Potential health risks of nanoparticles in foods, beverages and nutraceuticals
Abstract:
Nanotechnology is the twenty-first-century discipline concerned with studying and engineering manufactured materials with one dimension less than 100 nm that possess unique properties because of their size. The purpose of this chapter is to overview the occurrence and safety implications of this diverse group of materials used in foods, food packaging, beverages and nutraceuticals. Topics to be dealt with include formal definitions, aspects of their origin, properties and potential biological effects on human consumption of nanomaterial containing food products as well as strategies for risk assessment. Naturally occurring versus manufactured materials, as well as materials specifically designed to take advantage of unique physical properties inherent to the nanoscale will be presented, as well as how these factors impact the development of risk assessment models to avoid health effects.
2.1 Introduction
Nanotechnology is the twenty-first-century discipline concerned with studying and engineering manufactured materials with one dimension less than 100 nm. These substances range from the discipline-defining spherical buckyball (C60) of < 1 nm diameter, to polymer pharmaceuticals, lactalbumin nanotubes, nano-silver antimicrobials to nanowires of nm diameter but lengths of cm and longer. This chapter will overview the occurrence and safety implications of this diverse group of nanomaterials when used as food additives or employed in food packaging. The first section deals with formal definitions that place topics in the proper context. Aspects of their origin, detection and potential biological effects on human health will be presented. This will include discussions on naturally occurring versus manufactured or engineered materials, as well as materials specifically designed to take advantage of the unique physical properties inherent to the nanoscale. The extremely high surface area to mass ratio of nanoparticles results in their surface properties having a large influence in their biological behavior as well as the ability for detection. One issue unique to nanomaterials is their tendency to self aggregate and/or agglomerate with natural biomolecules, a phenomenon that determines their biological activity. How these factors impact regulations will be briefly reviewed since this area is still developing rapidly. The goal of this chapter is to review this emerging discipline relative to safety issues related to their use in human food or food packaging materials coming into contact with food.
2.2 Nanoscale materials
Nanomaterials are materials that have a physicochemical structure on a scale greater than atomic/molecular dimensions but less than 100 nm that exhibits physical, chemical and/or biological characteristics associated with its nanostructure. True nanotechnology involves substances that have novel properties because of their size, and if based on natural molecules (e.g. engineered biomolecules) function differently to that intended in nature (Linkov and Steevens, 2008; Narlikar and Fu, 2010; NRC, 2006). This is the so-called nanoeffect where unique or enhanced physical properties (strength), reactivity or biological interactions occur below a specific particle size threshold currently estimated at < 100 nm. The first highly studied particle was the C60 carbon fullerene buckyball which is a single molecular cage composed solely of carbon and hydrogen atoms. Engineered nanomaterials can exhibit a variety of unique and tunable chemical and physical properties that have applications in energy, electronics, medicine and aerospace technology. Many of these unique attributes are ideally suited to be exploited in the food industry. So-called nanofoods have been defined as any food which has been cultivated, produced, processed or packaged using nanotechnology techniques or tools or to which manufactured nanomaterials have been added (Neethirajan and Jayas, 2011).
One simple physical property that is often exploited is their exceptionally large surface area to mass ratio. It is their unique characteristics that have made engineered nanoparticles central components in an array of emerging technologies that have already led to commercialized products. A now classic example of a unique particle is the quantum dot (QD), a colloidal semiconductor nanocrystal, which possesses stable fluorescence and has been used in a number of biomedical applications. Similarly, carbon-based nanotubes and wires have been designed with unique properties of electrical conductivity and have become central to making exceedingly strong carbon-based materials and nanosensors. Nanosized formulations of titanium dioxide have been marketed as super-efficient sunscreen formulations. A wide range of base materials are used in these applications including carbon, silica, metal oxides and numerous polymers and composite materials. The wide literature base should be consulted for specific applications.
