1
Food Frying: The Concept
1.1 Introduction
Frying is a food preparation technique that involves foods and hot oil or fats. It is one of the fastest and simplest techniques for cooking food with pleasant, attractive properties. The food frying process consists of four main components: first, specific conditions such as temperature and pressure; second, a utensil or fryer; third, foods; and fourth, frying oil (referred to as ‘frying medium’ from this point onward). The fryer may be a simple pan or a complex industrial technology. The frying medium is usually an oil or an animal‐derived fat. The temperatures used for frying are in the 150–200 °C range. High temperatures promote reactions between food components like proteins and carbohydrates, surface dehydration of the crust, and oil uptake (Gertz 2014). In contrast to boiling in hot water, the heating of foods at elevated temperatures provides a desirable appearance (colour), texture (crispness), flavour, and taste (Perkins 2007). Frying is a more efficient process than other cooking methods, and has gained great popularity in both restaurants and industry because of its speed and operational simplicity.
Even though deep frying is an old and very popular process, it is still poorly understood. Proper frying practice and the most appropriate frying oil are generally determined by experience. Good understanding of the frying process helps in optimizing the manufacturing processes with regard to the quality of the food, the use life of the fat, and energy consumption. To guarantee a good quality of the fried end product, it is necessary to install a management system which includes all critical points of the frying process (Gertz 2014).
1.2 History of Frying
Food frying is one of oldest known food preparation techniques. However, the exact date of its invention and who first used it is hard to trace. Some authors propose that it was invented by the ancient Chinese (Rossell 2001). The third book of the Old Testament, Leviticus, chapter 2, verses 4–7, written c. 600 BCE, distinguishes between bread baked in an oven and that cooked on the griddle or in the pan. Pliny (c. 1st century CE) provides a prescription for spleen disease that involves frying eggs in vinegar (Morton 1998). Knowledge of frying was common in the fourteenth century, but scientific writing on the subject was still rare. Table 1.1 provides a brief history and timeline of the frying process (Stier 2004). The German Fat Society, ‘Deutsche Gesellschaft fuer Fettwissenschaft’ (DGF) may be considered a pioneer of fat science and frying. The DGF held symposia on frying fats in 1973 and 1979. The first conference on the subject, ‘The Frying of Foods’, was held in Madrid in 1986. These conferences and symposia were considered the prime motivator for attracting scientists' attention to frying science and technology. The European Society for Lipid Science and Technology has contributed much to the understanding of frying. In recent times, significant scientific breakthroughs have occurred, resulting in a huge sum of knowledge on frying that it is not possible to accommodate in a single book.
Table 1.1 Timeline of the frying process.
Source: Modified and reproduced with kind permission of John Wiley & Sons (Stier 2004).
| Year | Event |
| 3000 BCE | Chinese frying of meat |
| 1300 BCE | Hebrews fry flat breads |
| 1537 CE | Potatoes introduced in Europe |
| 1600–1700 | French fried potatoes emerged |
| 1853 | Potato chip invented in Saratoga Springs, NY by George Crum |
| 1890s | Potato chip industry begins in the United States |
| 1897 | Hydrogenation of edible oils invented |
| 1906 | Commercial oil roasting of shelled peanuts by Planters |
| 1908 | J. P. Dushesues founds Leominster Potato Chip Company |
| 1926 | Laura Scudder develops first potato chip bag of waxed paper |
| 1927–1930 | Cellophane begins to be used for potato chip bags |
| 1929 | Clarence Birdseye develops new commercial freezing technologies |
| 1932 | First tortilla chips (Doolin and Filler) produced in San Antonio, TX |
| 1933 | Dixie wax paper introduces pre‐printed glassine bag. Cracker barrel marketing of chips comes to an end |
| 1930–1935 | National Potato Chip Institute tells consumers that chips are not fattening if eaten in small amounts |
| 1938 | H. W. Lay Co. founds Lay's Potato Chip Company in Atlanta, GA |
| 1946 | First automatic packaging machine for chips developed |
| 1945–1950 | Extruded snacks introduced. MacBeth introduces continuous immersion cookers |
| 1950 | Under‐pan fired cookers introduced |
| 1950–1952 | Fryers with external heat exchangers with oil circulation introduced; Pork rinds introduced |
| 1950–1955 | Laminate bags with polypropylene/cellophane and polypropylene glassine introduced |
| 1953 | Simplot scientists develop a technique for par‐frying potato slices |
| 1957 | Heat & Control introduces ‘Big Goose’, a 1600 lb. capacity continuous system |
| 1958 | Urshel develops new slicers for potato chip manufacture |
| 1961 | Frito‐Lay merger |
| 1969 | Potato chip controversy develops with the introduction of Pringles and Chippos |
| 1970–1975 | 7000 lb. capacity fryers introduced |
| 1973 | 1st DGF Symposium. Germany proposes regulations based on oxidized fatty acids for restaurant frying oil |
| 1979 | 2nd DGF Symposium. Polar materials provides index of restaurant frying oil quality |
| 1987 | Blumenthal publishes surfactant theory of frying |
| 2000 | 3rd DGF Symposium. Principle quality index should be sensory parameters of food being fried |
| 2004 | 4th DGF Symposium |
| 2004 | 4th International Symposium on Deep‐Frying |
| 2011 | 6th International Symposium on Deep‐Frying, Germany |
| 2013 | 7th International Symposium on Deep‐Frying, United States |
| 2015 | 8th International Symposium on Deep‐Frying |
| 2016 | 1st International Symposium on Lipid Oxidation and Antioxidants, Portugal |
| 2017 | 9th International Symposium on Deep Frying, China |
1.3 Mechanism of Frying
Frying involves essential components such as foods and frying medium. As a general rule, frying medium is initially preheated to about 160–200 °C and then desired foods are either kept in it or immersed in it. Several physical and chemical reactions occur that result in the formation of fried foods. Figure 1.1 shows the experimental results of oil distribution during the frying and cooling of a potato slice. During frying, a small amount of oil is absorbed by the potato, while during cooling, the internal oil content increases at a fast rate for the first minute, while surface oil decreases until an equilibrium is reached after 4 minutes. In order to understand the fundamentals of frying, the following concepts are important.

Figure 1.1 Oil content absorbed or remaining on the surface of French fries during frying (170 °C) and cooling (20 °C).
Source: Reproduced with kind permission of John Wiley & Sons (Gertz 2014).
