Chapter 7
Advances in High-Performance Polymers Bearing Phthalazinone Moieties
Jinyan Wang1,2,3, Cheng Liu1,2,3, Shouhai Zhang1,2,3 and Xigao Jian1,2,3*
1State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, China
2Liaoning High Performance Resin Engineering Research Center, Dalian University of Technology, Dalian, China
3Department of Polymer Science & Materials, Dalian University of Technology, Dalian, China
*Corresponding author: [email protected]
Abstract
High-performance polymer materials have excellent performance at high temperatures and are indispensable in aerospace, electronics, electrical engineering, high-speed rail, and other important high-tech fields. Improving the processing performance of polymers without sacrificing their heat-resistant property is a research hot spot. Phthalazinone-containing high-performance polymers are a new class of high-performance polymer materials. This article reviews progresses in the synthesis and performance of phthalazinone-containing poly(aryl ether)s (including poly(phthalazinone ether sulfone ketone)s, poly(phthalazinone ether nitrile sulfone ketone)s, poly(phthalazinone ether sulfone ketone ketone)s, and poly(triaryl triazine ring)s), polyamides, polyimides, polyarylates, and polybenzimidazoles. Because the phenyl-phthalazinone structure is a twisted, non-coplanar, and fused ring, the above polymers are not only heat resistant but also soluble. The processing methods are diverse and include both thermoforming (molding, extrusion, injection, etc.) and solution processing. Hence, these polymers have a wide range of applications.
Keywords: Poly(aryl ether), polyamides, polyimide, polyarylates, polybenzimidazoles, phthalazinone
7.1 Introduction
It has been known that wholly aromatic heterocyclic polymers, whose exploitation can be traced back to the 1960s, possess unsurpassed thermal stability, resistance to radiation, good chemical stability, and good mechanical properties. Their superior performance characteristics can meet ultimate and harsh demands in aerospace, aviation, electronics, and other industries. However, their difficult processability, such as poor solubility and usually higher fusion temperature than their thermally decomposed temperature, limits their large-scale production except polyimide in the result of the limited applications in numerous fields. Other aromatic heterocyclic polymers such as polybenzimidazole, polybenzoxazole, poly(phenyl triazine), and polypyrrolone require harsh synthetic conditions and are difficult to scale [1].
Researchers have devoted a great deal of attention to develop the processing or solubility of the above aromatic heterocyclic polymers. One approach introduces an aryl ether linkage into the polymer backbone [2] to increase the flexibility of the molecular chain and produce amorphous polymers. This improves their moldability. However, this method also reduces the glass transition temperature (Tg) of the resulting polymers. For example, the first high molecular weight poly(ary lether) containing an aromatic heterocyclic structure was prepared by bisphenol A and 2,5-bis(4-fluorophenyl)–1,3,4-oxadiazole via a step-growth polymerization of aromatic nucleophilic substitution. Its Tg is only 180 °C [2]. Thus, improving the processing properties of the aromatic heterocyclic polymers without sacrificing their heat resistance has been becoming a major goal of material scientists.
A novel wholly aromatic heterocyclic polyarylethers containing phthalazinone moieties were firstly reported by Hay’s research group, and then by Jian in 1993 [3–5]. These polymers show the desired heat resistance with Tg values of more than 250 °C and reasonable solubility in some organic solvents. This paper discusses the relationship between the chemical synthesis and structure of phthalazinone-containing high-performance polymers with their properties.
7.2 A New Mmonomer: 1, 2-Dihydro-4-(4-hydroxyphenyl)-1-(2H)-phthalazinone
Phthalazinone and its derivatives are an important class of drug intermediates. Their -NH group can react with halogenated benzene derivatives via nucleophilic substitution [6]. A 4-hydroxyphenyl structure was introduced to the 4 position of the phthalazine ring to synthesize a novel phthalazinone derivative—1,2-dihydro-4-(4-hydroxyphenyl)–1-(2H)-phthalazinone (DHPZ) monomer. Firstly, Hay’s group used phenolphthaleins as the raw materials to synthesize the DHPZ and its substituted derivatives through four steps of the chemical reaction [3]. We just used cheap and accessible phenol and phthalic anhydrides as the starting materials. The target DHPZ monomer could be easily obtained in two-step gentle reaction conditions with quantitative yield [4, 5]. The structure of DHPZ is shown in Figure 7.1a.

Figure 7.1 (a) DHPZ and (b) its three-dimensional model in comparison with (c) imide ring.
Figure 7.1c shows a five-member imide ring which is the functional group of polyimide. It is well known that polyimides are a class of high-performance polymers with excellent comprehensive performance such as high temperature, high strength, and good insulation. However, wet conditions, especially high-temperature and high-humidity environments, are irritable to their hydrolytic imide ring cleavage reaction to go back to the polyamic acid, resulting in the clearly noticeable decrease of their performance. By comparing Figure 7.1a and c, we can find that the structure of phthalazinone has a N-heterocyclic six-member ring other than the five-member ring of imide group. This has significantly better chemical stability than five-member rings. Although the dissociation energy of the N-N bond is low, because of the conjugate stability of the phthalazine ring, phthalazinone has a very good thermal stability and thus retains the excellent performance of aromatic heterocycles in polyimide. It also overcomes the shortcomings of five-member imide rings, i.e., poor thermal hydrolysis stability.
Figure 7.1b shows the spatial structure of DHPZ simulated with Chemdraw software. In DHPZ, the benzene and phthalazine rings are not in the same plane and twisted, leading to the non-coplanar spatial structure of DHPZ. The degree of the twist angle between the benzene and phthalazine rings in DHPZ could be adjusted by changing the number and/or type of substituents near the phenolic hydroxyl group in the benzene ring. Fourier transform infrared spectroscopy of DHPZ [7] has revealed the resonance absorption peaks of the –NH group and the –OH group at 3300 and 3200 cm–1, respectively. Its 1H-NMR data show that the proton chemical shift of N-H group occurs at 12.74 ppm, whereas that of O-H group occurs at 9.81 ppm.
According to the molecular structure of DHPZ, it may have three resonant structures (Figure 7.2). Consequently, in the polymerization reaction with dihalogen monomers when treated with base, there may be two possible structures of the structural unit of resultant polymers. One is the polymer containing C-N linkages (Figure 7.3a) and the other one is enolized to produce a polymer with all C-O linkages (Figure 7.3b).

Figure 7.2 Resonance formula of 4-(4-hydroxylphenyl)-2,3-phthalazin-1-one.

Figure 7.3 Two kinds of possible structure of the structural unit of polymers derived from 4-(4-hydroxylphenyl) –2,3-phthalazin-1-one and activated dihalogenated monomers.
Hay et al., synthesized the model compounds and then examined them by infrared (IR), 1H-NMR, and 13C-NMR spectroscopy [8]. His research results demonstrate that the polymerization of DHPZ and halide monomers occurs by the reaction of the corresponding nitrogen anion and the absorption of the carbonyl group at 1660 cm–1 in the lactam gives a potent supporting. In other words, the products has the phthalazinone structure (Figure 7.3a). Our research is in agreement with Hay’s on the base of our in-depth exploitation on the synthetic route, synthetic kinetics, and polymerization reaction mechanism of DHPZ [9].
Hence, DHPZ behaves like bisphenol monomers and is capable of polymerizing with dihalogen monomers to yield polymers.
7.3 Synthesis and Properties of Phthalazinone-Containing Poly(aryl ether)s
7.3.1 Poly(phthalazinone ether sulfone ketone)s (PPESKs)
PPESKs were the first reported polymers containing phthalazinone moiety [3]. They were prepared by the classical nucleophilic displacement step-growth polymerization reaction of DHPZ and 4,4’-dichloro diphenyl sulfone and/or 4,4’-difluoro benzophenone in aprotic solvents (such as N,N-dimethylacetamide) catalyzed by potassium carbonate, as illustrated in Figure 7.4.