What is unique in the field of nanotechnology applications to food is the tendency for natural products such as lactalbumin, starch or corn zein to be used as substrates for nanostructures. Short natural nanotubes have been used to control viscosity and as gelation agents in foods. Existing food components such as omega-3 fatty acids or canola oil may be formulated as lipid nanoparticles or nanoencapsulated particles to improve stability or bioavailability. Additional applications include those in nutraceuticals which encapsulate a wide variety of additives, nutrients and vitamins in nanocapsules to improve delivery in foods and beverages; good examples being nanoencapsulated curcumic from turmeric and eugenol from cloves. Sophisticated biosensors are being developed to incorporate into packaging materials to detect microbial or fungal contamination or spoilage. In fact, due to their high surface area to mass ratio and high surface absorbing properties, nanoparticles have been developed to absorb ethylene gas to retard spoilage. Similarly, silver nanoparticles have been incorporated into packaging to prevent microbial contamination from developing in the first place. Nanobarcodes are being developed to improve traceability and food security. Nanolaminates are being developed to improve moisture retention and gas impermeability of beverage containers. Many of these applications have been extensively reviewed in the literature (Boowmeester et al., 2009; Chen et al., 2006; Nair et al., 2010; Ozimek et al, 2010).
Figure 2.1 displays micrographs depicting the appearance of six different nanomaterials to illustrate how diverse such particles can be. The water-soluble nC6 0, similar to particles that could occur in aqueous environments, nicely illustrates material heterogeneity (Xia et al., 2010a). The multiwalled carbon nanotubes illustrate larger and more complex engineered materials, with these specific materials shown to be taken up into viable cells in culture (Monteiro-Riviere et al., 2005).





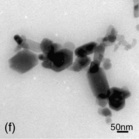
Fig. 2.1 Transmission electron micrographs of a selection of nanomaterials illustrating the large diversity in size, shape and crystalline form. (a) nC60; (b) multiwalled carbon nanotubes; (c) 20 nm silver; (d) 80 nm aluminum; (e) TiO2; (f) ZnO.
In a targeted chapter such as the present one, the focus must be on general unifying concepts that could be applied to a wide range of nanomaterials, including those used in food and food packaging. The first such concept is that size alone can be a misleading metric. A distinction must be made between a particle that just falls into a nanoscale range versus something that is truly nanoscale (< 100 nm manufactured with unique properties). Figure 2.2 illustrates two such scenarios. In the first case (a), this is a size distribution of particles with a mean diameter of 46 nm and a distribution that is entirely within the accepted nanoparticles range of 100 nm. In contrast, case (b) is actually a microsized particle with all of its size distribution above 100 nm. As seen with this case, there are particles with sizes measured in nm; however, the consensus definition is using a cut-off of 100 nm to relate to unique physical ‘nanoeffects’ only seen when one scale is < 100 nm. When dealing with many chemical additives, this is a scenario that can be encountered but which does not properly fall under the umbrella of nanotechnology. This confusion is especially relevant to milled food ingredients where the bulk of the material may be microsized and only a fraction spilling into the nm range, but not < 100 nm.
![]()

Fig. 2.2 Size distributions ofmodel nanomaterials determined using dynamic light scattering. (a) True nanomaterial whose size distribution is centered at 46 nm and represents all nanosized material. (b) A broader population of more dispersed material that bridges nano-and microparticles. The focus of most research related to nanomaterials is on particles with size distributions seen in (a). Although the particles in (b) are technically nanometer-sized material, most definitions would exclude them from being considered nanoparticles.
These are then purported to be ‘nanomaterials’ when in reality they are no different to conventional milled microsized particles. These types of materials probably do not warrant the attention that true nanoscale novel materials deserve.
A similar situation occurs when true nanosized particles aggregate (bind to one another) and/or agglomerate (bind to one another and other biomolecules such as proteins) into larger sized complexes. Because of the large surface area to mass ratio of these materials, this is a common phenomenon of nanotechnology. Although the constituent particles are nanosized, the stable complexes may not be. Such clumping can be seen in Fig. 2.1(a), (c), (d), (e) and (f). This phenomenon is often very sensitive to concentration, pH and local ionic strength. Exposure may occur to individual nanoparticles, but when encountering biological mediums, agglomeration may occur and their sizes change. When this happens, particles do not exhibit the relatively unique properties associated with the nanoscale. Depending on the strength of the molecular forces involved, the process can also be reversible. It is the scenario of larger microparticles, with some spillage into the nanorange, and aggregates/agglomerates that may predominate in nanomaterial exposure in food.
The final attribute which should be assessed are the biological implications of nanoparticles composed of inert manufactured materials versus those made from natural substances or biodegradable polymers. For example, some nanomaterials are formed from natural ingredients such as milk protein nanotubes, cellulose nanocrystals, starch, lipid nanoparticles and nanostructured liquids; natural materials that probably do not present unique concerns relative to interactions with biological systems. This is similar to the situation discussed above with micro-sized milled products whose particle size only spills over into the nm range and are not expected to possess unique properties. It is predominantly these types of materials that are being proposed for use in the food industry.
