Persisting issues with the most recognized building material health risks
Lead and asbestos
Emina Kristina Petrović, Victoria University of Wellington, Wellington, New Zealand
Abstract
This chapter uses the examples of lead and asbestos to evaluate whether there are any examples of successful elimination of harmful substances, and if so, which steps were needed in the process. The chapter reviews patterns in very slow recognition and action towards reductions and elimination of exposures to lead and asbestos. It proposes that even after 2–3 decades of regulation around these most known substances of concern, they are still not fully eliminated, and a more sophisticated understanding of just how harmful they are is still emerging.
Keywords
Known hazards; lead (Pb); asbestos; user health; building materials
6.1 Introduction
This chapter takes a detailed look at lead and asbestos, two examples of substances in the third stage of recognition of health risks: they are well known, have been known about for a long time, and have even been legislated against for a reasonably long time. In fact, when the health risks associated with building materials are mentioned many people immediately think of these two, well-known hazardous materials. Both lead and asbestos have been used since prehistoric times. The adverse health effect of lead was clearly observed during the time of the Ancient Greeks, while for asbestos the effects were only noted in the late 19th century. Thus, both lead and asbestos were already in the first stage of the early recognition of risks before the start of the 20th century. Yet, despite this, both materials have seen prolific increase in use during the 20th century. This historical reality, or paradox, of a high increase in the use of materials which were already in part recognized as health hazards is very important. It clearly establishes the idea that during much of the 20th century human health was not valued as much as the physical properties both materials offered. As this chapter shows, the evolution of the increased recognition of the risks they posed and gradual regulation against them also tells an important story of repeatedly slow reactions, tendencies to dismiss strong indications of a health problem as being based on insufficient evidence, and general continuation of favoring the physical properties of material and economic efficiency over human health. These are good examples of a disregard for the precautionary principle.
6.2 Issues with lead
6.2.1 Lead: general overview
Lead (Pb) has been used by human society for at least 4000–5000 years (Brown and Margolis, 2012). Lead is a naturally occurring metal which, historically, has been used in architecture for its pliability in forming the glazing beads (cames) in lead windows, lead roofs, flashings, and plumbing. During the 20th century it was mainly used in architecture as a stabilizer for lead-based paints.
As early as 370 BC, Hippocrates made some of the earliest recorded observations of health issues related to lead and other heavy metals (Philp, 2001, Chapter 6). Some sources have proposed that lead poisoning influenced the fall of the Roman Empire (Hayes, 2012). Better understanding of the risks eventually led to the passing of the first Food and Drugs Act by the British Parliament in 1875, but despite this cast lead soldiers and other lead toys were fairly common until the late 1940s and early 1950s (Philp, 2001, Chapter 6). Therefore, it is possible to observe that lead was already in the first stage of recognition of health risks before the start of the 20th century, but that the real action against it, indicative of transition to the second stage, did not start until mid-20th century.
Lead can negatively affect almost every organ, system, and process in the human body, including the cardiovascular, gastrointestinal, hemolymphatic, urinary, immune, nervous, and reproductive systems, and can cause tumors in laboratory animals (Carlisle et al., 2009). Of all the systems listed, the main target for lead toxicity is the central nervous system (Sanders et al., 2009). For painters with high lead exposure peripheral neuropathy and cognitive impairment have been recorded, starting with problems with the upper limbs (Krishnan et al., 2012). However, lower levels of on-going exposure can lead to inhibition of several enzymes involved in heme synthesis, influencing functions of the peripheral and central nervous system and increasing blood pressure (Jakubowski, 2011).
Young and unborn children are more at risk from lead than adults because their brains and nervous systems are developing, and because their bodies absorb much higher proportions of ingested lead than adult bodies (Sanders et al., 2009). The blood–brain barrier recognizes one common form of lead in the body as calcium thus allowing its entry into the brain, where lead can take over the functional role of calcium, altering neurochemistry and behavior, especially in the immature brain (Sanders et al., 2009). Acute lead poisoning in children starts with vertigo and irritability, progressing to delirium, vomiting, and convulsions (Philp, 2001, Chapter 6). Studies have shown that lead exposure in children persists into adulthood, and has been associated with brain damage, mental retardation, behavioral problems, developmental delays, violence, and death at high levels of exposure (Sanders et al., 2009).
The half-life for lead in blood is generally 30–35 days, while in bones it is 25 years; bones, hair, and teeth are where most of the free blood lead deposits (Philp, 2001, Chapter 6; Carlisle et al., 2009). Lead in the bones may be remobilized back into circulation at times of stress and tension, such as pregnancy, illness, traumatic life events, and aging (Zahran et al., 2009; Machida et al., 2009). Although the human body can eliminate lead, most of it stays in the body for a long time, contributing to the total body load.
Lead can come in different forms, as elemental lead, in organic lead compounds, and inorganic lead compounds. It can also change forms, e.g., from organic to inorganic (American Cancer Society, 2014). In buildings the most likely form of lead is in inorganic lead compounds. Although, carcinogenicity of substances differs from their toxicity, lead is recognized as a risk in both areas, and all forms of lead are toxic. However, the International Agency for Research on Cancer (IARC) recognizes different forms of lead as varying in their carcinogenicity: they classify inorganic lead compounds in Group 2A (probably carcinogenic to humans), elemental lead in Group 2B (possibly carcinogenic to humans), while organic lead compounds are classified as Group 3 (not classified for carcinogenicity to humans) (IARC, 2016).
