Chapter 6
Thermoplastic Polyimide (TPI)
Xiantao Feng1 and Jialei Liu2,*
1School of Chemistry and Pharmaceutical engineering, Huanghuai University, Zhumadian, China
2BeijingKey Laboratory of Thermal Science and Technology, Beijing, China, Technical Institute of Physics and Chemistry, CAS Key Laboratory of Cryogenics, Beijing, China
*Corresponding author: [email protected]
Abstract
This chapter is mainly focused on the polymerization, processing, properties, and applications of thermoplastic polyimides (TPIs). The polymerization and properties are introduced by their basic polymer units, such as BEPA, PMDA, BTDA, ODPA, BTDA, etc. The blends, composites, and nanocomposites of TPI are also described in this chapter, including the compounding with other molecules of TPI. The environmental impact and recycling is discussed briefly at the end of the chapter.
Keywords: Thermoplastic polyimide, polymerization, processing, properties, applications, blends, composites, compounding, environmental impact and recycling
6.1 Introduction and History
Polyimide (PI) is a polymer of imide monomers. In those of polymers, phthalimide polymer is the most important one.
According to the composition of their main chain, polyimides can be classified into three kinds as follows:
Aliphatic polyimides (linear polyimides), semiaromatic polyimides, and aromatic polyimides. The last ones are the most used polyimides because of their good thermostability such as the phthalimide polymers mentioned above. According to the type of interactions between the main chains, there are two different polyimides: thermosetting polyimides and thermoplastic polyimides. Thermosetting polyimides polymerized from pyromellitic dianhydride (PMDA) and bis(4-aminophenyl)ether (ODA) have a long history of roughly 40 years since commercialization under DuPont. Thermosetting polyimides are known for thermal stability, good chemical resistance, excellent mechanical properties, and characteristic orange/yellow color. The polyimide cannot be melted or injection-molded and therefore has some limitations for complicated design and productivity.

Figure 6.1 Structure of imide circle.
From the 1970s, the National Aeronautics and Space Administration (NASA) firstly launched the research and development of high-performance, high-temperature-resistant adhesive. This work is aimed to develop high-temperature (200 °C to 300 °C) organic adhesive in continuous use, such as aircraft structural components. The TPI resin is one of the key research areas. Compared with thermosetting polyimide material, TPI resin has good toughness, great damage tolerance, and repairable. TPI also can be used as matrix resin for continuous carbon fiber–reinforced resin composites; cryopreservation is no need for the prepared prepreg, thus significantly reducing the cost of manufacturing composite materials and improve the impact toughness of the composites, etc. On this basis, TPI materials which can be directly melted were developed, such as molding, extrusion, and injection molding processable TPI materials. The melt processable property of TPI can not only greatly reduce the costs, improve production efficiency, stabilize product quality, but also produce high value-added products with special performance and functions.
6.2 Polymerization and Fabrication
6.2.1 Thermoplastic Polyimides Based on BEPA
ULTEM is one of the typical bisphenol A bisether-4-diphthalic anhydride (BEPA)–based thermoplastic polyimides; it was commercialized by General Electric Company in 1982. ULTEM can be processed by conventional techniques such as injection molding at 360–380 °C. However, the thermos-oxidative stability of ULTEM is relatively poor owing to the presence of the thermally unstable. Takekoshi et al., [1] reported that ULTEM is produced via nitrodisplacement of bisnitroimides (scheme.6.1) with bisphenol A in polar aprotic solvents.

Scheme 6.1 Synthesis route of ULTEM.
Shi et al., [2] synthesized another several BEPA-based TPI with bisphenol A and a variety of diamines Table 6.1.
Table 6.1 Abbreviations and Structures of the Diamines.
| Abbreviations | Structures |
| PDA | |
| m-PDA |  |
| 4,4’-ODA | |
| 3,4’-ODA |  |
| p-BAPS |  |
| m-BAPS |  |
| TPER |  |
| TPEQ | |
| BAPB | |
| PTPEQ |  |
| o-TOL |  |
| m-TOL |  |
TPIs based on BEPA were prepared with various aromatic diamines in the conventional two-step process via poly(amic acid) (PAA) polymerization and successive thermal imidization. A typical procedure of PAA polymerization is as follows: to a solution of 8 mmol 1,3-phenylenediamine in 40 ml of DMAc, 8 mmol BEPA powder was gradually added. The solution was stirred at room temperature for 24 h. The viscous PAA solution obtained was cast with a doctor blade and dried in an oven at 70 °C for 3 h. The dried PAA films were then thermally imidized upon a stepwise heating process of 150 °C/1h + 200 °C/1h + 250 °C/1h + 280 °C/2h under vacuum in a free-standing state. The thickness of PI films ranged from 20 to 40 µm. To obtain the homopolyimide powder, the PAA solutions were refluxed at 170 °C for 4 h and then poured into a large excess of methanol for precipitation. The obtained powder was postcured under vacuum at 250 °C/h or 280 °C/h to ensure complete imidization.
6.2.2 Thermoplastic Polyimides based on PMDA
Pyromellitic dianhydride (PMDA) is one of the massive production and cheapest aromatic dianhydrides. Developing TPI based on PMDA could effectively lower the fabrication cost. But there are few TPIs based on PMDA due to their strong rigid structures. In the late 1980s, Mitsui Chemicals, Inc. began investigating and developing a thermoplastic polyimide to meet industry needs. As a result of these efforts, Aurum was launched. This material is synthesized from pyromellitic dianhydride (PMDA) and 4,4-bis (3-aminophenoxy)biphenyl and has a high heat resistance with glass transition temperature Tg = 250 °C. AURUM is an injection-moldable semicrystalline polyimide, but it has a very slow crystallization rate. The part obtained through injection molding is amorphous, not crystalline, although Aurum is a semicrystalline polymer. The postcuring after injection molding enabled crystallization, but control of the tight dimension was not sufficient [3].
AURUM has better heat resisting property than ULTEM, but the raw material 4,4-bis (3-aminophenoxy)biphenyl is much more cost which limited its yield and applications. E.I. du Pont de Nemours and Company developed another PMDA-based TPI called VESPEL. Its Tg is up to 385 °C. S. Tarmai and A. Yamaguchi used PMDA and ether diamines to synthesize a series of TPI. The typical polymerization is as follows: A mixture of 0.0582 mol of PMDA, 0.0600 mol of diamines, 0.0036 mol of phthalic anhydride, which was a termination of a polymer chain end, 0.009 mol of 7-picoline in 123 g of m-cresol was stirred at 150 °C for 4 h, and then after cooling down, the reaction mixture was poured into methanol. The precipitated polyimide was collected by filtration, followed by thorough washing with methanol and dried in a forced air oven to a temperature around the Tg of the polyimide. The other polyimide powders were prepared by the same method as mentioned above.
Scheme 6.3 shows the typical synthesis route of PMDA based TPI. The structures of the ether diamines can be found in Table 6.2.

Scheme 6.2 Synthesis route of AURUM.

Scheme 6.3 Synthesis route of TPI based on PMDA.
Table 6.2 The structures of the etherdiamines.
| Abbreviations | Structures |
| 4d-m |  |
| 4d-p | |
| 4f-m |  |
| 4f-p |  |
| 4g-m |  |
| 4g-p |  |
| 4e-m | 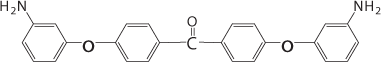 |
| 4e-p | |
| 4b-m |  |
| 4b-p | |
| 4c-m |  |
| 4c-p |  |
6.2.3 Thermoplastic Polyimides Based on BTDA
V. Ratta et al., used 3,3’,4,4’-biphenonetetracarboxylic dianhydride (BTDA) and an 1,3-bis(4-aminophenoxy) benzene (TPER diamine) to synthesize one kind of high-temperature semicrystalline thermoplastic polyimides. The polyimide displays a Tg at ca. 230 °C and two prominent melting endosperms at 360 °C and 460 °C, respectively, with a sharp recrystallization exotherm following the lower melting endotherm.
The reaction vessel was a three-neck round bottom flask equipped with a mechanical stirrer, nitrogen inlet, and a drying tube. Sufficient N-methylpyrrolidinone (NMP) was added to achieve a 10% solid concentration, and the solution was allowed to stir for 24 h, to afford a homogenous poly(amic acid) solution as shown in Scheme 6.4. A stepwise thermal imidization procedure was utilized. The first step was the casting of the poly(amic acid) precursor on the Pyrex glass plates. These plates were placed in the vacuum oven overnight until smooth nontacky films were obtained. Thermal imidization was achieved by raising the temperature to 100, 200, and 300 °C and holding at each of these temperatures for 1 h. The time to go from one temperature to the next was ca. 1 h each at the fastest heating rate available with the oven. After the completion of the cycle, the plates were allowed to cool to room temperature before being removed from the oven. The films were stripped off the glass plates in hot water and stored in a desiccator before use. Tapas koley et al., [6]. used a diamine monomer, 1, 4-bis-[{2’-trifluromethyl 4′-(4″-aminophenyl)phenoxy}] benzene (HQA), and BTDA to synthesize one kind of BTDA-based TPI (Figure 6.2). The polyimides showed reasonably high glass-transition temperature (Tg280 °C) and high thermal stability (Td, 558 °C).

Scheme 6.4 Synthesis route of TPER-BTDA-PA.

Figure 6.2 The structure of the HQA-BTDA-based TPI.
The bis(ether amine) monomer was reacted with BTDA according to two-step conventional procedure to obtain poly(ether imide) membranes. A representative polymerization procedure is as follows: BPADA (0.338 g, 0.65 mmol) was added in portions into the diamine (HQA) (0.377 g, 0.65 mmol) solution in dry DMF (7.0 mL) containing in a 25 mL round bottom flask equipped with a guard tube. The mixture was left for stirring at room temperature while it is transformed to a highly viscous solution of polyamic acid (PAA) within 45–60 min. In the second step, viscous PAA solution was spread onto a clean and dry flat-bottomed Petri dish and kept in oven initially at 80 °C for overnight for slow removal of the solvent. Finally, the thermal cyclization of the PAA to PI was achieved by sequential heating at 100, 150, 200, 250, and 300 °C, each for 1 h and at 350 °C for 30 min in an oven under nitrogen atmosphere. The resulting polyimide membranes were removed from the Petri dishes by immersing in hot water after that the membranes were dried under vacuum at 140 °C for 26 h.
6.2.4 Thermoplastic Polyimides Based on ODPA
Shanghai Research Institute of synthetic resins developed one TPI with 4,4’-oxy-diphthalic anhydride (ODPA) and 4,4’-oxybisbenzenamine (4,4’-ODA) called Ratem-YS-20 in 1970s [7]. NASA used ODPA and 3, 4’-oxybisbenzenamine (3, 4’-ODA) to synthesize another TPI called LaRC-IA. The structures of these two TPI based on ODPA could be found in Figure 6.3 [8, 9].

Figure 6.3 The structures of Ratem-YS-20 and LaRC-1A.
Shi et al., [2]. also synthesized and characterized a serial TPI with ODPA and a series of diamines (4,4’-ODA and 3, 4’-ODA included). The synthesis route of ODPA-based TPI is just the same as the BEPA-based ones as Shi et al., reported.
6.2.5 Thermoplastic Polyimides Based on BPDA
In 2001, S. Tamai et al., synthesized 3,3’,4,4’-biphenyltetracarboxylic dianhydride (BPDA)-based TPI. The synthesis procedure is as follows: 3,4’-oxydianiline (3,4’-ODA) polyimide was synthesized with 28.10 g (95.5 mmol) of BPDA, 20.03 g (100 mmol) of 3,4’-ODA, 1.33 g (9 mmol) of PA, which was used for terminating the polymer chain end. A four-neck round-bottom flask equipped with a mechanical stirrer, nitrogen pad, thermometer, and a condenser with a Dean–Stark trap was used as the reaction vessel. All monomers were added to a reaction vessel and then 3-methylphenol was added to achieve a 10% solids concentration. This solution was stirred and heated under nitrogen atmosphere for 12 h at 202 °C, to afford the PA end-capped polyimide. During the reaction, the by-product, water, was removed by nitrogen flow. After cooling down, methylethyl ketone was added to the reaction mixture. The precipitated polyimide was collected by filtration, followed by thorough washing with methylethyl ketone and dried in a forced air oven at 300 °C for 4 h under nitrogen atmosphere. The other polyimide obtained from 1,3-bis(4-aminophenoxy)benzene (TPER), and BPDA was also prepared by almost the same method as mentioned above.
6.2.6 Thermoplastic Copolyimides
E.I. du Pont de Nemours and Company published a patent which described the synthesis of thermoplastic copolyimides in 2001. The polymers were the reaction products of components comprising an aromatic dianhydride component, an aromatic diamine component, and an end-capping component. The aromatic dianhydride component is selected from the group consisting of BPDA and BTDA, BPDA is preferred.
The aromatic diamine component consists of a first aromatic diamine and a second aromatic diamine. The first aromatic diamine is selected from the group consisting of APB-134 and 3,4’-ODA. The second aromatic diamine is selected from the group consisting of APB-133, 4,4’-ODA, MPD, APB-144,BAPS, BAPB, BAPE, BAPP, and 4,4’-ODA, MPD in combination, and 4,4’-ODA and PPD in combination; wherein APB-133,APB-144, 4,4’-ODA,BAPS, and 4,4’-ODA and MPD in combination are preferred. 4,4’-ODA, and 4,4’-ODA and MPD in combination are most preferred.
Suitable end-capping components when diamines are in excess include, but are not limited to, phthalic anhydride and naphthalic anhydride. These copolyimides have a stoichiometry in the range from 93% to 98 %, exhibit a melting point in the range of 330 °C to 385 °C, and exhibit recoverable crystallinity as determined by differential scanning calorimetry analysis.
A typical synthesis procedure is as follows:
Preparation of Polyimide Based on BPDA//3,4’-ODA/APB-134//PA 95//70/30//10—(95% of stoichiometric dianhydride)
Into a 250 mL round bottom flask equipped with a mechanical stirrer and nitrogen purge were charged 5.3695 g (0.02681 mole) of diamine 3,4’-ODA, 3.3596 g (0.01149 mole) of diamine APB-134 and 60 ml of NMP. After dissolution of the diamines, 10.7073 g (0.03639 mole) of dianhydride BPDA and 0.5674 g (0.00383 mole) phthalic anhydride were added with stirring under nitrogen and rinsed in with 20 ml NMP. The following day, 14.46 ml (0.153 mole) of acetic anhydride (4 × moles of diamine) and 21.36 ml (1.53 mole) of triethylamine (4 × moles of diamine) were added to the poly(amic acid) solution to effect imidization. After about 10 minutes the polymer precipitated, any clumps were broken up by manual manipulation of the mechanical stirrer, and stirring was continued for about 6 hours. The resulting polymer slurry was then added to methanol in a blender to complete precipitation and remove NMP. The polymer was separated by filtration, washed with methanol, and then dried at ca. 200 °C overnight under vacuum with a nitrogen bleed. DSC analysis (10 °C/min.) of the resulting polyimide showed a melting point of 345 °C during the first heating scan, a crystallization exotherm upon the subsequent cooling scan at 296 °C, and a melting point at 346 °C during the subsequent reheat scan, indicating recoverable crystallinity from the melt.