Figure 7.4 Synthetic route of poly(phthalazinone ether sulfone ketone)s (PPESKs).
As known, during nucleophilic substitution step-growth polymerization of DHPZ with activated aromatic bis(halides), bisphenol monomers first react with carbonate (M2CO3) to form a salt. This step is an equilibrium reaction. The water generated during the reaction should be rapidly removed by azeotropic distillation in order to drive the reaction equilibrium toward the direction of the salt products and reduce the side hydrolysis reaction. Toluene, chlorobenzene, or xylene always is used as the water-carrying agent. Jian et al., reported that [10] fast speed of removal of water results in the wide molecular weight distribution of polymers when chlorobenzene is chosen as azeotropic agent, whereas xylene is favorable factor for high molecular weight of the polymer with relatively narrow polydispersity. The unsymmetric reactivity of -NH and -OH in DHPZ with base like potassium carbonate and the twisted, non-coplanar structure of DHPZ can make it have the cyclization tendency to form cyclic compounds [11]. The content of cyclic compounds or low molecular weight by-products can be suppressed by increasing the initial concentration in the polymerization system [10]. Jian optimized the reaction conditions. Tetramethylene sulfone is chosen as the reaction mediate to update the reaction at 200 °C. The polymerization can be completed for shorter time like 5 h with a high yield, compared with N,N-dimethylacetamide (DMAc) system [3] at atmospheric pressure. And the purified polymers can be easily achieved by washing the polymer with hot water for several times. The solvents involved in the reaction medium and purifying the resulting polymers can be recycled by fractional distillation.
The properties of PPESK (R1=R2=R3=H, in Figure 7.4) are shown in Table 7.1. This resin has excellent heat resistance and mechanical properties. Tgs of PPESK ranges from 263 °C to 305 °C and can be adjusted by the molar ratio of sulfone group and ketone group in the main chain of PPESK. The heat distortion temperature of PPESK with a sulfone to ketone ratio of 1:1 is 100 °C higher than that of commercialized poly(ether ether ketone) (PEEK) which is 152 °C. Its tensile strength at 250 °C is 1.5 times more than that of PEEK under the same conditions indicating much more excellent high-temperature mechanical performance of PPESK. It is worth noting that PPESK can be selectively soluble in some organic solvents including chloroform, DMAc, and N-methylpyrrolidone (NMP). These improved properties of PPESK are due to the introduction of wholly-aromatic, twisted, and non-coplanar structures of phenyl-phthalazinone moieties. This fundamentally solves the technical problems of the traditional high-performance engineering plastics being high-temperature resistant but not soluble or vice versa.
Table 7.1 Comparison of physical properties between PPESK and PEEK.
| Property | PPESK |
PEEK (450 G) |
|
| Glass transition temperature (Tg)(°C) | 263–305 |
143 (Tm = 334) |
|
| Starting temperature for 5% thermal decomposition (Td5%) (°C, in N2) | >500 |
>500 |
|
| Heat distortion temperature (1.8 MPa) (°C) | 253 (S/K = 1:1) |
152 |
|
| Tensile strength (MPa) | r.t. | 90–122 |
93 |
| 250 °C | 32 (S/K = 1:1) |
12 |
|
| Elongation at break (%) | 11–26 |
50 |
|
| Flexural strength (MPa) | 153–172 |
170 |
|
| Flexural modulus (GPa) | 2.9–3.3 |
3.3 |
|
| dielectric permittivity | 3.46 |
3.5 |
|
| Density (g/cm3) | 1.31–1.34 |
1.32 |
|
| Solubility | NMP, DMAc, chloroform |
Concentrated sulfuric acid |
|
However, rigid and twisted backbone of PPESKs also give them fairly difficulty during their melting processing. Their high melting viscosity (higher than 104 Pa·s) [12], caused by the entangled molecular chains, and high Tgs really limit them to apply for injection processing. Some efforts have been taken to improve their processability. One of the attempts is to blend them with low-melt-viscosity polymers, such as PPESK or commercialized poly(ether sulfone) oligomers [12] and aromatic liquid crystalline polymers (LCP) [13]. The results proved that the addition of low-melt-viscosity poly(aryl ether)s is an effective method to reduce the PPESK’s melt viscosity, although the slight decrease in Tgs and mechanical properties with reserved excellent thermal stability. LCP acts as a processing aid as well as in situ reinforcing agent in the blend of PPESK/LCP. The copolymerization of greater flexible bisphenols than DHPZ, for instance bisphenol A [14] or 4,4’-thio bisphenol [15], or rod-like hydroquinone [14] or biphenol [10] (Figure 7.5), was also carried out. It is noted that the tendency to decrease in melt viscosity of PPESK is obscure even though the content of bisphenols with greater flexibility is higher than 50%. Xiao used the equilibrium torque of the HAAKE Rheomix, which greatly depends on the melt viscosity of the polymers, to determine the processability of the copolymers (named as BP-COPPES) derived from DHPZ, biphenol, and bis(4-chlorophenyl) sulfone [10]. In this system, the equilibrium torques were slightly decreased with increasing the content of linear biphenyl structure and ether linkage to a considerable degree. Moreover, decreasing the molecular weight of BP-COPPES can further reduce its melt viscosity.