6.2.2 Regulations against lead
During the 1970s, systematic actions against lead exposure started in many parts of the world. In this period the main focus was leaded petrol and lead-based paints. Recognitions of the health risks associated with lead in petrol increased during the 1970s leading to removal of leaded petrol in many countries during the 1980s and 1990s (O’Grady and Perron, 2011). In the United States, the Lead Paint Poison Prevention Program was introduced in 1970 (Philp, 2001, Chapter 6). This was followed with the 1977/78 US ban of lead-based paint (Roberts et al., 2012). The level of restriction varied in different countries at different times, e.g., the 1976 Canadian limit for lead in interior paints was set eight times higher than the US restrictions 2 years later (O’Grady and Perron, 2011). In France, the sale of lead carbonate, which was used in paint, was officially only banned in 1993 (Lucas et al., 2012). Therefore, for many developed countries during the 1970s lead has gone through the second stage of recognition of risk, leading to bans from the late 1970s.
Unfortunately, these trends are not shared by all countries. In the last 10 years, high levels of lead in new paints have been found in 20 countries from five continents (Ewers et al., 2011). Most are developing countries where legislation limiting lead content either does not exist or is ineffectively enforced, but some are more developed countries such as India and Taiwan (Khan et al., 2010; Ewers et al., 2011). For example, the study of Ewers et al. (2011) tested newly purchased paints in Taiwan and found that the median lead concentration was more than 30 times the current US standard and less than half of the paint samples had lead content within the current US standard (Ewers et al., 2011). They also observed that when samples from China were tested these had lower levels of lead.
6.2.3 Impact of low levels of lead on human health
Since the introduction of more strict regulations against lead, new studies have measured the impact of low levels of lead on human health. Evaluation of such low levels was generally not possible before regulative action, because most people already had higher levels in their system. Therefore, this section shows the issues associated with attempts at comprehensive assessments of a problem substance when the exposure level in the general population is already elevated: the subtler impacts are impossible to study. This further indicates the importance of action to limit exposure even before a more sophisticated understanding of the issues is possible.
Current research indicates that even very low levels of blood lead in children can cause a measurable negative impact on their development. Levels of safe exposure changed in the later decades of the 20th century. In 1991, the US Centers for Disease Control and Prevention decreased earlier action blood lead levels to 10 μg/dL for children under the age of six (Roberts et al., 2012), and the same measure was established in many other countries soon after (Oulhote et al., 2011). (10 μg/dL equates to 100 μg/L, both measures are found in the literature.) Good indicative examples of earlier action blood lead levels of concern include the 1960 US level of 60 μg/dL (Jakubowski, 2011) and the 1987 Canadian level of 25 μg/dL (O’Grady and Perron, 2011), clearly indicating a general trend towards lowering acceptable levels.
The action blood lead level of 10 μg/dL for children reflected the knowledge available at that time, which demonstrated a decline in IQ points with every incremental elevation of blood lead levels greater than 10 μg/dL (Roberts et al., 2012, p. 1). In 1978, the US national blood lead level in children aged 1–5 years was over 10 μg/dL for 13.5 million children or almost 90% of American children (Brown and Margolis, 2012). By the early 1990s that figure had dropped to about 10% and continued dropping to approximately 1.5% of all American children by 2007–08 (Fig. 6.1; Brown and Margolis, 2012). Similarly, in the late 1970s, the geometric mean blood lead level for all American children was 15 μg/dL, and in 2012 it was under 2 μg/dL (Brown and Margolis, 2012). This significant decrease in blood lead has enabled more subtle studies, and also indicates that in some countries, even after the official bans on use of lead as a sign of the third stage of recognition, some of the features of the second stage continued, creating a need to decrease regulated levels to help children specifically at risk. Nevertheless, more recent estimates still assert that in the United States 25% of the population are at risk from high lead exposure (Jordan et al., 2003), and lead poisoning in children costs the nation $43.4 billion a year, compared to $2.0 billion for childhood asthma (Ferguson et al., 2012).
