, or electrode compartment, and oxidation of the fuel (hydrogen) and reduction of the oxidizing agent (oxygen) takes place with the release of energy in the form of electric current to an external load.
In redox batteries, essentially the same process takes place but with different reagents and the singular difference that the reaction products can be regenerated externally to be reused in the cell an unlimited number of times. In actuality, the water produced in a fuel cell can also be “reused” by dissociation into H2 and O2. However, in the redox system the intent is to reuse the reagents rather than discard them.
Energy density of an electrochemical couple is obviously one of the many important properties to be considered when evaluating it as a potentially viable means of storing energy. Because of the large difference in available energy per unit weight of electrochemical processes as compared to other currently employed sources of energy, a different level of standards must be adopted in their assessment. A comparison between batteries and fossil fuels shows a vast difference in energy levels.
This situation is illustrated in Table 5.1, which shows the performance numbers for fossil fuels. The remarkable aspects of these sources of energy are that they are in convenient liquid form, easy to handle, and they make use of the surrounding oxygen in the atmosphere as oxidant. Because of these features, petroleum products have risen rapidly to their prominent position as universal fuels for most mobile and remotely powered machines ranging from electric power generators to automobiles and aircraft.
Table 5.1 Heats of combustion for some hydrocarbons.
| Fuel type (Phase) | Formula | kWh/Kg | kWh/lb |
| Ethane (G) | C2H6 | 14.9 | 6.78 |
| n-Pentane (L) | C5H12 | 13.45 | 6.11 |
| n-Hexane (L) | C6H14 | 13.35 | 6.06 |
| n-Heptane (L) | C7H16 | 13.34 | 6.06 |
| n-Octane (L) | C8H18 | 13.26 | 6.02 |
| Methanol (L) | CH3OH | 6.2 | 2.8 |
| Ethanol (L) | C2H5OH | 8.27 | 3.76 |
In applications where power, energy capacities, safety, and “portability” are critical, there is no rival. Only in very specialized situations such as in naval vessels, where the ability to remain at sea for great distances and for long periods of time without refueling, has nuclear power competed. Also, in many instances where air pollution is a concern, or where fossil fuels and hydropower are not options, nuclear power is competitive.
A quick comparison of some of the better-known energy sources for doing our work is presented below. Relevant details about gasoline as our universal fuel are also illustrated below.
5.2 Hydrocarbon Fuel: Energy Density Data
The following is an outline of the energy densities of various common hydrocarbon fuels that have been employed for the propulsion of vehicles via internal combustion engines. The most common among these fuels are the petroleum derivative polymers, heptane and octane.
Table 5.1 shows the total energy of combustion for some of these fuels when combustion is complete and when it takes place at 20°C. These hydrocarbons may be represented as having the general molecular form of CxHyO2.
When complete combustion takes place, assuming no formation of nitrogen compounds, the general form of the reaction is
(5.2)

where
(5.3)

Such complete combustion is an idealized situation since some of the products are CO and the process is invariably not isothermal. However, these data do give representative maximum values for their useful available energy.
Octane rating of gasoline is usually the percentage of octane to heptane in the fuel mixture. A 100-octane fuel might then be all octane.
Ethanol fuel’s energy density of over 8 kWh/Kg is a very high value, especially when compared to most other processes for storing energy in a controllable and usable form. However, when one examines the other factors associated with its actual application to the conditions necessary to its use and the characteristics of engines, we see this number significantly diminished.
A look at the amount of energy available from a gallon of octane for the mechanical energy necessary to propel a vehicle gives the following figures.
Maximum energy conversion efficiency of a tuned, constant speed I.C. engine is in the order of 20%. Hence, at a specific gravity of about 6 pounds per gallon, only 1/5 of the 35.7 kWh per gallon is actually available, or about a 7 kWh/gallon maximum.
Burning 1 gallon, 6 pounds, of octane completely requires 24 pounds of oxygen for the reactions. That amount of O2 has a volume at STP of 260 ft3. Since only 20% of the composition of air is oxygen, a minimum volume of air of 5 × 260 = 1300 ft3 is required to “burn” 1 gallon of octane.