1.3.1 Heat and Mass Transfer
Foods can be prepared at an elevated temperature in different ways, such as by pan frying, shallow frying, or deep frying, while temperatures of 100–103 °C can be achieved when cooking in water. With the use of fat or hot air as a heating medium, a higher temperature can be generated without exceeding the boiling temperature of the water inside the food (Vitrac et al. 2000). As the temperature difference between the heating medium and the food increases, the heat transfer within the cooking process runs much faster. Simultaneous with the heat transfer, mass is transferred from the food to the frying medium and vice versa (Gertz 2014).
A food is a solid body with holes and pores filled with water and air. Immediately after its immersion in hot oil, traces of free water at the surface evaporate very rapidly, causing a violent bubbling and drying of the surface. When the vaporization of water is faster than the ability of the surrounding oil to remove the steam by convection, the heat transfer rate from oil to food surface is zero, due to the heat resistance of the steam evaporating from the surface. The introduction of the food into the hot oil and the sudden evaporation of moisture from the food cause a violent bubbling. Bubbling enlarges the contact area between air and oil. Thus, the heat transfer rate between oil and air increases, accelerating the oxidative degradation of the oil (Costa et al. 1999). By lowering the oil temperature, reducing the quantity of food to be fried, or pre‐drying the food, rapid evaporation of the water from the surface of the food and intense bubbling can be avoided (Sobukola et al. 2010). With increasing frying time, the bubbling becomes less intense and the evaporating water steam has a more protective effect (Dana et al. 2003), creating a steam blanket above the oil surface and reducing the headspace air flow, providing protection against oxidation by avoiding contact with air.
The heat is transferred by convection from the oil to the surface of the product and by conduction to the centre of the product. The water inside the product is heated to boiling point, resulting in an increased pressure. As a consequence, water at the surface leaves the product and that in the interior of the food migrates from the central position radially outward to the walls (Vitrac et al. 2000). This water transport is responsible for providing cooling in the external region of the product after the first period of frying, ensuring that the food is not burnt or charred. The moisture in the inner part of the food is heated to boiling, inducing gelatinization of starch and denaturation of proteins (Gertz 2014).
It has been shown by Manglik (2006) that by adding small quantities of surface‐active soluble agents, the interfacial tension and surface tension (oil–solid interfacial, oil–vapour tension) can be altered and the heat transfer improved. A simple practical test at 170 °C with 10 × 10 × 500 mm potato pieces demonstrates the heat transfer capacities of different oils (Table 1.2). The measured times till the central temperature reaches 100 °C are all different due to the different physical and chemical properties of the oils. In frying tests, it has been observed that potatoes fried in beef tallow and palmolein contain higher amounts of acrylamide than those fried in sunflower or rapeseed oil for the same period, due to the accelerated heat transfer (Gertz et al. 2003).
Table 1.2 Formation of acrylamide during deep frying in various oils.
Source: Reproduced with kind permission of John Wiley & Sons (Gertz 2014).
| Time needed to reach 100 °C in the score | FOS‐unit | Acrylamide level (µg kg−1) of French fries (40 g) prepared in oil (fryer capacity: 2 l); heating time: 3, 5 min, 170 °C | |
| Palmolein | 65 | 3.5 | 594 |
| Beef tallow | 74 | 2.5 | 301 |
| Sunflower oil | 80 | 0.8 | 205 |
| Rapeseed oil | 80 | 0.5 | 203 |
| Groundnut oil | 82 | 0.4 | 190 |
| Groundnut oil hardened | 84 | 0.0 | 192 |
Palmolein and beef tallow contain more polar components, such as mono‐ and diacylglycerols and medium‐chain triacylglycerols (TAGs), than do oils such as rapeseed oil, sunflower oil, and groundnut oil. To compare the relative polarities, fresh oils were measured with FOS (Food Oil Sensor). The reading units of the FOS measurements are related to their dielectric constants and polarity (Wegmuller 1994). It is possible that these more polar compounds reduce the surface tension between oil and food surface or oil and water steam; however, Gil and Handel (1995) have not observe any effect of diacylglycerols or fatty acids on the surface tension in frying oils. Blumenthal (1991) published a monograph proposing a surfactant theory of frying. As oil degrades, more surfactant materials are formed, causing increased contact between oil and food. Those materials cause a better heat transfer at the oil–food interface and reduce the initially high surface tension between these two immiscible zones. The so‐called ‘Frying Oil Quality Curve’ (Figure 1.2) demonstrate the relationship between the degradation of frying oil and the chemical changes in the oil. This curve shows five stages of oil degradation, and relates them to food quality. The goal must be to extend the optimum frying window.
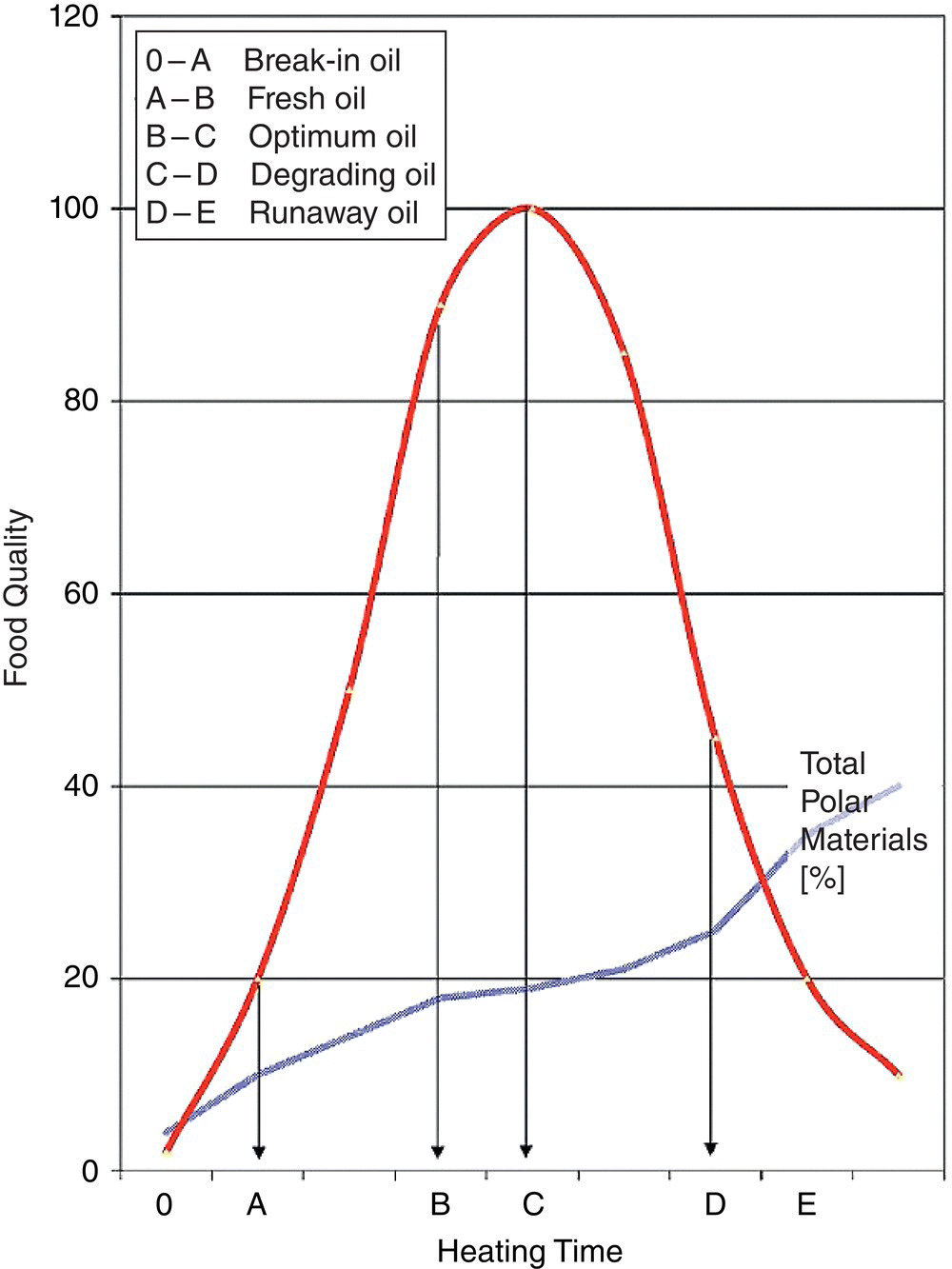
Figure 1.2 Frying oil quality curve according to Blumenthal (1991).