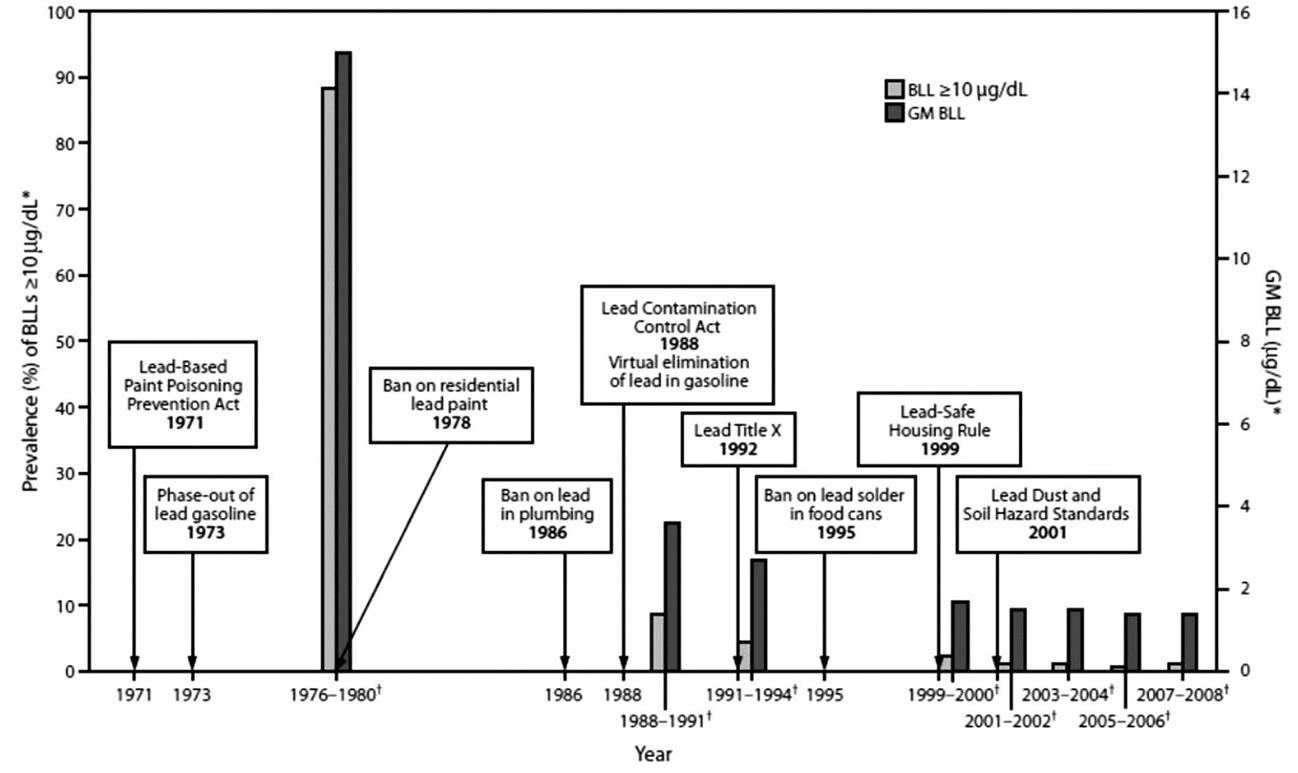
Research since the 1970s has demonstrated that the greatest rate of decline in IQ occurs in blood lead levels of between 3 and 8 μg/dL (Roberts et al., 2012, p. 1). Therefore, in 2000 the World Health Organization Regional Office for Europe recommended efforts be made to ensure that at least 98% of an exposed population of all ages has blood lead levels under 10 μg/dL, which is supported by the current median blood lead level of under 5.4 μg/dL (Jakubowski, 2011). A very similar level and rationale were set in 2012 by the US Centers for Disease Control (Roberts et al., 2012, p. 1). A French survey from 2007 involved 3800 children and estimated a geometric mean for blood lead level of 1.5 μg/dL, with 0.11% of children having a higher blood lead level of above 10 μg/dL (Oulhote et al., 2011). A study from 2007 to 2008 compared blood lead levels for children in six European cities (mainly central European) and three non-European cities. It showed that blood lead levels in children varied very little between European countries but were noticeably higher in the non-European cities they studied, these being in China, Ecuador, and Morocco (Hrubá et al., 2012). This implies that many parts of the developed world have lead exposure under reasonable control, but that this is not a trend shared worldwide.
Earlier studies, such as one from 1995, suggested a mean decrease in full-scale IQ of the order of 2 IQ points for a change in mean blood lead level from 10 to 20 μg/dL (Jakubowski, 2011). Subsequent studies have established that in fact much of the decrease of IQ occurs for exposures under 10 μg/dL: for a change of exposure from 2.4 to 10 μg/dL, IQ tends to change by 3.9 points, while for a change of exposure from 20 to 30 μg/dL, IQ changes by 1.1 points (Jakubowski, 2011). Similarly, in the 1990s, research established strong links between IQ and blood lead levels in children of 2 years of age, while subsequent research, even of the same cohort, suggests that exposures of school-age children to lead may be more strongly related to performance in cognitive testing (Mazumdar et al., 2011). Several studies have found that poorer performance in these types of test correlate to higher levels of lead, even for levels as low as 2 μg/dL (Jakubowski, 2011; Zahran et al., 2009; Amato et al., 2012).
The current low and moderate blood lead levels in many parts of the Western population have also been shown to have a significant impact on other aspects of children’s development. Moderate blood lead levels are correlated to lower growth rate, lower height, and lower weight for age (Yang et al., 2012). Levels of lead from 5 μg/dL have been found to alter reproductive hormones in young girls (Gollenberg et al., 2010). Lead has contributed to attention-deficit hyperactivity disorder (ADHD), and this was even a trend with children who are asymptomatic for ADHD (Winneke, 2011). It has been suggested that lead and other heavy metal prenatal exposure impairs the development of visual processing (Ethier et al. 2012). Further, blood lead level has been found to be correlated to increased stress responses at currently “normal” levels under 10 μg/dL (Gump et al., 2009). Concerns have been raised that lead poisoning can cause nerve damage to the sense organs and nerves controlling the body, increasing the chance of neurodegenerative diseases like Alzheimer and Parkinson diseases later in life (Sanders et al., 2009; Li et al., 2010).