Under ideal conditions, a vehicle with a mileage performance of 30 mpg traveling along a roadway at 60 mph would be consuming 2 gallons of octane per hour. That vehicle engine would be taking in a minimum of 2,600 ft3 of 20°C air per hour for complete combustion to occur. The air cleaner and fuel system must be able to handle airflow rates at least in the range of 3,000ft3/hour, or 50 ft3/minute.
After over a century of development, we have learned how to accomplish these feats with what have become extremely reliable machines. However, two very important facts should be borne in mind:
- Only a small fraction of the stored energy is utilized in the current internal combustion engines.
- A much larger amount of oxygen in both weight and volume must be available from the surrounding atmosphere for the fossil-fueled I.C. engine to function.
An interesting fact should be noted here about energy densities. The extremely attractive energy density of petroleum products is made possible only because it is not necessary to carry the oxidizer on board the vehicle. The oxygen is supplied by the immediately surrounding environment and is available when needed at any demand rate. If it were necessary to carry the oxygen on board the vehicle, the number would be quite different. An amount of oxygen about four times the weight of fuel is needed.
Thus, if the practical 20% efficiency of conversion is taken into account, 1.2 kWh/lb of fuel would be realized, and if we add the four times weight of oxygen, the figure becomes 240 Wh/lb.
If now we also include the weight of nitrogen in the air, we obtain the low value of 40 Wh/lb for the energy density of a power source system that not only had to carry the fuel but also its own air comprised of only 20% O2.
All of the proceeding may seem a bit unfair in assessing the “true” energy density of a hydrocarbon fuel, but in actuality this is the situation with which any contending energy system must compete. The “unfairness” lies mainly in the fact that these fossil fuel systems breathe air and do not have to carry the weight of the oxidant along. By virtue of the same fact, the combustion products are exhausted into the atmosphere, thus increasing pollution problems in some instances.
To eliminate air pollution entirely, it is necessary to devise energy producing systems that do not involve combustion products of any sort, other than perhaps water vapor, into the surrounding environment. This essentially means that a non-combustion method of producing heat or power must be substituted for the burning of fossil fuels. In fact, the only combustible materials are either carbon or compounds of carbon, all of which yield CO2 as the ultimate product of the process.
The necessity of carrying not only the fuel or reducing agent on board the system but the oxidizers as well does present a severe handicap when attempting to develop a competing high energy density system.
5.3 Electrochemical Cells
Electrochemical cells suffer not only from the lower energy densities associated with such processes but also from the necessity of carrying all of the chemical reagents necessary to the available energy reactions. This situation does enable the cell to remain independent of the external surroundings during its operation. These factors contribute to energy densities of electrochemical reactions, which are at least an order of magnitude lower than those of hydrocarbons.
Among the most energetic of electrochemical reactions are those between metals and halogens. The highest specific energy capacity is that between lithium and fluorine because of their low atomic weights and high reactivity, or free energy of combination. The reaction of the formation of solid salt, Li + F → LiF, occurs with a free energy of 140 kcal/gm mol. wt. and at a potential of over 6 volts. This corresponds to an energy density of 3,100 Wh/Kg for the direct or electrochemical reaction of the elements. This value is quite attractive and even competitive with fossil fuels. However, there are no practical ways of handling the highly reactive components. Both the metal lithium and the element fluorine react energetically with water and most other substances associated with the construction of electrochemical cells. Hence, in order to make an operable cell employing this couple, it is necessary to find some non-aqueous, probably polar solvent in which the salt LiF is soluble and that will not react with either of the two reagents.
Fused salt electrolytes are possibilities, but they involve high temperatures, difficult operating conditions, and increased hazards. There is also the problem of storing free fluorine as an available reagent for a charged cell. These problems are formidable, and no short-term, practical solutions are in sight.
5.4 Metal-Halogen and Half-Redox Couples
The term redox has been used in recent times in a manner that is not entirely clear when applied to electrochemical cells. All such processes involve oxidation and reduction. The fuel (reducing agent) is oxidized, and the oxidizing agent is reduced in the process, thus producing energy in a hopefully usable fashion. The term redox, as applied to electrochemical systems, refers to a system that employs reducing reactants and oxidizers that are passed through the cell, or reaction chamber, for the production of electric power. The reactants are subsequently regenerated at some location external to the reactor cell.
