Gas-Fired Power Plants and the Environment
Abstract
The combustion of natural gas can have a significant environmental effect. The combustion reaction generates large quantities of carbon dioxide as well as nitrogen oxides and carbon monoxide. Meanwhile methane itself is a potent greenhouse gas and its release during the production and delivery of natural gas contributes to global warming. The production of nitrogen oxides can be controlled either by staged combustion and the design of low NOx combustors for gas turbines or by removing it from the exhaust gases using a catalytic reduction process before the gases are released into the atmosphere. Carbon monoxide can also be catalytically removed. The production of carbon dioxide in gas turbines and combined cycle plants is not legally controlled but it may become necessary for plant operators to introduce carbon capture and storage over the next decade to meet new emission regulations.
Keywords
NOx; greenhouse gases; carbon dioxide; staged combustion; low NOx burners; selective catalytic reduction; catalytic oxidation; carbon capture and storage
Natural gas is a fossil fuel and its combustion has a significant environmental impact. Combustion releases large quantities of carbon dioxide into the atmosphere and this contributes to the increase in atmospheric concentrations of the gas which are blamed for global warming. Methane, the major constituent of natural gas as supplied by pipeline, is on its own a potent greenhouse gas so any inadvertent release of this will also be important. According to the US Environmental Protection Agency, 3.8% of greenhouse gas emissions in the United States between 1990 and 2009 came from methane released by oil and natural gas systems. Most of this is released during oil and gas production but transmission and distribution accounts for around 30% of the annual total.
In addition to these greenhouse gas emissions associated with natural gas-fired power plants, there are damaging noxious emissions from the combustion process itself. The most important of these for a gas plant is the generation of nitrogen oxides from the nitrogen in air at the high combustion temperatures typical of natural gas combustion. These emissions have been linked to a range of environmental problems including a serious impact on human health. Depending on the combustion conditions, combustion of natural gas can also generate carbon monoxide as well as some unburnt hydrocarbons. Both must be controlled to meet modern emission regulations.
Unlike coal, natural gas contains very little sulfur and so this has no significant environmental impact. It contains no heavy metals either. However, it will release waste heat to the environment both in the form of hot exhaust gases through the plant stack and warm water if local water supplies are used in the steam turbine condenser of a combined cycle plant. Heat emissions of this type are not usually considered particularly harmful but they can change local environmental conditions.
Like any other power plant, there will be some local disruption due to additional traffic movements associated with the plant. The fuel, gas, will be delivered by pipeline so this will cause little disruption once it has been built. Overall, a natural gas-fired power station is likely to be the most benign of any fossil-fuel powered plant.
9.1 Nitrogen Oxide Emissions
The combustion of fossil fuels including natural gas leads to the production of quantities of nitrogen oxides, often collectively referred to as NOx. The main constituents of NOx are nitrogen oxide (or nitric oxide, NO), nitrogen dioxide (NO2), and nitrous oxide (N2O). In power plant combustion furnaces the main NOx constituent generated is nitrogen oxide which is the most stable oxide at these temperatures. It probably accounts for 90% or more of the NOx from a gas-fired plant.
Nitrogen oxides are controlled because of the range of environmental problems they can cause. They are a major source of ground level ozone which can cause severe respiratory problems. The gas can react with other components in the atmosphere to create an acid aerosol which is also harmful. NOx is responsible in part for the haze that falls over urban areas and it can, together with sulfur dioxide, cause acid rain which has been responsible for destroying forests and life in lakes as well as damaging buildings. An abundance of nitrogen from nitrogen oxides is a cause of massive algae growths in waterways. Finally, nitrous oxide is a greenhouse gas.
Table 9.11 shows a selection of national and regional standards for the emission of nitrogen oxides from power plants. In all cases the standards have been tightened in recent years as the dangers of NOx emissions have become clearer. In China all new plants have to adhere to a limit of 100 mg/m3. For existing plants, those built after 2005 also have to meet this standard but older plants have less stringent limits. In the European Union the limit is 200 mg/m3 for all plants after 2016 but older plants can emit 500 mg/m3 before that date, although they must comply with the new limit from the start of 2016. In the United States the standard is 117 mg/m3 for plants built after 2005 but earlier plants can release up to 640 mg/m3. All gas-fired combined cycle power plants within these jurisdictions must comply with these limits. Similar limits apply elsewhere.
Table 9.1
International Emission Standards for Nitrogen Oxides from Power Plants
| Country/Region | Emission Limit for New Plants (mg/m3) | Emission Limit for Existing Plants (mg/m3) |
| China | 100 | 160–640 for plants built before 2006 |
| European Union | 200 | 500 for plants built before 2016 |
| United States | 117 | 200 for plants built before 2004 |
Source: AirClim.