Source: Reproduced with kind permission of John Wiley & Sons (Gertz 2014).
After the external zone is dehydrated, the crust–crumb interface starts moving towards the centre from the food. This porous dried region (crust) is created and continues to increase in size as long as water is migrating from the central position of the food radially outward to the surface. The temperature profile in the crust is a function of oil temperature, whereas the evolution of the core temperature is independent of the oil temperature so long as water is transferred from the inner part of the food to the surface (Figure 1.3); it cannot be changed to accelerate the frying process.

Figure 1.3 Scheme of heat and mass transfer during deep frying.
Source: Reproduced with kind permission of John Wiley & Sons (Gertz 2014).
The thickness of the crust increases with frying time up to about 0.3–2.0 mm (Ngadi et al. 1997; Gertz 2014). When frying potato chips (crisps), the crust region enlarges quickly and the core zone disappears. The low thickness, lack of liquid water to be evaporated, falling pressure, and high heat transfer raise the temperature of the material above 100 °C very quickly.
Models developed to calculate the transient temperature, moisture content, and oil content during the frying process use many simplifying assumptions. Dincer (1996) propose a single‐phase model using a discretization of heat and mass transfer. To simplify the calculations, it is assumed that there is no effect of mass transfer on heat transfer and vice versa. Farid and Chen (1998) found a good agreement between the predicted and experimental temperature distribution, except at the end of the frying period, where the central temperature of the potato chips exceeded the boiling temperature of water.
A two‐phase or moving‐boundary model has been proposed to describe the mechanism of heat and mass transfer during frying by Farkas et al. (1996), among others. These authors proposed the existence of two regions, separated by an interface: the core (unfried) and the crust (fried) regions. During immersion frying, heat is transferred from the frying oil to the core via the crust region. Water is evaporated at the moving boundary at 100 °C. The heat conduction equation was used to describe the heat transfer in this region. The temperature difference is the driving force of heat transfer. The changing physical properties due to increasing fat degradation made the system too complex for a realistic model to be developed. Also, the effects of crust formation on physical properties were neglected. This model is comprehensive and helps to explain the frying process. The simplified scheme of heat and mass transfer during the actual frying of food is depicted in Figure 1.3.
1.3.2 Oil Uptake
During the frying process, food takes up oil contents. Oil uptake seems to be independent of frying temperature, but is significantly affected by frying time, moisture loss, and the structure of the products to be fried (Gamble et al. 1987; Alvarez et al. 2000). The initial moisture content of food can be reduced by pre‐treatments such as pre‐drying at temperature 70–75 °C, air or vacuum drying (Mariscal and Bouchon 2008), osmotic dehydration (Bunger et al. 2003) and blanching (Sobukola et al. 2010), or else in combination with a post‐treatment (Mariscal and Bouchon 2008). In the food industry, the use of hydrocolloids such as carboxymethylcellulose, pectin, sodium alginate, powdered cellulose, and modified starch is very common to retard the moisture loss (Holikar et al. 2005; Saha and Bhattacharya 2010; Gertz 2014).
The initial superficial vaporization and subsequent in‐depth vaporization create a porous, dried, and overheated region which is generically called ‘crust’. The pores can be small voids, molecular interstices, or large caverns which are filled with water and air. They may be interconnected or nonconnected. The water should be able to travel throughout the entire porous structure, as if it were a network of pipes. For this reason, it is important not to overheat the product when food is immersed into hot oil. A good structured crust helps to retard the loss of moisture; otherwise, the pores will be too large or will be destroyed due to the high vapour pressure. When the porosity of the material is low, the increase in pressure can significantly reduce the drying rate. For materials with weak structures due to high water content and/or the absence of cell structure, water transport can be so intense that liquid water escapes the surface without vaporization (Gertz 2014).
Fried foods like yeast‐raised doughnuts have more oil accumulated at the surface due to their thin crust layer. Unlike in deep frying, these foods float on the surface during frying (i.e. shallow frying). The heated water in the core starts boiling and increases the volume of the food. Only a little water finds its way through the crust. For this reason, the crust is very thin. Another effect is that volatiles remain in the hot oil, producing off‐flavours like rancidity, because not enough steam is leaving the crust and stripping them off (Gertz 2014).
1.3.3 Mechanism of Oil Absorption
Oil uptake is a complex mechanism that is still not clearly understood. The initial product structure, the various interchanges between the product and the heating medium, and the variations of product and oil properties are the factors which complicate this phenomenon (Ziaiifar et al. 2008).