In primary cells where only the discharge process takes place, the oxidation process occurs at the negative electrode, and the reduction of a chemical occurs at the positive electrode. In the case of the LeClanche (dry) cell, zinc is oxidized to zinc-oxygen compounds, and manganese dioxide is reduced. Secondary, or electrically rechargeable cells, have dual process electrodes. During charging from an external electrical source, the reduction occurs at the negative electrode and oxidation at the positive (Figure 5.1).

Figure 5.1 Enhanced concentration cell operation.
Half-redox implies that the processes at one of the two electrodes in a cell involve reagents that remain mobile as liquids or gases so that they may be introduced and removed from the respective electrode region. The general form of such reactions is M+n + nX–1 → MXn.
Metal-halogen cells are in the half-redox category. Halogens are liquid or gas at room temperature and can be caused to flow over the surface of an electrode as needed to sustain the reaction processes or to remove reaction products. If the product of oxidation, such as the salt, ZnBr2, in the case of the zinc/bromine couple, is soluble in the electrolyte, there are no solid products formed onto or removed from the surface of the (+) electrode during charging or discharging.
Since the reactant and reaction products can, in principle, be supplied during discharge to the electrode surface and removed for regeneration at some external location, the bromine electrode qualifies as a redox type reagent.
The importance of this aspect of redox behavior in this case is not primarily because of its ability to be removed from the cell for storage or reconversion elsewhere but because it reacts directly with the reducing agent, zinc, to produce the salt. Furthermore, its storage at the electrode or in a reservoir is mobile and can be made uniform. Cells with reagents that will recombine by direct union offer the possibility of an extremely long life.
Another couple that has received little attention and has the attributes of half-redox cells and homogeneous cation is the Fe/Fe+3 couple. Oxidation/reduction reaction is Fe0 + 2Fe+3 → 3Fe+2.
The diagram in Figure 5.2 shows the rather interesting and unique characteristics whereby one can make use of the two oxidation states of the single element iron. The disadvantages of this system are the facts that iron is an electronically conductive solid with poor plating properties, and that hydrogen gas evolves not only upon charging but at times due to the necessarily low pH of the electrolyte.

Figure 5.2 Energy level diagram for the iron redox cell.
However, with further development in such matters as the use of non-aqueous electrolytes and improvements in plating quality, the iron-redox system could become practical for some applications where cost is important and size is relatively unimportant.
Of the four halogens (fluorine, chlorine, bromine, and iodine), bromine has been selected for serious evaluation as an oxidant because of its more favorable physical and chemical properties. For example, it is much more reactive than iodine and has lower costs, but it is not nearly as volatile and ill behaved as a storable component in a cell as either chlorine or fluorine. Chlorine will hydrolyze much more rapidly in water than bromine will, and it does not form reversible complexes as well as bromine does. Fluorine is an extremely difficult and costly material to work with in elemental form.
A number of companies in recent years have produced some reversible cells and batteries on a laboratory prototype basis, in which the chlorine was stored as a clathrate (frozen hydrate) in a zinc/chlorine battery. Because of life and hazard considerations, among others, the battery has not emerged as a practical secondary source for widespread use to date. These factors contribute to the difficulties of achieving practical battery designs using higher energy density reagents such as those above.
Figure 5.3 is a chart that compares the free energy of reaction per kilogram of reactants for a range of metal-bromine couples. These values were calculated from the free energies of the reaction and sum of the half-cell potentials available from the literature.