Figure 7.5 Synthesis of ternary copoly(phthalazinone ether ketone).
7.3.2 Poly(phthalazinone ether ketone ketone) (PPEKK) and Its Copolymers
Poly(ether ketone ketone) (PEKK), commercialized by BASF and Hoechst, has higher Tg of 153 °C than PEEK, commercialized by ICI in the late 1980s, and thus can be used at higher temperature. However, poor solubility and high cost of PEKK still hinder it to find extensive application. For synthesis of poly(aryl ether ketone) (PAEK), a high-activity fluorinated aromatic ketone monomer, such as 4,4’-difluoro benzophenone, is always used as the raw material to react with bisphenol monomer via nucleophilic displacement reaction for enough high molecular weight. However, high-cost fluorinated aromatic ketone monomer makes the resulting PAEK expensive. Researchers attempted to synthesize PAEKs from activated dichloro monomers instead of difluoro monomers [16–19]. The reactivity of 4,4-dichloro-diphenyl ketone is so low that no high molecular weight polymers can be obtained when it reacts with the bisphenol. Hergenrother et al., reported that 1,4-bis(4-chlorobenzoyl) benzene (BCBB) and bisphenol monomer could produce high molecular weight PAEK via a nucleophilic substitution reaction [20].
Our group successfully synthesized the high molecular weight PPEKK with inherent viscosity of 0.61 dL/g by nucleophilic aromatic substitution polycondensation of BCBB and DHPZ as shown in Figure 7.6 [21]. As we expected the incorporation of the rigid phthalazinone ring into its backbone imparts PPEKK good thermal stability with 249 °C of Tg and 507 °C of the temperature for 5% weight loss in nitrogen (Table 7.2). Meanwhile, the values of tensile and flexural strength are 102 MPa and 168 MPa, respectively, which is a typical range for high-performance polymers. PPEKK is soluble in chloroform and NMP, and partly soluble in DMAc. The results of testing the friction coefficient and friction wear coefficient of PPEKK show that the friction coefficient of PPEKK is almost the same as that of nylon and higher than that of PTFE, but the friction wear coefficient of PPEKK is much lower than that of PTFE and nylon under the same experimental conditions, indicating that PPEKK is candidate of the high-performance abrasive composites matrix. The melt viscosity of PPEKK is still high to 105 Pa·s, but sensitive to shear rate. Increasing temperature and shear rate, such as at 350 °C and lower than 20/s of shear rate, can effectively decrease the melt viscosity of PPEKK to 104 Pa·s.
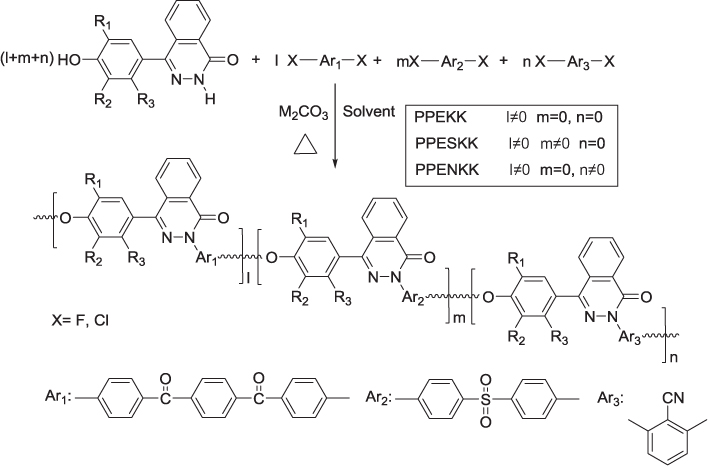
Figure 7.6 Synthetic route of poly(phthalazinone ether ketone ketone)s (PPEKK) and its copolymers.
Table 7.2 Physical Properties of PPEKK and Its Copolymers.
| Property | PPEKK |
PPESKK (S/KK = 1:1) |
PPENKK (N/KK = 1:1) |
| Tg (°C) | 249 |
266 |
273 |
| Td5% (°C in N2) | 506 |
510 |
516 |
| Heat distortion temperature (1.8 MPa) (°C) | 240 |
256 |
260 |
| Tensile strength (MPa) | 102 |
92 |
116 |
| Elongation at break (%) | 12 |
11 |
12 |
| Flexural strength (MPa) | 168 |
157 |
189 |
| Flexural modulus (GPa) | 2.9 |
3.2 |
3.2 |
| Volume resistivity (Ω·cm) | 6 × 1016 |
5 × 1016 |
5 × 1016 |
| Oxygen index | 34 |
36 |
39 |
| Density (g/cm3) | 1.33 |
1.33 |
1.33 |
| Solubility | NMP, DMAc, chloroform |
||
Copolymerization of BCBB and DHPZ with 4,4’-dichloro diphenyl sulfone or 2,6-dichlorobenzonitrile gave a series of high molecular weight polymers including poly(phthalazinone ether sulfone ketone ketone) (PPESKK) [22, 23] and poly(phthalazinone ether nitrile ketone ketone) (PPENKK) [24], respectively. The reaction formula is shown in Figure 7.6 and the physical properties of them are in Table 7.2. Introducing sulfone group or nitrile group into the main chain of PPEKK can further improve their thermal properties.
7.3.3 Poly(phthalazinone ether nitrile sulfone ketone)s (PPENSKs)
Poly(aryl ether nitrile)s (PAENs), whose characteristics is pendant cyano groups, have been identified as high performance thermoplastics [25]. Their pendent cyano groups give them several favorable properties such as higher thermooxidative and thermal stability than non-cyano-containing poly(aryl ether)s [26], good flame retardancy, good adhesion to many substrates due to interaction with other functional groups through polar interaction [27, 28], and also provide a potential site for polymer cross-linking [27, 29, 30], or for further functionalization. However, there was only one kind of PAENs firstly commercialized by Idemitsu Kosan Company Limited in 1986 [31–33]. Its trade name is PEN-ID300 (PENTM). PENTM is a semicrystalline polymer and is prepared by resorcinol and 2,6-difluorobenzonitrile via step-growth nucleophilic substitution polymerization. Its molecular formula is shown in Figure 7.7. PENTM with Tg of 148 °C and a melting point (Tm) of 340 °C can be used under 260 °C and has high tensile strength up to 132 MPa. These merits make PEN attract many researches for applying in the fields of aerospace, industry, and automobile. However, its semicrystalline nature resulting in poor solubility in common organic solvents except concentrated sulfuric acid often restricts its use for wide range applications. Furthermore, its modulus decreases dramatically when environmental temperature is higher than 150 °C owing to its relatively low Tg, which hardly qualifies the composites for use at the elevated temperatures. These also lead to its harsh synthesis condition and relatively high cost.

Figure 7.7 The chemical structure of PEN™.
We also devote our attention to exploiting PAENs containing phthalazinone moiety. Firstly, the reactivity of cheap 2,6-dichlorobenzonitrile (DCBN) was studied. We found that it is impossible to obtain high molecular weight PAENs in the polymerization of DHPZ and 2,6-dichloro-benzonitrile and/or 4,4’-dichloro-diphenyl sulfone due to the low reactivity of 2,6-dichloro-benzonitrile. Adding a small amount of anhydrous halide salt like potassium fluoride to the polymerization system can push the reaction to get high molecular weight polymers. Thus, in the presence of special composite catalysts, ternary or quaternary copolymerization of the low-cost 4,4’-dichloro-benzophenone or 1,4-bis (4-chlorobenzoyl) benzene has been found to be successful. In DHPZ/ DCBN/4,4’-difluoro benzophenone (DFK) polymerization system, DHPZ firstly polymerized with low reactive DCBN for a given time at polymerization temperature after removal of water produced during reaction, followed by addition of high reactive DFK. Thus a series of high molecular weight poly(phthalazinone ether nitrile ketone (PPENK) [34], PPENSK, and poly(phthalazinone ether nitrile sulfone ketone ketone) (PPENSKK) were successfully prepared, as shown in Figure 7.7. The introduction of a carbonyl group (-CO-) or terephthaloyl group ![]() into the main chain of the polymer offers major advantages including high temperature resistance, high strength, and moderate cost. Our group has completed a 100 tons/year pilot engineering project and then a 500 tons/year industrial test. Table 7.3 shows the main physical properties of our typical products in comparison to that of PENTM.
into the main chain of the polymer offers major advantages including high temperature resistance, high strength, and moderate cost. Our group has completed a 100 tons/year pilot engineering project and then a 500 tons/year industrial test. Table 7.3 shows the main physical properties of our typical products in comparison to that of PENTM.