1.3.3.1 Water Escape and Oil Uptake
Most authors agree that during frying, heat and mass transfer are controlled by heat transfer at the surface of the product. The rate of vaporization is proportional to the temperature difference between the oil and the boiling point of water (Vitrac et al. 2002). Numerous works propose a simple description based on a convective mass transfer approach that is too simple. Farkas et al. (1996) were the first scientists to propose a physical description of frying. They stated that this process should be described as a complex Stephan problem, because of the coupled heat and mass transfer resulting in the displacement of a moving vaporization front that separates two dynamic regions: a dehydrated crust and a humid core. As the crust presents low thermal conductivity, it affects heat, and mass transfer and is partly responsible for the decrease in the dehydration rate.
In general, we can say that the more the water is removed from the surface, the more the oil is absorbed. Figure 1.4 plots the relationship between oil content and moisture loss. When mass transfers in deep fat frying are studied, the escape of water is usually linked to oil absorption. Indeed, Gamble et al. (1987) found that moisture loss and oil uptake were inter‐related and were both linear functions of the square root of frying time. They hypothesized that the oil entering the slice would lie in the voids left by the escaping water. Hence, in addition to quantitative aspects, water loss can become an explanatory variable for transformation and especially oil uptake, because water escape is at the origin of very diverse material phenomena such as the creation of cavities (Vitrac et al. 2000). Indeed, as dehydration occurs at a temperature above 100 °C, water steam finds selective weaknesses in the cellular adhesion that lead to the formation of capillary pathways, increasing surface porosity. Furthermore, some of this vapour may be trapped within the pores as a result of restrictive intercellular diffusion and expand, becoming superheated, distorting the pore walls, and contributing to product porosity. Accordingly, some studies have examined the increase of porosity during frying and correlated it to the amount of oil uptake (Pinthus and Saguy 1994; Moreira et al. 1997). Characterization of the product microstructure thus appears to be a determining factor in the description of transfers at the macroscopic scale, such as oil uptake.
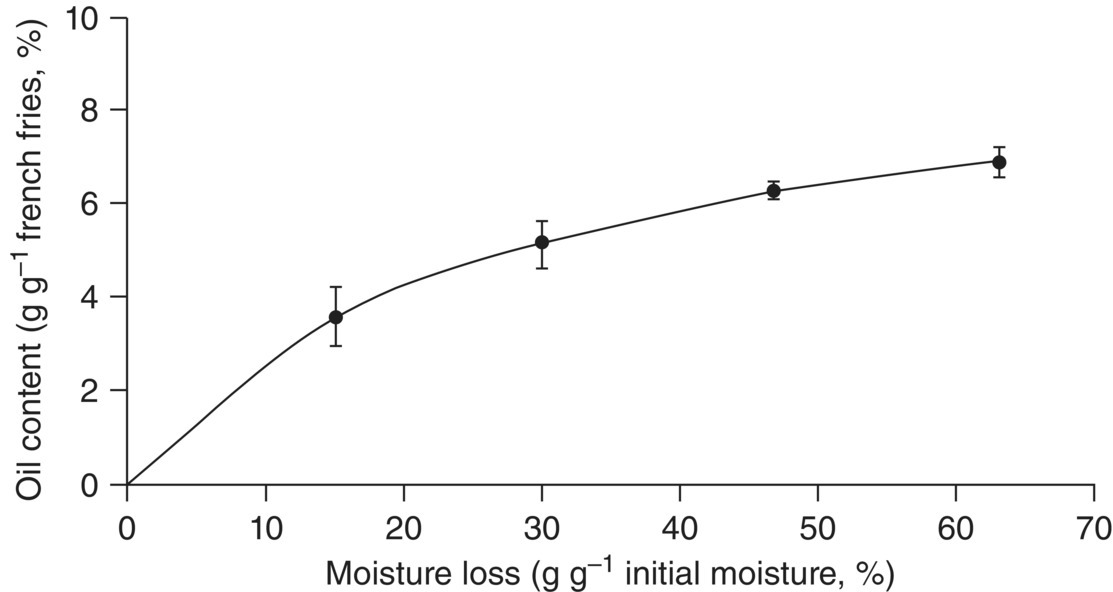
Figure 1.4 Oil content versus moisture loss in French fries during frying at 155 °C.
Source: Reproduced with kind permission of John Wiley & Sons (Ziaiifar et al. 2008).
1.3.3.2 Capillary Pressure and Oil Uptake
Moreira et al. (1999) introduced a physical relation between oil absorption and porosity, stating that the mechanism of oil uptake may be caused by capillary forces. Indeed, when a fluid displacement such as oil absorption occurs in microcanals like crust pores, surface phenomena such as viscosity or capillary forces become very important. Capillarity is the ability of a narrow pore to draw a liquid upwards. It occurs when the adhesive intermolecular forces between a liquid and a solid are stronger than the cohesive intermolecular forces in the liquid. This causes a concave meniscus to form where the liquid is in contact with the vertical surface. This phenomenon creates a difference of pressure between the two sides of the curbed interface, as expressed by the Laplace law (Figure 1.4):
where Pi is the pressure at the point i (Pa), γ is the surface tension of the oil (N m−1), ϑ is the wetting angle between the oil and the solid (rad), and r is the pore radius (m).
In addition, P2−P3 = −ρgh, according to the hydrostatic pressure difference (Figure 1.5), where ρ is the oil density (kg m−3), g is the acceleration gravity (m s−2), and h is the height of the capillary motion (m).
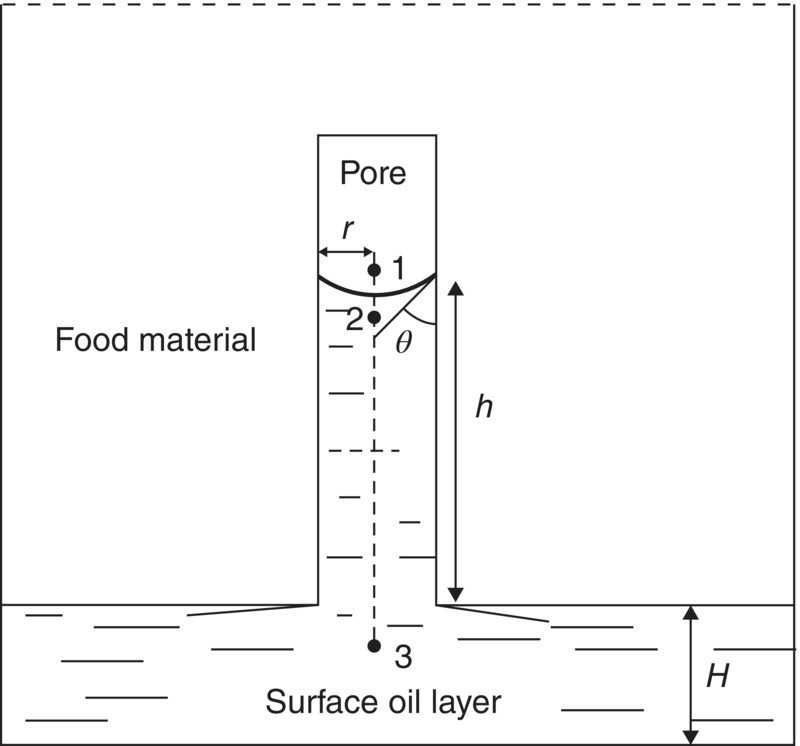
Figure 1.5 Diagram of oil flowing into a pore.