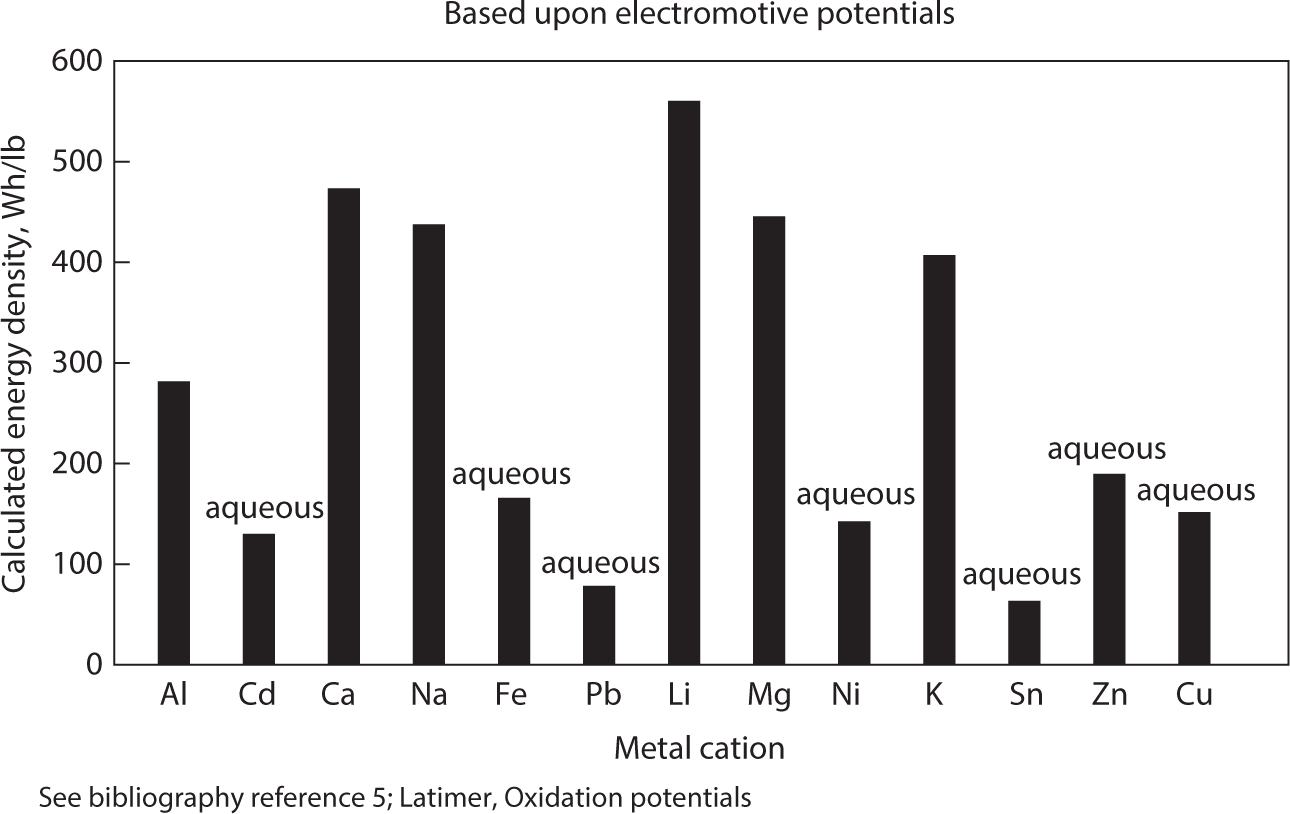
Figure 5.3 Energy densities of metal-bromine couples.
The most attractive couple, strictly from an energy standpoint, is that of Li/Br. Unfortunately, there is currently no technology that allows us to either electro-deposit lithium metal out of aqueous solution or even-exist in an air/water environment. Non-aqueous solvents present problems not only of hermetic sealing but limited life, simultaneous chemical compatibility with free bromine, and low electrolytic conductivity. Fused salt electrolytes must be operated at high temperatures, and cells experience many other types of problems ranging from chemical durability, thermal expansion/contraction, mechanical strength, and sealability. A high temperature metal/halogen system seems quite impractical for any application at any time in the foreseeable future.
High temperature fused salt cells, such as the Na/S system, have been constructed with some degree of success, but they do not employ free halogen as the oxidizer.
All of the couples that employ aluminum and alkali metals have higher energy densities because of their high reactivity as chemical agents. This greater reactivity also results in greater problems of cell containment and reaction rate control.