There are two principle strategies to control the NOx emissions from a gas-fired power plant. The first is by careful control of the combustion conditions when the natural gas is burnt and the second is by removal of the NOx from the exhaust gas stream of the power plant after it has been produced. Most natural gas-fired power plants need to use both strategies to comply with emissions regulations.
9.2 NOx Production Pathways
Natural gas contains no significant amount of nitrogen, unlike coal, and so all the NOx produced in a gas-fired power plant is the consequence of the oxidation of nitrogen from air at the very high temperatures generated during the combustion process. There are probably hundreds of different chemical pathways that can lead to NOx formation, many involving different fragmentary reactants that are generated during the combustion.
Among this multitude of possibilities, two broad pathways have been identified, one called thermal generation and the other prompt generation. Thermal generation involves a reaction during the combustion process which releases oxygen atoms from oxygen molecules in air. The freed oxygen atoms then react with molecular nitrogen to generate nitrogen oxide. This reaction is important once the combustion temperature rises above around 1300°C and will become the major source of nitrogen oxide above 1600°C when both molecular nitrogen and molecular oxygen start to dissociate spontaneously.
The prompt pathway—which is a catch-all for the other ways NOx can be produced—normally involves molecular fragments called hydrocarbon radicals generated from the breakup of hydrocarbon molecules. These react with molecular nitrogen producing a range of nitrogen-containing radicals that are easily converted to nitrogen oxide. As with thermal production of nitrogen oxides, the higher the temperature the more likely they are to be produced.
Since the speed of all these reactions depends on the combustion temperature, so does the quantity of NOx that is produced during combustion. The higher the temperature, the greater the quantity produced. At the same time the efficiency of a heat engine depends on temperature; high efficiency requires high working gas temperatures. A high efficiency engine must therefore lead to conditions that are ideal for the production of large quantities of NOx.
9.3 Low NOx Burners
In spite of these competing requirements, it is possible to limit the amount of NOx produced during the high temperature combustion process. The most important strategy is to control the amount of oxygen that is available in the hottest part of the combustion flame.
Methane (CH4) and its constituents, hydrogen and carbon, are all more reactive towards oxygen than nitrogen. Each molecule of methane requires two molecules of oxygen for complete combustion. If the amount of oxygen is limited to this amount or less, methane molecules will preferentially react with the oxygen that is available, limiting the amount that of oxygen can be turned into NOx. If the amount of oxygen is less than is required2 the conditions will lead to incomplete combustion of the natural gas. The reaction must eventually be allowed to go to completion if all the energy from methane combustion is to be released so more air is added to the combustion chamber later, when the combustion gases have cooled a certain amount and the likelihood of NOx production is reduced.
In a gas-fired steam plant, this strategy is called staged combustion. Air and natural gas are mixed in less than stoichiometric proportions and then ignited in the boiler to create the main fireball. Further air is then admitted to the hot combustion gases in the region above the fireball where the temperature is lower. It is also possible to admit additional natural gas above the main combustion zone to preferentially scavenge oxygen from nitrogen oxides, reducing them to nitrogen again. In order for the combustion reaction to continue fully it is normal to add an excess amount of air above that needed for stoichiometry. The excess air also helps cool the combustion gases. For natural gas, the air to natural gas ratio, by volume, required for a stoichiometric amount of oxygen to be present is 1:8.43.
The situation is slightly more complex in a gas turbine combustor because the total amount of air that is mixed with the natural gas has to be controlled in order to keep parasitic losses resulting from the compression of excess air to a minimum. Nevertheless a similar type of staged combustion can be achieved, with air and fuel mixed in less than stoichiometric proportions for initial combustion while additional air is admitted through the combustor liner at later stages. The method used in most advanced gas turbine combustors is slightly different, a process called premixed combustion in which air and natural gas are carefully and thoroughly mixed before entering the combustor with just enough oxygen present to react with the combustible constituents of the natural gas and none left for nitrogen production. As with staged combustion, this process must be carefully and continuously controlled. Any changes in natural gas composition must be monitored so that the amount of air can be adjusted as necessary. Too little oxygen will lead to production of carbon monoxide and unburnt hydrocarbon fragments which are potentially harmful. Too much oxygen and NOx levels will rise. With complex fuel nozzle configurations and careful air/fuel mixing the production of NOx can be limited to 15–25 ppm for large industrial gas turbines and under 10 ppm in some small turbines designs.
The injection of water or steam into the combustion chamber has also been used to limit the production of NOx by cooling the combustion gases so that the reaction rate for nitrogen compounds is reduced. This can compensate for limited amounts of excess air. Water/steam injection also has an impact on the overall cycle performance. It is not normally used in large industrial turbines but is used in some aero derivative gas turbines.