Source: Reproduced with kind permission of John Wiley & Sons (Ziaiifar et al. 2008).
Therefore, the pressure difference ΔP at the two points 1 and 3 of the pore is:
Oil absorption is therefore dependent on pore radius. Small pores cause higher capillary pressures and thus higher oil content (Moreira et al. 1997). In addition, the lower the contact angle between the oil and the product surface, the higher the adhesion forces and the oil uptake. Finally, the higher the surface tension of the liquid, the higher the oil uptake. Moreira and Barrufet (1998) stated that γ decreases with increasing temperature, resulting in a capillary pressure reduction. This fact contributes to limiting oil uptake during frying.
The main difficulty with capillarity motion determination at the end of frying is the determination of pore radii that are nonhomogenous in shape. Furthermore, pores can be filled with liquid, water vapour, or air depending on the conditions at the end of frying. Thus, the wetting property of oil towards the solid matrix is nonhomogenous and difficult to determine in such complex and multiple fluid phase systems. Moreover, capillary motion equations are mostly used in their static form, resulting in the expression of the equilibrium positions of fluids. This simplification comes from the fact that other forces, such as vacuum effect and the weight of absorbed oil, are involved in oil absorption.
1.3.3.3 Vapour Condensation and Vacuum Effect
During frying, intense drying occurs at a temperature greater than the temperature of water ebullition. The solid matrix of the food is an obstacle to water bubble growth, leading to a pressure gradient in the food. Overpressure was evaluated in an experimental work by Vitrac et al. (2000), who measured an inner overpressure of 45 kPa during the frying of an alginate gel containing 10% starch. Overpressure depends on the initial structure of the material. Indeed, the more resistant the structure is towards fluid dilatation, the higher the pressure inside the material. However, some structures are not sufficiently resistant to pressure and can break, allowing liquid water to escape. This phenomenon of water loss in both steam and liquid forms was observed during the deep fat frying of apple slices (Vitrac et al. 2003).
Consequently, during frying, when the product still presents high free water content susceptible to evaporate, the escape of water and the associated overpressure in the material is an obstacle to oil absorption. In opposition, when the product is removed from the fryer, the core temperature decreases, steam condenses, and the pressure in the product abruptly decreases. As a consequence, the important difference between the inner and outer pressures creates a ‘vacuum effect’, resulting in the penetration of the surface oil into the product (Gamble and Rice 1987). Moreover, Vitrac et al. (2000) found the depression in a food model gel to be 35 kPa a few seconds after the product had been removed from the oil bath; they therefore stated that this vacuum is the most important force acting on oil uptake in the porous media.
1.3.3.4 Adherence and Drainage of Oil
Oil absorption involves a balance between adhesion forces (capillary and water condensation) and drainage of oil during the cooling period (Ufheil and Escher 1996). In the case of frying, adherence, which is an important factor in oil uptake, is the ability of the oil to stick to the outer surface of the product. Drainage is the removal of surface oil as a result of gravity forces.
Theoretically, when a solid is removed from a bath of wetting liquid, it drags out a liquid film the thickness (H) of which (Figure 1.4) is given by the Landau–Levich–Derjaguin relation as (Krozel et al. 2000):
where μ is the oil viscosity (Pa s), γ is its surface tension (N m−1), U is the speed of removal (m s−1), ρ is the oil density (kg m−3), and g is the gravity due to acceleration (m s−2).
Experimental data on fibre coating show that the film formed in the case of slow withdrawals from pure viscous liquids such as oils fits the Landau law. At high withdrawal velocities, the thickness decreases with the velocity, because the solid can only drag the viscous boundary layer. Furthermore, the presence of surface‐active compounds may cause a thickening of the film (Quéré and de Ryck 1998).
1.3.4 Product Properties Affecting Oil Uptake
1.3.4.1 Size, Shape, and Surface of the Product
As oil uptake is a surface phenomenon, the specific dimensions of a food will determine the amount of oil that can be taken up. Results show that oil absorption increases significantly when product thickness is reduced and product surface is increased (Ziaiifar et al. 2008). For instance, French fries absorb less oil than chips because they have a smaller surface/volume ratio, as shown in Table 1.3 (Paul and Mittal 1997). A linear relationship has been set between surface area and oil content (Gamble and Rice 1987). As most of the fat penetrates the food through the pores in the crust, the structural properties of the outer layer of the food are important. Indeed, cells broken during cutting are a privileged location for oil absorption (Dana and Saguy 2006). Using good‐quality blades for cutting can, therefore, reduce the surface roughness of the product and thus the surface area, resulting in lower oil uptake.
Table 1.3 Water and fat content in various raw and deep‐fried products.
Source: Modified and reproduced with kind permission of John Wiley & Sons (Ziaiifar et al. 2008).