In contrast, exposure to the so-called hard engineered nanomaterials, such as carbon nanotubes, exotic particles like quantum dots and nanosensors, and certain metals, may result in unique biological effects not seen with other natural materials. The main concern is that after systemic absorption, there are limited mechanisms for novel inert materials to be metabolized or excreted from the body, resulting in bioaccumulation. Their unique surface properties may also result in adverse biological effects related to membrane disruption, oxidation, adsorption of essential nutrients and biomolecule agglomeration. In contrast, with biodegradable polymers, natural substrates and most nanomedicines being developed, elimination occurs thereby avoiding long-term bioaccumulation. This issue is further discussed in the health and safety section below.
Table 2.1 lists some of the physical characteristics that have been developed to date to characterize nanomaterials. As this discipline develops, it is important that all such materials be fully characterized so that important attributes can be linked to biological effects. At this stage in this emerging discipline, significant research needs to define the relationship between these parameters and potential hazard and exposure. Most of the characterization metrics reflect physical structure, chemical properties or crystalline form, attributes often determined under very ‘non-biological’ artificial conditions (high temperature, high vacuum, etc.). What is lacking are metrics that relate to biological interactions, such as the partition coefficient used to gauge tissue deposition for chemicals. Due to issues of colloid stability and aggregation, partition coefficients cannot be easily determined for nanoparticles. Similarly, what are the particle size and reactivity when mixed with gastrointestinal contents and/or food or after absorption in blood or intracellularly in cytosol? It is these conditions that are relevant to predict biological effects. Our laboratory has recently established a metric, termed the biological surface adsorption index (BSAI), to characterize the nature of nanoparticles interactions with biological systems (Xia et al., 2010b).
2.3 Potential health risks of nanoparticles in foods, beverages and nutraceuticals
At this point, true manufactured nanomaterials have not made it into the agricultural and food sectors. The first materials encountered are microsized powders and dusts which include in their size distributions some nanoscale material (e.g. Fig. 2.2(b)). Nanostructured liquids, polymer-based additives and nanoscale liposomes may be encountered but should not present unique toxicological hazards. Their major effect may be to alter the rate and extent of absorption or biodistribution of the composite material after exposure in food.
Based on existing projections, the following would be examples of potential areas in the future where nanomaterials would be encountered in food systems (Boowmeester et al., 2009; Chen et al., 2006; Kuzma and VerHage, 2006; Nair et al, 2010; NRC, 2009; Ozimek et al, 2010; Riviere, 2007):
• nanoencapsulation of pesticide formulations to improve stability or specifically target organisms (e.g. only released in insect gut);
• drugs formulated as block-polymers or dendrimers for increased stability and delivery;
• short nanotubes made from natural proteins (lactalbumin, zein) to control colligative properties of foods;
• nanoencapsulated food ingredients to mask taste and increase storage stability (e.g. fish oils) or increase water solubility (dietary polyphenols);
• nanosized nutritional supplements to improve delivery (lipids, vitamins, nutraceuticals);
• packaging material – bio-nanocomposites to improve performance (decreased gas permeation with nanoclays), biodegradability, nanosensors to detect spoilage or organic contaminants, nanoparticles containing antifungal and antimicrobial agents;
• microbial contamination – sensors or engineered particles directed toward removal of specific organisms; antimicrobial silver nanoparticles;
• contaminants from crops – nanomaterials used in bioremediation of farm pollutants, crop fertilizer, nanosensors used to monitor soil condition;
• natural nanomaterials – volcanic dust, anthropogenic forms such as combustion products.
As can be appreciated from the existence of relatively vague definitions of nanomaterials, it is difficult to project what may actually be encountered and termed a nanomaterial. However, it is clear that significant research in nanotechnology is occurring in the food sector and as products and formulations develop, they may eventually enter directly into food or packaging or be used in crops or the food animal feed cycle.
A great deal of research has been focused on applications to crop production. Carbon nanotubes have been shown to penetrate tomato seeds and zinc oxide nanoparticles can enter the root tissue of ryegrass (Khodakovskays et al., 2009; Lin and Xing, 2008). These findings suggest that nanoformulations of essential nutrients and metal fertilizer additives for food crops may best be delivered using nanotechnology (DeRosa et al., 2010). These findings also strongly suggest that nanomaterial contaminants in the environment could end up in crops, and thus potentially food derived from them. Should this occur, then focus must also be directed to the potential uptake of nanoparticles into food animals being exposed to nanoparticles taken up into plant material components of feed or fodder.