Figure 6.2 The structures of TPI based on BPDA.
The synthesis of other compolyimides was similar to the typical manner.
Table 6.3 Abbreviations of the Diamines.
| Abbreviations | Diamines |
| APB-133 | 1.3-Bis(3-aminophenoxy)benzene |
| APB-134 | 1.3-Bis(4-aminophenoxy)benzene (=RODA) |
| APB-144 | 1.4-Bis(4-aminophenoxy)benzene |
| BAPB | 4.4-Bis(4-aminophenoxy)-biphenyl |
| BAPE | Bis(4-[4-aminophenoxy]phenylether(=4,4’-Bis(4-aminophenoxy)-diphenylether) |
| BAPP | 2.2-Bis(4-[4-aminophenoxyl]phenyl)propane |
| BAPS | 4,4’-Bis(4-aminophenoxy)diphenyl sulfone |
| MPD | 1.3-Diaminobenzene |
| PPD | 1.4-Diaminobenzene |
| 3,4-ODA | 3,4-Oxydianiline |
| 4,4-ODA | 4.4-Oxydianiline |
| 3,3-ODA | 3,3’-Oxydianiline |
| RODA | 1,3-Bis(4-aminophenoxy)benzene(=APB134) |
6.3 Properties
In overall, TPIs have the excellent properties as follows:
- The outstanding characteristics of TPI are excellent heat resistance, the long-term use temperature is about 230–240 °C, and the Tg is up to 250 °C.
- Superdimensional stability. The thermal expansion coefficient of polyimide is only 50PPM/ °C, and it has good creep resistance.
- Excellent mechanical properties. Tensile strength is about 100 MPa, impact strength is about 260 KJ/m2.
- Good flame retardancy. The oxygen index is 36–46, low smoke rate, strong self extinguishing property.
- Excellent electrical insulation performance.
- Excellent oil resistance and solvent resistance.
- High viscosity.
6.3.1 TPI Based on BEPA
ULTEM (Scheme 6.1) is one of the typical bisphenol A bisether-4-diphthalic anhydride (BEPA)-based thermoplastic polyimides; it was commercialized by General Electric Company in 1982. The properties of ULTEM are the worst of the TPI family. But the advantages of lower cost, easy to process made it popular in the market. Its global yield achieved ten thousand tons these years. The properties of ULTEM are listed in Table 6.4
Table 6.4 Properties of ULTEM 1000 (Pure TPI, Without Additives, No Complexes or Blends).
| Properties | Units | Conditions | ULTEM 1000 |
| Density | g/cm3 | 1.27 |
|
| Water absorption | % | 23 °C,24h | 0.25 |
| 23 °C, saturated impregnation | 1.25 |
||
| Heat deflection temperature | °C | 18.6 kg/cm2 | 200 |
| Coefficient of liner expansion | K-1 | 23 °C | 6.2*10-5 |
| Tensile strength | MPa | 23 °C | 107 |
| Elongation at break | % | 23 °C | 60 |
| Tensile modulus | MPa | 23 °C | 60 |
| Bending strength | MPa | 23 °C | 3060 |
| Flexural modulus | MPa | 23 °C | 148 |
| Compressive strength | MPa | 23 °C | 3370 |
| Compressive modulus | MPa | 23 °C | 143 |
| Cantilever impact strength | Kg.cm/cm | 23 °C, no gap | 2960 |
| 23 °C, with gap | 370 |
||
| Rockwell hardness | 109 |
||
| Friction factor | Between self’s | 0.19 |
|
| With steel | 0.2 |
||
| Wear rate | mg | CS17, 1 kg, 1000 C | 10 |
Shi et al., synthesized and characterized a serial TPI based on BEPA as we mentioned in Section 6.2.1. The properties of the TPI are as shown in Table 6.4.
Table 6.5 shows the properties of BEPA-based homopolyimides without any additives. The reduced viscosities of those PAAs ranged from 0.86 to 2.65 dl g–1, indicating the corresponding PIs probably had sufficiently high molecular weights. Generally speaking, the processing condition for the crystalline PIs is often limited in the range from Tm +α to Td. In many cases, aromatic PIs do not become a free-flowing liquid just above the Tm‘s in contrast to common semicrystalline polymers such as poly(ethylene terephthalate). This is probably due to surviving intermolecular interactions even just above the Tm‘s. Therefore, the thermo-processing temperatures for semicrystalline PIs are generally several tens of degrees higher than the Tm‘s. For example, semicrystalline PI AURUM (Tm = 388 °C) can be processed at 410 °C (α = 22 °C) [12]. However, in most cases, the upper temperature limit for the processing of aromatic PIs should be established below 400 °C in order to avoid undesirable thermal degradation such as crosslinking and also to use common molding machines. Thus, for designing TPI, the limited processing temperature range is a critical factor.
Table 6.5 The Properties of TPI Based on ΒΕΡΑ. the Melt Flowability Was Performed as a Qualitative Test on a Temperature-Regulated Hotplate at 400 °C. Tg (The Glass Transition Temperature); Tm (Melting Point); Td (Thermal Decomposition Temperature); Td5 (5% Weight Loss Temperature).
| 1 | TPI | ηred of PAA (dl.g-1) | Tg (°C) | Tm (°C) | Td5 (°C) In N2 in air | Melt flowability | |
| ΒΕΡΑ/PDA | 1.86 |
233 |
no | 474 |
449 |
Poor | |
| 2 | ΒΕΡΑ /m-PDA | 1.30 |
215 |
no | 489 |
475 |
Good |
| 3 | BEPA/4,4’-ODA | 1.76 |
211 |
no | 511 |
501 |
Poor |
| 4 | BEPA/3,4’-ODA | 0.83 |
198 |
no | 510 |
508 |
Good |
| 5 | BEPA /TPEQ | 2.65 |
205 |
no | 503 |
491 |
Poor |
| 6 | BEPA /TPER | 0.86 |
189 |
no | 504 |
484 |
Good |
| 7 | BEPA /BAPB | 1.57 |
217 |
no | 514 |
491 |
Poor |
| 8 | BEPA /PTPEQ | 1.28 |
195 |
no | 511 |
504 |
Good |
| 9 | BEPA /p-BAPS | 1.00 |
229 |
no | 474 |
486 |
Poor |
| 10 | BEPA /m-BAPS | 1.06 |
194 |
no | 472 |
478 |
Good |
| 11 | BEPA /o-TOL | 2.05 |
253 |
no | 484 |
494 |
Poor |
| 12 | BEPA /m-TOL | 2.01 |
238 |
no | 493 |
491 |
Poor |
The Tg‘s of BEPA-based PIs ranged from 189 to 253 °C depending on the diamine structures, and they are 17–93 °C lower than those of the ODPA systems. The melt flowability in BEPA-based PIs varied significantly depending on the diamine structures. As shown in 16.5, m-diamines usually have good melt flowability, whereas p-diamines have poor melt flowability. It is worth noticing that all of the BEPA polyimides in this case have no Tm points. They are completely amorphous even in the PI powder prepared via reflux of the PAA solutions. This is most likely attributed to the presence of the bent and bulky isopropylidene groups in the backbone.
Among the BEPA-based PIs, only BEPA/m-PDA (ULTEM type) simultaneously satisfied the demands of a high Tg (>200 °C) and good melt flowability. This PI was the earliest developed by the General Electric Company, as an injection-moldable PI.
6.3.2 Thermoplastic Polyimides Based on PMDA
In the late 1980s, Mitsui Chemicals, Inc. developed a TPI called AURUM which is based on PMDA. Its synthesis and structure could be found in Scheme 6.2.
S. Tarmai and A. Yamaguchi used PMDA and ether diamines to synthesize a series of TPI. They tested the inherent viscosity (η) and Tg of the PMDA-based TPI [4].
6.3.3 TPI Based on ODPA
Shanghai Research Institute of synthetic resins developed a series TPI called Ratem-YS-20 and its properties were shown in Table 6.11.
NASA used ODPA and 3,4’-oxybisbenzenamine (3, 4’-ODA) to synthesize another TPI called LaRC-IA. This TPI possesses good agglutinating property and thermal oxidation stability. Its properties are shown in Tables 6.12 and 6.13 [7–9].
Table 6.6 Properties of PL450c and SP-1 (PL450c Is One of the Product Models of AURUM, SP-1 Is One of the Product Models of VESPEL, Unfilled).
| Properties | Units | PL450c | SP-1 |
| Density | g/cm3 | 1.33 | 1.43 |
| Molding shrinkage | % | 0.83 | |
| Water absorption | % | 0.34(23 °C,24h) | 0.24(23 °C,24h) 0.72(50 °C,48h) |
| Moisture absorption | % | 0.24(24h) | |
| Tensile strength | MPa | 92 (23 °C) | 88 (23 °C) |
| 58(150 °C) | 42.3(260 °C) | ||
| Elongation at break | % | 90(23 °C) | 7.5(23 °C) |
| 90(150 °C) | 6.0(260 °C) | ||
| Bending strength | MPa | 137(23 °C) | 112.7(23 °C) |
| 88(150 °C) | 63.4(260 °C) | ||
| Flexural modulus | GPa | 2.94(23 °C) | 3.17(23 °C) |
| 2.55(150 °C) | 1.76(260 °C) | ||
| Compressive strength | MPa | 120(23 °C) | 25.3(Deformation rate 1%) |
| 76(150 °C) | 135.9(Deformation rate 10%) | ||
| Impact strength | J/m | 90(With gap) | |
| Tm | °C | 388 | 388 |
| Tg | °C | 250 | 250 |
| Melting index | g/(10min) | 4.5–7.5 (385 °C/2.3lbs) |
4.5–7.5 (385 °C/2.3lbs) |
| Coefficient of thermal expansion | 10-6K-1 | 5.5(MD) | 54(MD) |
| Heat deflection temperature | °C | 238 | 460 |
| Heat conductivity | W/(m.K) | 0.17 | |
| 0.24(23 °C) | 0.35(40 °C) | ||
| Specific heat capacity | Cal/(g. °C) | 0.24(100 °C) | |
| 0.34(300 °C) | |||
| Dielectric constant | 1kHz | 3.2 | 3.2 |
| 1MHz | 3.1 | 3.1 |
Table 6.7 Long-Term Thermal Stability of AURUM [13].
| Properties | Retention at 250 °C/% | |||
100h |
500h |
1000 |
2000 |
|
| Tensile strength | 110 |
95 |
90 |
90 |
| Elongation at break | 100 |
90 |
90 |
90 |
| Tensile modulus | 90 |
95 |
100 |
100 |
| Weight loss | <0.1 |
<0.1 |
0.3 |
0.8 |
| Properties | Retention at 270 °C/% | |||
100h |
500h |
1000 |
2000 |
|
| Tensile strength | 105 |
95 |
95 |
90 |
| Elongation at break | 100 |
90 |
90 |
85 |
| Tensile modulus | 85 |
95 |
100 |
100 |
| Weight loss | <0.1 | <0.1 | 0.3 |
0.9 |
| Properties | Retention at 290 °C/% | |||
100h |
500h |
1000 |
2000 |
|
| Tensile strength | 95 |
70 |
60 |
40 |
| Elongation at break | 90 |
65 |
45 |
35 |
| Tensile modulus | 100 |
100 |
100 |
100 |
| Weight loss | <0.1 |
0.1 |
0.5 |
1.3 |
Table 6.8 Friction Properties of AURUM [14].
| Normal load/N | Coefficients of friction | Wear rates | ||||||
0.3m/s |
0.6m/s |
0.9m/s |
1.2m/s |
0.3m/s |
0.6m/s |
0.9m/s |
1.2m/s |
|
50 |
0.48 |
0.38 |
0.33 |
0.30 |
2.4 |
12.4 |
15.3 |
144 |
100 |
0.40 |
0.42 |
0.46a |
25.3 |
20.2 |
201 |
||
150 |
0.38 |
0.40a |
— |
— |
27.8 |
165 |
— |
— |
200 |
0.40a |
0.43 |
— |
— |
18.3 |
666 |
— |
— |
aShort test (<15,000 m sliding distance) due to overload;
(–)not tested due to immediate overload.
Table 6.9 Friction Properties of VESPEL-SP-1 [13].
| Normal load/N | Coefficients of friction | Wear rates | ||||||
0.3m/s |
0.6m/s |
0.9m/s |
1.2m/s |
0.3m/s |
0.6m/s |
0.9m/s |
1.2m/s |
|
| 50 | 0.51 |
0.41 |
0.37 |
0.40 |
7.7 |
12.3 |
13.5 |
18.2 |
| 100 | 0.47 |
0.41a |
0.38a |
0.38a |
19.5 |
36.5 |
34.8 |
33.9 |
| 150 | 0.45 |
0.42a |
0.40a |
0.40a |
31.7 |
59.7 |
65.0 |
68.9 |
| 200 | 0.41 |
0.44a |
0.48a |
0.48a |
50.9 |
100 |
126 |
137 |
aShort test (<15,000 m sliding distance) due to overload.
Table 6.10 Effect of Amino Substituted Position in Ether Diamine Having Four Benzene Rings on Tg of Polyimide.
| Dianhydride | Diamines abbreviations |
η(dlg-1)a |
Tg(°C)b |
| PMDA | 4d-m |
0.48 |
208 |
| PMDA | 4d-p |
0.49 |
288 |
| PMDA | 4f-m |
0.54 |
218 |
| PMDA | 4f-p |
0.51 |
304 |
| PMDA | 4g-m |
0.49 |
218 |
| PMDA | 4g-p |
0.53 |
310 |
| PMDA | 4e-m |
0.51 |
230 |
| PMDA | 4e-p |
0.46 |
289 |
| PMDA | 4b-m |
0.49 |
250 |
| PMDA | 4b-p |
I.Sc | 310 |
| PMDA | 4c-m |
0.49 |
254 |
| PMDA | 4c-p |
0.47 |
320 |
aInherent viscosity (η) determined on 0.5% solutions in a solvent (p-chlorophenol/phenol = 9/1 wt/wt) at 35 °C
bD.s.c. at a heating rate of 16 °C/min
cInsoluble in a solvent for η measurement
Table 6.11 Properties of Ratem-YS-20 (Pure TPI, Without Additives, No Composites or Blends).
| Properties | Units | Conditions | Ratem-YS-20 |
| Density | g/cm3 | 1.4 |
|
| Tensile strength | MPa | 130 |
|
220 °C |
65 |
||
| Elongation at break | % | 7 | |
| Bending strength | MPa | 23 °C |
131 |
220 °C |
62 |
||
| Flexural modulus | GPa | 23 °C |
3.35 |
220 °C |
1.6 |
||
| Compression modulus | GPa | 1.5 |
|
| Impact strength | kJ/m2 | 250 |
|
| HDT | °C | 1.82MPa |
239 |
| TEC | °C | 20-230°C |
1–5 |
| Coefficient of friction | ≤0.3 |
||
| Dielectric constant | 1MHz | 3.1–3.5 |
|
| Dielectric loss | 1MHz | 0.0038 |
|
| Surface resistance | Ω | 1015–1016 |
|
| Volume resistivity | Ω.cm | 1016–1017 |
Table 6.12 The Properties of LaRC-IA.
| Usage of phthalic anhydride (%) | Calculating relative molecular weight | Experimental relative molecular weight | Hinh/(dL/g) | Apparent viscosity / (Pa.s) |
| 1 | 48158 |
14023 |
0.697 |
35 |
| 2 | 23958 |
14000 |
0.595 |
22 |
| 3 | 15892 |
12765 |
0.422 |
14 |
| 3.5 | 13586 |
10830 |
0.413 |
12.6 |
| 4 | 11858 |
12730 |
0.372 |
9.2 |
| 4.5 | 10513 |
11513 |
0.351 |
7.4 |
Table 6.13 The Properties of TPI Based on ODPA. the Melt Flowability Was Performed as a Qualitative Test on a Temperature-Regulated Hotplate at 400 °C.
| TPI | ηred of PAA (di.g-1) | Tg (°C) | Tm (°C) | Tm/Tg (K/K) | Td5 (°C) In N2 in air | Melt flowability | ||
| 1 | ODPA/PDA | 2.54 |
326 |
506 |
— |
580 |
571 |
Poor |
| 2 | ODPA/m-PDA | 1.32 |
262 |
423 |
— |
541 |
533 |
Poor |
| 3 | ODPA/4,4’-ODA | 1.77 |
250 |
390 |
1.27 |
550 |
538 |
Poor |
| 4 | ODPA/3,4’-ODA | 1.52 |
232 |
318 |
1.17 |
536 |
514 |
Good |
| 5 | ODPA/TPEQ |
2.41 |
236 |
415 |
1.35 |
560 |
549 |
Poor |
| 6 | ODPA/TPER |
1.05 |
211 |
338 |
1.26 |
547 |
518 |
Good |
| 7 | ODPA/BAPB | 2.81 |
244 |
377 |
1.26 |
565 |
555 |
Poor |
| 8 | ODPA/PTPEQ | 1.17 |
212 |
no |
— |
557 |
553 |
Good |
| 9 | ODPA/p-BAPS | 1.61 |
258 |
418 |
— |
501 |
515 |
Poor |
| 10 | ODPA/m-BAPS | 1.03 |
216 |
no |
— |
488 |
510 |
Good |
| 11 | ODPA/o-TOL | 1.25 |
345 |
no |
— |
522 |
498 |
Poor |
| 12 | ODPA/m-TOL | 2.50 |
298 |
no |
— |
510 |
507 |
Poor |
Shi et al., synthesized and characterized a serial TPI based on ODPA as we mentioned in Section 6.2.4. The properties of the TPI are as shown in Table 6.13.
Table 6.13 shows the properties of ODPA-based homopolyimides without any additives. Compared to BEPA polyimides, ODPA-based homopolyimides possess higher Tm, Tg, Td, and the viscosities. For ODPA/4,4’-ODA, ODPA/3,4’-ODA, ODPA/TPEQ, ODPA/TPER, and ODPA/BAPB, the PI powder prepared via reflux of the PAA solutions displayed melting peaks in the DSC scan, indicating that these PIs are semi-crystalline. However, one notices that the Tm‘s of some ODPA-derived PIs are too high for processing, for example Tm = 415 °C for ODPA/TPEQ and 390°C for ODPA/4,4’-ODA. In ODPA/PDA and ODPA/m-PDA, both without flexible ether linkages, no Tm‘s in the DSC heating process were exhibited up to 450 °C. Therefore, we estimated their Tm‘s from a Tm/Tg relation. As illustrated in Table 6.13, the ratios Tm/Tg range from 1.17 to 1.35 for semicrystalline ODPA-based PIs, clearly in agreement with an empirical equation for a series of PI systems: Tm≈4.3Tg (in kelvin) [15]. The calculated Tm‘s from this relation are 506 °C for ODPA/PDA, 423 °C for ODPA/m-PDA, and 418 °C for ODPA/p-BAPS, respectively. These Tm‘s are much higher than 400 °C, implying that these PIs are also removed from the TPI candidate list. However, according to the Tm/Tg relationship, the molecular design toward lowering Tm inevitably brings about an undesirable Tg decrease. This is the main problem in obtaining high-performance TPIs possessing both high Tg‘s and low Tm‘s for semicrystalline PIs. On the other hand, noncrystalline PIs are expected to have a much wider thermoprocessing window than semicrystalline PIs; ODPA/PTPEQ and ODPA/m-BAPS are classified in this category.
6.3.4 Thermoplastic Polyimides Based on BPDA
S Tarmai et al., reported the synthesis and characterization of high thermally stable semicrystalline polyimide based on 3,4’-oxydianiline and 3,3’,4,4’-biphenyltetracarboxylic dianhydride, end-capped with phthalic anhydride. The quenched film of this polyimide displayed a glass transition temperature and a melting temperature at 251 °C and 402 °C, respectively, by differential scanning colorimeter measurement. Isothermal differential scanning colorimeter studies ascertained this polyimide to be a polymer having first crystallization kinetics. Thermogravimetric analyses and isothermal melt viscosity studies evidenced excellent thermal stability (thermo-oxidative stability and melt viscosity stability) for this polyimide. The ηinh of the two TPI were 0.62 and 1.65, respectively. The weight loss temperature could be found in Table 6.14.
Table 6.14 Weight Loss Temperatures of the 3,4’-ODA Polyimide and the TPER Polyimide.
| TPI | Atmosphere | Weight loss temperature (°C) | |
| 1wt.% | 5wt.% | ||
| 3,4’-ODA polyimide | Nitrogen | 548 |
580 |
| air | 550 |
580 |
|
| TPER polyimide | Nitrogen | 558 |
577 |
| air | 515 |
572 |
|
6.3.5 Thermoplastic Copolyimides
E.I. du Pont de Nemours and Company published a patent which described the synthesis of thermoplastic copolyimides in 2001. The structures and properties could be found in Table 6.14.
6.4 Chemical Stability
In overall, TPIs are stable for dilute acid, but most of the TPIs are hydrolyzed materials, especially in the alkaline solution. And this makes TPI special to the other polymers. We could recycle the dianhydrides and diamines by alkaline hydrolysis reaction. For example, the recycling rate is up to 80–90 % through the hydrolysis for Membrane Kapton. Although, like the other aromatic polymers, TPIs are not stable for concentrated sulfuric, concentrated nitric acid, and halogens.
6.4.1 Hydrolytic Stability
Because of their excellent thermal stability, polyimides are considered as strong candidates for use as interlevel dielectrics in a variety of advanced packaging applications. It is important to determine whether or not degradative reactions that might occur in processing or during use could cause any significant deterioration in these dielectric properties. One such question is whether or not polyimides are susceptible to any hydrolytic reactions during long-term use.
The reported research of the TPI hydrolysis properties was most focused on the PMDA- and ODA-based TPI, such as Kapton series. Kapton is prepared via the polycondensation of pyromellitic dianhydride (PMDA) and oxydianiline (ODA) in, N.,N-dimethylacetamide (DMAc), forming the corresponding polyamic acid. The polymer solution is chemically imidized using the appropriate chemicals, followed by a heat treatment to remove solvent and conversion chemicals and to also complete the imidization. DeIasi et al., [15] reported the effect of an aqueous environment on the properties of Kapton polyimide film. Immersion of specimens in distilled water at 25 °C to 100 °C for time periods ranging from one hour to several hundred hours resulted in a decrease in the ultimate tensile strength of the polymer from 157.32 MPa to approximately 95.76 MPa, and a corresponding decrease in elongation to failure from 38% to approximately 5%. The kinetics of this decrease in mechanical properties is second order and yields activation energy of approximately 15.6 kcal/mole. The reaction is slightly dependent on pH in the range 2.0 to 12.0 but is highly dependent on the pH in the range 0.4 to 2.0. The decrease in mechanical properties at pH 2.0 to 6.0 appears to be due to hydrolysis of either uncyclized amic acid linkages or diamide functional groups present in the polyimide, whereas that at pH below 2.0 is probably the result of hydrolysis of both imide and amide bonds. Prolonged reflux of the polyimide in water resulted in the extraction of a water-soluble, amide-containing material.
Table 6.15 Structures and Properties of Thermoplastic Copolyimides Based on BPDA.
| Copolyimides | Proportion | Tm/°C |
Tc/°C |
Tm/°C |
Tg/°C |
| BPDA//3,4’-ODA/APB-134//PA | 95//70/30//10 | 345 |
296 |
346 |
— |
| 95//75/25/10 | 354 |
298 |
354 |
— |
|
| 95//80/20//10 | 362 |
295 |
362 |
— |
|
| BPDA//3,4’-ODA/APB-144//PA | 95//70/30//10 | 359 |
320 |
358 |
— |
| 95//75/25/10 | 360 |
318 |
354 |
— |
|
| 95//80/20//10 | 363 |
306 |
363 |
— |
|
| BPDA//3,4’-ODA/4,4’-ODA//PA | 95//80/20//10 | 382 |
302 |
382 |
— |
| BPDA//3,4’-ODA/4,4’-ODA/M-PDA//PA | 95//80/10/10//10 | 350 |
255 |
||
| BPDA//APB-134/APB-144//PA | 95//80/20//10 | 390 |
344 |
390 |
— |