While the negative impact of lead on memory, learning, and IQ has often been studied, there is also evidence that lead influences other behaviors such as mood (depression), anxiety, schizophrenia, and violence/aggression (Sanders et al., 2009). It has been noted that lead-exposed 4–5-year-old children exhibit an increase in aggression, and from there lead exposure has been associated with juvenile delinquency and criminal behavior (Sanders et al., 2009, Narag et al., 2009). Between 1979 and 1984, a Cincinnati-based study recruited pregnant women living in impoverished neighborhoods with a high concentration of older, lead-contaminated housing (Wright et al., 2009). It measured prenatal and early childhood exposure to lead, and subsequently compared that data with the local criminal justice records on arrests of the same children by 2005, then 19–24 years of age. Increased blood lead levels before birth and during early childhood were associated with higher rates of arrest than the control group. Also, average childhood blood lead was significantly associated with higher risk of arrest involving violent crime (Wright et al., 2009). In a similar New Zealand study, 1265 Christchurch children were studied from birth (in 1977) to age 21 (Fergusson et al., 2008). Their dental lead levels at ages 6–9 were significantly associated with both officially recorded violence/property convictions and self-reported violence/property offending.
More recent research on lead has attempted to identify more subtle ways in which lead is harmful for human bodies. For example, lead has also been researched in relation to body processes such as oxidative stress, cell membrane biophysics, and signaling and neurotransmission (Verstraeten et al., 2008). Another study has established that the presence of lead in cells can trigger the sequence of preprogrammed bodily processes called apoptosis, which leads to the elimination of cells without releasing harmful substances into the surrounding area (Yedjou et al., 2010). While this is a healthy process that protects the body from unhealthy cells or substances, Yedjou et al. noted that the mechanisms by which lead induces this elimination remain unclear. However, their study has demonstrated how the presence of lead nitrate can trigger cellular processes that lead to the formation of human leukemia (HL-60) cells (Yedjou et al., 2010). A 2011 study established that there are genetic biomarkers of increased neurotoxic risk, indicating that boys with a particular genetic expression are most likely to be affected (Sobin et al., 2011). Research of this nature could lead to development of targeted prevention programs, particularly for those who are more at risk.
More recent studies have also made much progress with identifying both possible sources of high exposure, such as certain food plants that tend to absorb and carry higher concentrations of lead (for mushroom varieties particularly prone to absorbing lead see Petkovšek and Pokorny, 2013) or foods, such as brown rice, that might be able to help reduce mammal lead-induced toxicity (Zhang et al., 2010). Furthermore, it has been observed that blood lead levels seem to fluctuate in relation to the seasons, yet without a proper understanding of what influences these fluctuations (Havlena et al., 2009).
The results of these studies of more recent, postregulation issues associated with lead clearly indicate that when the general level of exposure is very high, it is very hard for the research community to evaluate the important thresholds for adverse effects on human health accurately. This conclusion is very disturbing, because it indicates that researchers could be facing similar inability to fully evaluate adverse effects for any other substances which are highly prevalent within the population. Thus, the same could be now taking place with chemicals which are in the second or even in the first stage of recognition of risk.
6.2.4 Issues facing recovery from lead contamination
Some significant sources of lead have continued even after its official bans. The US Center for Disease Control and Prevention lists these as: lead-contaminated soil, food, tap water, lead paint, folk remedies, pottery, and dust (Brown and Margolis, 2012). During the late 1970s and early 1980s, when recognition of the risks associated with lead exposure culminated in various bans, all of these sources were likely to contain lead. Since then, there has been much improvement: lead in tinned food and lead in new paint have been eliminated in developed countries, and much work has been done to reduce the prevalence of lead pipes in public water supplies (Brown and Margolis, 2012). However, the historical use of lead has left a legacy of contamination in the natural environment, since elemental lead does not degrade (Carlisle et al., 2009). Furthermore, lead continues to be newly harvested, and is often used in electronic items, which creates new potential lead pollution at recycling points for electrical waste (Yang et al., 2012). In addition, due to poorer regulations in less developed countries, imported items manufactured in such countries can introduce new lead contamination (Guney and Zagury, 2012). Unfortunately, even in very recent years hidden lead has been found in internationally available toys and low-cost jewelry (Guney and Zagury, 2012).
Nevertheless, the most significant concern associated with lead in developed countries is lead in paint in older housing. In the United States this is increasingly recognized as a problem that comes together with poverty, because US children from low-income families are about three times more likely to have high blood lead levels than children from middle- and high-income families; and similarly, children from the inner city, with concentrations of older housing, are four times more likely to have elevated blood lead levels than children in other areas (Sanders et al., 2009). One comprehensive US intervention specifically targeted windows because they have the highest likelihood of containing lead paint and the highest amounts of lead dust (Dixon et al., 2012). Surveying 189 homes from four cities, they had a group where all windows were replaced, a group where some were replaced, and a group with no replacement windows. All windows that were not replaced were repaired and maintained. Their results show that 12 years after the intervention homes with all windows replaced had interior floor dust lead and window sill dust lead levels almost half those in nonreplacement homes, even when controlling for other factors (Dixon et al., 2012). The homes where some windows were replaced also exhibited a decrease, which was about half the decrease from complete replacement (Dixon et al., 2012). Furthermore, one study found a child with a blood lead level of 23 μg/dL who lived in a house with lead-containing paint which was observed to have excessive dust on the windowsills, dirt on the carpet, and a dirt-covered yard but no evidence of peeling paint (Roberts et al., 2012), indicating that the overall level of cleaning in older houses can contribute to the problem.