| Food | Water content (g 100 g−1 wet basis) | Oil content (g 100 g−1 wet basis) | Water content (g g−1 nonfat dry matter) | Oil content (g g−1 nonfat dry matter) | Reference |
| Raw products | |||||
| Plantain | 60 | 0.1 | 1.53 | 0.005 | (Rojas‐Gonzalez et al. 2006) |
| Potato | 80 | 0.1 | 4 | 0.005 | (Talburt 1987) |
| Eggplant | 95 | 0.1 | 19.38 | 0.005 | (Kalogeropoulos et al. 2006) |
| Cassava | 60 | 0.1 | 1.50 | 0.005 | (Vitrac et al. 2000) |
| Tortilla dough | 47 | 1.5 | 0.88 | 0.03 | (Moreira et al. 1999) |
| Fried products | |||||
| Plantain cylinders | 32 | 7 | 0.80 | 0.15 | (Rojas‐Gonzalez et al. 2006) |
| French fries | 44 | 13 | 1.05 | 0.30 | (Talburt 1987) |
| Potato chips | 2 | 40 | 0.03 | 0.69 | (Talburt 1987) |
| Eggplant cubes | 50 | 40 | 3.85 | 2.71 | (Kalogeropoulos et al. 2006) |
| Cassava chips | 2 | 25 | 0.05 | 0.35 | (Vitrac et al. 2000) |
| Tortilla chips | 2 | 25 | 0.025 | 0.35 | (Moreira et al. 1999) |
1.3.4.2 Composition and Density of the Product
As shown in Table 1.3, from a humid raw product, deep fat frying provides more or less high‐fat content products depending on their initial composition and thickness. Indeed, initial solid content in the product is a factor that influences oil uptake during frying because of the relationship between water loss and oil uptake (Pinthus and Saguy 1994; Moreira et al. 1997). For a final fried product that exhibits an intermediary water content (i.e. thick product) such as plantain cylinders, French fries, and eggplant cubes, the higher the initial water content, the higher the final oil uptake (Table 1.3). Similarly, a higher potato density (1103 kg m−3 compared with 1093 kg m−3) can reduce oil content by about 10% (Ufheil and Escher 1996), because of the relationship between a product's density and its initial water content (Paul and Mittal 1997). Table 1.3 shows that the important moisture loss that occurs during frying of thin products (i.e. chips) leads to considerable fat uptake, because of the extensive void volumes created by the water escape (Gamble et al. 1987). Finally, the ability of a raw material to present or develop high porosity during frying, mainly because of a high level of initial water content or extensive water loss, will control oil uptake. However, the typical behaviour of eggplant during frying has to be noticed. Indeed, even if the residual water content is still quite high after frying (only 50% of initial water is removed), oil uptake is as high as in totally dehydrated products like chips. This phenomenon can be explained by the fact that eggplant is an aqueous nonstarchy product whose structure is very weak and spongy (Kalogeropoulos et al. 2006).
1.3.5 Frying Oil Properties and Oil Uptake
1.3.5.1 Oil Type
The effect of oil type differs greatly depending on its composition. Kita and Lisińska (2005) wrote that fat absorption is higher when the amount of unsaturated fatty acid increases in oil. On the other hand, Vitrac et al. (2000) showed that oil uptake is weaker with an unsaturated oil such as cotton oil than with palm oil, because of the former's weak viscosity during cooling and its ability to drain easily. These contradictions could be explained by the fact that oil viscosity is very influential in the oil absorption mechanism but is involved in both adhesion and draining dynamics (Eq. 1.3). Moreover, the frying oil may contain a portion of fat that solidifies upon cooling, making it harder to drain or shake from the food, as well as less likely to penetrate deeply into crust pores. Fat content can be considered to be a sum of both fat penetrations into the crust and fat crystallization on the surface.
The higher the oil viscosity, the slower the oil migration. Initial oil viscosity depends not only on the oil type but also on the temperature and oil quality. As shown in Figure 1.6, oil viscosity decreases with a decreasing temperature, following the Arrhenius equation. The oil's initial superficial tension is also an important factor to consider in the capillary action leading to oil uptake (Eq. 1.1). An increase in interfacial tension leads to an increase in oil uptake. Therefore, the addition of surfactants (surface‐active agents or wetting agents) such as Tween80 (polysorbate) and Span80 (sorbitan mono‐oleate) in the frying oil could change surface properties and modify oil content in the final product (Pinthus and Saguy 1994). However, these products are not widely approved for food use.

Figure 1.6 Viscosity changes in fresh and used soybean oils.
Source: Reproduced with kind permission of John Wiley & Sons (Ziaiifar et al. 2008).
1.3.5.2 Oil Ageing
The result of oil ageing is an increase in viscosity due to polymer formation (Figure 1.6) and a decrease of contact angle due to the formation of polar compounds. The increase in viscosity could contribute to an increase in oil quantity on the food surface, according to Eq. (1.3), while the decrease in contact angle could increase the wetting properties of the oil, both of which would result in higher oil content. For this reason, Tseng et al. (1996) argued that the amount of surface oil on tortilla chips increased with oil degradation. Still, some studies prove that the total final oil content of tortilla chips is not significantly affected by the change in oil quality between the first and the thirtieth frying operation (Mehta and Swinburn 2001). Nevertheless, this chemical evolution, which can have adverse effects on oil quantity and quality, can be slowed by the addition of natural or synthetic antioxidants (Man and Jaswir 2000; Houhoula et al. 2004). In conclusion, oil ageing plays a role in oil uptake, but its effect is less than expected.
1.3.6 Process Factors
A wide spectrum of process factors, including the conditions of pre‐processing, frying, and post‐frying, has been reported to affect oil absorption in fried foods, as shown in Figure 1.7. Some of these process steps and conditions have been patented as means of decreasing oil uptake.

Figure 1.7 Important factors involved in the frying operation affecting oil uptake.
Source: Reproduced with kind permission of John Wiley & Sons (Ziaiifar et al. 2008).
1.3.6.1 Pre‐processing Factors Affecting Oil Uptake
The most popular processes applied before frying of products at the industrial scale are blanching, air drying, osmotic dehydration, steam baking, and surface treatment (coatings). Blanching is a process of food preparation wherein the food substance is plunged into boiling water or steam in order to deactivate its enzyme and micro‐organism contents. The effect of this pre‐frying process on final oil uptake is quite ambiguous, because of the different conditions applied. Some authors (Rimac‐Brnčić et al. 2004) state that blanching before frying decreases the dry mass content of the product because of the migration of water‐soluble components from the product to the blanching water. As a result, this phenomenon increases water content and thus final oil content (Alvarez et al. 2000; Pedreschi and Moyano 2005). On the other hand, other studies suggest that the surface starch gelatinization that occurs during blanching could form a firm thin layer that protects the food from oil absorption. In addition, blanching can activate pectin esterase enzymes which make surface cell walls collapse, causing a decrease in the porosity and oil content of the product (Aguilera‐Carbó et al. 1999).
1.3.6.2 Post‐frying Conditions
When the product is removed from the frying medium, its temperature immediately starts to decrease. Below 100 °C, water vapour condenses and the internal pressure drops, resulting in the creation of a positive pressure vacuum favouring oil uptake. The most important factors affecting oil absorption during post‐frying are the cooling conditions. Indeed, temperature influences oil viscosity and the interfacial tension involved in oil uptake phenomena. Matz (2012) stated that if the product is removed from the fryer while its temperature is still increasing, oil uptake will decrease. The hydrodynamics of cooling is also important: vigorously shaking the basket of fried products immediately after removal from the fryer can drain the surface oil if it is still liquid and has not yet been sucked into the pores. The oil that can penetrate the pores is thus limited (Thanatuksorn et al. 2005).