Scheme 6.5 Hydrolysis reaction of TPI.
DeIasi [16] also researched the thermal regeneration of the tensile properties of hydrolytically degraded polyimide film. Prior to degradation, the film had an ultimate tensile strength of 182 MPa and an elongation to failure of 62.0%. The regenerated polyimide exhibits a much improved hydrolytic stability over the untreated material and specimens heat treated directly without prior aqueous degradation. Pawlowsk [17] found that a 6.7 M/1.9 M KOH/ K2CO3 solution had a 40–50 % faster etch rate at 5- and 30-sec exposures compared to a 6.7 M KOH solution. The faster etch rate, along with the visual observation of a thinner gel layer. and less flocculant etch by-product in solution, suggested that the K2CO3 additive enhances the dissolution of the hydrolyzed polyimide. As the K2CO3 concentration was increased, the etch rate increases when the KOH concentration was maintained at 2.7 M. The maximum etch rate observed at 1.9 M K2CO3 when the KOH concentration was held at 6.7 M probably results from solubility limits. Pryde [18]used IR to research the hydrolytic stability of TPI membranes. Hydrolyses were carried out at 90 °C / 95% relative humidity by placing the samples over a saturated solution of K2SO4 at 90 ± 2 °C. Table 6.16 shows the hydrolytic stability of TPI on different substrates.
Table 6.16 Hydrolytic Stability of TPI.
| TPI | Substrate | Cure temperature (°C) | Hydrolysis time (hours) | Hydrolysis (%) |
| BTDA-ODA | Si | 300 |
680 |
6.4 |
| BTDA-ODA | Si | 400 |
680 |
6.0 |
| BTDA-ODA | AgCl | 400 |
680 |
6.2 |
| PMDA-ODA | Si | 300 |
400 |
1 |
| PMDA-ODA | Si | 400 |
680 |
1 |
Stephans [19]studied the alkaline hydrolysis of a polyimide (PMDA-ODA) surface as a function of time, temperature, and hydroxide ion concentration. Quantification of the number of carboxylic acid groups formed on the modified polyimide surface was accomplished by analysis of data from contact angle titration experiments. Using a large excess of NaOH, pseudo-first-order kinetics were found, yielding kobs ≈ 0.1–0.9 min-1 for conversion of polyimide to poly(amic acid) depending on [OH-]. Conversion of the polyimide surface to one of poly(amic acid) was found to reach a limiting value with a formation constant, K, in the range 2–10 L/moL.
6.4.2 Oxidative Stability
The low Earth orbit, 200–700 km above the Earth surface, sees a harsh space environment with hazards such as atomic oxygen, ultraviolet radiation, ionizing radiation, high vacuum, micrometeoroids, and detritus as well as severe temperature cycling, which may cause considerable damages to spacecraft materials. Atomic oxygen (OA) is predominant among those hazards. OA is formed by solar ultraviolet radiation, which dissociates oxygen molecules into free atomic oxygen in the outer ionosphere at an altitude higher than 100 km. The concentration of OA is approximately from 106 to 109 atoms/cm3. When the aircraft travels at 8 km/s, the OA flux is 1012–1015 atoms/(cm2.s). The energy release of OA crash is about 5 eV, which is adequate to break the C-O and C-C bond; this is so called OA attack [22]. It causes micrometer roughness when the surface of the PI attacked by OA. Inorganic coats are usually used to improve the OA attack resistance, such as ITO, silicon resin. Li et al., [23] researched the nano-SiO2-doped PI; it was found that the OA attack resistance was greatly improved when the contents of SiO2 were 20%.
6.5 Compounding
Efforts have been devoted to compounding TPI with other molecules to provide them with enhanced solubility, optical transparency, photosensitivity, photorefractivity, and nonlinear optical properties, while maintaining their excellent thermal and intrinsic properties. Several compounding methods will be introduced below.
6.5.1 Chloromethylation
Chloromethylation of PEI chains [24] was carried out to introduce the ATRP initiators onto their backbones and the surface, including the pore surface, of the resulting microporous membranes. Amphiphilic poly-(poly(ethylene glycol) methyl ether methacrylate)-block-poly-(2,2,2-trifluoroethyl methacrylate), or P(PEGMA)-b-P(TFEMA), copolymer brushes were incorporated via consecutive surface-initiated ATRPs from the alkyl halide sites on the PEI microporous membrane. The chloromethylation of PEI is as follows.
In a 100 mL, three-necked, round-bottom flask equipped with a condenser (with a drying tube attached at the top) and an argon inlet, 5 g of PEI (equivalent to 8.4 mmol of the repeat units) was dissolved in 40 mL of dichloroethane under stirring at 60 °C. Paraformaldehyde (1.5 g), phosphorus trichloride (10 g), and zinc chloride (5 g) were added slowly to the reaction mixture under an argon atmosphere. The mixture was stirred at 60 °C for 48 h and then poured into 400 mL of methanol. The precipitate was filtered, redissolved, and reprecipitated into a water/methanol mixture (50/50, v/v) and then dried under reduced pressure at 80 °C for at least 24 h until a constant weight was obtained. The chloromethylated PEI, or PEICl, so-obtained was further purified by dissolving in N,N-dimethylformamide and then reprecipitating in methanol.

Scheme 6.6 Chloromethylation of PEI.
Chloromethylation of 6FDA-ODA PI [25–26]
A typical chloromethylation procedure was as follows. In a 100-mL, three-necked, round-bottom flask equipped with a condenser (with a drying tube at the top) and a nitrogen inlet, 6FDA-ODA PI (0.290 g, 0.5 mmol of the repeating unit) was dissolved in 15 mL of chloroform. The solution was heated to 60 °C, and 0.20 mL of chloromethyl methyl ether (1.5 mmol) and 0.18 mL of tin(IV) chloride (1.5 mmol) were slowly added to the reaction mixture under a nitrogen atmosphere. The mixture was stirred at different allocated time intervals and then poured into 300 mL of methanol. The precipitate was filtered, reprecipitated into a water/methanol mixture (50/50 v/v), and dried in a vacuum oven at 60 °C for 2 days. The maximum degree of chloromethylation was 1.81. To attempt further functionalization, Zhang et al., explored the esterification after chloromethylation of 6FDA-ODA PI with cinnamic acid [28].
The esterification was performed in the presence of K2CO3 and TBAB, which acted as a reaction catalyst and a phase-transfer catalyst, respectively. From the NMR spectra, it was confirmed that the reaction was almost quantitative. The esterification procedure is as follows: To a completely dissolved dimethylformamide (DMF) solution (5 mL) of chloromethylated 6FDA-ODA PI (0.25 g) and cinnamic acid (1.2 equiv to OCH2Cl), one equiv (to OCH2Cl) of K2CO3 and TBAB were added. The reaction mixture was stirred for 24 h at 40 °C under a nitrogen atmosphere. The solution was poured into water, and the precipitate was collected by filtration. Reprecipitation from THF into methanol was repeated three times, and the resulting polymer was dried in a vacuum oven for 48 h at 50 °C. In addition, after chloromethylation of TPI, quaternization reaction could be introduced onto the chloromethanol group [27].