9.4 Selective Catalytic Reduction
While controlled combustion can limit the production of NOx it is unlikely to reduce the levels low enough to meet emissions regulations. Therefore an additional procedure is required. The most commonly used is selective catalytic reduction (SCR). This process involves mixing the exhaust gases with a suitable reducing reagent and then passing them over a specially formulated metallic catalyst. The catalyst promotes the reaction between the reducing reagent and nitrogen oxides in the exhaust gas, turning them back into molecular nitrogen.
The reducing agents used in the catalytic reduction process are either ammonia, which is the most common reagent, or urea. The reagent must be mixed with the exhaust gases at a temperature that is suitable for the reaction to proceed. For the SCR process3 this is typically between 250°C and 430°C but can fluctuate by around 90°C either side of this range and still be effective. The layout of an SCR system is shown in Fig. 9.1.
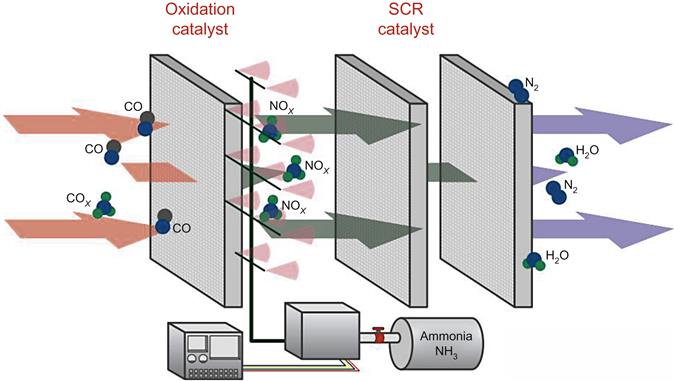
The other key ingredient is the catalyst. This is usually an active metal or ceramic with a highly porous structure. A typical catalyst for SCR might contain around 80% titanium dioxide as the main substrate with vanadium pentoxide, tungsten trioxide, and a small amount of aluminum. However, most catalysts are proprietary and compositions vary. The catalyst normally has a honeycomb structure which presents a large surface area to the exhaust gases which pass through the catalytic modules. The catalyzed reaction takes place on the surface of the catalyst so the larger the area available, the more effective it is.
In order for the exhaust gases and reducing reagent to enter the catalyst module at the correct temperature, the SCR system is often installed within the heat recovery steam generator of a combined cycle plant. For an open cycle gas turbine system it may be necessary to cool the exhaust gases before they enter the catalytic system. Once the flue gases are cool enough, they are mixed with ammonia from a spray system in the flue gas ductwork. The catalytic modules which catalyze the reaction are placed downstream, where the NOx molecules are converted into nitrogen and water vapor. These harmless products are then carried away in the flue gases and exhausted to the atmosphere.
The overall efficiency of the SCR process depends on the concentration of NOx in the exhaust gases. At around 20 ppm the efficiency is close to 70% but may fall at lower concentrations. This efficiency will rise with concentration up to around 150 ppm. High efficiency SCR systems can achieve greater than 90% conversion efficiency.
It is important to control the amount of ammonia that is added to the exhaust gases during SCR because if there is too much, some will remain in the flue gases that are released into the environment, creating a new emissions problem. To control this “ammonia slip” requires monitoring of both the quantity of NOx in the exhaust gases and the ammonia that remains in the flue gases after the SCR reactor. If sulfur is present, the SCR reactor can turn it into sulfur trioxide which will react with water to produce highly corrosive sulfuric acid. This is generally not a problem with natural gas but could be in a dual-fuel gas turbine plant if it switches to a liquid fuel that contains any sulfur.
The SCR catalyst is sensitive to certain flue gas constituents that can poison it, reducing its effectiveness. However, natural gas contains few, if any, constituents that will harm the catalyst and this techniques is successfully and economically applied to most large gas turbine and combined cycle plants. Too high a temperature can also impair its effectiveness so the flue gas temperature must be controlled. When the efficacy of the catalyst modules falls below a certain level they must be removed and replaced. The exhausted modules will generally be regenerated or their catalytic constituents recycled for reuse.
9.5 Carbon Monoxide
Natural gas combustion in a gas turbine can lead to high levels of carbon monoxide during controlled combustion. If there is insufficient oxygen present then the combustion reaction does not run to completion and CO is formed instead of CO2. Carbon monoxide production can also increase when the gas turbine is operating at part load because the temperature in the combustion chamber is lower, again leading to incomplete combustion. At the same time, incomplete combustion can also produce unburnt hydrocarbon fragments known as volatile organic components or VOCs.