Figure 7.8 Diagram for synthesis of poly(phthalazinone ether nitrile)s.
Table 7.3 Physical Properties of Poly(phthalazinone ether nitrile)s and PENTM.
| Property | PPENS (N/S = 1:1) |
PPENK (N/K = 1:1) |
PPENSK (N/K/S = 2:1:1) |
PENTM |
| Tg (°C) | 301 |
277 |
290 |
Tg = 148 Tm = 340 |
| Td5% (°C) | ≥500 |
≥500 |
≥500 |
≥500 |
| Heat distortion temperature (1.8 MPa) (°C) | 280 |
270 |
275 |
165 |
| Tensile strength (MPa) | 90 |
130 |
105 |
132 |
| Elongation at break (%) | 10 |
12 |
10 |
10 |
| Flexural strength (MPa) | 158 |
194 |
175 |
194 |
| Flexural modulus (GPa) | 3.3 |
3.8 |
3.5 |
3.8 |
| Oxygen index | 35 |
38 |
38 |
40 |
| Density (g/cm3) | 1.33 |
1.32 |
1.30 |
1.32 |
| Solubility | NMP, DMAc, chloroform |
Concentrated sulfuric acid |
||
As seen from Table 3, amorphous phthalazinone-containing poly(aryl ether nitrile)s exhibit excellent heat resistance far superior to that of PENTM. Their Tg values are more than 270 °C, and the 5% thermal decomposition temperatures are all above 500 °C. Their heat distortion temperatures are higher than 270 °C, which is 100 °C higher than that of PENTM. In addition, they are soluble in some organic solvents including chloroform, DMAc, and NMP. These properties of poly(phthalazinone ether nitril e)s can be regulated by changing the content ratios of the sulfone groups, nitrile groups, and carbonyl groups in their main chain. They display electrical surface resistivity of 1013 Ω and dielectric coefficient of 3.45 with the dielectric loss of 0.004 detected at 1 MHz, indicating their potential application in electronic field. Their mechanical properties are excellent and close to PENTM. These characteristics indicate these phthalazinone-containing poly(arylene ether nitrile)s can be considered not only as new candidates for processable high-performance engineering plastics but also as functional materials.
To extend these polymers’ application, PPENK was developed to be a new high-performance magnet wire [35]. PPENK magnet wire, manufactured through a conventional magnet wire coating process, exhibits excellent electrical properties with a mandrel flexibility of no break on one diameter and excellent moisture resistance as well, compared to polyamide and polyimide insulated resins. The typical wire can be easily made on 0.9 mm Cu wire at 380 to 480 °C without pinholes. The resulting wire has heat resistance to thermal shock (30 min, 470 °C, two diameter) and can be used in a high-temperature (above 220 °C) electric insulation system. Therefore, PPENK magnet wire can be widely used in contemporary heavy-duty electrical devices.
7.3.4 Polyarylether Containing Aryl-s-triazine and Phthalazinone Moieties
The resonance energy of s-triazine nucleus is 82.4 kcal/mol, far higher than that of benzene (36 kcal/mol). Hence, polymers containing triaryl s-triazine ring structure have more excellent thermal stability, higher tensile strengths, and modulus at elevated temperatures, relative to the conventional polymers, as well as chemical resistance, radiation resistance, flame retardance, and self-extinguishing capability [36]. These outstanding comprehensive properties allow triaryl s-triazine-containing polymers to be used as the matrix resins for high-temperature coatings, adhesives, films, and composite materials in aviation, aerospace, microelectronics, and other high-tech fields.
Traditionally, the trimerization of cyano-containing polymers had to be promoted at a temperature in the range of 200–500 °C under a pressure of 30–50,000 atmospheres catalyzed by acid or without catalyst system [37]. he superhigh pressure extremely restricts their applications for the lager volume of bulky equipment. Linear polymers containing triaryl s-triazine ring structure still have poor solubility due to their chain rigidity and intermolecular strong charge transfer between the phenyl and s-triazine ring. In addition, the softening temperature of these polymers is too high to melting processing.
A series of new heat-resistant and soluble s-triazine-containing poly(aryl ether)s were firstly prepared by incorporating sulfone and phthalazinone groups into the polymer backbones, respectively. The polymers (named as PPESPs) were derived from the nucleophilic displacement polycondensation reaction of 2,4-bis(4-fluorophenyl)–6-phenyl-1,3,5-triazine (BFPT), 4,4’-dichloro-diphenyl sulfone, and DHPZ (Figure 7.9) [38]. They are soluble in organic solvents including NMP, DMAc, and dimethylformamide (DMF) at room temperature and are soluble in hot DMSO and sulfolane. Differential scanning calorimetry (DSC) test results show that all PPESPs copolymers have only one Tg ranging 269–305 °C, suggesting that poly(phthalazinone ether phenyl-s-triazine) (PPEP) and PPES chains have good compatibility. The Tg value of the copolymers gradually increases with sulfone group content. The investigated polymers exhibit high decomposition temperatures for 5% weight loss (T5%: 503–536 °C) and high char yields at 800 °C (all above 53%), confirming their excellent thermal stability. The thermal decomposition temperature and char yields at 800 °C both increase as the amount of phenyl-s-triazine in the polymer increases since the sulfone group is considered as the less thermal stable moiety relative to phenyl-s-triazine. These phenomena are in agreement with the fact that the introduction of phenyl-s-triazine groups impart polymers with better thermal stability than other groups, most possibly due to the presence of s-triazine that brings its polymers higher resonance energy along the main chain [39, 40]. Poly(arylene ether s-triazine)s containing alkyl-, aryl-, and chloro-substituted phthalazinone moieties in the main chain were also investigated (Figure 7.10) [41]. The side groups connected to phenyl-phthalazinone segment endow the resultant polymers with good solubility while maintaining other attractive properties. The solubility of the polymers obtained is further improved than nonsubstituted analogue [38] except chloro-polymers due to the pendent methyl or phenyl groups in the polymer side chains that help to enlarge the average intermolecular distance of those polymers. Their good solubility in common organic solvents illustrates that they may be promising for practical applications in the fields of high-temperature membranes, structural coatings, and adhesives.

Figure 7.9 Synthesis of PPESPs.

Figure 7.10 Synthetic route of s-triazine-containing poly(aryl ether)s with substituents.
To broaden the application of phthalazinone-containing polymers at higher temperature, we designed and synthesized cyano-terminated poly(phthalazinone ether amide)s (CN-PPEAs) (Figure 7.11). The trimerization of the terminal cyano groups in CN-PPEAs is activated by the electron-withdrawing carbonyl groups in the para-substitution (Figure 7.12) [42]. There were some reports to demonstrate the moderate trimerization of the cyano-containing polymers in the presence of Lewis acids such as zinc chloride at normal pressure [27, 43]. A small quantity of terephthalonitrile (TPH, 2.9 wt% relative to the CN-PPEAs) was used to increase the concentration of cyano groups in the curing system, and zinc chloride (ZnCl2, 1.9 wt% relative to the CN-PPEAs) was selected as catalyst. The thermal cross-linking reaction of CN-PPEAs occurred at moderate temperature, such as at 278 °C for low molecular weight of CN-PPEAs and at 314 °C for higher molecule weight of CN-PPEAs. However, the curing time should be prolonged for completing the curing reaction. The addition of TPH and ZnCl2 to the curing system was confirmed to be effective in facilitating the thermal curing of CN-PPAs by the fact that no obvious thermal cross-linking exothermic peak of neat CN-PPEAs was observed and their Tg values detected by the first DSC run were very close to those tested by the second run. Cured CN-PPEAs show much higher thermal stability, including high thermal decomposition temperatures (the T5% values higher than 460 °C) and higher char-yielding (Cy values in the range of 67–75% at 800 °C) under the protection of nitrogen. Other cyano-end-capped samples, such as aromatic bis(ether nitrile)s [44] and oligomeric phthalazinone-base poly(arylene ether nitrile)s with different terminal cyano contents (PPEN-DC) [30], were also prepared and their curing reactions were investigated elaboratively. The pendant cyano groups of PPEN-DC are observed to be less reactive to trimerize, while the terminal cyano groups demonstrate much higher reactivity in s-triazine-forming reaction [30]. Although their cross-linking behavior is similar to that of CN-PPEAs, they exhibit more excellent thermal and oxidative stability than CN-PPEAs.

Figure 7.11 Synthetic route of CN-PPEAs.

Figure 7.12 Proposed thermally activated cross-linking of PPEN-DCs with TPh.
It has been demonstrated that cyano concentration, mobility, and reactivity are all important factors for the trimerization, and especially the cyano reactivity plays a dominant role [30]. The phthalonitrile resin has been proven to be a unit of high efficiency for raising cyano concentration and improving reactivity [45, 46]. Aromatic diether-linked phthalonitrile monomers/polymers [47–49], phenolic novolac [50], and polybenzoxazine [51] resins modified by either the introduction of phthalonitrile units into the polymer chains or the addition of bisphthalonitriles into the curing systems have been developed in the application of structural materials such as reinforced composites or electronic components including printed circuit boards.
We also engage in exploiting phthalonitrile-functional phthalazinone resins for reinforced composites by the incorporation of phthalonitrile end-groups aiming to increase the cyano concentration and thus to promote their trimerization. Firstly, an unsymmetrical phthalonitrile-functional phthalazinone (Ph-HPPZ), namely 4-(4-(4-(3,4-dicyanophenoxy) phenyl)–1-oxophthalazin-2(1H)-yl) phthalonitrile was designed and synthesized, followed by the curing reaction with the presence of different aromatic diamines [52]. The polymerization of bisphthalonitrile Ph-HPPZ can be thermally performed in one step to a prepolymer with desired viscosity (B stage resin, Po-HPPZ) or can be directly advanced to cross-linked networks with the charge of amine additives (Figure 7.13). The structure of B stage resin Po-HPPZ was analyzed by IR and 1H-NMR spectra. The results demonstrated that certain polymerizations of Ph-HPPZ occurred at 250 °C, resulting in prepolymers containing iminoisoindolenine structure. Keller also postulated that the amine, when present in minute quantities, probably initially attacks the nitrile components of bisphthalonitrile to afford an N-substituted-3-iminoisoindoline intermediate [45, 46]. The B-staged resins, having the Tg values in the range of 180–190 °C depending on the diamine nature, are stable at room temperature and are all easily soluble in chloroform and aprotic polar solvents (dimethyl sulfoxide (DMSO), NMP, DMAc, and N,N-dimethyl formylamine (DMF)) as well as warm acetone, ethanol, and methanol. They can be easily shaped and formed via thermoplastic processing. Using low-activity 4,4’-diamino diphenyl sulfone as the curing agent, the processing window could be expanded moderately. For this reason, these kinds of B-staged resins could be promising in applications other than the conventional phthalonitrile resins, such as polymer composites, adhesive, or coating applications.