As we descend lower in specific energy density, the couples become increasingly easier to manage as an operating cell. However, their attractiveness as sources of energy also diminishes. One must then look for a compromise, hoping that a delightful selection can be found that is easy to mange, well-behaved, and has useful energy and power densities.
To increase the likelihood of applicability of a couple, it is important to stay as much within ambient conditions as possible. A cell that is operable at near room temperature ranges is compatible with an aqueous environment and is also compatible with materials of desirable construction. Also, electrodes and separators that are chemically resistant and have reasonable costs are required.
Why explore and attempt to select a metal-halogen couple in the first place? The following two reasons can explain why:
- This category of reagents as electrochemical cells offers high energy density and perhaps higher power density.
- There is the possibility of long cycle life because of the totally reversible nature of the cell, in principle.
Of all of the couples compatible with aqueous electrolyte systems and ambient conditions, the zinc-bromine has appeared most attractive. Zinc is the metal furthest from hydrogen in the electro- motive series as a reducing agent that can be plated out of solution in water. Bromine is similarly compatible in water and has a relatively low vapor pressure at 25°C. In the absence of catalysts, bromine hydrolyzes at a low rate, and equilibrium is attained at very low concentrations of HBrO in acidic solutions.
The reactions at the negative and positive electrodes during discharge are Zn → Zn+2 + 2e at 0.76 volts and Br2 + 2e → 2Br–1 at 1.07 volts.
The whole net reaction is simply Zn+2 + 2Br–1 → ZnBr2 at 1.82 volts.
The high reaction potential that is actually realized is slightly over 1.8 volts per cell. Almost 200 watt-hours per pound of reactants are available for the reaction if it goes to completion and no other electrical losses are incurred. These figures are quite attractive for many application possibilities. Lead-acid cells have a theoretical energy density for the energy storing reagents alone of 70 to 80 Wh/lb, or about 200 Wh/kg, as compared to the upper limit offered by the Zn/Br2 couple of 440 Wh/kg.
The zinc/bromine system has some very fundamental problems, such as zinc plating being spongy and dendritic, zinc reacting in an acidic solution evolving hydrogen, and bromine being extremely oxidizing, difficult, and expensive to store.
The second of the attributes of metal-halogen couples is their ability to return to the initial conditions of the discharged state as secondary cells. When the couples are discharged completely, the chemistry has returned to the initial conditions the cell possessed when first fabricated, and any remaining reagents will react directly if given the opportunity in cell design. Assuming no deterioration of electrodes, etc., and no loss of components from the system, the cell has no permanent memory, in principle. Obviously, if it is possible to “tame” the reaction in a manner rendering it reasonably safe, low cost, and long-life, applications such as load leveling, peaking, and even the electric vehicle are possibilities.
An interesting comparison of the performances of a variety of common energy systems is provided in the Ragone type diagram in Figure 5.4.

Figure 5.4 Ragone plot for energy storage and generation systems.
We see that the performance on both an energy and power basis is far superior for heat engines such as the turbines and internal combustion engines. Fuel cells are significantly better performers on an energy basis than other batteries or electrochemical devices, but are not yet competitive on power density.
5.5 Full Redox Couples
There are couples, other than metal/halogen, that are compatible with aqueous solutions and are entirely redox in character. However, the selection is not very attractive when one imposes the usual conditions of low cost, environmentally benign, well-behaved, long life, usable energy, and power densities. Table 5.2 is a partial list of the better-known reducing and oxidizing agents that could be utilized in an electrochemical cell as a source of electrical energy.
Table 5.2 Redox cell reactants at room temperature.
| Oxidizer | Potential | Reducer | Potential |
| O2 | –1.23 | SO3–2 | 0.93 |
| F2 | –2.87 | ||
| Cl2 | –1.36 | Cr+2 | 0.41 |
| Br2 | –1.07 | S | 0.50 |
| I2 | –0.54 | ||
| S2O8–2 | –2.01 | ||
| Fe+3 | –0.77 | Fe+2 | –0.77 |
| MnO4–1 | –1.51 | ||
| V+4/V+5 | –0.25 | V+2/V+3 | 1.1 |
Oxidizers and reducing agents (fuels) are listed as either chemical elements or ions (radicals) depending on their form in a cell at the beginning of “discharge,” or at the start of the energy-producing mode. The potentials are given in volts above or below hydrogen. Following convention, positive volts are above hydrogen, and negative volts are below hydrogen in the electromotive series.