Combustor design can help promote full combustion but that may not be sufficient to keep the production of CO below regulatory emission limits. If that is the case then a carbon monoxide control system must be installed. This is normally a catalytic oxidation system. As with the SCR system described above, a catalytic oxidation system has catalytic modules that are placed in the exhaust gas path. These contain metallic catalysts that catalyze the reaction of carbon monoxide with some of the oxygen remaining in the flue gases, converting it to carbon dioxide. The same catalyst will usually be effective in oxidizing VOCs too, rendering them harmless.
In order to prevent interaction between different catalytic systems a catalytic oxidation system will usually be placed before the SCR system, as shown in Fig. 9.1. Oxidation catalysts include metals like rhodium, platinum, palladium, ruthenium and iridium. As with SCR catalytic modules, these metals will be carried in a ceramic honeycomb that presents a very large surface area to encourage rapid reaction of the carbon monoxide and VOCs as the pass through the modules.
9.6 Carbon Dioxide
Carbon dioxide is one of the normal combustion products when a hydrocarbon such as methane is burnt in air or oxygen. The basic combustion reaction of methane in air is:
While methane does not produce as much carbon dioxide as coal does for each unit of electricity produced, the greenhouse gas is still a major product that is released into the atmosphere from all natural gas-fired power plants. In the United States in 2014 natural gas-fired power plants accounted for 22% of power sector carbon dioxide emissions and around 27% of generated power according to the US Energy Information Administration. While the shift to natural gas has not been as pronounced in most other regions as it has in the United States, emissions will still be considerable.
The control of carbon dioxide emissions from power stations is not mandated anywhere although restrictions are under discussion.4 In consequence there are as yet no commercial systems that will remove carbon dioxide from the flue gases of a natural gas-fired power plant. However, there are ways in which these emissions could be controlled and these may well be required by the middle of the third decade of the 21st century.
There are three ways of reducing the carbon dioxide emissions from a natural gas-fired power plant, postcombustion capture, precombustion capture and oxyfuel combustion. Application of any of them will reduce the efficiency of a gas-fired combined cycle power plant by 7 to 17 percentage points compared to a plant with no carbon capture. (The discussion of the three processes below is primarily concerned with gas turbine combined cycle plants since these represent the bulk of natural gas-fired power plants. However, similar techniques can be applied to natural gas-fired steam plants.)
Postcombustion capture is conceptually the simplest scheme to apply. It involves fitting a chemical processing plant to the power station, a processing plant that scrubs the exhaust gases to remove carbon dioxide. This technique is already used in the oil and gas industry and the technology for capture is well understood. One of its major advantages is that it can be retrofitted to existing plants. However it has never been applied to a commercial combined cycle power plant.
The process involves passing the flue gases up through a tall tower into the body of which is sprayed a reagent that will selectively react with and remove the carbon dioxide from the gases. The concentration of carbon dioxide in the flue gases of a combined cycle plant are typically 3–4%, relatively low when considering the reaction mechanics of the capture process. In consequence this reagent needs to form a chemical bond with the carbon dioxide to be effective. Once the reagent has captured the carbon dioxide, it is cycled through a regeneration plant where it is heated to release the carbon dioxide. The latter is then compressed ready for transportation and storage, while the reagent is ready for reuse. This regeneration process is energy intensive because of the chemical bond between the CO2 and the scrubbing reagent and regeneration is responsible for the larger part of the energy losses resulting from carbon capture. The most common capture reagent is monoethanolamine but other reagents are being developed. The efficiency penalty for postcombustion capture is around 7–8 percentage points.5
Precombustion capture is essentially chemical reforming of natural gas to convert it into hydrogen, followed by capture of the carbon dioxide produced during the reforming process. Reforming is a partial oxidation process and it normally requires an oxygen plant when carbon capture is being applied because otherwise the gases resulting from the reaction will be diluted with nitrogen from air, making the separation of carbon dioxide more difficult. When an oxygen plant is used, the final product is virtually pure hydrogen which is burnt in a gas turbine combined cycle plant. The efficiency penalty for this process is much higher than for postcombustion capture, with recent International Energy Agency estimates putting it at 16–22 percentage points.
The final scheme, oxyfuel combustion, replaces the combustion air in the gas turbine with oxygen. This means that the product of combustion is mainly carbon dioxide and water vapor, allowing the carbon dioxide to be separated easily. However, it too requires an oxygen plant. In effect oxyfuel combustion replaces the separation of carbon dioxide from nitrogen in the exhaust gases of a plant for the separation of oxygen from nitrogen in air before combustion. In addition the use of oxygen instead of air changes the operating conditions of the gas turbine both by significantly increasing the combustion temperature compared to combustion in air and by changing the mass flow through the turbine. This complicates the design of a combined cycle power plant with oxyfuel combustion. Oxyfuel combustion is seen as attractive in coal-fired power stations, and may therefore also be attractive in natural gas steam plants but it is probably less cost-effective than postcombustion capture in a combined cycle plant.