What type of nanomaterial properties potentially could alter how a nanoformulation of a compound would require different consideration than the chemical itself, from the perspective of bioavailability and subsequent biodistribution? Initial work in this field has been heavily skewed to inhalational studies and developed along the principles of particle and fiber toxicology, since this was the primary route for human occupational and environmental exposure. From this work, there have been a number of studies on biodistribution of absorbed nanomaterial and a wealth of in vitro studies on cellular toxicity which will not be reviewed here (Monteiro-Riviere and Tran, 2007). Since the bioavailability of nanomaterials from animal feed is probably limited, and data as to in vivo biological effects are almost nonexistent for even model materials, any discussion of potential adverse effects in humans consuming nanomaterial-contaminated meat or milk would be purely speculative.
A number of issues important to nanomaterial risk assessment depend on having knowledge of post-absorption nanomaterial biodistribution and defining which specific tissues nanomaterials become sequestered in. A unique aspect of nanoparticle biodistribution is their interaction with proteins to form a so-called corona. The formation of an enveloping corona upon exposure to biological environments is a unique phenomenon of nanomaterials compared to chemical and small molecule behavior. Recent studies have suggested that it is the properties of the nanoparticle corona, when present, that often determines biological activity (Lynch and Dawson, 2008; Lynch et al., 2007). This nanoparticle–protein interaction precedes its delivery to a cellular target, at which point properties of the nanoparticle may determine the nature of any toxicity seen (e.g. oxidation of cellular target, DNA binding, etc.) (Monteiro-Riviere and Tran, 2007).
Published reviews of the field of nanoparticle pharmacokinetics and biodistribution suggest that there is sporadic literature on the pharmacokinetics of nano-particles in laboratory animals and no studies are available in larger animals or humans (Alexis et al., 2008; Li and Huang, 2008; Riviere, 2009). This is a serious deficit in the knowledge needed to assess nanomaterial behavior in food. Most research has focused on development of nanosized drug polymers for applications in cancer chemotherapy and vaccine delivery or by using degradable nanosub-strates as carriers of small organic drugs. The use of nanosized formulations of traditional drugs to increase dissolution via enhanced surface area is a common theme, though not relevant to the relatively stable carbon- and metal-based manufactured nanomaterials and devices. These studies are relevant to nanoformulations of nutraceuticals in food and beverages; however, the matrices studied for delivery are simple compared to most food.
The nanomedicine literature deals with biodegradable polymers and cell-specific targeting of drugs. These studies generally suggest that nanoparticle size, surface charge and surface chemistry are important factors to consider. One reason that nanomedicine has such potential is that the larger size of nanoparticles, coupled with their tendency to form protein coronas, is generally expected to restrict tissue distribution except in so-called leaky capillary beds (pore size ≈ 100 nm), which include the liver, spleen, bone marrow and tumors, making use of nanoparticles for cancer chemotherapy possible. Nanoparticles are also delivered to the liver, spleen and bone marrow because of interaction with opsonin proteins as well as antibodies and complement, which allow macrophages to engulf and sequester them. Neutral nanoparticles tend to avoid this interaction. Smaller sized nanoparticles (5–50 nm) that bind to other plasma proteins may penetrate other tissue beds and avoid this fate. Anionic quantum dots have been shown to directly enter endothelial cells in phagocytic-poor tissues both in vivo and ex vivo (Lee et al., 2007; Praetner et al., 2010).Recall that particulate lipoproteins characterized by different densities (e.g. low and high density cholesterol carriers) are essentially nanoparticles that circulate in the body and are taken up into cells by specific endocytic pathways, providing a transport mechanism for nanoparticle uptake (Zhang and Monteiro-Riviere, 2009). These studies suggest that should nanomaterials be absorbed into the body after oral exposure in the diet, nanoparticles will be present in tissues and thus require consideration relative to potential residue exposure in edible products from food animals.