Scheme 6.7 Chloromethylation of 6FDA-ODA PI.

Scheme 6.8 Esterification after chloromethylation of 6FDA-ODA PI.

Scheme 6.9 Sulfonation of PEI.
6.5.2 Sulfonation
Sulfonation of PEI by chlorosulfonic acid [27, 29].
PEI (20 g) was dissolved in 100 mL of 1,2-dichloroethane at 60 °C, and subsequently the PEI solution was kept at 30 °C. Chlorosulfonic acid mixed with 75 mL of 1,2-dichloroethane was added to the PEI solution within 1 h with vigorous stirring. After being reacted for a definite period, the reaction product, which precipitated in the reaction medium, was dissolved in DMAc at 50 °C, coagulated with excess isopropanol, filtered, washed with isopropanol, and dried at 40 °C in a vacuum oven. The sodium salt form of the product was obtained by soaking it in excess 0.1 mol/L NaOH aqueous solution for 2 days. Moreover, TPI also can be sulfonated by concentrated H2SO4 and SO3 under differential conditions [30, 31].The gas phase concentration of SO3 can be calculated according to the literature [32].
6.5.3 Phosphorylation
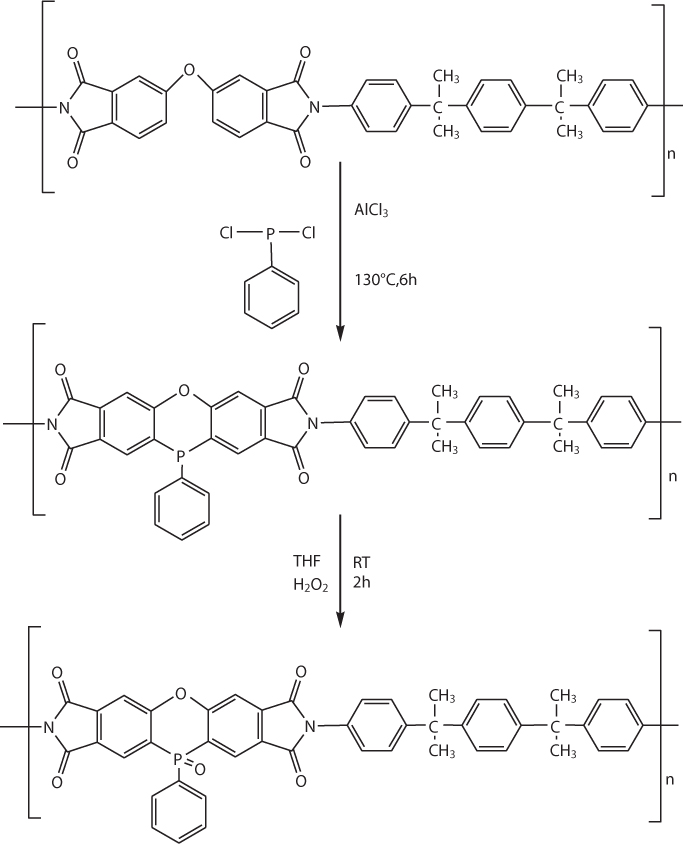
In a nitrogen atmosphere, 1 g of PIA, 10 g of dichlorophenylphosphine, and 1 g aluminum chloride were stirred at 130 °C for 6 h. After cooling, the reaction mixture was poured into 400 g of crushed ice. The solid was filtered and washed with icy 10% sodium hydroxide aqueous solution and water. The product (PIA-P) was then dried under vacuum. PIA-P was then dissolved in THF and treated with aqueous H2O2. The product (PIA-PO) was obtained by reprecipitation from methanol. The introduction of phosphorus-containing groups was found to slightly reduce the thermal stability of the polyimides and conspicuously increase their flame retardance.
6.5.4 Bromination
Brominated Matrimid was prepared as shown in Scheme 16.9. The bromination was conducted by the addition of bromine (6 mL, 18.72 g, 117.13 mmol) to a yellowish solution of PI (2.00 g, 3.62 mmol) dissolved in chloroform (13.3 mL, 15% w/v) in a 100-mL, round-bottom flask equipped with a condenser and a magnetic stirrer. The dark red solution was vigorously stirred for 6 h, during which time a dark gum was precipitated from the reaction mixture. Methanol (70 mL) was poured into the flask under vigorous stirring to precipitate the yellow, modified polymer. The precipitated polymer was filtered and stirred vigorously again in fresh methanol. This was repeated until no more bromine could be extracted, this being indicated by colorless methanol. The brominated PI was purified by redissolution in chloroform and precipitation from 95% ethanol. After vacuum drying, a yield of 90% (2.06 g) was obtained.
Scheme 6.10 Phosphorylation of TPI.

Scheme 6.11 Bromination of Matrimid TPI.

Scheme 6.12 Triarylsulfonium salt forming reaction.
The brominated polymer retained the good solubility and thermal properties that it possessed before modification. Compared with unmodified PI, brominated Matrimid had a lower decomposition temperature but an increased Tg, most likely attributable to decreased chain mobility in the presence of bulky bromine atoms. The brominated polymer exhibited approximately 1.6-fold increases in gas permeabilities for O2 and CO2, with some concurrent decreases in permselectivity of approximately 7–8% for the O2 /N2 and CO2 /CH4 gas pairs.
6.5.5 Arylation
High molecular weight polymers containing triarylsulfonium salt groups as part of the main chain could be prepared by arylation of sulfur-containing aromatic polyimides. The experimental procedure is as follows:
Sulfur-containing TPI (10.64 g repeating units) was dissolved in 50 mL hot chlorobenzene and then arylated using 0.03 g cupric benzoate and 1.278 g diphenyliodonium hexafluorophosphate at 130 °C for 3 h under an inert atmosphere of N2. The resulting polymer solution was poured into methanol and the precipitated polyimide–sulfonium salt, the product was isolated by filtration, washed with methanol, and dried in vacuum. Such polymers were photosensitive, undergoing photodegradation when irradiated in the presence of perylene as a photosensitizer.
6.6 Processing
After the polymerization and the fabrication of the TPI, some processing procedures are needed to make the raw TPI polymers into products. The processing procedures include injection molding, compression molding, extrusion molding, coating, and spinning.
6.6.1 Injection Molding
Injection molding is a manufacturing process for producing parts by injecting material into a mold. Injection molding can be performed with a host of materials mainly including metals (for which the process is called die casting), glasses, elastomers, confections, and most commonly thermoplastic and thermosetting polymers. Material for the part is fed into a heated barrel, mixed, and forced into a mold cavity, where it cools and hardens to the configuration of the cavity.
When thermoplastics are molded, typically pelletized raw material is fed through a hopper into a heated barrel with a reciprocating screw. Upon entrance to the barrel, the temperature increases and the Van der Waals forces that resist relative flow of individual chains are weakened as a result of increased space between molecules at higher thermal energy states. This process reduces its viscosity, which enables the polymer to flow with the driving force of the injection unit. The screw delivers the raw material forward, mixes and homogenizes the thermal and viscous distributions of the polymer, and reduces the required heating time by mechanically shearing the material and adding a significant amount of frictional heating to the polymer. The material feeds forward through a check valve and collects at the front of the screw into a volume known as a shot. A shot is the volume of material that is used to fill the mold cavity, compensate for shrinkage, and provide a cushion (approximately 10% of the total shot volume, which remains in the barrel and prevents the screw from bottoming out) to transfer pressure from the screw to the mold cavity. When enough material has gathered, the material is forced at high pressure and velocity into the part forming cavity. To prevent spikes in pressure, the process normally uses a transfer position corresponding to a 95–98% full cavity where the screw shifts from a constant velocity to a constant pressure control. Often injection times are well under 1 second. Once the screw reaches the transfer position, the packing pressure is applied, which completes mold filling and compensates for thermal shrinkage, which is quite high for thermoplastics relative to many other materials. The packing pressure is applied until the gate (cavity entrance) solidifies. Due to its small size, the gate is normally the first place to solidify through its entire thickness [37]. Once the gate solidifies, no more material can enter the cavity; accordingly, the screw reciprocates and acquires material for the next cycle while the material within the mold cools so that it can be ejected and be dimensionally stable. This cooling duration is dramatically reduced by the use of cooling lines circulating water or oil from an external temperature controller. Once the required temperature has been achieved, the mold opens and an array of pins, sleeves, strippers, etc. are driven forward to demold the article. Then, the mold closes and the process is repeated.
Briefly speaking, the injection molding includes the following steps:
- Drying the pellets;
- Loading into moulding machine;
- Heating and shearing material into a melt;
- Injecting the melt into the mold cavity;
- Part cooling, solidification;
- Ejecting the finished part from the mold.
6.6.2 Compression Molding
Compression molding is a method of molding in which the molding material, generally preheated, is first placed in an open, heated mold cavity. The mold is closed with a top force or plug member, pressure is applied to force the material into contact with all mold areas, and heat and pressure are maintained until the molding material has cured. The process employs thermosetting resins in a partially cured stage, either in the form of granules, putty-like masses, or preforms. Compression molding is a high-volume, high-pressure method suitable for molding complex, high-strength fiber glass reinforcements. Advanced composite thermoplastics can also be compression molded with unidirectional tapes, woven fabrics, randomly orientated fiber mat, or chopped strand. The advantage of compression molding is its ability to mold large, fairly intricate parts. Compression molding produces fewer knit lines and less fiber-length degradation than injection molding.
Table 6.17 Thermoplastic Moulding Conditions of ULTEM.
| Item | Conditions | Item | Conditions |
| Drying | 150 °C, more than 4h | Injection pressure | 70–126 MPa |
| Resin temperature | 340–425 | Holding pressure | 56–105 MPa |
| Pyramis | 325–410 | Back pressure | 0.4–3 MPa |
| Forepart | 320–405 | Compression ratio | 1.5–3.0 |
| Middle-part | 315–395 | Aspect ratio | 16/1–24/1 |
| After-part | 310–325 | Clamping force | 50–80 MPa |
| Mold temperature | 65–175 | Molding shrinkage | 0.5–0.7 % |

Figure 6.5 Injection molding machine.
The compression molding starts, with an allotted amount of plastic or gelatin placed over or inserted into a mold. Afterward the material is heated to a pliable state in and by the mold. Shortly thereafter the hydraulic press compresses the pliable plastic against the mold, resulting in a perfectly molded piece, retaining the shape of the inside surface of the mold. After the hydraulic press releases, an ejector pin in the bottom of the mold quickly ejects the finished piece out of the mold and then the process is finished. Depending on the type of plunger used in the press, there will or won’t be excess material on the mold [38].
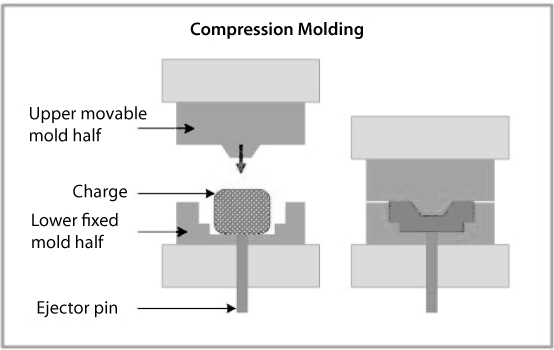
Figure 6.6 Compression molding procedure.
6.6.3 Extrusion Molding
Extrusion is a manufacturing process used to make pipes, hoses, drinking straws, curtain tracks, rods, and fibers. The granules melt into a liquid which is forced through a die, forming a long “tube-like” shape. The shape of the die determines the shape of the tube. The extrusion is then cooled and forms a solid shape. The tube may be printed upon and cut at equal intervals. The pieces may be rolled for storage or packed together. Shapes that can result from extrusion include T-sections, U-sections, square sections, I-sections, L-sections, and circular sections. One of the most famous products of extrusion molding is the optical fiber cable. Extrusion is similar to injection molding except that a long continuous shape is produced.
6.6.4 Coating
A coating is a covering that is applied to the surface of an object, usually referred to as the substrate. The purpose of applying the coating may be decorative, functional, or both. The coating itself may be an all-over coating, completely covering the substrate, or it may only cover parts of the substrate.
Coating processes may be classified as follows:
Chemical vapor deposition;
Physical vapor deposition;
Chemical and electrochemical techniques;
Spraying;
Roll-to-roll coating processes.
Among these processes, chemical vapor deposition is preferred for TPI. Masayuki Iijima et al., prepared polyimide thin films which were on the whole surface of a substrate using a high-temperature vapor deposition polymerization method (VDP-H). In this method, pyromellitic dianhydride (PMDA) and oxydiamine (ODA), which are monomers of polyimides, are introduced into a process chamber from separate evaporation sources through inlet tubes.
The apparatus for the VDP-H (ULVAC Japan model VEP-3040) is shown in Figure 6.7. The temperature of the process chamber 1 (450 mm × 650 mm) was kept at about 200 °C with a mantle heater by a temperature controller. A substrate set up on the substrate holder 12 was rotated at 1 rpm to ensure a uniform distribution of film thickness. The evaporation source ovens for PMDA and ODA joined to the side of the chamber were kept at about 200 °C and 181 °C, respectively. A reflection board was employed to extend the time that the monomers remained in the chamber. The pressure during deposition was kept at about 0.1 Pa. Under these conditions, the deposition rate of the polymer films was 54 nm min-1. The monomer inlet tubes six were designed to supply the monomers uniformly. Film thickness was controlled by changing the amounts of monomers in the source chamber. For example, 10 g of each monomer afforded films of 4 µm thickness. Deposition rate and film thickness were monitored by an optical monitor.