While window replacement is a fairly expensive strategy and can often be impracticable, improvements in management of household cleaning can come at no or very low cost, and some studies have evaluated the effectiveness of different educational initiatives on lead risks and strategies to reduce exposure, including effective and safe cleaning in housing with lead paint (Ferguson et al., 2012; Jordan et al., 2003, 2004, 2007; Brown et al., 2006 Hilts et al., 1998).
Early studies on the effectiveness of educational interventions related to lead were in highly polluted areas. For example, during the 1990s, the town Trail in Canada has been actively monitored because of the active lead/zinc smelter in the area, and because many children had blood lead levels at the individual intervention level, set then at 15 µg/dL (Hilts et al., 1998). The actions on site included indoor dust control trials, ongoing soil treatment experiments, close monitoring of community blood lead levels, and included community education (Hilts et al., 1998). Even when controlled for other factors, the researchers concluded that community education was making a measurable difference in children’s blood lead levels, especially when comparing outcomes of years with and without such programs (Hilts et al., 1998).
When comprehensive peer studies of educational interventions on children’s blood lead levels were conducted, findings were mixed, with many pointing to measurable reductions (Hilts et al., 1998; Jordan et al., 2003; Ferguson et al., 2012), and others not observing such a clear difference (Brown et al., 2006). For example, when children’s blood and household lead dust levels were comprehensively monitored and compared in paired groups either receiving the standard level of education or a more intense 20-visit intervention, the latter reduced the risk of blood lead levels over 10 µg/dL by approximately 34% (Jordan et al., 2003). Others have observed no such difference between the intervention and nonintervention groups and concluded that once the blood lead level is elevated, it is hard for educational interventions to help to reduce it (Brown et al., 2006).
Research on the outcomes of educational interventions has identified a wide range of factors that influence possible outcomes, decreasing causal clarity. For example, the participants of the nonintervention group generally also receive additional information about their lead exposures, either through measured blood lead levels, or through measured lead content in household dust, or both (Jordan et al., 2003; Brown et al., 2006), and some participants have reported this information as inspiring positive change (Jordan et al., 2003, 2004). Additionally, it is hard to evaluate all possible influential factors, which are as diverse as the level of rainfall in the months leading to the study, which influences the lead in outdoor dust (Hilts et al., 1998), or the type of floor cleaner used for floors painted with lead paint, which can influence greater or lesser release of lead (Jordan et al., 2004).
Some educational interventions monitored neither blood lead level nor household dust, but self-reported change. For example, a broad-scale educational intervention on lead exposure in Arkansas with surveys immediately after the educational intervention and 36 months later showed 45% of participants reported some changes to prevent exposing a child to lead, while 53% reported planning to change something (Ferguson et al., 2012). Such programs have been recognized as increasing the level of lead awareness through education by empowering individuals with knowledge that can aid in reducing blood-lead levels in at-risk children (Ferguson et al., 2012). Such programs are also very cost-effective (Ferguson et al., 2012).
Based on this review it is possible to conclude that the issues facing recovery from lead contamination still seem complex. Lead contained in existing buildings is at the core of this problem and more education and more financial assistance are needed to support a decrease in health risks from lead exposure. Absence of regulations and monitoring of activities in homes, however, makes systematic changes difficult, because many activities from basic DIY projects to cleaning practices can influence the release of lead.
6.2.5 Lead: summary
This overview shows that early regulations against lead exposure were delayed after the articulated recognition of the risks. Although it is hard to give a clear date when the first stage of recognition started for lead, because it probably dates to prehistoric times, it is easy to assert that lead was established in the first stage of recognition before the start of the 20th century. Yet, no significant efforts towards regulation can be observed in the first half of that century. During the 1970s many Western countries experienced the greatest change in treatment of lead through the proposed elimination of leaded petrol and leaded paints, and this decade can be seen as an accelerated second stage of recognition of risk. The third stage then emerged by the end of the 1970s with the ban on leaded paint, which was followed during the 1980s–90s with the ban on leaded petrol. Despite these bans in Western countries, many less developed countries are still in the very early stages of risk recognition.
Furthermore, for lead, research and subsequent decreasing “action levels,” generally characteristic of the second stage, are still on-going, long after the bans. It is now known that the average blood lead levels common in US children of the 1970s have probably negatively impacted many aspects of their development, which was impossible to observe while the overall level of lead pollution was much higher. Similarly, the main current source of contamination is old housing with lead paint, which in the past was a relatively minor risk, compared to new application of such paints, lead water pipes, and the pollution from leaded petrol. Furthermore, the total impact is still unknown, because the release of blood lead stores in the later stages of life has generally not yet happened for the children of the 1970s. More sophisticated understanding of the adverse effect lead has on the human body will continue to be produced in years to come. For instance, a recent cost–benefit analysis suggests that for every dollar spent to reduce lead hazards $17–$220 is saved, which compares well to immunization costs (Brown and Margolis, 2012). Therefore, lead still does not appear to be a cold case.