Figure 7.13 Polymerization and cross-linking reactions of Ph-HPPZ.
When the reaction temperature was increased to 325 °C or higher temperature, the B-staged resins continued to react with diamine and phthalonitrile. Thermal data of the cured polymers indicate their excellent thermal and thermooxidative stability. They can retain 95% weight at 506–514 °C and 90% weight at 547–561 °C under a nitrogen atmosphere, respectively. Their anaerobic char yields are more than 70%. Therefore, these phthalazinone-based phthalonitriles, their prepolymers, and networks may be considered as promising base materials in a wide range of applications. These results also encourage us to develop other phthalonitrile-functional poly(phthalazinone ether)s.
7.4 Polyamides and Polyimides Containing Phthalazinone Moieties
Wholly aromatic polyamides are an important class of high-performance engineering plastics. The typical commercialized product, aramid fiber, having an ultra-high strength and modulus as well as high temperature resistance and light weight, is widely used in various fields such as aerospace and electronics. Aramid fiber resin matrix composites have been used in aerospace, rockets, and aircrafts to reduce the weight of aircrafts in the result of increasing payload and saving fuel. Thus, the world market’s demand for high-performance all-aromatic polyamide is increasing. However, high rigidity of the backbone and strong hydrogen bonding interchains result in their insolubility in most organic solvents and also give rise to their high softening temperatures. These properties make them generally difficult and expensive to be processed. For example, Kevlar®, whose structure is poly(p-phenylene terephthalmaide) (PPTA), can only be spinned from its conc. H2SO4 solution. In our research, we attempted to prepare organic soluble lyotropic liquid crystalline aromatic copolyamides with other desired properties by incorporating the twisted and non-coplanar phthalazinone structure into the rigid main chain of PPTA for the first time. The synthesized system was established by composing p-phenylenediamine (PPD), 4,4’-diamino diphenylether (DAPE), terephthaloyl dichloride (TPC), and novel diamine, 1,2-dihydro-2-(4-aminophenyl)–4-[4-(4-(aminophenoxyl) phenyl)](2H)phthalazin-1-one (DHPZ-DA) (Figure 7.14) through low-temperature solution polymerization [53]. The key diamine DHPZ-DA was readily prepared by the condensation of DHPZ with p-chloronitrobenzene in the presence of potassium carbonate to give a dinitro compound at high yield, followed by reduction with hydrazine monohydrate/Pd-C.

Figure 7.14 Synthesis of soluble phthalazinone-containing copolyamides.
In the polymerization reaction, hydrogen chloride, generated from the polycondensation reaction between diamine and diacid chloride, was quickly absorbed by pyridine to prevent the side reaction of the diamine with it to form salt resulting in an obstacle of the polymerization. In addition, pyridine hydrochloride can increase the solubility of the resulting polymer. Lithium chloride (LiCl) was added into the polymerization system to enhance the solubility of the copolyamides. In this system, the addition of DHPZ-DA was optimized at 10% of the total amount of diamine monomers (p-103060) to improve the solubility in NMP solutions containing 1% LiCl and maintain the properties of aramid, for instance, with Tg of 348 °C, and a 5% thermal decomposition temperature 511 °C. Importantly, a slightly higher amount of dichloride than diamine was adopted to give the resultant copolyamide the inherent viscosity of 5.5 dL/g.
The resulting copolyamides were treated by heating above the Tg (360 °C) for 2 h before slow cooling. Their wide angle X-ray diffraction (WAXD) shows clear crystallization peaks. In addition, increasing the content of phthalazinone moiety and the ether bond in the copolymers gives rise to enlarge the distance between the polymer chains in the polymers calculated according to the data of WAXD. For example, when the content of DHPZ-DA was added up to 20% of the total diamine monomers, this distance between the polymer chains in the resulting copolymer p-202060 was about 10% larger than that in p-103060 with 10% DHPZ. his further demonstrates that the twisted, asymmetric phthalazinone structure prevents the main chain of the copolyamides from forming perfect regular alignment leading to effectively improved solubility.
We examined the lyotropic liquid crystalline behavior of the prepared copolyamides and their crystal structure [53, 54]. The copolymers were dissolved in concentrated sulfuric acid or NMP +1% LiCl at 2–30%. The solution was coated on a glass slide and then covered with a cover slide. The upper cover slide was shifted to apply shearing force to the polymers, which were then studied with a polarizing microscope (Figure 7.15). At specific concentrations, the solution shows a clear sector-shaped or a mesh-like texture with obvious optical anisotropy. The luminance and the range of the mesh-like structure become larger with increasing shear rate, indicating a larger liquid crystal area. his result shows that under certain traction forces, the rigid chains of phthalazinone-containing copolyamides can be orderly aligned, suggesting the strong effect of the intermolecular hydrogen bonds. his finding offers a good prospect for the application of polyamides bearing phthalazinone moieties in fibers and films.

Figure 7.15 Photomicrographs of polyamides solution taken at room temperature: (a) P2-103060 at 22wt % in 100% H2SO4 solution (100×), (b) P2-103060 at 24wt % in 100% H2SO4 solution (100×), (c) P2-103060 at 8wt % in NMP/1%LiCl solution (100×), (d) P2-103060 at 8wt % in NMP/1%LiCl solution (400×).
A series of high molecular weight copolyamides derived from the reaction of DHPZ-DA and its substituted derivatives, with commercial diamine as the comonomers, and terephthalic acid under the synergistic catalysis of triphenyl phosphite (TPP), pyridine, and anhydrous calcium chloride, were also prepared (Figure 7.16) [55]. All the copolyamides with the inherent viscosity in the range of 0.43–2.38 dL/g were soluble in NMP and DMAc. Their Tg values decreased slightly with increasing phthalazinone content but remained above 300 °C (306–346 °C). Meanwhile, improvement in the tensile strength of the copolymers above 67 MPa was manifested by yield behavior and ductile fracture with increasing phthalazinone moiety. This suggests that the incorporation of the phthalazinone structure increases the solubility of polyamides, maintains their excellent heat resistance, and improves their mechanical properties.

Figure 7.16 Synthesis of copolyamides with phthalazinone moieties.
Polyamide-imides, generated by introducing amide groups into the main chain of the polyimide, have heat resistance similar to polyimides with improving the solubility and processing performance relative to polyimide. The polyamide-imides are widely used in insulating paints, enamel insulated wires, and other fields. We have synthesized a series of phthalazinone-containing polyamide-imides [56] with the structure shown in Figure 7.17.

Figure 7.17 Structure diagram of polyamide-imides containing phthalazinone moieties.
The unique structure of the phthalazinone-containing polyamide-imides gives these copolymers excellent thermal properties. Their Tg is 317–355 °C with 5% thermal decomposition temperatures above 450 °C. They have good mechanical properties (film tensile strength higher than 130 MPa), electrical insulation properties (volume resistivity greater than 1016 Ωcm), and are soluble in the aprotic polar solvents NMP and DMAc.
The organosoluble polyimides were then prepared from phthalazinone-containing dianhydrides [57]. Four novel non-coplanar and asymmetric dianhydrides were synthesized on condensation of asymmetric heterocyclic bisphenol-like compounds with N-phenyl-4-chloro-phthalimide in presence of potassium carbonate, respectively, giving corresponding diimides, followed by hydrolyzed and dehydrated as usual. The “one-step” solution polymerization of the above four dianhydrides obtained with various aromatic diamines were undertaken successfully in m-cresol in the presence of a catalytic amount of isoquinoline at 200 °C (Figure 7.18). Polyetherimides having intrinsic viscosities of 0.45–1.81 dL/g in NMP or m-cresol were obtained. These polyetherimides are readily soluble in many common organic solvents such as DMAc, NMP, m-cresol, and chloroform. Their Tg values are in range of 279–355 °C, as determined by differential scanning calorimetry. Polyetherimide derived from BPA dianhydride and DHPZ-DA was also synthesized by the same polymerization as above [58]. Its Tg was 40 °C higher than commercially available Ultem’s Tg, derived from BPA-dianhydride and m-phenylenediamine.