Figure 6.7 Apparatus for VDP-H: 1, process chamber; 2, vessels for monomer; 3, heaters for evaporation; 4, substrate holder; 5, film thickness monitor; 6, monomer inlet tube; 7, reflection board; 8, monomer trap.
As the process chamber was kept at about 200 °C, neither monomer could deposit on the substrate. Because the temperature of the substrate was higher than the evaporation temperature of the monomers, only polymerized films were deposited on the substrate. Infrared spectroscopy indicated that the VDP-H films were about 50% imidized. When the film thickness was less than 1 µm, uniform step coverage was observed, and above 2.5µm a patterned surface was reduced to a planar surface. Film thickness was controlled by changing the amounts of monomers in the evaporation sources. The dielectric constant (ε = 3.5), dissipation factor (tanδ=0.002), thermal characteristics (5% weight loss temperature = 551 °C, and tensile strength (170 MPa) of the VDP-H films cured for 60 min at 300 °C in vacuum were the same as those reported for Kapton® films. The modulus of elasticity (4.0 GPa) was larger than that of Kapton®, and it was found that the VDP-H films showed less elongation than Kapton® [39].
6.6.5 Spinning
Spinning is a manufacturing process for creating polymer fibers. It is a specialized form of extrusion that uses a spinneret to form multiple continuous filaments. There are many types of spinning: wet, dry, dry jet-wet, melt, gel, and electrospinning. First, the polymer being spun must be converted into a fluid state. If the polymer is a thermoplastic, then it can be simply melted; otherwise it is dissolved in a solvent or chemically treated to form soluble or thermoplastic derivatives. The molten polymer is then forced through the spinneret, and then it cools to a rubbery state, and then a solidified state. If a polymer solution is used, then the solvent is removed after being forced through the spinneret.
6.7 Applications
Because of their excellent performances, TPI continues to gain importance in a wide variety of applications like membranes, high-temperature adhesives, composites, and engineering plastics, etc.
6.7.1 Membranes
Dupont developed one kind of TPI membranes called IMIDEX which is based on AURUM. Its thickness is 25 µm–1mm, and wideness is 660 mm. The properties of IMIDEX were shown in Table 6.18 [41].
Table 6.18 Properties of IMIDEX Membranes.
| Properties | Value | Properties | Value | ||
| Mechanical properties | Flexural modulus/GPa | 3.779 |
Thermal properties | Continues use temperature/°C | 230 |
| Tear strength (original)/Kg | 22.9 |
Thermal expansion/(10-6K-1) | 55 | ||
| Tear strength (processing)/Kg | 1.3 |
Thermal conductivity/[W/(m.K)] | 0.18 | ||
| Elongation at break/% | 110 |
Tg/°C | 255 | ||
| Impact strength/(J/cm) | 55 |
Tm/°C | 388 | ||
| Tensile modulus/GPa | 3.06 |
Flammability (UL94) | VTM-0 (25 um) | ||
| Tensile modulus/MPa | 118 |
||||
| Electric properties | Dielectric constant/1 kHz | 2.5 |
Other properties | Density/(g/cm3) | 1.33 |
| Dielectric loss/1 kHz | 0.0014 |
Water absorption/% (24h, 50 °C, 75%RH) | 1.0 |
Affix Technology Co., Ltd. developed ESD Polyimide Tapes. This Tape is constructed to fill the requirements of a high-performance thermoplastic (Polyimide Backing Materials). It is manufactured from polyimide film backing with high-efficient silicone adhesive. The ESD additive in the tape acts to reduce the electrostatic discharge that occurs upon tape removal. The product’s low static properties can eliminate circuit board degradation due to electrostatic discharge.

Figure 6.8 Low Static Kapton® Tapes.
The typical product is Low Static Kapton® Tapes; these tapes are made of 1 millimeter Kapton® film backed by silicone adhesive. This tape can withstand temperatures of up to 500 °F/260 °C. Adhesive is 1.6 millimeter thick. Total thickness of this product is 2.6 ± 1 mil. Color is Amber and is packaged in a 36 yds roll. This tape is ideal for environment where static discharge is a concern [42].
6.7.2 Adhesives
As the adhesives, TPI could strongly cohere with several substrates, such as metals, nonmetals, and polymers. TPI-based adhesives were widely used in aerospace and electronics industries. Among the different kinds of TPI, BTDA-based TPI is the best adhesive material and mostly used these years. Strong contributions to this area from the workers of NASA Largely Research Center have led to a variety of TPI adhesives. One typical adhesive is the LaRC-TPI (Largely Research Center Thermoplastic Polyimides), which is prepared with BTDA and 3,3’-DABP.
Another typical BTDA-based TPI adhesive is the LaRC-CPI (Largely Research Center Crystalline Polyimides). As a crystalline thermoplastic, LaRC-CPI could be used as a high-temperature adhesive.
Titanium alloys are widely used in aerospace; they have very high tensile strength and toughness. They are light in weight and have extraordinary corrosion resistance and the ability to withstand extreme temperatures. Hergenrother et al., [43] used LaRC-CPI for the adhesion of the titanium alloys. They have researched the influences of relative molecular weight and different adhesive conditions including pressure on the adhesive performance. The results suggest that the adhesive performance is no good when the molecular weight is too higher or lower. Commonly, low temperature and high pressure will lead to good adhesive performance.

Figure 6.9 Structure of LaRC-TPI.

Figure 6.10 Structure of LaRC-CPI.
Hergenrother also studied the adhesion of titanium with LaRC-CPI2. LaRC-CPI2 is synthesized from with 4,4’-oxy-diphthalic anhydride (ODPA). Different from LaRC-CPI, in the range of the researched relative molecular weight, the original adhesive performance of LaRC-CPI2 was not changed a lot. After the aging test at 204 °C, the lower molecular weight LaRC-CPI2 possesses better adhesive performance. In contrast, after the aging test at 300 °C, the higher molecular weight LaRC-CPI2 would possess better adhesive performance. Higher adhesive pressure (1.38 Mpa) would lower the performance of the LaRC-CPI2 [44]. Other LaRC adhesive series like LaRC-8515 [45], LaRC-MPEI [46] (modified phenylethynyl terminated polyimide), and their applications could be seen according to the literature.
6.7.3 Composites
Whether it is bismaleimide (BMI) resin or polymerization of monomer reactants (PMRs) resin, the fracture toughness cannot meet the needs of some applications, although the fracture toughness is increased by using of linear polymers, but usually, Tg, the important property would be decreased in the same time. This is unacceptable in the application of advanced composite materials. In order to obtain high fracture toughness composite materials, researchers have spent great efforts to explore the high-performance thermoplastic resin for advanced composites in the 1980s and 1990s. The most typical one is based on the poly-etherketone resin composites; however, the Tg of this kind of polymer is hardly to excess above 200 °C. So, even the required temperature is 177 °C, this kind of resin-based composite materials could not be used in HSCT project (High Speed Civil Transport, NASA). During this period, NASA led the development of advanced composite materials based on the thermoplastic polyimide resin. Some of the main products and its applications are introduced below.
6.7.3.1 Skybond
Skybond is developed for composite resin of aircrafts polyimide varnish. The Skybond® product line encompasses a number of products usable as matrix resins for manufacture of high thermal stability structural composites. Composites based on the Skybond® family of resins have continuous use temperatures in excess of 600° F with short-term capability greater than 1000° F. Prepreg has been successfully manufactured from all common high-performance substrates, such as E-glass, high modulus carbon, and quartz. Two new classes of matrix resins have been developed in the Skybond® family. The first can be processed by RTM molding techniques to bring the outstanding thermal resistance of polyimide resins to RTM molded composites. The second new class of Skybond® matrix resins is the two-stage cure resins that when used in prepreg applications allow the manufacture of very low void level composites by autoclave processing (<0.5% voids) combined with excellent thermal stability of polyimide resins. More specific properties and applications could be found in Skybond® 700 Technical Bullitin [47].
6.7.4 Engineering Plastics
High-performance engineering plastics are a kind of plastic materials that have better mechanical and/or thermal properties than the more widely used commodity plastics (such as polystyrene, PVC, polypropylene, and polyethylene). Because of their high thermal stability and easy machinability, TPIs were also wildly used in high-performance engineering plastics, such as VESPEL, ULTEM, AURUM, and Ratem.
6.7.4.1 VESPEL Plastics
VESPEL is a range of durable high-performance polyimide-based plastics which are manufactured by DuPont. It is synthesized from PMDA and ODA. The basic structure is shown in Figure 6.11.

Figure 6.11 Basic structure of VESPEL.
This high-performance polymer is mostly used in aerospace, semiconductor, and transportation technology. It combines heat resistance, lubricity, dimensional stability, chemical resistance, and creep resistance, to be used in hostile and extreme environmental conditions.
Unlike most plastics, it does not produce significant outgassing even at high temperatures, which makes it useful for lightweight heat shields and crucible support. It also performs well in vacuum applications, down to extremely low cryogenic temperatures. However, VESPEL tends to absorb a small amount of water, resulting in longer pump time while placed in a vacuum. For different applications, special formulations are blended/compounded. The product number and its applications are shown in Table 6.19.
Table 6.19 Applications of VESPEL.
| Product no. | Instructions | Application area |
| SP-1 | Unfilled base polyimide resin | Aerospace, electronics, sealing |
| SP-21 | 15% graphite filled | Bearings, thrust pad |
| SP-22 | 40% graphite filled | Bearings, wear strip |
| SP-211 | 15% graphite and 10% PTFE filled | Bearings, wear strip, gasket |
| SP-3 | 15% MoS2 filled | Vacuum and dry circumstances sealing, bearings |
6.7.4.2 ULTEM Plastics
ULTEM is a family of PEI products manufactured by SABIC as a result of acquiring the General Electric Plastics Division in 2007, developed by Joseph G. Wirth in the early 1980s. ULTEM resins are used in medical and chemical instrumentation due to their heat resistance, solvent resistance, and flame resistance. ULTEM 1000 (standard, unfilled polyetherimide) has a high dielectric strength, inherent flame resistance, and extremely low smoke generation. ULTEM has high mechanical properties and performs in continuous use to 340 °F (170 °C). Ultem 1000 has typical thermal conductivity of 0.22 W/m·K (but some sources give 0.122 W/m·K).
6.7.4.3 AURUM Plastics
AURUM Plastics is a thermoplastic polyimide resin that is melt-processable lending itself to fabrication methods such as injection molding and extrusion. Its synthesis and structures could be found in 6.2.2.
AURUM Plastics offering continuous use temperatures to 240 °C (465 °F), AURUM parts exhibit low outgassing, superior radiation resistance, excellent wear performance, and resistance to chemicals. Components made from AURUM resin are excellent replacements for metals, ceramics, and other plastics. Typical applications of AURUM Plastics include thrust washers and seal rings for automotive and off-road vehicle transmissions, thermal insulators and stripper fingers for high-speed copiers, jet engine components, check valve balls, spline couplings, heat-resistant gears, vanes, wear strips, valve seats, carriers for aluminum hard disks and silicon wafers, journal bearings, and bearing retainers. AURUM parts can be recycled because of its thermoplastic nature.
6.7.4.4 Ratem Plastics
Ratem is the polyimide engineering plastics developed by Shanghai Research Institute of Synthetic Resins. Its typical structure could be found in 6.2.4. Ratem includes YS-10, YS-20, YS-260, YS-30, etc., which are in the rod, plate, and pipe shapes.
All of the products of this series can be used for a long period of time at 180–280 °C. Some of them can even be used at the temperature up to 490 °C for a short period of time. They have good properties, such as good mechanical property, good thermal stability and antioxidation property, low thermal conductivity and friction coefficient, high wearing resistance, excellent chemical resistance, and radiation resistance. These excellent properties make them now widely applied in the industries of mechanics, instruments, electric appliance, office appliance, automobile, aerospace, and nuclear power.
6.8 Blends of Thermoplastic Polyimide (TPI)
There are two ways to fabricate the TPI blends: the one is polyimide blends with polyimide; the other is polyamic acid blending method (TPI must be soluble). As the second way, due to the amino acid exchange reaction, the formation of copolymers is inevitable, and there are many impact factors on the properties of the TPI blends, such as blending conditions, time, temperature, etc. In case to avoid the formation of copolymers, polyimide usually blends with polyamic acid, or blending polyester amides together which will not cause amino acid exchange reaction.
The Tg of the polymer blends could be calculated by the Fox equation (6.1), Gorden-Tayler equation (6.2), and Couchman equation (6.3).
(6.1)

Tg, glass transition temperature of blends; Tg1 and Tg2 are the glass transition temperatures of the components 1 and components 2, respectively; ω1 and ω2 are the mass proportions of the components 1 and components 2, respectively.
(6.2)
κ=Δβ2/Δβ1, Δβ = coefficient change of volume thermal expansion at Tg.
(6.3)

ΔCp1 and ΔCp2 is the thermal capacity change of components 1 and components 2, respectively.
Miscibility is crucial to the performance of the resulted blends, e.g., molecular composites, where rigid and flexible components are miscible, which enables to enhance simultaneously conflicting properties such as modulus and toughness. The miscibility can also be determined by these three equations. Several TPI blends will be simply introduced as follows.
6.8.1 TPI Blends with TPI
Ma et al., [52], reported the blends of New-TPI(AURUM) and ULTEM 1000 (PEI). New-TPI with a weight-average molecular weight (Mw) of ca. 30000 and PEI (ULTEM 1000, Mw, ca. 30000) was used as a raw material for the blends. Seven melt-quenched New-TPI/PEI blends (New-TPI/PEI = 100/0; 80/20. 60/40, 50/50, 40/60, 20/80, 0/100) were supplied by Mitsui-Toatsu Chemicals, Inc. The blend samples were prepared by using a single screw extruder. The phase behavior and semicrystalline morphology of the blends were studied by using DSC and SAXS. As their results: melt-quenched amorphous New-TPI/PEI blends are miscible over the whole composition range. Phase separation occurs when melt-quenched New-TPI/PEI blends are heat treated at 260 °C; crystallization does not occur in this case. Semicrystalline structures with phase-separated amorphous regions develop during heat treatment of the New-TPI/PEI blends at temperatures of 290–340 °C. Semicrystalline structures with miscible amorphous regions develop when the melt-quenched New-TPI/PEI blends are heat treated at temperatures above 350 °C. Similar structures are also found in the melt-crystallized New-TPI/PEI blends. PEI segments are incorporated in the amorphous layer between the New-TPI crystals, regardless of phase separation (Table 6.20). The structures of two TPIs can be found in scheme 6.1 and scheme 6.2.
Table 6.20 Tg of New-TPI/PEI Blends.
| Ultem 1000 (%) | Tg /°C(melt-quenched) | Tg / °C(260°C treated) | Tg / °C(360 °C treated) |
| 0 | 250 |
250 |
248 |
| 20 | 240 |
244 |
240 |
| 40 | 232 |
218,244 |
230 |
| 50 | 229 |
220,243 |
223 |
| 60 | 226 |
220,241 |
222 |
| 80 | 220 |
220 |
220 |
| 100 | 217 |
217 |
216 |
Goodwin [53] also studied the properties of New-TPI/PEI blends. It was found that the New-TPI/PEI blends were single-phase blends by mixing and quenching the two polyimides according to the variation in amorphous Tg, as monitored by DSC and DMA. Annealing of the blends above the Tg did not induce shifts in the Tg of any composition, nor did it produce any changes in the magnitude and location of the endothermic overshoot arising from physical ageing. Thus, we concluded that the blends did not phase separate.
6.8.2 Polyamic Acid Blending
Mazinani et al., [54] reported the intermolecular interactions and miscibility behavior of two polyimide blend systems, Extem/Matrimid and Extem/U-Varnish. DSC results for the Extem/U-Varnish system showed the existence of a single glass transition temperature (Tg) in each composition, suggesting the miscibility of the blends, whereas DSC analysis of Extem/Matrimid system indicated immiscibility but compatibility between two polymers. Interactions between studied polymer systems and four aprotic solvents including N-methyl-2-pyrrolidone (NMP), dimethylacetamide (DMAc), dimethylformamide (DMF), and dimethyl sulfoxide (DMSO) were assessed on the basis of the difference between their solubility parameters. Among the selected solvents, DMAc showed the highest affinity with both blend systems. XRD patterns and rheological behavior of Extem/U-Varnish system revealed that the crystalline nature and viscosity of the blend polymers decreases as the ratio of Extem/U-Varnish increases. In conclusion, Extem and U-Varnish were found to constitute a miscible pair at a molecular level over the entire composition range whereas Extem and Matrimid could not form a miscible blend.
In order to study the specific interactions between Extem and U-Varnish polymers, the Tgs of the polymer blends were estimated by theoretical equations and compared with experimental data. The empirical Tg values formed a concave curve as a function of composition and exhibited a positive deviation from the linearity, indicating the presence of specific interactions between Extem and U-Varnish polymer chain.
Tong et al., [55] reported the preparation of rodlike/flexible polyimide blends is feasible by utilizing poly(amic acid) amine salt precursors, which are free from the intermolecular transamidation reaction. Compared to poly(amic ester)s, the preparation of poly(amic acid) amine salts is much more straightforward and easier. In addition, poly(amic acid) amine salts as PI precursors are used more and more in practice. Typical preparation method of poly(amic acid) amine salt precursors is as follows: a mixture of a dianhydride and a respective diamine in NMP was stirred for 6 h at room temperature under nitrogen, and an appropriate amount of TEA was added to the above poly(amic acid) solution to neutralize all the carboxylic acid groups.