The limited literature of the pharmacokinetics of carbon-based material including C60 fullerenes and carbon nanotubes is primarily focused on therapeutic applications. A classical pharmacokinetic study was conducted in Sprague–Dawley rats using a water-soluble C6 0 derivative with antiviral properties (Rajagopalan et al.; 1996). Terminal half-life was approximately 7 h, volume of distribution was 2 L/kg consistent with extensive distribution to the liver and spleen, and there was no evidence of urinary excretion. These C60 were 99% protein bound in plasma, illustrating the protein corona concept. Yamago et al. (1995) studied a 14C-labeled lipophilic yet water-soluble C60 after intravenous (IV) and oral administration, with very little oral absorption detected. After IV administration, only 5% of the compound was excreted, all by the fecal route, with most of the label retained in the liver after 30 h. In a recent study of 14C-labeled C60 injected into pregnant rats, C60 accumulated in the liver but also crossed the placenta and was transmitted to offspring via the dam’s milk and subsequently absorbed systemically (Sumner et al., 2010). This has implications both for nanomaterial transfer to a suckling infant from an exposed lactating mother and for human exposure to residues in milk should a similar phenomenon occur in dairy cows or goats. Local aggregation and agglomeration of distributed nanoparticles, precipitated by local tissue pH and osmolarity, could also result in pockets of tissue accumulation after absorption.
There are systemic disposition studies reported using inherently fluorescent quantum dots derivatized for medical imaging. Similar to carbon materials reviewed above, they accumulate in the liver and spleen (Akerman et al., 2002). Imaging studies in mice clearly show that quantum dots surface coatings alter their disposition and pharmacokinetic properties (Ballou et al., 2004). These coatings determined in vivo tissue localization, with retention of some quantum dots occurring up to 4 months. Our group has published a physiologically based pharmacokinetic model based on reported quantum dots studies in rats (Lee et al., 2009). Quantum dots had extensive distribution to tissues including the liver and kidney; however, minimal excretion occurred from the body, demonstrating bioaccumulation concerns. Choi et al. (2007) showed small QD with a hydrodynamic radius less than 5.5 nm could be excreted by the kidney, zwitter ionic and neutral coatings reduced plasma protein corona formation, and larger sizes were not excreted. Finally, pharmacokinetic studies have been conducted with nanodimension drug block co-polymers and dendrimers. As described above for other nanomaterials, the physical/chemical properties of the drug and polymer carrier effects disposition and circulating half-life (Kabanov et al., 2002).
All these mechanisms of tissue deposition have toxicological relevance for nanomaterials. Should significant nanomaterial be absorbed systemically after administration in foods or from migration from packaging materials, there would be a potential for biodistribution to tissues. As seen in the rodent studies reviewed above, transfer to milk may also be possible. Recently, a body of research has defined the ability of orally dosed nanoparticle proteins and vaccines to be systemically bioavailable (de Rieux et al., 2006), a finding consistent with the C60 rat studies mentioned above. Titanium dioxide nanoparticles (25 and 80 nm groups), often used as a white pigment in food products, were orally dosed to mice and absorption was compared to microsized particles (155 nm). Nanoscale material preferentially accumulated in the liver, spleen, lung and kidneys induced adverse effects in the liver (Wang et al., 2007). Zinc oxide nanoparticles were also shown to be bioavailable to snails after environmental exposure (Croteau et al., 2010), raising the concern of inadvertent dietary exposure from environmental contamination of fodder. However, there is no direct research on nanomaterial exposure in the complex matrix of processed foods.
2.4 Risk assessment of nanomaterials present in foods, beverages and nutraceuticals
The regulatory status of nanomaterials is not well defined across applications and regulatory jurisdictions. There is developing consensus that nanosizing a material may introduce new properties to the material and thus chemical identity alone cannot be used to assure comparability. This has obvious implications on GRAS (Generally Regarded As Safe) status and extension of food additive approvals to nanoformulated material. Lack of consistent definitions applicable across multiple product areas delays regulations from being made which in turn hampers realistic risk assessments from being developed (NRC, 2009; Taylor, 2008). The US Food and Drug Administration does not impose new regulations based on how the material is manufactured; however, resulting differences in chemical properties do influence evaluation strategies. As discussed above, many existing nano-particles are natural and many so-called nanomaterials are really not ‘nano’ at all. Definition and characterization become crucial.