6.3 Issues with asbestos
6.3.1 Asbestos: general overview
Asbestos use began 4500 years ago, but significantly increased during the 20th century, and peaked during the mid-1970s (Park et al., 2012). Asbestos is the general commercial name for a group of naturally occurring mineral silicate fibers of the serpentine and amphibole series (Fig. 6.2; Park et al., 2012). Asbestos minerals are crystalline with weaknesses in the crystal structure which cause long thin fibers to be released along fracture planes and become airborne when the rock is stressed (Donaldson and Poland, 2012). Normally, asbestos is defined as a mineral fiber with lengths of 5–10 µm, proportionally small fiber diameters of usually under 1 µm, and with an aspect ratio >3:1 (Park et al., 2012). While its mineral chemical composition gives asbestos its recognized properties against fire, in the building industry it was also often used to reinforce a surrounding material, such as cement, due to the properties of these mineral fibers. In many Western countries, asbestos-containing products were widely used in construction between the 1920s and mid-1980s, in products such as roof tiles, wall claddings, vinyl flooring, sprayed fire protection, decorative ceilings, roofing membranes, adhesives, and paints (Level, 2016).
Unfortunately, the same feature that gave asbestos fiber good applicability in the construction industry is the foundation of the health problems it causes, and in contrast to lead, this somewhat narrows down the range of impacts asbestos has on the human body. Although much research has been undertaken over the last few decades, the precise molecular mechanisms involved with asbestos are not yet fully understood (Liu et al., 2013). However, it is well known that asbestos exposure can lead to a series of different lung diseases, such as pulmonary fibrosis (asbestosis), pleural abnormalities (effusion and plaques), and malignancies (bronchogenic carcinoma and mesothelioma) (Liu et al., 2013). Further, due to their small diameter, proportionally long length, and biopersistence, these fibers are easy to inhale, and once in the body they can cause a number of processes in the lung that could lead to carcinogenesis (Donaldson and Poland, 2012). Long biopersistent fibers can generate free radicals chronically that directly damage DNA, leading to long accumulation of dose and interaction with cells of the immune system. In addition, the long fibers create a series of processes that inhibit positive cell functioning and trigger the defensive mechanism, which becomes chronic due to cellular inability to expel pollution of the size and proportion of asbestos fibers (Donaldson and Poland, 2012).
One of the challenges with research on asbestos is the long latency period between exposure to asbestos and presentation of health concerns, ranging from 15 to 40 years (Liu et al., 2013). This is related to the damage which asbestos fibers create due to the body’s inability to eliminate them effectively, which keeps certain protective and eliminative processes in a state of chronic overuse, thus gradually creating health problems (Sanchez et al., 2009; Donaldson and Poland, 2012). Delays like this have historically made conclusive research more difficult and contributed to blurring of the recognition of the problem.
The evaluation of the health impact of asbestos is further complicated by its natural variability which, at least on a theoretical level, justifies the assumption that its impact could vary to reflect this. Generally, chrysotile asbestos has been seen as potentially less harmful due to various estimates that once in the body it disintegrates, which makes it less pathogenic than the amphiboles (Donaldson and Poland, 2012). This could potentially be very significant, given that chrysotile asbestos represented as much as 95% of the asbestos used worldwide since 1900 (Park et al., 2012). Unfortunately, recent reviews strongly assert this is a misconception (Kanarek, 2011), and concur with the IARC, that all forms of asbestos are recognized human carcinogens and should be banned (Paglietti et al., 2012). Furthermore, in 2009, having asserted the need for a complete ban on all asbestos use, the IARC concluded that several nonpulmonary forms of cancer can be caused by exposure to asbestos, and that asbestos has been associated with risk of systemic autoimmune disease (Park et al., 2012).
There is also recognition that occupational exposure to asbestos means the substance has often made its way into workers’ homes through the dust brought home on clothing, which then exposes families to risk (Peretz et al., 2008). More recently, cases of long-latent asbestos-related health problems are becoming apparent in adults who were exposed to asbestos as children through their parents’ work (Reid et al., 2013; Peretz et al., 2008). Similarly, longitudinal studies of people who were exposed to asbestos outdoors as children in the 1970s because of local mining are now giving conclusive results that childhood exposure to asbestos increases cancer incidence rates, mainly but not exclusively due to an increase in rates of mesothelioma (Reid et al., 2013). The number of individual cases of this nature is likely to continue increasing because of the long latency period.
In contrast to lead where evaluations of bodily exposures are possible (as seen through measured blood lead level and bone lead level), none of the articles found during this review reported such measures for asbestos. Rather, many research articles used questionnaires and interviews to obtain descriptive accounts of possible asbestos exposure (Peretz et al., 2008; Olsen et al., 2011), or, when possible, measured prevalence of asbestos in air (Reid et al., 2013). This, together with the long latency period, makes accurate studies very difficult, and this is especially the case for lower levels of exposure. Recent research has, through studies of the children of asbestos workers or children who lived in affected neighborhoods, established that their exposure, which was probably much lower than that of the asbestos workers themselves, has also led to adverse health effects (Reid et al., 2013; Peretz et al., 2008). Thus, it seems that for asbestos indirect indications will be needed to evaluate the extent of adverse effects from lower levels of exposure. Studies of removal work carried out now could become especially useful in showing the impact even lower levels of exposure have on human health, but this is likely to mean waiting for 40–50 years before enough evidence accrues given the long latency period.