Figure 6.12 Basic structures of Matrimid, U-Varnish, and Extem.
Table 6.21 Tg Values of Extem/U-Varnish Blend.
| Extem (%) | Experimental(/°C) | Fox equation(/°C) | Gordon Taylor equation(/°C) |
| 100 | 262.58 |
262.58 |
262.58 |
| 80 | 268.34 |
265.92 |
268.17 |
| 50 | 273.91 |
271.09 |
274.03 |
| 20 | 278.07 |
276.47 |
278.10 |
| 0 | 280.18 |
280.18 |
280.18 |
Table 6.22 Tg Values of Extem/Matrimid Blend.
| Extem (%) | Experimental(/°C) | Fox equation(/°C) | Gordon Taylor equation(/°C) |
| 100 | 262.58 |
262.58 |
262.58 |
| 80 | 261.72 |
272.87 |
276.66 |
| 50 | 265.14 |
289.91 |
267.08 |
| 20 | 265.42 |
309.23 |
263.07 |
| 0 | 323.61 |
323.61 |
323.61 |
6.9 Composites of Thermoplastic Polyimide (TPI)
Whether it is bismaleimide (BMI) resin or polymerization of monomer reactants (PMR) resin, the fracture toughness cannot meet the needs of some applications, although the fracture toughness is increased by using of linear polymers, but usually, Tg, the important property would be decreased in the same time. This is unacceptable in the application of advanced composite materials. In order to obtain high fracture toughness composite materials, researchers have spent great efforts to explore the high-performance thermoplastic resin for advanced composites in the 1980s and 1990s. The most typical one is based on the poly-etherketone resin composites; however, The Tg of this kind of polymer is hardly to excess above 200 °C. So, even the required temperature is 177 °C; this kind of resin-based composite materials could not be used in HSCT project (High Speed Civil Transport, NASA). During this period, NASA led the development of advanced composite materials based on the thermoplastic polyimide resin. Some of the main products and their applications are introduced below.
6.9.1 LaRC Composites
LaRC thermoplastic polyamides products series are researched and developed by Langley Research Center of NASA. LaRC is the oldest of NASA’s field centers, established in 1917, located in Hampton, Virginia, United States. A series of TPI composites named after Langley Research Center of NASA will be introduced as follows.
LaRC-TPI/AS4 composites: LaRC-TPI is prepared with BTDA and 3,3’-DABP, its composites properties with AS4 glass cloth are shown in Table 6.23
Table 6.23 Properties of LaRC-TPI/AS4 Composites.
| Tg/ °C | 250 | |
| Bending strength/Mpa | 23 °C |
1972 |
177 °C |
1372 |
|
| Bending modulus/Gpa | 23 °C |
97 |
177 °C |
92 |
|
| Short-beam shear strength/Mpa | 23 °C |
95 |
150 °C |
73 |
LaRC-8515/IM7: LARC-8515 (Langley Research Center-8515) is an aromatic polyimide based on 3,3’,4,4’-biphenyltetracarboxylic dianhydride (BPDA) and an 85:15 molar ratio of 3,4’-oxydianiline (3,4’-ODA) and 1,3-bis(3-amnophenoxy)benzene (APB) [56]. Its composite properties with IM7 carbon fiber are shown in Table 6.24.
Table 6.24 Properties of LaRC-TPI/IM7 Composites.
| Properties | Test temperature/°C | PA:4% | PA:5% |
| Short-beam shear strength/MPa | RT |
104.5 |
130.7 |
93 |
93.6 |
108.8 |
|
150 |
82.1 |
75.3 |
|
177 |
74.4 |
79.2 |
|
| 0 ° flexural strength/MPa | RT |
1789 |
1972 |
93 |
1639 |
1763 |
|
150 |
1451 |
1574 |
|
177 |
1434 |
1505 |
|
| 0 °C flexural modulus/GPa | RT |
138 |
156 |
93 |
136 |
149 |
|
150 |
141 |
128 |
|
177 |
151 |
153 |
|
| 0 ° compression strength/MPa | RT |
1042 |
1430 |
177 |
1021 |
1141 |
|
| 0 ° open hole compression (OHC) strength/MPa | RT |
401 |
415 |
177 |
316 |
272 |
LARC-IAX-3 [57] (Langley Research Center™-improved adhesive experimental resin-3) aromatic polyimide, based on oxydiphthalic anhydride, 3,’4-oxydianiline (3,’4-ODA), and 1,4-phenylenediamine (p-PDA), was evaluated as a matrix for high-performance composites. T H HouT and L St Clair characterized the composite mechanical properties such as short-beam shear strength, longitudinal and transverse flexural strength and flexural modulus, longitudinal tensile strength and notched and unnotched compression strengths measured at room temperature (RT) and elevated temperatures. Similar properties were obtained independent of the carrier solvent used during matrix resin synthesis. These mechanical properties were superior to those previously measured for IM7/LARC-IA and IM7/LARC-IAX composites. The enhanced mechanical properties were attributed to the substitution of 25% 3,’4-ODA by p-PDA in the LARC-IA imide backbones. The properties of the LARC-IAX composites are shown in Table 6.25.

Figure 6.13 Chemical structure of LaRC-IAX-3.
Table 6.25 Properties of LaRC-IA/IM7 Composites Series.
| Properties | Test temperature / °C | IAX-3 5%,GBL | IAX-3 4%NMP | IAX-3 5%NMP | IA 4%NMP | IAX 5%NMP |
| Short-beam shear strength/MPa | RT |
119 |
130 |
132 |
133 |
109 |
93 |
88 |
98 |
98 |
119 |
87 |
|
150 |
59 |
72 |
70 |
110 |
67 |
|
177 |
41 |
55 |
55 |
66 |
54 |
|
| 0° flexural strength/MPa | RT |
1899 |
1938 |
1902 |
1555 |
1465 |
93 |
1679 |
1372 |
1598 |
1131 |
1192 |
|
150 |
1344 |
1429 |
1300 |
1112 |
906 |
|
177 |
1157 |
1190 |
1112 |
1048 |
720 |
|
| 0° flexural modulus/GPa | RT |
141 |
139 |
141 |
98 |
128 |
93 |
143 |
126 |
140 |
96 |
109 |
|
150 |
146 |
140 |
130 |
103 |
112 |
|
177 |
138 |
132 |
130 |
105 |
104 |
|
| 90° flexural strength/MPa | RT |
143 |
172 |
150 |
209 |
|
93 |
77 |
121 |
103 |
198 |
||
150 |
58 |
108 |
68 |
165 |
||
177 |
41 |
100 |
45 |
152 |
||
| 90° flexural modulus/GPa | RT |
4.8 |
5.1 |
4.9 |
4.1 |
|
93 |
4.1 |
4.5 |
4.4 |
4.0 |
||
150 |
4.2 |
4.2 |
4.0 |
3.7 |
||
177 |
3.2 |
3.7 |
3.7 |
3.7 |
||
| 0° tensile strength/MPa | RT |
2690 |
2902 |
2422 |
2416 |
|
177 |
2588 |
2639 |
2321 |
2346 |
||
| 0 ° compression strengths/MPa | RT |
1378 |
1400 |
1282 |
||
177 |
1227 |
|||||
| 0 ° open hole compression (OHC) strength/MPa | RT |
412 |
402 |
399 |
||
177 |
246 |
243 |
256 |
In continuation of an effort to develop TPI for use in aerospace applications, P M Hergenrothert and S J Havens [44] developed a new polyimide. LARC-CPI 2, This semicrystalline polyimide was prepared by the reaction of 4.4’-oxydiphthalic anhydride with 1,4-bis(4-aminophenoxy-4’-benzoyl)benzene. In high-molecular-weight form, this material has a Tg of 223 °C and a crystalline melt temperature of approximately 350 °C. Controlled-molecular-weight end-capped versions of LARC-CPI two were used to fabricate adhesive panels and composites that exhibited good mechanical properties at temperatures as high as 200 °C. The properties of the LARC-CPI two composites are shown in Table 6.26.
Table 6.26 Properties of LaRC-CPI 2/AS4 Composites.
| Test temperature/ °C | Postcu ring treatment | Flexural strength/MPa | Flexural modulus | Short-beam shear strength /MPa |
| 25 | No | 1786 |
117 |
83.7 |
| 25 | 300/ °C, 16h | 1717 |
119 |
51.7 |
| 177 | No | 1255 |
115 |
53.1 |
| 204 | 300/ °C, 16h | 1076 |
117 |
46.2 |
| 204 | No | 1089 |
92 |
41.4 |
LARC-SCI [58] (Langley Research Center—Semi-Crystalline polyImide) is an aromatic polyimide based on 3,4’-oxydianiline and 3,3’,4,4’-biphenyl tetracarboxylic dianhydride. This polyimide was synthesized and evaluated for use as a neat resin and a matrix resin for advanced composites. Three 30% w/w solids polyamic acid/N-methypyrrolidone solutions were prepared using 2, 3, and 4% stoichiometric imbalances end-capped with phthalic anhydride to provide polyimides with theoretical number average molecular weights of approximately 22,800, 15,100, and 11,400 g mol–1, respectively. The composites properties of IM7 carbon fiber with these three resins are shown in Table 6.27.
Table 6.27 Properties of LaRC-SCI/IM7 Composites Series.
| Properties | Test temperature / °C | PA, 2% | PA,3% | PA, 4% |
| Short-beam shear strength/MPa | RT |
98.0 |
90.1 |
106.5 |
93 |
81.5 |
81.1 |
94.4 |
|
150 |
68.8 |
73.4 |
82 |
|
177 |
67.0 |
61.5 |
70 |
|
| 0° flexural strength/MPa | RT |
1930 |
2076 |
|
93 |
1642 |
1926 |
||
150 |
1522 |
1436 |
||
177 |
1338 |
1360 |
||
| 0° flexural modulus/GPa | RT |
145.8 |
150.0 |
|
93 |
147.2 |
152.1 |
||
150 |
144.4 |
150.7 |
||
177 |
138.7 |
133.1 |
||
| 90° flexural strength / MPa | RT |
166.2 |
||
93 |
119.7 |
|||
150 |
126.1 |
|||
177 |
129.6 |
|||
| 90° flexural modulus/GPa | RT |
7.75 |
||
93 |
4.93 |
|||
150 |
4.58 |
|||
177 |
4.44 |
|||
| 0° tensile strength/MPa | RT |
2690 |
||
177 |
2493 |
|||
| 0 ° compression strengths / MPa | RT |
1152 |
||
177 |
1151 |
|||
0 ° open hole compression (OHC) strength/MPa |
RT |
388 |
LARC-SI (NASA Langley Research Center-Soluble Imide) is an aromatic thermoplastic polyimide. LARC-SI is synthesized from equimolar amounts of oxydiphthalic anhydride (ODPA), 3, 3’, 4, 4’-biphenyltetracarboxylic dianhydride (BPDA) and the equivalent amount of 3, 4’-oxydianiline (3, 4’-ODA). Phthalic anhydride (PA) was used as an end-capper to control molecular weight. The composites properties of IM7/LaRC SI are shown in Table 6.28.
Table 6.28 Properties of LaRC-SI/IM7 Composites Series.
| Properties | Test temperature / °C |
PA, 3% |
PA,3% normlization |
| Short-beam shear strength/MPa | RT |
103 |
95 |
93 |
79 |
74 |
|
150 |
60 |
56 |
|
177 |
48 |
44 |
|
| 0° flexural strength/MPa | RT |
1698 |
1597 |
93 |
1568 |
1475 |
|
150 |
1381 |
1299 |
|
177 |
1285 |
1209 |
|
| 0° flexural modulus/GPa | RT |
142 |
134 |
93 |
142 |
135 |
|
150 |
144 |
136 |
|
177 |
143 |
134 |
|
| 90° flexural strength/MPa | RT |
121 |
144 |
93 |
97 |
115 |
|
150 |
116 |
138 |
|
177 |
102 |
12 |
|
| 90° flexural modulus/GPa | RT |
4.2 |
4.8 |
93 |
3.6 |
4.3 |
|
150 |
3.4 |
4.0 |
|
177 |
3.0 |
3.7 |
|
| 0° tensile strength/MPa | RT |
2712 |
2504 |
177 |
2605 |
2404 |
|
| 0 ° compression strengths/MPa | RT |
1340 |
|
177 |
848 |
||
| 0 ° open hole compression (OHC) strength/MPa | RT |
309 |
296 |
208 |
200 |
||
compression after impact (CAI) strength/MPa |
RT |
316 |
348 |
LaRC-ITPI [59] is an isomeric variation of the better known LaRC-TPI which is based on 4,4’-isophthaloydiphthalic anhydride and 1,3-phenylenediamine. It was evaluated as a matrix for high-performance composites. Its structure is shown in Figure 6.14. The composite properties of LaRC-ITPI/IM7 are shown in Table 6.29. As seen in the table, 80% of the room temperature OHC strength was retained at 177/ °C, indicating that the LaRC-ITPI is an excellent high-temperature matrix material for selected future aerospace applications where solvent resistance is not a key requirement.
Table 6.29 Properties of LaRC-ITPI/IM7 Composites.
| Properties | Test temperature / °C |
LaRC-ITPI/IM7 |
| Short-beam shear strength/MPa | RT |
88.4 |
177 |
67.8 |
|
| 90° flexural strength/MPa | RT |
108.9 |
93 |
83.4 |
|
150 |
73.1 |
|
177 |
75.8 |
|
| 90° flexural modulus/GPa | RT |
3.38 |
93 |
2.83 |
|
150 |
2.48 |
|
177 |
2.83 |
|
0° tensile strength/MPa |
RT |
2371 |
0° tensile modulus/GPa |
RT |
137.8 |
0° compression strengths/MPa |
RT |
1088 |
0° compression modulus/GPa |
RT |
124.0 |
| 0° open hole compression (OHC) strength/MPa | RT |
337.7 |
177 |
261.9 |
|
Compression after impact (CAI) strength/MPa |
RT |
292.9 |
Compression after impact (CAI) modulus/MPa |
RT |
49.5 |
| Interlaminar shear strength/MPa | RT |
146.8 |
177 |
111.6 |
|
| Interlaminar shear modulus/GPa | RT |
17.09 |
177 |
12.06 |