Introduction of new technologies into food production is fraught with potential difficulties and public anxiety, as is evidenced in the debates on regulation of genetically modified organisms and food irradiation. In July 2010, the European Parliament proposed suspension of sale of food containing ingredients derived from nanotechnology. The United Kingdom has recommended that products be tracked and research specific to applications in food be pursued (Anon, 2010). In the United States, the National Nanotechnology Initiative has specifically been established to attempt to coordinate activities across all components of the federal government, from research and funding perspectives. As definitions become more focused for specific applications (e.g. powders that have nanosized constituents and natural nanomaterials versus novel engineered nanomaterials), agencies responsible for regulating nanomedicines will develop guidelines to define areas of concern. The Environmental Protection Agency and the US Department of Agriculture have likewise addressed resources to define potential hazards. There is little doubt that guidelines and regulations will be issued and then revised as relevant knowledge becomes available and products enter commerce.
2.5 Future trends
As can be appreciated from the tone of this chapter, there is minimal information currently available on the presence or behavior of nanomaterials in food and beverage products. This is typical of an emerging discipline such as nanotechnology. Many of the materials initially considered are either natural or extensions of microsized material widely used in foods. Packaging materials will use guidelines developed for ‘normal’ materials. Major nano issues would be detection and differentiation of mobile nanoparticles from immobile large aggregates. The specific properties of the nanomaterial would then determine its absorption profile and subsequent biodistribution in food. However, there is great activity on developing novel applications in microbial and chemical contaminant detection using nanosensors, packaging, crop applications such as fertilizers, as well as nutraceutical, vaccine and drug delivery. As we gain experience with such substances, developments in detection and our knowledge of biological effects will no doubt also become more advanced.
2.6 Sources of further information and advice
Nanotechnology is a rapidly developing field and new discoveries occur on a daily basis. Major research journals such as Nature Nanotechnology, Nano Letters, Small and Nanotoxicology, as well as review journals including Nano Today and Wiley Interdisciplinary Reviews of Nanomedicines and Nanotechnology, are potential sources of information. Discipline-oriented journals should also be scanned since the majority of applied nanomaterial research is covered in this fashion. Government and nongovernment organization websites are always useful sources. This author has found the following websites to be helpful: www.nano. gov, cordis.europa.eu/nanotechnology, www.nano.org.uk,www.nanowerk.com and www.safenano.org. A particularly useful site that tabulates nanomaterials in global commerce is www.nanotechproject.org/inventories/consumer/.
2.7 References
Akerman, M.E., Chan, W.C.W., Laakkonen, P., Bhatia, S.N., Ruoslahti, E. Nanocrystal targeting in vivo. Proceedings of the National Academy of Sciences USA. 2002; 99:12617–12621.
Alexis, F., Pridgen, E., Molnar, L.K., Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Molecular Pharmaceutics. 2008; 5:505–515.
Anonymous. Editorial: Nanofood for thought. Nature Nanotechnology. 2010; 5:89.
Ballou, B., Lagerholm, B.C., Ernst, L.A., Bruchez, M.P., Waggoner, A.S. Noninvasive imaging of quantum dots in mice. Bioconjugate Chemistry. 2004; 15:79–86.
Boowmeester, H., Dekkers, S., Noordam, M.Y., Hagens, W.I., Bulder, A.S., De Heer, C., Ten Voorde, S.E.C.G., Wijnhoven, S.W.P., Marvin, H.J.P., Sips, A.J.A.M. Review of health safety aspects of nanotechnologies in food production. Regulatory Toxicology and Pharmacology. 2009; 53:52–62.
Chen, H., Weiss, J., Shahidi, F. Nanotechnology in nutraceuticals and functional foods. Food Technology. 2006; 60:30–36.
Choi, H.S., Liu, W., Misra, P., Tanaka, E., Zimmer, J.P., Itty Ipe, B., Bawendi, M.G., Frangioni, J.V. Renal clearance of quantum dots. Nature Biotechnology. 2007; 25:1165–1170.
Croteau, M.N., Dybowska, A.D., Luoma, S.N., Valsami-Jones, E., A novel approach reveals that zinc oxide nanoparticles are bioavailable and toxic after dietary exposure. Nanotoxicology 2010; 5:79–90, doi: 10.3109/17435390.2010.501914.
Derosa, M., Monreal, C., Schnitzer, M., Walsh, R., Sultan, Y. Nanotechnology in fertilizers. Nature Nanotechnology. 2010; 5:91.
Des Rieux, A., Fievez, V., Garinot, M., Schneider, Y.J., Préat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. Journal of Controlled Release. 2006; 116:1–27.