As is evident, there are relatively few choices for chemical reagents that satisfy the prerequisites of acceptable voltage, chemical stability, and being liquid in both states of oxidation, relatively safe, low-cost, and compatible with ambient conditions.
Of all of the above, and more that are not listed here, the oxidizers that show most promise are still ferric ions, bromine, and vanadium in acid solutions. Of the even fewer choices available for reducing agents, it seems that sulfur (complexed) and vanadium are most likely to yield some practical systems. We have elected to pursue sulfur as the most attractive element for the reducing agent in a room temperature aqueous electrolyte. The properties that are most attractive, and quite remarkable when considered in light of its use as the reagent in the negative side of a cell, include the following:
- Plentiful supply
- Low cost
- High solubility as a polysulfide complex in water solutions
- Low equivalent weight
- Reasonable potential
- Well-behaved electrochemically
- Low volatility.
Many oxidizing agents have been tested in conjunction with sulfur as the negative electrode material. Bromine has been of most interest because of the potential for a completely reversible cell. Looking back into past decades, we see that other redox couples have been explored intensively in the past, such as that of Cr+2/Fe+3. The NASA Lewis laboratory has probably contributed most toward its research. One of the problems associated with its cycle life and coulombic efficiency is that of maintaining separation between the chromium and iron ionically in solutions. Imperfect separation is achieved by employing ion-exchange membranes between cell compartments, but cross diffusion inexorably results in deterioration of the electrolytes. Since both ions have positive electric charges, they cannot be separated during charging as in a metal-halogen couple with oppositely charged cations and anions. Anion membranes with low resistance, low cost, and very high transport number ratios for the transport of chloride ions in the NASA cell are difficult to fabricate.
At present, the vanadium redox couple is receiving a fair amount of attention because of a number of interesting features. The fact that it is homogeneous, regarding the active reagent vanadium, and the reasonable solubility in water of the vanadium salts make the system attractive as a long-life energy storage mechanism. However, the negative aspects are the high cost of vanadium and its compounds, high molecular weights of the reagents, the necessity for operating electrolytes at very low pH, and the complexities of operating a system that requires full flow electrolytes. The potential market for such a system appears to be mainly as load leveling or standby emergency power sources.
5.6 Possible Applications
In selecting electrochemical couples to investigate and perhaps develop into operable systems and products, it is vitally important to keep the intended application criteria constantly in mind. The application potentials impose their own needs for cost, life, reliability, and energy and power density.
Some application possibilities for full flow redox systems include the following:
- Utility load leveling at generating and customer sides
- Peaking at user or customer side of power lines
- Emergency energy source in cases of power failure
- Portable power for convenience, or for remote locations
- Perhaps for electric vehicles and small boats.
With each area of application, there are certain requirements for a secondary battery power source that determine its usefulness. For example, in the case of load leveling, battery weight or energy density and power density are not directly significant factors because they are in stationary locations. These parameters are meaningful only when converted into cost. Usually, larger physical equipment (batteries) has larger costs because of the required ground area and support structures, such as buildings. Hence, higher energy density batteries tend to be more attractive because of their associated costs. More materials of construction and a larger amount of floor space (footprint) and building needed to house the energy storage battery system increases the initial cost. However, longer cycle life systems will tend to amortize initial costs. Cost would naturally be greater as a consequence for lower energy density (ED) batteries.
Storing energy output from generating equipment during low demand times to use for later peak demand periods can save in the cost of hydrocarbon fuels and generating capital equipment, but only if the economics of the battery are favorable. Storing for peak power demand periods at the user end of the power line can also save in the capital cost of transmission lines and transformers to the energy user.