1.3.7 Chemical and Physical Changes of the Frying Medium
During frying, a series of complex changes and reactions take place. The nature and rate of decomposition of the products depend, among other things, on the composition of the oil (fatty acids pattern, unsaponifiable matter content), the mode of frying (intermittent or continuous; shallow, pan, or deep frying), the frying temperature, the length of the frying process, and the type of food being fried. To cover these physical and chemical changes, many analytical methods are proposed in the literature (Croon et al. 1986; Gertz et al. 2000). Increasing acidity, total polar materials (TPM), and polymerized TAGs (PTG), darkening colour, and decreasing iodine value and polyunsaturated fatty acid content are typical indices of oil degradation at elevated temperatures. In 2000, the DGF recommended determining both the sum of all polar compounds and polymerized TAGs:
Analysis of suspect frying fats and oils should use two tests to confirm abuse. Recommended analyses should be total polar materials (TPM) (<24%), polymeric triglycerides (PTG) (<12%). Determination of TPM and PTG are now considered to be the most reliable and objective tests for assessing deterioration (DGF 2000).
A number of nations have set limits for both criteria for evaluating used frying oils. The group of polar compounds includes oxidized fat degradation products, di‐ and oligomerized TAGs, and naturally occurring polar compounds such as mono‐ and diacylglycerols (Gertz 2014). The determination of TPM and PTG using the standard methods or by NIR requires laboratory equipment. Thus, these indices are more often used in research and governmental laboratories than in regular in‐house quality control. Many quick tests are now available on the market to identify polar materials by measuring the dielectricity constant. Besides free fatty acid (FFA), these quick tests are increasingly being applied in the frying industry for quality control.
1.4 Why We Fry Foods
The basic question is, why are we frying foods? Historically, some foods were not digestible or easily consumable. Normal cooking with water and heat consumes a lot of time, and frying speeds this process. Some of the reasons why we fry our foods include:
- Frying increases the speed of food preparation.
- Frying converts indigestible foods to digestible one.
- Frying is a more efficient process than other cooking methods.
- Frying can form foods with specific tastes and textures.
- Frying is responsible for significant odour formation in foods.
- Frying increases the acceptability of foods.
- Frying is easy and economical compared to other food preparation methods.
1.5 Key Concepts
- Frying is one of the fastest and simplest food preparation techniques.
- Frying consists of four main components: specific conditions, frying equipment, foods, and frying medium.
- Frying is one of oldest food preparation techniques, although it is hard to trace exactly when it was invented.
- The mechanism of frying consists of heat and mass transfer, oil uptake, and physical and chemical reactions.
- Food bodies are made of holes and pores filled with water. The water evaporates according to the temperature and timing of frying. Subsequently, the holes and pores are occupied by oil.
- The dehydration of the food surface in frying produces a crust/crumb, which increases with frying time.
- Oil uptake can be reduced by several pre‐treatment procedures to produce foods of acceptable quality.
- The size, shape, composition, density, and surface of the food product can all affect oil uptake.
- Pre‐ and post‐frying conditions significantly affect the frying of foods.
- Physical and chemical changes occur in foods and the frying medium, affecting flavour, taste, and sensory characteristics.
References
- Aguilera‐Carbó, A., Montañez, J.C., Anzaldúa‐Morales, A. et al. (1999). Improvement of color and limpness of fried potatoes by in situ pectinesterase activation. European Food Research and Technology 210: 49–52.
- Alvarez, M.D., Morillo, M.J., and Canet, W. (2000). Characterization of the frying process of fresh and blanched potato strips using response surface methodology. European Food Research and Technology 211: 326–335.
- Blumenthal, M. (1991). A new look at the chemistry and physics of deep‐fat frying. Food Technology (USA) 45: 68–71.
- Bunger, A., Moyano, P., and Rioseco, V. (2003). NaCl soaking treatment for improving the quality of french‐fried potatoes. Food Research International 36: 161–166.
- Costa, R.M., Oliveira, F.A.R., Delaney, O., and Gekas, V. (1999). Analysis of the heat transfer coefficient during potato frying. Journal of Food Engineering 39: 293–299.
- Croon, L.B., Rogstad, A., Leth, T., and Kiutamo, T. (1986). A comparative study of analytical methods for quality evaluation of frying fat. Fette, Seifen, Anstrichmittel 88: 87–91.
- Dana, D., Blumenthal, M.M., and Saguy, I.S. (2003). The protective role of water injection on oil quality in deep fat frying conditions. European Food Research and Technology 217: 104–109.
- Dana, D. and Saguy, I.S. (2006). Review: mechanism of oil uptake during deep‐fat frying and the surfactant effect‐theory and myth. Advances in Colloid and Interface Science 128–130: 267–272.
- DGF (2000). Recommendations for the 3rd International Symposium on Deep‐Fat Frying – Optimal Operation. European Journal of Lipid Science and Technology 102: 594.
- Dincer, I. (1996). Modelling for heat and mass transfer parameters in deep‐frying of products. Heat and Mass Transfer 32: 109–113.
- Farid, M.M. and Chen, X.D. (1998). The analysis of heat and mass transfer during frying of food using a moving boundary solution procedure. Heat and Mass Transfer 34: 69–77.
- Farkas, B.E., Singh, R.P., and Rumsey, T.R. (1996). Modeling heat and mass transfer in immersion frying. I, model development. Journal of Food Engineering 29: 211–226.
- Gamble, M.H. and Rice, P. (1987). Effect of pre‐fry drying of oil uptake and distribution in potato crisp manufacture. International Journal of Food Science and Technology 22: 535–548.
- Gamble, M., Rice, P., and Selman, J. (1987). Relationship between oil uptake and moisture loss during frying of potato slices from cv record UK tubers. International Journal of Food Science and Technology 22: 233–241.
- Gertz, C. (2014). Fundamentals of the frying process. European Journal of Lipid Science and Technology 116: 669–674.
- Gertz, C., Klostermann, S., and Kochhar, S.P. (2000). Testing and comparing oxidative stability of vegetable oils and fats at frying temperature. European Journal of Lipid Science and Technology 102: 543–551.
- Gertz, C., Klostermann, S., and Parkash Kochhar, S. (2003). Deep frying: the role of water from food being fried and acrylamide formation. OCL 10: 297–303.
- Gil, B. and Handel, A.P. (1995). The effect of surfactants on the interfacial tension of frying fat. Journal of the American Oil Chemists' Society 72: 951–955.
- Holikar, M.V., Annapure, U.S., Singhal, R.S., and Kulkarni, P.R. (2005). Pectin and calcium chloride treatment for low‐fat fried green gram splits. Journal of the Science of Food and Agriculture 85: 1677–1680.