6.3.2 Regulations against asbestos
Around 1977, when the demand for asbestos peaked internationally, some 25 countries produced a total of 4.8 million metric tons per year, to be used in 85 countries (Park et al., 2012). Although the use of asbestos has since been banned in many industrialized countries, early in the 21st century, global use was still 2.1 million metric tons per year, primarily in Asian and less developed countries, where most of it is used to manufacture asbestos cement building materials (Park et al., 2012). This prolific industrial use of asbestos during the 20th century should be seen in contrast with early recognitions of its negative health impact. In 1898, early in the period of the industrialized use of asbestos, Adelaide Anderson, Principal Lady Inspector of Factories in the British Home Office, accurately noted that “[t]he sharp jagged edge of the insoluble mineral dust had undoubtedly occasioned much illness and death from respiratory disease” (Department of Labour, 2006, p. 38). Early in the 20th century, the relationship between asbestos dust exposure and fibrosis of the lungs and asbestosis was already recognized, and by the 1950s and early 1960s the relationship between lung cancer and mesothelioma and asbestos exposure was confirmed (Department of Labour, 2006, p. 38). Yet, the production was at that time still internationally increasing. This makes asbestos a good example of a material that has been used for a long time since first being recognized as a hazard, thus since the first stage of recognition of risk.
Although early regulations that deal with asbestos date back to 1931 with the formation of the British Asbestos Industry Regulations (Department of Labour, 2006, p. 38), it was in the 1980s that much change took place and many countries started to ban the use of blue and brown asbestos (Department of Labour, 2006, pp. 35–36). However, the complete ban of asbestos generally took place later: Italy in 1992 (Paglietti et al., 2012); New Zealand in 2002 (Waikato District Health Board, 2012); Australia in 2003 (Olsen et al., 2011); and Japan in 2005 (Park et al., 2012). By 2012, 52 countries had completely banned use of any form of asbestos (Paglietti et al., 2012). Therefore, for asbestos the start of the third stage came in two stages. For many countries there were about 20 years between the start of banning and complete bans, which often occurred at the start of the 21st century, more than 100 years since articulation of the first stage of recognition of risk.
6.3.3 Issues facing recovery from asbestos contamination
Although still in use in many less developed countries, it is now possible to start assessing the legacy of asbestos since its official ban in many developed countries. Unfortunately, this reveals new types of concern. Olsen et al. (2011) discuss the impact of asbestos in three waves: first the workers mining and milling raw asbestos and manufacturing asbestos products; second the workers who used asbestos products in industry, such as the building industry; and finally the third wave consisting of people diagnosed with asbestos-related diseases after a short-term and/or low-level exposure in the home or workplace, often due to activities related to home maintenance or renovation involving asbestos-containing building products.
The amounts of asbestos used varied greatly between products. For example, sprayed asbestos varied between 5% and 95% asbestos content (Dumortier and De Vuyst, 2012). Similarly, the prevalence of asbestos-containing products in different countries more or less reflected the ease of its availability. For example, in Australia, where naturally occurring asbestos was mined until the 1960s, 25% of all new homes were clad in asbestos cement (Olsen et al., 2011). Generally, asbestos-containing materials that are in good condition and are not the subject of everyday wear and tear, pose little risk. In other cases, the risk can be very high. For example, asbestos-vinyl flooring tiles have been shown to suffer the on-going release of asbestos fibers in normal use (Sebastien et al., 1982). Thus, they are a health hazard both while installed and if removed. These great differences make it hard to make any general estimates on possible exposure levels.
The problem is that even after the new use of asbestos was banned, much of it remained in existing structures, often as asbestos cement materials used in cladding, roofing, or piping (Olsen et al., 2011), but also in or underneath vinyl and linoleum floors and as a sprayed compound (Level, 2016). Thus, not surprisingly, the clearance of installed asbestos from buildings is now recognized as a major construction business in developed countries (Dumortier and De Vuyst, 2012), and one of the problems is that much of that removal work is carried out by low-skilled casual labor (Department of Labour, 2006, pp. 25–26). In 1992, an audit of floor sanders in Christchurch, New Zealand, revealed work practices which included a failure to prevent spread of dust to other rooms, poor respiratory protection for sanders, sanders transferring asbestos dust to their vehicle and home on their work clothes and tools, lack of a thorough “clean up” after sanding, and even the careless disposal of sanding dust (Department of Labour, 2006, p. 25). Similar problems with poor compliance with established and regulated procedures for the safe removal of asbestos have been noted in other countries (Dumortier and De Vuyst, 2012). Unskilled DIY removal of asbestos can be a particular problem because it is very hard to regulate and monitor.
6.3.4 Other asbestos-related risks
Current knowledge focuses on risks associated with inhalation of asbestos. Ingestion and dermal exposure have so far received very limited research attention. One area of concern is asbestos in drinking water (Kjærheim et al., 2005; Koumantakis et al., 2009; Wei et al., 2013). Consideration of the presence of asbestos in water is especially important because between the 1920s and the 1980s, asbestos cement was commonly used in mains water supply systems in many countries (Browne et al., 2005; Department of Labour, 2006, p. 36). To remediate problems with taste, asbestos-cement pipes were sometimes lined with vinyl-based coatings (Spence et al., 2008). As these networks age they become prone to frequent pipe bursts. Additionally, asbestos can enter water systems from mines or natural occlusion making its way into the rivers, lakes, and water table reserves in measurable quantities (Koumantakis et al., 2009; Wei et al., 2013). However, studies to date generally do not provide strong evidence of an association between exposure to asbestos in drinking water and the presence of gastrointestinal or respiratory cancers (Kjærheim et al., 2005; Browne et al., 2005; Koumantakis et al., 2009), but the cases that have been found indicate a 20-year or longer delay between exposure and development of cancer (Kjærheim et al., 2005). Additional problems have also emerged: asbestos in river water has been associated with a negative impact on the behavior of fish and other water life, and results indicate that homes supplied with asbestos-contaminated water are associated with an increase of airborne asbestos (Koumantakis et al., 2009). Therefore, it is possible to anticipate that studies of total risks from asbestos could enter a new phase with a heightened recognition of these other risks.