Figure 6.14 Structure of LaRC-ITPI.
6.9.2 Skybond
Skybond [60] is developed for composite resin of aircrafts polyimide varnish. The Skybond® product line encompasses a number of products usable as matrix resins for manufacture of high thermal stability structural composites. Composites based on the Skybond® family of resins have continuous use temperatures in excess of 600° F with short-term capability greater than 1000° F. Prepreg has been successfully manufactured from all common high-performance substrates, such as E-glass, high modulus carbon, and quartz. Two new classes of matrix resins have been developed in the Skybond® family. The first can be processed by RTM molding techniques to bring the outstanding thermal resistance of polyimide resins to RTM molded composites. The second new class of Skybond® matrix resins is the two-stage cure resins that when used in prepreg applications allow the manufacture of very low void level composites by autoclave processing (<0.5% voids) combined with excellent thermal stability of polyimide resins. The properties of Skybond/glass cloth composites are shown in Table 6.30. The results shown in Tables 6-30 are representative of laboratory data on 1/8 inch thick laminates. Laminates were made using 181 glass cloth with A-1100 soft finish. The thermal aging was in air. The testing temperatures were the same as the exposure condition temperatures in most cases. Testing temperatures are noted in parentheses.
Table 6.30 The Properties of Skybond/glass Cloth Composites.
| Flexural strength/PSI(24 °C) | 7.5–8.5104 |
| Flexural modulus/PSI(24 °C) | 3.12 × 106 |
| Tensile strength/PSI(24 °C) | 5.7 × 104 |
| Dielectric strength/(kV/mm) | 5.6–7.2 |
| Dielectric constant (1MHz) | 4.1 |
| Dielectric loss (1MHz) | 0.0045 |
| Volume resistivity (Ω.cm) | 2.47 × 1015 |
| Weight loss/% (371°C 100h) | 3 |
| Water absorption/% (24h immersion) | 0.7 |
| Elongation/% | 1.9 |
6.9.3 PAI Polyamide–Imide Composites
PAI: Polyamide-imide [61] is a kind of new engineering plastics containing amide group. Polyamide-imides display a combination of properties from both polyamides and polyimides, such as high strength, melt processibility, exceptional high heat capability, and broad chemical resistance. Polyamide-imide polymers can be processed into a wide variety of forms, from injection or compression molded parts and ingots, to coatings, films, fibers and adhesives. Generally these articles reach their maximum properties with a subsequent thermal cure process.
Solvay Company developed a series of PAI-based composites called TORLON. Table 6.31 shows the simple description and their processing methods. The specific properties of these composites could be found on the Solvay Company websites.
Table 6.31 Description and Processing Methods of TORLON Composites.
| Products | Description | Processing methods |
| Torlon® 4275 | Good wear resistance at high velocities, contains PTFE and graphite | Injection molding; machining; profile extrusion |
| Torlon® 4301 | General purpose wear resistance, contains PTFE and graphite | injection molding; machining; profile extrusion |
| Torlon® 4435 | Good wear resistance at high velocities and high pressures, contains PTFE and graphite | injection molding; machining; profile extrusion |
| Torlon® 4630 | Excellent wear resistance in dry environments, contains PTFE and graphite | Injection molding; machining; profile extrusion |
| Torlon® 4645 | Excellent wear resistance in lubricated environments, contains PTFE and carbon fiber | Injection molding; machining; profile extrusion |
These composites could be used in the production of high-tech equipment, precision parts, and requiring high wear resistance, such as no lubrication bearing, seal and bearing retainer and reciprocating compressor parts, electronics and semiconductor industries. Used in high temperature, high vacuum, strong radiation, ultralow temperature conditions, work products, mold used for aircraft parts, reciprocating compressor impeller, the bearing retainer, the jet engine parts for combustion system Chipset and socket welding pedestal, cup body. Used for production of precision parts, cases such as electronics and semiconductor industry, oil drilling equipment and so on.
6.10 Nanocomposites of Thermoplastic Polyimide (TPI)
Nanoparticles draw intensively attention these decades because of their special properties compared to the bulk materials. Many kinds of nanomaterials were reported and thoroughly studied, such as metal nanomaterials, carbon nanomaterials, oxide nanomaterials, organic nanomaterials, etc. Nanocomposite is a multiphase solid material where one of the phases has one, two, or three dimensions of less than 100 nanometers (nm), or structures having nanoscale repeat distances between the different phases that make up the material. In the case of TPI nanocomposites, TPI is used as the matrix; the nanoparticles are used as the modifier to disperse in the TPI matrix. Compared with the thermosetting polymide nanocomposites, the research and development of TPI nanocomposites are relatively less reported.
6.10.1 TPI/silver Nanocomposite
Khalil Faghihi and Meisam Shabanian [62] reported a TPI/silver nanocomposite. The soluble polyimide–silver nanocomposite containing chalcone moieties as a photosensitive group was synthesized by a convenient ultraviolet irradiation technique. A precursor such as AgNO3 was used as the source of the silver particles. The soluble polyimide was synthesized by the one-step synthesis of polyimide from polycondensation reaction of 4, 4’- diamino chalcone with pyromellitic anhydride in the presence of isoquinoline solution.

Figure 6.15 Chemical structure of the soluble polyimide.
Table 6.32 Thermal Properties of PI/silver Nanocomposite.
| Polymer | Tg/ °C |
T5/°C |
T10/°C |
Char yield/% |
| Pure PI | 149 |
370 |
410 |
53.89 |
| PI-silver nanocomposite | 176 |
410 |
420 |
56.19 |
The temperatures of 5% and 10% wt losses and also the char yield at 800 °C of PISN were higher than the pure PI. The higher thermal stability of nanocomposite can be attributed to the presence of inorganic silver nanoparticles in the PI matrix. Also, the Tg of nanocomposite was higher than pure PI. The increase in the Tg can be attributed to the strong interaction between the silver nanoparticles and polar imide chain.
The silver nanoparticles were homogeneously dispersed in the PI matrix. In the UV–vis absorption spectra of the PISN, the absorption peak due to the SPR of silver particles was observed at 418 nm. Due to the presence chalcone moieties in polymer backbone and good thermal properties, these silver/PI nanocomposites can be photosensitive and have the potential for the use in microfabrication of conductive components in microelectronic industry.
Thanh Hoai Lucie Nguyen etc. [63] reported another TPI/silver nanocomposite, which is based on silver nanowires and thermoplastic polyamide. The typical fabrication procedure of the composite is as follows: A volume of Ag NW suspension was added to the PI/NEP solution under sonification. This suspension was film coated on a glass plate and placed in an oven at 80 °C during 30 min to evaporate the solvent. The film was removed from the glass plate and heated at 300 °C during 2 h in order to evaporate the residual traces of NEP. The formed composite was a film with a thickness around 50 µm. PI/Ag NW composites were elaborated with a volume fraction varying from 0 to 3 vol%.
For the neat PI, the Tg is near 330 °C. The glass transition temperature exhibits a very slight modification with the filler content. This variation is not significant. It is suggested that the addition of Ag NWs does not have impact on PI physical structure.
The addition of Ag NWs into the PI matrix increased the conductivity by 16 orders of magnitude and reached a conductivity value of 102 S·m–1.
Table 6.33 Thermal Properties of TPI/Ag NWs Nanocomposite.
| Volume fraction of ag NWs (vol.%) | 0 |
0.5 |
1.0 |
1.5 |
2.0 |
2.5 |
3 |
| Tg(°C) | 329 ± 2 |
331 ± 2 |
326 ± 2 |
327 ± 2 |
327 ± 2 |
327 ± 2 |
327 ± 2 |
6.10.2 TPI/Fe-FeO Nanocomposite
Jiahua Zhu et al., [64] used the commercialized TPI Matrimid 5218 to fabricate the TPI-Fe-FeO nanocomposite. Pure PI and Fe-FeO/PI nanocomposite fibers with various Fe@FeO nanoparticle loadings (5, 10, 20, and 30 wt %) are fabricated by the electrospinning process.
Higher voltage is required for the fabrication of nanocomposite fibers at high nanoparticle loadings owing to the increased viscosity of the nanocomposite solutions. The nanoparticles in the nanocomposite fibers become magnetically harder with a significant increase of coercivity from 62.3 Oe (as-received Fe@FeO nanoparticles) to 188.2 Oe. Meanwhile, an increase of about 7.4% in the nanoparticle shell thickness is deduced during the high-voltage electrospinning fiber fabrication process.

Figure 6.16 Chemical structure of Matrimid 5218.
Table 6.34 Thermal Properties of Fe@FeO/ PI Nanocomposite.
| Polymer | Tg/ °C |
Tm/ °C |
| Pure PI | 62.4 |
169.7 |
| 10wt%Fe@FeO/ PI | 72.9 |
184.2 |
| 20wt%Fe@FeO/ PI | 74.4 |
185.1 |
| 30wt%Fe@FeO/ PI | 76.5 |
186.9 |
Table 6.35 Thermal Stability Performance of Fe@FeO/ PI Nanocomposite.
| Polymer | T5/°C |
Wt residue at 400 °C |
| Pure PI | 473.8 |
98.3 |
| 5wt%Fe@FeO/PI | 480.6 |
99.8 |
| 10wt%Fe@FeO/PI | 481.9 |
99.7 |
| 20wt%Fe@FeO/PI | 482.9 |
99.6 |
| 30wt%Fe@FeO/PI | 484.3 |
99.9 |
TGA and DSC results indicate an enhanced thermal stability with an increased glass transition (Tg) temperature of the nanocomposite fibers as compared to that of the pure PI fibers. The Tg and Tm of the nanocomposite does not much increase when the loading amount increased from 10wt% to 30wt%.
6.10.3 TPI/Carbon Nanocomposites
A kind of TPI/carbon nanocomposites [65] were reported by M Lebróncolón in 2010. These nanocomposites were based on the SWCNT, pyrene derivative, and the BPADA thermoplastic polyimide.
(1-pyrene-N-(4-N’-(5-norbornene-2,3-dicarboxyimido)phenyl butanamide)
It has been found that polyimide films prepared with the SWCNT/1 complexes showed higher tensile strengths and storage moduli than those of the neat polyimide film and polyimide nanocomposite films prepared with pristine SWCNTs at the same loading levels. The films fabricated with the TPI nanocomposites also had higher Tg and better high-temperature stability than both the neat polyimide films and corresponding nanocomposite films prepared with pristine SWCNTs. Furthermore, DC volume conductivities increased significantly with addition of these complexes and were higher than nanocomposite films prepared with uncomplexed SWCNTs. These materials could be suitable for use as multifunctional materials in applications where the combination of good mechanical properties and electrical conductivity is required.

Figure 6.17 Chemical structure of pyrene derivative 1.

Figure 6.18 Chemical structure of BPADA thermoplastic polyimide 2.
Table 6.36 Thermo Properties of the Nanocomposites.
| Additives | No |
1.5wt% SWCNTs |
3.5wt% SWCNTs |
5.5wt% SWCNTs/1 |
1.5wt% SWCNTs/1 |
3.5wt% SWCNTs/1 |
5.5wt% SWCNTs/1 |
| T5/°C | 388 |
408 |
410 |
489 |
486 |
481 |
510 |
| Tg/°C | 169 |
168 |
188 |
185 |
184 |
188 |
197 |
VE Smirnova et al., [66] reported TPI/carbon nanocomposite. They have studied the effects of additives of single-walled carbon nanotubes prepared via electric-arc synthesis and carbon nanofibers produced via gas-phase synthesis on the crystallization capacities and mechanical and electric properties of composite films. The thermoplastic polyimide (PI R–ODFO) matrix was synthesized from 1,3-bis-(3,3’,4,4’-dicarboxyphenoxy) benzene and 4,4’-bis-(4-aminophenoxy)biphenyl.
The typical fabrication of TPI/carbon nanocomposite is as follows: A homogeneous suspension of a calculated amount of CNTs and CNFs in NMP was prepared with an IL 100–6 ultrasonic dispenser (a generator capacity of 0.5 kW, ultrasonic treatment times of 2 h and 30 min for dispersing the CNFs and single-walled CNTs, respectively). The dispersion was poured into a solution of PAA in NMP, and the resulting mixture was stirred with a mechanical stirrer (1500 rpm) for 4 h. The solutions were poured on a glass substrate and dried at 60 °C for 20 h. Thermal cyclization was performed in a stage heating mode at 100, 200, and 300°C for 1 h at each temperature. To standardize the initial state of the samples, they were amorphized via annealing at 360 °C for 10 min followed by cooling to room temperature at a rate of 10 K/min.
The introduction of CNFs into the matrix of the thermoplastic PI R–ODFO increases the degree of crystallinity achieved during annealing. An increase in the filler concentration leads to an increase in the crystallinity of the material. Sharp decreases in the volume resistivities of the composite films are observed at concentrations of 0.1–0.5 wt % single-walled CNTs and carboxylated single-walled CNTs and 3 wt % CNFs because of the formation of a percolation cluster.
Zhang and Du [67] introduced another CNT-CF/TPI nanocomposite. Carbon nanotubes (CNTs) were introduced into carbon fiber (CF) by chemical vapor deposition and then were incorporated in the GCTPTM thermoplastic polyimide composites. CNT-coated CF was shown to be significant with elevated tensile properties of CNT-CF/TPI hybrid composite. In contrast with the neat CF/TPI composite, CNT-CF/TPI composite has shown enhanced tensile strength increased to approximately 30%. The justification of supreme tensile properties of hybrid composite was explained to be dependent on fiber–matrix adhesion incorporated by interfacial CNT. In 2015, S. V. Larin et al., [68] also studied and reported structural properties of TPI/carbon nanocomposites based on thermoplastic polyimides filled with surface-modified carbon nanotubes (CNT) by means of fully atomistic molecular dynamics simulations.