- Houhoula, D.P., Oreopoulou, V., and Tzia, C. (2004). Antioxidant efficiency of oregano in frying and storage of fried products. European Journal of Lipid Science and Technology 106: 746–751.
- Kalogeropoulos, N., Grigorakis, D., Mylona, A. et al. (2006). Dietary evaluation of vegetables pan‐fried in virgin olive oil following the Greek traditional culinary practice. Ecology of Food and Nutrition 45: 105–123.
- Kita, A. and Lisińska, G. (2005). The influence of oil type and frying temperatures on the texture and oil content of French fries. Journal of the Science of Food and Agriculture 85: 2600–2604.
- Krozel, J.W., Palazoglu, A.N., and Powell, R.L. (2000). Experimental observation of dip‐coating phenomena and the prospect of using motion control to minimize fluid retention. Chemical Engineering Science 55: 3639–3650.
- Man, Y.B.C. and Jaswir, I. (2000). Effect of rosemary and sage extracts on frying performance of refined, bleached and deodorized (RBD) palm olein during deep‐fat frying. Food Chemistry 69: 301–307.
- Manglik, R.M. (2006). On the advancements in boiling, two‐phase flow heat transfer, and interfacial phenomena. Journal of Heat Transfer 128: 1237–1242.
- Mariscal, M. and Bouchon, P. (2008). Comparison between atmospheric and vacuum frying of apple slices. Food Chemistry 107: 1561–1569.
- Matz, S.A. (2012). Snack Food Technology. Berlin: Springer Science+Business Media.
- Mehta, U. and Swinburn, B. (2001). A review of factors affecting fat absorption in hot chips. Critical Reviews in Food Science and Nutrition 41: 133–154.
- Moreira, R.G. and Barrufet, M.A. (1998). A new approach to describe oil absorption in fried foods: a simulation study. Journal of Food Engineering 35: 1–22.
- Moreira, R.G., Castell‐Perez, M.E., and Barrufet, M.A. (1999). Deep Fat Frying: Fundamentals and Applications. Gaithersburg, MD: Aspen.
- Moreira, R.G., Sun, X., and Chen, Y. (1997). Factors affecting oil uptake in tortilla chips in deep‐fat frying. Journal of Food Engineering 31: 485–498.
- Morton, I. (1998). Geography and history of the frying process. Grasas y Aceites 49: 247–249.
- Ngadi, M.O., Watts, K.C., and Correia, L.R. (1997). Finite element method modelling of moisture transfer in chicken drum during deep‐fat frying. Journal of Food Engineering 32: 11–20.
- Paul, S. and Mittal, G.S. (1997). Regulating the use of degraded oil/fat in deep‐fat/oil food frying. Critical Reviews in Food Science and Nutrition 37: 635–662.
- Pedreschi, F. and Moyano, P. (2005). Effect of pre‐drying on texture and oil uptake of potato chips. LWT – Food Science and Technology 38: 599–604.
- Perkins, E.G. (2007). Volatile odor and flavor components formed in deep frying. In: Deep Frying: Chemistry, Nutrition and Practical Application (ed. M.D. Erickson). Urbana, IL: AOCS Press.
- Pinthus, E.J. and Saguy, I. (1994). Initial interfacial tension and oil uptake by deep‐fat fried foods. Journal of Food Science 59: 804–807.
- Quéré, D. and de Ryck, A. (1998). Le mouillage dynamique des fibres. Annals of Physics 23: 1–149.
- Rimac‐Brnčić, S., Lelas, V., Rade, D., and Šimundić, B. (2004). Decreasing of oil absorption in potato strips during deep fat frying. Journal of Food Engineering 64: 237–241.
- Rojas‐Gonzalez, J.A., Avallone, S., Brat, P. et al. (2006). Effect of deep‐fat frying on ascorbic acid, carotenoids and potassium contents of plantain cylinders. International Journal of Food Sciences and Nutrition 57: 123–136.
- Rossell, J. (2001). Frying: Improving Quality. Sawston: Woodhead Publishing.
- Saha, D. and Bhattacharya, S. (2010). Hydrocolloids as thickening and gelling agents in food: a critical review. Journal of Food Science and Technology 47: 587–597.
- Sobukola, O.P., Awonorin, S.O., Oladimeji, S.L., and Olukayode, B.F. (2010). Optimization of pre‐fry drying of yam slices using response surface methodology. Journal of Food Process Engineering 33: 626–648.
- Stier, R.F. (2004). Frying as a science – an introduction. European Journal of Lipid Science and Technology 106: 715–721.
- Talburt, W.F. (1987). Potato Processing. New York, NY: Van Nostrand Reinhold.
- Thanatuksorn, P., Pradistsuwana, C., Jantawat, P., and Suzuki, T. (2005). Effect of surface roughness on post‐frying oil absorption in wheat flour and water food model. Journal of the Science of Food and Agriculture 85: 2574–2580.
- Tseng, Y.C., Moreira, R., and Sun, X. (1996). Total frying‐use time effects on soybean‐oil deterioration and on tortilla chip quality. International Journal of Food Science and Technology 31: 287–294.
- Ufheil, G. and Escher, F. (1996). Dynamics of oil uptake during deep‐fat frying of potato slices. LWT – Food Science and Technology 29: 640–644.
- Vitrac, O., Dufour, D., Trystram, G., and Raoult‐Wack, A.‐L. (2002). Characterization of heat and mass transfer during deep‐fat frying and its effect on cassava chip quality. Journal of Food Engineering 53: 161–176.
- Vitrac, O., Raoult‐Wack, A.L., and Trystram, G. (2003). Influence of liquid water transport on heat and mass transfer during deep‐fat frying. In: Transport Phenomena in Food Processing (ed. J. Welti Chanes, J. Véles‐Riuz and G.V. Barbosa‐Cànovasn). Boca Raton, FL: CRC Press.
- Vitrac, O., Trystram, G., and Raoult‐Wack, A.L. (2000). Deep‐fat frying of food: heat and mass transfer, transformations and reactions inside the frying material. European Journal of Lipid Science and Technology 102: 529–538.
- Wegmuller, F. (1994). Polar components of frying fats derived from data of dielectric measurements. Zeitschrift für Lebensmittel‐Untersuchung und ‐Forschung 199: 51–54.
- Ziaiifar, A.M., Achir, N., Courtois, F. et al. (2008). Review of mechanisms, conditions, and factors involved in the oil uptake phenomenon during the deep‐fat frying process. International Journal of Food Science and Technology 43: 1410–1423.