Replacements for asbestos are generally called manmade mineral fibers and often take a similar form of being long, thin fibers. Although generally these are considered to break transversely, rather than longitudinally like asbestos, concerns have been raised that a similar pathology could be observed with these materials. Carbonari et al. (2011) studied the in vitro impact of glass fibers, ceramic fibers, and Wollastonite fibers (a form of asbestos), and found that naturally occurring Wollastonite fibers induced blood vessel formation in a similar fashion to that observed for other asbestos, while the glass and ceramic fibers did not exhibit such similarities. They concluded that the size and shape of the fibers and their chemical composition and biopersistence are important factors influencing the development of adverse health effects and that very thin, persistent fibers are the most harmful (Carbonari et al., 2011). When evaluating the impact of dermal exposure to ceramic fibers introduced as an asbestos replacement, a high level of dermal irritation was observed (Keić-Świerczyńska and Wojtczak, 2000).
6.3.5 Asbestos: summary
The limitations in the available knowledge about risks associated with asbestos indicate that it is still a less recognized and understood health risk than lead. An understanding of the important mechanisms of response to asbestos within the human body is still missing. Once these are more understood, this new knowledge could change the way asbestos is considered. This situation reinforces the reality that many discoveries associated with the second stage of risk recognition continue into the third stage, but also that it is essential to take actions to reduce exposure to hazards based on early knowledge, rather than waiting for the full picture to emerge.
Furthermore, just as with lead, asbestos poses concern in Western countries through the “third wave” of exposures to asbestos already built into structures. Because exposure to asbestos is harder to measure, and personal reports are often used to evaluate exposure, it is even harder than it is for lead to quantify the prevalence and extent of exposures from unskilled DIY, or even simple maintenance actions (such as abrasive washing of sheet materials containing asbestos). Yet, the examples of lead and asbestos clearly suggest that once hazardous materials are contained in the existing housing stock they remain a permanent issue for the lifespan of these structures.
6.4 Conclusion
The examples of lead and asbestos show that many issues remain unresolved more than three decades since serious regulative efforts have attempted to control their use in Western countries. As discussed, scientific research is still revealing the exact mechanisms of the adverse health impacts these substances have. Some of the longer-term and across-generation observations are only now becoming available for exposure to the substances in the 1970s. As seen for asbestos the latency period could be 40 years, which makes it difficult to obtain clear causal evidence, and thus, conclusive evidence. In both cases newer research shows that much lower levels and shorter exposures than assumed in the 1970s or 1980s also have strong adverse health effects. The levels that were used and built-in during the 20th century now seem both high and hazardous.
Therefore, it is clear that the most significant pattern observed when evaluating lead and asbestos, the two substances most recognized for their adverse effect on human health, is delays in responding to recognition of significant indications of high health risks. As Olsen et al. (2011) explained, many Western countries are now facing the “third wave” of adverse health impacts from both lead and asbestos, through the removal, maintenance, or poor use of already built-in materials. Although these do not impact on everybody in contemporary society, the potential of hidden sources of exposure is present throughout society. Poor regulative control of DIY activities and a high presence of built-in hazards regularly contribute to the creation of new, although completely preventable, diseases through exposure to these well-known hazards. Current regulations are often powerless here.
Further successful removal of health hazards from already built-in materials that contain lead and asbestos is unlikely to be achieved through the use of regulation. Regulative changes have been excellent in removing hazards from new building, but existing built-in hazards also have to be addressed. It is irresponsible to allow continued unregulated, unpredictable exposures from well-known hazards through unskilled activities. To make improvements in this area on a broad-scale education is more likely to be effective in changing the attitudes people have towards the materials in their homes.
Furthermore, the way 20th century society has approached the use and regulation of lead and asbestos shows serious problems with the paradigm of waiting for a scientific proof, and only then regulating. Solid indications existed long before complete evidence, and in the case of lead and asbestos these indications were clear even before the start of the 20th century. The time lapse, of about a century, between solid indications and action, enabled prolific increased use over a very long period, thus unjustifiably increasing the levels of exposure of many people. For the health of the general population a more precautionary principle has to be adopted. It is essential to develop a way of recognizing the risks as being potentially serious much sooner. This chapter proposes the examples of lead and asbestos are evidence for advocating for a paradigm shift, towards adopting a more precautionary principle attitude.
Finally, the examples of lead and asbestos are in many ways easy to discuss. They are well known as harmful for the human body in almost any form of exposure. They are also very persistent, both having a very long half-life, and are very hard to eliminate from the environment properly. While these characteristics make them highly undesirable, they also make that undesirability very obvious. Therefore, the only partial elimination of the health hazards associated with lead and asbestos is very disappointing, and sets a grim agenda for some of the other substances considered here. The next chapter deals with the less obvious hazards of formaldehyde, plasticizers, and other volatile organic compounds. These chemicals are more reactive and much faster to change their form, making it hard to collect permanent data on them. These features make obtaining accepted scientific knowledge even more difficult.