Cell imbalance in arrays of large numbers of battery cells can become a severe problem, especially for deep discharge cycling. Deep cycling is desirable because it reduces the amount of battery needed to store the usable energy. However, because battery cells are not identical in structure and performance, deep cycling can result not only in a decreasing usable output of stored energy but also in permanent damage to cells that have been reversed. Lead-acid batteries are particularly prone to damage by reverse charging. To compensate for this cycle life limitation, lead-acid batteries are usually not subjected to deep cycling, thus resulting in the necessity to build and purchase more energy storage capacity than will be in actual use.
Safety, though always a serious consideration in the design and use of energy systems, is not as important in bulk energy storage applications because proper provisions can be made to cope with potentially hazardous situations by proper containment and trained personnel.
In the case of consumer product areas such as the electric vehicle or portable power for tools and recreational purposes, safety is one of the prime concerns. In order to appreciate the directions in which battery development efforts are, or should be, headed some of the principle features or aspects of battery sources are identified below.
For Bulk Storage
- High energy turnaround efficiency
- Flat discharge voltage versus current curve
- Minimum or absence of cell imbalance
- Competitive costs of capital equipment amortized over cycle life
- Low maintenance because of cost considerations
For Portable or Electric Vehicle Use
- Low capital cost
- Safe operation
- Highest energy density possible
- Highest power density possible
- Compactness in size as well as small weight
- Minimum maintenance
- Chemical recharge ability desirable
Except for the higher sensitivity to energy efficiency in bulk energy applications, consumer applications and particularly the electric vehicle have even more difficult application requirements to meet. The latter has especially difficult criteria of safety and compactness imposed upon the battery design that exceed those for bulk energy uses. The lead-acid battery has been in use extensively for over a century, and it still retains its position of prominence in industrial and consumer applications. Even though its energy density is lower than what is desired in many instances, its familiarity, dependence over a wide range of operating conditions, and acceptable cycle life give rise to its universal character. Other contenders as large storage batteries include the Edison (nickel-iron) cell and the Junger cell (nickel cadmium). Because of either cost or performance factors, their application is much more limited than the lead-acid. With a maximum specific energy density ranging between 12 and 18Wh/lb, or 26 to 40Wh/kg, the lead-acid battery leaves much to be desired in electric vehicle applications. The usual range of such Pb-Ac power vehicles is 50 to 70miles maximum. Doubling the energy density of a battery would certainly be a welcome improvement, but it would still limit a vehicle to a 100 to 150 mile range between lengthy charging times.
The redox battery systems do offer the possibility of chemical “re-fueling,” thus extending the usable range of an electric vehicle indefinitely as long as sources or fueling stations of the chemical reagents are available en route.
Numerous electrochemical couples making use of oxygen in the atmosphere as an oxidant have been experimented with and prototyped over recent decades. Perhaps the most common of these are the alkaline versions of metal-air cells. The more common versions include, Mg-air, Al-air, and Zn-air. Most suffer from problems of available power density and the deterioration of air electrode catalysts due to poisoning contaminants. And when these devices are considered for use as chemically rechargeable cells, the complexities and inconveniences of removing and replacing many depleted metallic electrodes and handling caustic electrolytes containing the spent products are severe. Consequently, they also have not gained a position in any of the large-scale consumer/industrial application areas.
To summarize, the three areas of metal-halogen, half-redox, and redox, and more specifically, the zinc/bromine, iron-redox, and sulfur/bromine have been selected for investigation over past years at TRL, and they are presented here because of certain inherent characteristics. These include the following:
- Zinc/bromine couple – high energy density, well-behaved, completely reversible
- Iron/ferric couple – low cost, long life, completely reversible, great safety
- Sulfur/bromine couple – low cost, long life potential, no cell imbalance, chemically rechargeable.
In all the cases above, reverse charging has little or no permanent, negative consequences on the cells. There are, however, some serious negative factors that have precluded their extensive use. These include such issues as safety encountered with the use of strong oxidizing agents such as bromine, difficulty with managing metal plating, the generation of hydrogen gas and the decomposition of water, rising acidity of electrolytes causing the evolution of gasses, and the compatibility of materials of construction in the presence of halogens.