Figure 7.18 Synthesis of homopoly(ether imide)s.
7.5 Phthalazinone-Containing Polyarylates
Wholly aromatic polyesters have good heat resistance, excellent mechanical strength, UV resistance, and creep resistance, and have been used as electronic materials and optical materials that play important roles in aerospace, electronics, and telecommunications. For example, U-polymer synthesized via a copolycondensation of bisphenol A and terephthalic acid/isophthalic acid mixture has good weather resistance and transparency with utility in lampshades and mirrors in electrical engineering. However, although the U-polymer can be subjected to melt processing, its melt viscosity is high leading to poor processing properties. It is particularly difficult to process large parts. In addition, its Tg is only 190 °C. Hence, poly(aryl ether)s with a high Tg and good solubility still need to be developed to expand their range of applications. We have also incorporated the phthalazinone structure into the main chain of the polyarylates to improve their heat resistance and solubility.
Firstly, the phthalazinone-containing diacid compound, 4-[4-(4-carboxy-phenoxy)phenyl]–2-(4-carboxyphenyl)–2,3-diaza-naphthalen-1-one (H-DA), was designed and prepared by nucleophilic substitution reactions of DHPZ and its substituted derivative with chlorobenzonitrile to give phthalazinone-containing dinitrile compounds, followed by hydrolysis at the present of base. Then high molecular weight polyarylates containing phthalazinone moiety were then obtained through the solution copolycondensation with various bisphenols [59] (Figure 7.19) at the present of a tosyl chloride (TsCl)/DMF/pyridine (Py) condensing agent, named as Higashi method [60–63]. This system is suitable for diacid systems that can dissolve in pyridine. In our system, H-DA can be soluble in pyridine by virtue of the twist, coplanar phthalazinone structure, although its structure is still rigid.

Figure 7.19 Synthesis of phthalazinone-containing poly(aryl ether) by the Higashi method.
The resulting polyarylates with the inherent viscosities of 0.15–0.58 dL/g have Tg valuesof 209–272 °C. In the bisphenol monomer, the isopropyl groups and sulfur atoms containing electron-donating groups have relatively high reactivity in the result of their high inherent viscosities. The Tg of the polyarylate synthesized by bisphenol A and H-DA is 40 °C higher than that of the commercial U-polymer derived from the copolycondensation reaction of bisphenol A, p-phthaloyl chloride, and m-phthaloyl chloride. They can be soluble in NMP, DMAc, pyridine, and chloroform at room temperature.
We also studied the copolymerization of bisphenol A and terephthalic acid with H-DA as the coreactant by Higashi method [64]. When the molar content of terephthalic acid was higher than 50% in the reaction system, no high molecular weight copolymers were found because the low molecular weight polymers were precipitated from the polymerization system. Thus controlling the molar content of terephthalic acid less than 50% successfully gave high molecular weight copolyarylates. Its 50 L pilot experiment was also performed. The inherent viscosities of the resulting polymers were all greater than 0.60 dL/g, and increasing phthalazinone content significantly improves the heat resistance and solubility of the resulting copolyarylates.
7.6 Phthalazinone-Containing Polybenzimidazole
Polybenzimidazole (PBI) has been recently attracted more attention to develop its application in fuel cell components because of its high mechanical properties, excellent thermal stability, and chemical resistance. Liu explored the effect of 4-phenyl phthalazinone moiety on the solubility, thermal properties, and structure of 4-phenyl phthalazinone-based PBIs [65]. The designed polybenzimidazoles having the inherent viscosities in the range of 1.10–2.05 dL/g, prepared by solution polycondensation in polyphosphoric acid (PPA) were found to be soluble in aprotic polar solvents such as NMP, DMAc, DMSO, and m-cresol without the addition of inorganic salts. And they also maintain the expected thermal properties such as high glass transition temperatures in the range of 398–408 °C and the temperatures for 5% and 10% weight loss of the polymers ranging from 516 to 594 °C and 560 to 672 °C, respectively. Then Li investigated the copolybenzoimidazole, synthesized by the polymerization reaction of H-DA, terephthalic acid, and 3,3’-diaminobenzidine in PPA, after doping with phosphoric acid [66]. Acid doping level is defined as the number of moles of absorbed phosphoric acid (mol H3PO4) per mole of PBI repeat unit. Results show that the doping level of polymers increases with the increasing content of phthalazinone moiety. The acid doped polymers having the highest doping level (15.2 mol H3PO4) give the best proton conductivity up to 0.13 S/cm at 160 °C, equivalent to the reported membranes at doping levels of 30–50 mol H3PO4 [67, 68]. This result is interpreted that the twisted non-coplanar phthalazinone structure, on the one hand, destroys the regularity of the polymer main chain and reduces the intra- and intermolecular force to weak hydrogen bonding, and on the other hand, the nitrogen atoms and carbonyl groups in phthalazinone structure also improve the absorption of phosphoric acid. This special structure affords interspaces to phosphoric acid and form a particular proton conduction pathway to facilitate proton transfer.
In order to enhance the chemical stability and mechanical property of her polybenzimidazoles containing phthalazinone moiety, Li further designed a kind of phthalazinone-based PBI with functional hydroxyl groups (PPBIOH) in the main chain to enhance the phosphoric acid absorbing and proton conductivity for high-temperature proton exchange membrane fuel cells [69]. PPBIOH exhibit high proton conductivities (>0.1 S/cm at temperatures above 120 °C) with just 10.1–12.2 mol H3PO4, good mechanical properties (stress of 1.7–2.9 MPa and strain of 28.1–68.0%), and high oxidative stability (breaking time in the range of 52–155 hr). The reasonable mechanical strength and oxidative stability, compared to nonhydroxyl group phthalazinone-containing PBI, can be due to cross-linking of hydroxyl groups in PPBIOH (Figure 7.20). Moreover, the PPBIOH membrane with relatively low acid doping level displays high proton conductivities.