Figure 6.19 Chemical structure of PI R–ODFO.
It is shown that the CNT surface area available for contacts with TPI matrix influences significantly the polymer matrix structure. Increase of CNT surface modification degree leads to reduced surface area of the filler available for the interaction with polymer chains. Thus, in the case of uniform distribution of functional groups on the CNT surface, no orientation ordering of the flat fragments of polyimide chains has observed even near the filler surface.
6.10.4 TPI/CF/TiO2 Nanocomposite
Li [69] reported a TPI/CF/TiO2 nanocomposite which was prepared through compression molding as milled CF/TPI mixtures without further melt mixing. The incorporation of TiO2 leads to a significant improvement in friction and wear properties of the CF/TPI composite. The scratches on the worn surface of the CF/TPI composite filled with TiO2 are considerably reduced. When the TiO2 particle content was less than 10 vol%, the wear rates of TiO2 filled composites decreased with increasing TiO2 filler content, which were lower than that of composites filled with the same content of CF. With further increase in TiO2 filler content, the wear rate of TiO2-filled composites sharply increased close to that of pure PI and higher than that of composites filled with CF.
6.11 Environmental Impact and Recycling
TPI is a kind of environmental friendly material, which is very low hazardous. Some of them could be used to make table ware and medical apparatus. Several TPIs have good biocompatibility, for instance, nonhemolytic and nonhazardous in the external cell experiment. In addition, because TPI could be melt processed, solvent is not necessary in the processing procedure, the environmental impact is very little.
As TPIs are hydrolyzed materials, especially in the alkaline solution. Dianhydrides and diamines could be recycled by alkaline hydrolysis reaction. For example, the recycling rate is up to 80–90 % through the hydrolysis for Membrane Kapton.
6.12 Conclusions
TPI has excellent comprehensive performance, it is easily processing, and almost no solvent evaporation in the processing process and higher production efficiency, and this makes it superior to the traditional thermosetting polyimide in the economy benefits and environmental protection. It has become a hot research spot. Through the development of TPI in recent years, the authors think that the development of TPI should be carried out in the following aspects:
- In view of thermal properties, moisture absorption and cost, the research should be focused on the development of ether anhydride, ketone anhydride, and biphenyl anhydride-based TPI.
- In view of processing, the relationship between heat resistance and processability of TPI should be balanced. Try to develop multi melt-processable TPI.
- In view of solvent resistance and high-temperature mechanical properties, research efforts should be focused on the development of high crystallinity and high crystallinity rate TPI.
- Readers should know that this chapter is not a thorough summary due to the enormous scientific reports on TPI.
References
1. Takekoshi, T., Wirth, G., Heath, D.R., Kochanowski, J.E., Manello, J.S., Webber, M.J., Polymer syntheses via aromatic nitro displacement reaction. J. Polym. Sci.: Polym. Chem 18, 3069, 1980.
2. Shi, Z., Hasegawa, M., Shindo, Y., Yokata, R., He, F., Yamaguchi. H., Ozawa, H., Thermo-Processable Polyimides with High Thermo-Oxidative Stability as Derived from Oxydiphthalic Anhydride and Bisphenol a Type Dianhydride. High Perform. Polym. 12, 377, 2000.
3. Morita, A., Thermoplastic Polyimide (TPI). In: Engineering Plastics Handbook: McGraw-Hill Companies, Inc., 2006.
4. Tamai, S., Yamaguchi, A., Ohta, M., Melt processible polyimides and their chemical structures. Polymer 37, 3683–3692, 1996.
5. Ratta, V., McGrath, J.E., Wilkes, G.L., Ayambem, A., Crystallization and multiple melting behavior of a new semicrystalline polyimide based on 1, 3-bis (4-aminophenoxy) benzene (TPER) and 3,3’,4,4’-biphenonetetracarboxylic dianhydride(BTDA. Polymer, 42, 6173, 2001.
6. Koley, T., Bandyopadhyay, P., Mohanty, A.K., Banerjee, S., Synthesis and characterization of new aromatic poly(ether imide)s and their gas transport properties. Eur. Polym. J 49, 4212–4223, 2013.
7. Shanghai Research Institute of Synthetic Resins. Product Instruction of Polyimides.
8. Hou, T.H., Jensen, B.J., St Clair, T.L., IM7/LARCTM-IA polyimide composites. High Perfom. Polym., 7, 105, 1995.
9. Hou, T.H., Jensen., B.J., St Clair, T.L., IM7/LARC™-IAX-3 Polyimide Composites. High Perfom. Polym., 10, 193, 1998.
10. Tamai, S., Kuroki, T., Shibuya, A., Yamaguchi, A., Synthesis and characterization of thermally stable semicrystalline polyimide based on 3,4’-oxydianiline and 3,3’,4,4’-biphenyltetracarboxylic dianhydride. Polymer, 42, 2373, 2001.
11. Auman, B. C., Summers, J. D., Corcoran, W. R., EP Patent. 1219664 March 27, 2002, to E.I. du Pont de Nemours and Company.
12. Sroog, C.E., 4th European Technical Symposium on Polyimides and High Performance Polymers, University Montpellier 2-France, 266–297, 1996.
13. Vespel Product Bulletin E-61477.
14. Samyn, P., Schoukens, G., The effect of processing method on dry sliding performance of polyimides at high load/high velocity conditions. Eur. Polym. J 44, 716, 2008.
15. Deiasi, R., Russull, J., Aqueous Degradation of Polyimides. Aqueous degradation of polyimides. J. Appl. Polym. Sci., 15, 2965, 1971.
16. Deiasi, R., Thermal regeneration of the tensile properties of hydrolytically degraded polyimide film. J. Appl. Polym. Sci.,16, 2909, 1972.
17. Pawlowski, W.P., Coolbaugh, D.D., Johnson, C.J., Malenda, M.J., Etch rate studies of base catalyzed hydrolysis of polyimide film. II. J. Appl. Polym. Sci 43, 1379–1383, 1991.
18. Coralie, A.P., Polymeric Materials for Electronics Packaging and Interconnection. Polyimide Hydrolysis Measurement by Fourier Transform-IR Spectroscopy. ACS symposium series 407, 57, 1989.
19. Stephans, L.E., Myles, A., Thomas, R.R., Kinetics of Alkaline Hydrolysis of a Polyimide Surface. Langmuir 16, 4706, 2000.
20. Harris, I.L., Chambers, A.R., Roberts, G.T., Preliminary results of an atomic oxygen spaceflight experiment. Mater. Lett., 31, 321, 1997.
21. Skurat, V.E., Barbashev, E.A., Dorofeev, Y.I., Nikiforov, A.P., Gorelova, M.M., Pertsyn, AI., Simulation of polymer film and surface behaviour in a space environment. Appl. Surf. Sci 92, (441), 1996.
22. Miyazaki, E., Tagawa, M., Yokota, K., Yokota, R., Kimoto, Y., Ishizawa, J., Investigation into tolerance of polysiloxane-block-polyimide film against atomic oxygen. Acta Astronautica 66, 922, 2010.
23. Duo, S., Li, M., Zhu, M., Zhou, Y., Resistance of polyimide/silica hybrid films to atomic oxygen attack. Surface and Coatings Technology 24, 6671–6677, 2006.
24. Cheng, Z., Zhu, X., Kang, E.T., Neoh, KG., Modification of Poly(ether imide) Membranes via Surface-Initiated Atom Transfer Radical Polymerization. Macromolecules 39, 1660–1663, 2006.
25. Zhang, A., Li, X., Nah, C., Hwang, K., Lee, M-H., Neoh, C.W., Lee, Y.H., Facile modifications of a polyimide via chloromethylation. I. Novel synthesis of a new photosensitive polyimide. J. Polym. Sci. A Polym. Chem., 41, 22–9, 2003.
26. Zhong, Z. X., Han, S.H., Lee, S.H., Lee, M.H., Facile modifications of polyimide via chloromethylation: II. Synthesis and characterization of thermocurable transparent polyimide having methylene acrylate side groups. Polym. Int., 54, 406 2005.
27. Shen, L.Q., Xu, Z.K., Yang, Q., Sun, H.L., Wang, S.Y., Xu, Y.Y., Preparation and characterization of sulfonated polyetherimide/polyetherimide blend membranes. J. Appl. Polym. Sci., 92, 1709, 2004.
28. Wang, G.G., Weng, Y.M., Zhao, J., Chen, R.R., Xie, D., Preparation of a functional poly(ether imide) membrane for potential alkaline fuel cell applications: Chloromethylation. J. Appl. Polym. Sci., 112, 721, 2009.
29. Lakshmi, R. T. P. S. M., Bhattacharya, S., Varma, I.K., Effect of Sulfonation on Thermal Properties of Poly (ether imide)2. High. Perform. Polym., 18, 115, 2006.
30. Ranucci, E., sandgren, A., Aandronova, N., Albberssson, A.C., Improved polyimide/metal adhesion by chemical modification approaches. J. Appl. Polym Sci 82, 1971, 2001.
31. Gygatgajyrta, S., Min, K.J., Anhydrous state proton conduction in sulfonated bisphenol a polyetherimide with 1H-1,2,4 triazole as proton solvent. Polym. Sci. Polym. Phys., 47, 2178, 2009.
32. Miles, F.D., Niblock, H., Wilson, G.L., The vapour pressure of oleum. Trans. Faraday Soc 35, 345, 1940.
33. Liu, Y. L., Hsiue, G.H., Lan, C.W., Kuo, J.K., Jeng, R.J., Chiu, Y.S., Preparation and characterization of sulfonated polyetherimide/polyetherimide blend membranes. J. Appl. Polym. Sci., 92, 1709, 2004.
34. Guiver, M.D., Robertson, G.P., Dai, Y., Bilodeau, F., Kang, Y.S., Lee, K.J., Jho, J.Y., Won, J., Structural characterization and gas-transport properties of brominated matrimid polyimide. J. Polym. Sci., Polym. Chem., 40, 4193, 2002.
35. Crivello, J.V., Lee, J.L., Conlon, D.A,. Synthesis and characterization of photosensitive polyimides. J. Polym. Sci., Polym. Chem., 25, 3293, 1987.
36. Injection Molding, Meridian Products Corporation, https://meridianproductscorp.com/index.php/molding, April, 2016.
37. Malloy, R.A., Plastic Part Design for Injection Molding. Munich, Vienna, New York: Hanser 1994.
38. Introduction to Compression Molding. http://www.efunda.com/home.cfm, March, 2013.
39. Masayukli, L., Yoshikazu, T., Electrical, thermal and mechanical properties of polyimide thin films prepared by high-temperature vapor deposition polymerization. High Perform. Polym., 5, 219, 1993.
40. Manufacturing: Synthetic and Cellulosic Fiber Formation Technology, http://www.fibersource.com/fiber-world-classroom/manufacturing/, June, 2018.
41. Ding, M.X., Polyimides-relationship between chemistry, structure and properties, as well as materials, p530.China Science Publishing & Media Ltd.
42. Low Static Kapton® Tapes. http://www.esdproduct.com/esd_tapes.php. June, 2018.
43. Hergenrother, P.M., Havens, S.J., Adhesive properties of LARC-CPI, a new semi-crystalline polyimide. Sampe. J., 24, 13, 1988.
44. Hergenrother, P.M., Havens, S.J., Preliminary adhesive and composite properties of LARC-CPI 2, a new semicrystalline polyimide. High Perf. Polym., 5, 177 1993.
45. Jensen, B.J., Hou, T.H., Wilkinson, S.P., Adhesive and composite properties of LARCTM-8515 polyimide. High Perf. Polym., 7,11 1995.
46. Jensen, B.J., Chang, A.C., Synthesis and Characterization of Modified Phenylethynyl Imides. High Perform. Polym., 10,175 1998.
47. Skybond® 700 Technical Bulletin, Industrial Submit Technology corporation. http://istusa.com/skybond/, June, 2017.
48. ULTEM Plastics. www.asco.com. June, 2017.
49. Takekoshi, T., Kochanowski, J. E., Manello, J.S., Webber, M. J., Polyetherimides. II. High-temperature solution polymerization. J. Polym. Sci. Ploym. Symp., 74, 93 1986.
50. AURUM Plastics. http://www.dupont.com/products-and-services/plastics-polymers-resins/parts-shapes/brands/vespel-polyimide/products/aurum-tpi-resin.html. June, 2017.
51. Ratem Plastics. http://www.chem-syn.com/en/product.asp?bclass=1. June, 2017.
52. Ma, S. P., Takahashi, T., Binary blends of a new wholly aromatic thermoplastic polyimide and poly(ether imide). Polymer., 37, 5589, 1996.
53. Goodwin, A.A., Thermal properties of a thermoplastic polyimide blend. J. Appl. Polym. Sci., 72,543 (1999).
54. Mazinani, S., Darvishmanesh, S., Ramezani, R., Bruggen, B.V., Miscibility of polyimide blends: Physicochemical characterization of two high performance polyimide polymers. React. Funct. Polym., 111 2016.
55. Tong, Y.J., Huang, X.D., Chung, T.S., A New Strategy To Prepare Rodlike/Flexible Polyimide Blends through Poly(amic acid) Amine Salt Precursors. Macromolecules., 34, 5748 2001.
56. Jensen, B.J, Hou, T.H., Wilkinson, S.P., Adhesive and composite properties of LARCTM-8515 polyimide. High. Perform. Polym., 7, 11 1995.
57. Stenzenberger, H.D., High-temperature composites from bismaleimide resins: A binder concept. Appl.Polym. Symp., 310,91 1977.
58. Hou, T.H., Bryant, R.G., Processing and Properties of IM7/LARC-SCi Polyimide Composites. High Perform. Polym., 9, 437 1997.
59. Hou, T.H., Siochi, E.J., Johnston, N.J., Clair, T.L.S., IM7/LARC TM -ITPI polyimide composites. Polym., 35, 4956 1994.
60. Skybond® 700 Technical Bulletin, Industrial Submit Technology corporation. June, 2017.
61. TPI/silver nanocomposite. http://www.solvayultrapolymers.com/en/data-sheets/torlon-pai-data.html.June, 2017.
62. Faghihi, K., Shabanian, M., Synthesis and Characterization of Polyimide–Silver Nanocomposite Containing Chalcone Moieties in the Main Chain by UV radiation. J. Thermoplastic Compos. Mater., 25, 89, 2012.
63. Nguyen, T.H.L., Cortes, L.Q., Lonjon, A., Dantras, E., Laca-banne, C., High conductive Ag nanowire–polyimide composites: Charge transport mechanism in thermoplastic thermostable materials. J. Non-Crys. Solid., 385, 34 2014.
64. Zhu, J.H., Wei, S.Y., Chen, X.L., Karki, A.B., Rutman, D., Young, D.P., Guo, Z. H., Conductive Polypyrrole/Tungsten Oxide Metacomposites with Negative Permittivity. J. Phys. Chem. C., 114, 8844, 2010.
65. Lebróncolón, M., Meador, M.A., Gaier, J.R., Solá, F., Scheiman, D.A., Reinforced thermoplastic polyimide with dispersed functionalized single wall carbon nanotubes. Acs. Appl. Mater. Interfaces., 2, 669, 2010.
66. Smirnova, V.E., Gofman, I.V., Ivan’Kova, E.M., Didenko, A.L., Krestinin, A.V., Effect of single-walled carbon nanotubes and carbon nanofibers on the structure and mechanical properties of thermoplastic polyimide matrix films. Polym. Sci. Series: A., 55, 268, 2013.
67. Zhang, J.G., Du, G., Mechanical properties of polyimide composite reinforced with carbon nanotubes and carbon fibers. J. Thermoplastic Compos. Mater., 28, 1250, 2015.
68. Larin, S.V., Glova, A.D., Serebryakov, E.B., Nazarychev, V.M., Kenny, J.M., Influence of the carbon nanotube surface modification on the microstructure of thermoplastic binders. RSC Advances., 5, 51621, 2015.
69. Li, Z.H., The effect of titanium dioxide on the tribological properties of carbon fiber-reinforced polyimide composites. J. Thermoplastic Compos. Mater., 28, 257, 2015.