Figure 7.20 Cross-linked structure of PPBIOH membranes.
7.7 Conclusions and Prospects
High-performance polymer materials have become indispensable materials in aerospace, electronics and electrical engineering as well as precision instrumentation, energy, machinery, transportation, petrochemistry, and other fields. Balancing their heat resistance and processibility has been pushing researchers to develop novel high-performance polymer materials having reasonable solubility and maintaining outstanding thermal stability.
Based on extensive experimental research, our research group has established a series of high-performance resins bearing phthalazinone moieties, including poly(aryl ether)s, polyarylates, polyamides, polyimide, polybenzimidazole, and poly(aryl s-triazine). The incorporation of twisted, non-coplanar, wholly aromatic phthalazinone structure into the polymer main chain can impart the polymers with both high temperature resistance and good solubility in the result of reducing their preparation cost with diverse processing methods, such as thermoforming and solution processing. Most importantly, they all exhibit excellent mechanical properties at elevated temperature. This greatly expands the potential applications of these resins in high temperature. Of the above resins, industrialized productions of PPESK, PPENSK, and PPESKK have been realized. In-depth study on these high-performance resins will be intensively undertaken to expand their applications in continuous fiber-reinforced thermoplastic composites, high-temperature-resistant functional membranes, high-temperature-resistant insulating paints, coatings, adhesives, etc.
Acknowledgments
We gratefully acknowledge the financial support of National Natural Science Foundation of China (21074017, 51273029, 59473019, 20604004) and Fundamental Research Funds for the Central Universities (No. DUT 14ZD219 and DUT16LK14), Liaoning Natural Science Foundation of China (No. 20170540175) and DUT16LK14), Liaoning Natural Science Foundation of China (No. 20170540175).
References
1. Alger, M. Polymer science dictionary. New York, Chapman & Hall, 1997.
2. Hergenrother, P.M., Connell, J.W., Labadie, J.W., Poly, H.J.L., Poly(arylene ether)s containing heterocyclic units. High Perform. Polym., 117, 67–110, 1994.
3. Berard, N., Hay, A.S., Polymers from hydroxyphenylphthalazinones. Polym. Prep., 34, 148–149, 1993.
4. Jian, X.G., Hay, A.S., Zheng, H.B., Poly(aryl ether sulfone) containing phthalazinone moiety and its preparation methods. Chinese patent, 1993.
5. Jian, X.G., Hay, A.S., Zheng, H.B., Poly(aryl ether ketone) containing phthalazinone moiety and its preparation methods. Chinese patent ZL93109179. 29, July, 1993.
6. Abubshait, S.A., Kassab, R.R., Al-Shehri, A.H., Abubshait, H.A., Synthesis and reactions of some novel 4-biphenyl-4-(2H)-phthalazin-1-one derivatives with an expected antimicrobial activity. Journal of Saudi Chemical Society, 15(1), 59–65, 2011.
7. Liu, Y.J., Liu, S.J., Jian, X.G., Synthesis and characterization of poly(ether sulfone ketone) containing phthalazinone moiety. Chin. Chem. Lett., 9, 257–258, 1998.
8. Berard, N., Paventi, M., Chan, K.P., Hay, A.S., Polymers from 4-(4-hydroxyphenyl)phthalazin-1-one. Macromol. Symp., 77(1), 379–388, 1994.
9. Jian, X.G., Zhu, X.L., Chen, L.Z., Study on polymerization mechanism of poly(aryle- ther)s containing phthalazinone moieties. J. Dalian. Univ. Technol., 39(5), 629–634, 1999.
10. Lihong Xiao., Cheng Liu., Gongxiong Liao., Yuan Zhang., Xigao Jian, Xiao, L.H., Liu, C., Liao, G.X., Zhang, Y., Jian, X.G., Synthesis and Characterization of Novel Copoly(aryl ether sulfone) Containing Phthalazinone and Biphenyl Moieties. High Performance Polymers, 22(3), 274–285, 2010.
11. Meng, Y., Hlil, A.R., Hay, A.S., Synthesis and thermal properties of poly(arylene ether ketone)s containing phthalazinone moieties. J. Polym. Sci. A Polym. Chem., 37(12), 1781–1788, 1999.
12. Meng, Y.Z., Hay, A.S., Jian, X.G., Tjong, S.C., Synthesis of novel poly(phthalazinone ether sulfone ketone)s and improvement of their melt flow properties. J. Appl. Polym. Sci., 66(8), 1425–1432, 1997.
13. Meng, Y.Z., Tjong, S.C., Hay, A.S., Morphology, rheological and thermal properties of the melt blends of poly(phthalazinone ether ketone sulfone) with liquid crystalline copolyester. Polymer, 39(10), 1845–1850, 1998.
14. Meng, Y.Z., Hay, A.A., Jian, X.G., Tjong, S.C., Synthesis and properties of poly(aryl ether sulfone)s containing the phthalazinone moiety. J. Appl. Polym. Sci., 68(1), 137–143, 1998.
15. Liao, G.X., Jian, X.G., He, W., Jin, Q.F., Li, Q., LH, W., Synthesis and properties of polyetherketone containing sulfide and phthalazinone moietie. Acta. Polym. Sin., 5, 641–646, 2002.
16. Hegenrother, P.M., Jenson, B.J., Poly(arylene ethers) from bis-1,3 and 1,4-(4-chlorobenzoyl) benzene. Polym. Prep., 26, 174–178, 1985.
17. Percec, V., Clough, R.S., Rinaldi, P.L., Litman, V.E., Reductive Dehalogenation vs Substitution in the Polyetherification of Bis(aryl chloride)s Activated by Carbonyl Groups with Hydroquinones: A Potential Competition between SET and Polar Pathways. Macromolecules, 27(6), 1535–1547, 1994.
18. Bhatnagar, A., Mani, R.S., King, B., Mohanty, D.K., Competing reactions for poly(aryl ether ketone) synthesis: 2. Preparationof high molecular weight polymer from hydroquinoneand 1,3-bis(4-chlorobenzoyl)benzene. Polymer, 35(5), 1111–1114, 1994.
19. Mani, R.S., Zimmerman, B., Bhatnagar, A., Mohanty, D.K, Poly, M.D.K., Poly(aryl ether ketone) synthesis via competing SNAR and SRN1 reactions: 1. Polymers derived from 1,3-bis(p-chlorobenzoyl)benzene and 1,3-bis(p-fluorobenzoyl)benzene with hydroquinone and 4,4’-isopropylidenediphenol. Polymer, 34(1), 171–181, 1993.
20. Hergenrother, P.M., Jensen, B.J., Havens, S.J., Poly(arylene ethers. Polymer, 29(2), 358–369, 1988.
21. Liu, Y.J., Jian, X.G., Liu, S.J., Synthesis and properties of novel poly(phthalazinone ether ketone ketone. J. Appl. Polym. Sci., 82(4), 823–826, 2001.
22. Liu, Y.J., Jian, X.G., Liu, S.J., Synthesis and characterization of poly(ether sulfone ketone ketone) containing phthalazinone moiety. Chin. J. Mater. Res., 14, 100–103, 2000.
23. Liu, Y.J., Jian, X.G., Zhang, J., Study on the rheological behaviors of a novel poly(ether sulfone ketone ketone) containing phthalazinone moiety. Eng. Plast. Appl., 26, 19–21, 1998.
24. Wang, M.J., Liu, C., Liu, Z.Y., Dong, L.M., Jian, X.G., Synthesis and characterization of phthalazinone-containing poly(aryl ether nitrile ketone ketone)s. Acta Polym Sin, 9, 833–837, 2007.
25. Rao, V.L., Saxena, A., Poly, N.K.N., arylene ether nitriles. J Macromol Sci Part C Polym Rev, 42, 513–540, 2002.
26. Matsuo, S., Murakami, T., Takasawa, R., Synthesis and properties of new crystalline poly(arylene ether nitriles. J. Polym. Sci. A Polym. Chem., 31(13), 3439–3446, 1993.
27. Haddad, I., Hurley, S., Marvel, C.S, Poly, M.C.S., Poly(arylene sulfides) with pendant cyano groups as high-temperature laminating resins. J. Polym. Sci. Polym. Chem. Ed., 11(11), 2793–2811, 1973.
28. Sivaramakrishnan, K.P., Marvel, C.S., Aromatic polyethers, polysulfones, and polyketones as laminating resins. II. J. Polym. Sci. Polym. Chem. Ed., 12(3), 651–662, 1974.
29. Saxena, A., Rao, V.L., Prabhakaran, P.V., Ninan, K.N., Synthesis and characterization of polyamides and poly(amide–imide)s derived from 2,2-bis(4-aminophenoxy) benzonitrile. Eur. Polym. J., 39(2), 401–405, 2003.
30. G.P, Y., Liu, C., Wang, J.Y., G.H, L., Han, Y.J., Synthesis, J.X.G., characterization, and crosslinking of soluble cyano-containing poly(arylene ether)s bearing phthalazinone moiety. Polymer, 51, 100–109, 2010.
31. Matsuo, S., Murakami, T., Polycyanoaryl ether films. European patent EP0192262, 1986.
32. Matsuo, S., Murakami, T., Nagatoshi, K., Bandou, T., Polycyanoaryl ether, method for preparing the same and uses thereof. European patent EP0243000, 1987.
33. Matsuo S.Murakami T1986Polycyanoaryl ether and method of preparing the sameEuropean patent EP0187638
34. Wang, J., Wang, M., Liu, C., Zhou, H., Jian, X., Synthesis of poly(arylene ether nitrile ketone)s bearing phthalazinone moiety and their properties. Polym. Bull., 70(5), 1467–1481, 2013.
35. Yan, Q.L., Wang, J.Y., Liu, Z.Y., Jian, X.G., S.Q, L., New coating for magnet wires. Wire Indust, 72, 177–180, 2005.
36. Anderson, D.R., Holovka, J.M, John, M.H., Thermally resistant polymers containing the s-triazine ring. J. Polym. Sci. A-1 Polym. Chem., 4(7), 1689–1702, 1966.
37. Keller, T.M., Dominguez, D.D., High temperature resorcinol-based phthalonitrile polymer. Polymer, 46(13), 4614–4618, 2005.
38. G.P, Y., Liu, C., Zhou, H.X., Wang, J.Y., Jian, X.G., Synthesis and characterization of soluble copoly(arylene ether sulfone phenyl-s-triazine)s containing phthalazinone moieties in the main chain. Polymer, 50, 4520–4528, 2009.
39. G.P, Y., Liu, C., G.H, L., Wang, J.Y., Jian, X.G., Thermal degradation kinetic of poly(aryl ether sulfone 1,3,5-triazine)s containing phthalazinone moieties. Thermochim. Acta, 514, 51–57, 2011.
40. G.P, Y., Liu, C., Wang, J.Y., T.S, G., Synthesis, J.X.G., characterization and properties of heat-resistant and soluble poly(aryl ether)s containing s-triazine units in the main chain. Polym. Degrad. Stab., 94, 1053–1060, 2009.
41. G.P, Y., Liu, C., Wang, J.Y., Xu, J., Jian, X.G., Synthesis and characterization of poly(arylene ether s-triazine)s containing alkyl-, aryl- and chloro-substituted phthalazinone moieties in the main chain. Polym. Int., 59, 1233–1239, 2010.
42. G.P, Y., Wang, J.Y., Liu, C., Lin, E.C., Jian, X.G., Soluble and curable poly(phthalazinone ether amide)s with terminal cyano groups and their crosslinking to heat resistant resin. Polymer, 50, 1700–1709, 2009.
43. Verborgt, J., Marvel, C.S., Aromatic polyethers, polysulfones, and polyketones as laminating resins. J. Polym. Sci. Polym. Chem. Ed., 11(1), 261–273, 1973.
44. G.P, Y., Liu, C., Wang, J.Y., X.P, L., Jian, X.G., Heat-resistant aromatic S-triazine-containing ring-chain polymers based on bis(ether nitrile)s: synthesis and properties. Polym. Degrad. Stab., 95, 2445–2452, 2010.
45. Keller, T.M., Synthesis and Polymerization of Multiple Aromatic Ether Phthalonitriles. Chem. Mater., 6(3), 302–305, 1994.
46. Keller, T.M., Dominguez, D.D., High temperature resorcinol-based phthalonitrile polymer. Polymer, 46(13), 4614–4618, 2005.
47. Sastri, S.B., Armistead, J.P., Keller, T.M., Sorathia, U., Phthalonitrile-glass fabric composites. Polym. Compos., 18(1), 48–54, 1997.
48. Dominguez, D.D., Keller, T.M., Properties of phthalonitrile monomer blends and thermosetting phthalonitrile copolymers. Polymer, 48(1), 91–97, 2007.
49. Dominguez, D.D., Jones, H.N., Keller, T.M., The effect of curing additive on the mechanical properties of phthalonitrile-carbon fiber composites. Polym. Compos., 25(5), 554–561, 2004.
50. Sumner, M.J., Sankarapandian, M., McGrath, J.E., Riffle, J.S., Sorathia, U., Flame retardant novolac–bisphthalonitrile structural thermosets. Polymer, 43(19), 5069–5076, 2002.
51. Lin, C.H., Cai, S.X., Leu, T.S., Hwang, T.Y., Lee, H.H., Synthesis and properties of flame-retardant benzoxazines by three approaches. J. Polym. Sci. A Polym. Chem., 44(11), 3454–3468, 2006.
52. G.P, Y., Liu, C., X.P, L., Wang, J.Y., Jian, X.G., Pan, C.Y., Highly thermostable rigid-rod networks constructed from an unsymmetrical bisphthalonitrile bearing phthalazinone moieties. Polym. Chem., 3, 1024–1032, 2012.
53. Liang, Q., Liu, P., Liu, C., Jian, X., Hong, D., Li, Y., Synthesis and properties of lyotropic liquid crystalline copolyamides containing phthalazinone moiety and ether linkages. Polymer, 46(16), 6258–6265, 2005.
54. Liu, P., Liang, Q., Liu, C., Jian, X., Hong, D., Li, Y., Preparation and characterization of lyotropic liquid crystalline aromatic copolyamides containing twisty and non-coplanar moiety. Polym. J., 38(5), 477–483, 2006.
55. Cheng, L., Jian, X.G., Mao, S.Z., Aromatic polyamides derived from unsymmetrical diamines containing the phthalazinone moiety. J. Polym. Sci. A Polym. Chem., 40(20), 3489–3496, 2002.
56. Zhu, X., Jian, X., Soluble aromatic poly(ether amide)s containing aryl-, alkyl-, and chloro-substituted phthalazinone segments. J. Polym. Sci. A Polym. Chem., 42(8), 2026–2030, 2004.
57. Wang, J.Y., Liao, G.X., Liu, C., Jian, X.G., Poly(ether imide)s derived from phthalazinone-containing dianhydrides. J. Polym. Sci. A Polym. Chem., 42(23), 6089–6097, 2004.
58. Wang, J.Y., Jian, X.G., Xiao, S.D., Synthesis and characterization of poly (ether imide)s containing phthalazinone and isopropyl moieties. Chin. Chem. Lett., 12, 593–594, 2001.
59. Gao, Y.R., Wang, J.Y., Liu, C., Jian, X.G., Synthesis of new soluble polyarylates containing phthalazinone moiety. Chin. Chem. Lett., 17, 140–142, 2006.
60. Higashi, F., Akiyama, N., Takahashi, I., Koyama, T., Direct polycondensation of aromatic dicarboxylic acids and bisphenols with tosyl chloride and N,N-dimethylformamide in pyridine. J. Polym. Sci. Polym. Chem. Ed., 22(7), 1653–1660, 1984.
61. Higashi, F., Ong, C.-H., Okada, Y., High-molecular-weight copolyesters of dihydroxybenzophenones by ?induced? copolyesterification using TsCl/DMF/Py as a condensing agent. J. Polym. Sci. A Polym. Chem., 37(18), 3625–3631, 1999.
62. Higashi, F., Yamada, Y., Hoshio, A., Direct polycondensations of hydroxybenzoic acids by use of diphenyl chlorophosphate in the presence of N,N-dimethylformamide. J. Polym. Sci. Polym. Chem. Ed., 22(9), 2181–2187, 1984.
63. Higashi, F., Takakura, T., Sumi, Y., Copolycondensation of various compositions of isophthalic acid and terephthalic acid with hydroquinones or bisphenols by tosyl chloride/dimethylformamide/pyridine. J. Polym. Sci. A Polym. Chem., 42(10), 2321–2328, 2004.
64. Liu, C., Chun, C.B., Wang, J.Y., Zhang, S.H., Jian, X.G., Synthesis and properties of polyarylates containing 4-phenylphthalazinone moiety. Acta Polym Sin, 2, 186–191, 2011.
65. Liu, C., Li, X., Xu, J., Jian, X., Synthesis and characterization of novel polybenzimidazoles containing 4-phenyl phthalazinone moiety. Eur. Polym. J., 47(9), 1852–1860, 2011.
66. X.P, L., Liu, C., Zhang, S.H., G.P, Y., Jian, X.G., Acid doped polybenzimidazoles containing 4-phenyl phthalazinone moieties for high-temperature PEMFC. J Membr Sci, 424, 423128–423135, 2012.
67. X.P, L., Liu, C., Zhang, S.H., Zong, L.S., Jian, X.G., Functionalized 4-phenyl phthalazinone-based polybenzimidazoles for high-temperature PEMFC. J Membr Sci, 442, 160–167, 2013.
68. Iwakura, Y., Uno, K., Imai, Y., Polyphenylenebenzimidazoles. J. Polym. Sci. A Gen. Pap., 2(6), 2605–2615, 1964.
69. Chen, X., Shao, Z.Z., Huang, Y.F., Zhou, P., T.Y, Y., Influene of crosslinking agent content on structure and properties of glutaraldehyde crosslinked chitosan membranes. Acta Chim Sin, 58, 1654–1659, 2000.