Kabanov, A.V., Batrakova, E.V., Alakhov, V.Y. Pluronic block copolymers as novel polymer therapeutics drug and gene delivery. Journal of Controlled Release. 2002; 82:189–212.
Khodakovskays, M., Dervishi, E., Mahmood, M., Xu, Y., Li, Z., Watanabe, F., Biris, A.S. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACSNano. 2009; 3:3221–3227.
Kuzma, J., Verhage, P. Nanotechnology in Agriculture and Food Production. Washington: Woodrow Wilson International Center for Scholars; 2006.
Lee, H.A., Imran, M., Monteiro-Riviere, N.A., Colvin, V.L., Wu, W., Riviere, J.E. Biodistribution of quantum dot nanoparticles in perfused skin. Nano Letters. 2007; 7:2865–2870.
Lee, H.A., Leavens, T., Mason, S., Monteiro-Riviere, N., Riviere, J. Comparison of quantum dot biodistribution with a blood-flow-limited physiologically based pharmacokinetic model. Nano Letters. 2009; 9:794–799.
Li, S.-D., Huang, L. Pharmacokinetics and biodistribution ofnanoparticles. Molecular Pharmaceutics. 2008; 5:496–504.
Lin, D., Xing, B. Root uptake and phytotoxicity ofZnO nanoparticles. Environmental Science & Technology. 2008; 42:5580–5585.
Linkov, I., Steevens, J. Nanomaterials: Risks and Benefit – Nato Science for Peace and Security Series C: Environmental Security. Dordrecht, Netherlands: Springer; 2008.
Lynch, I., Cedervall, T., Lundqvist, M., Cabaleiro-Lago, C., Linse, S., Dawson, K.A. The nanoparticle-protein complex as a biological entity: a complex fluids and surface science challenge for the 21st century. Advances in Colloid and Interface Science. 2007; 134:167–174.
Lynch, I., Dawson, K. Protein-nanoparticle interactions. Nano Today. 2008; 3:40–47.
Monteiro-Riviere, N.A., Tran, C.L. Nanotoxicology: Characterization. Dosing and Health Effects, New York: Informa Healthcare; 2007.
Monteiro-Riviere, N.A., Nemanich, R.J., Inman, A.O., Wang, Y., Riviere, J.E. Multi-walled carbon nanotube interactions with human epidermal keratinocytes. Toxicology Letters. 2005; 155:377–384.
Nair, H.B., Sung, B., Yadav, V.R., Kannappan, R., Chaturvedi, M.M., Aggarwal, B.B. Delivery of anti-inflammatory nutraceuticals by nanoparticles for the prevention and treatment of cancer. Biochemical Pharmacology. 2010; 80:1833–1843.
Narlikar, A.V., Fu, Y.Y. The Oxford Handbook ofNanoscience and Technology. New York: Oxford University Press; 2010.
National Research Council. A Matter of Size: Triennial Review of the National Nanotechnology Initiative. Washington: The National Academies Press; 2006.
National Research Council. Nanotechnology in Food Products. Washington: The National Academies Press; 2009.
Neethirajan, S., Jayas, D.S. Nanotechnology for the food and bioprocessing industries. Food andBioprocess Technology. 2011; 4:39–47.
Ozimek, L., Pospiech, E., Narine, S. Nanotechnologies in food and meat processing. Acta Scientiarum Polonorum, Technologia Alimentaria. 2010; 9:401–412.
Praetner, M., Rehberg, M., Bihari, P., Lerchenberger, M., Uhl, B., Holzer, M., Eichhorn, M.E., Furst, R., Perisic, T., Reichel, C.A., Welsch, U., Krombach, F. The contribution of the capillary endothelium to blood clearance and tissue deposition of anionic quantum dots in vivo. Biomaterials. 2010; 31:6692–6700.
Rajagopalan, P., Wudl, F., Schinazi, R.F., Boudinot, F.D. Pharmacokinetics of a water-soluble fullerene in rats. Antimicrobial Agents and Chemotherapy. 1996; 40:2262–2265.
Riviere, J.E. The future of veterinary therapeutics: a glimpse towards 2030. Veterinary Journal. 2007; 174:462–471.
Riviere, J.E. Pharmacokinetics of nanomaterials: an overview of carbon nanotubes, fullerenes and quantum dots. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2009; 1:26–34.
Sumner, S.C., Fennel, T.R., Snyder, R.W., Taylor, G.F., Lewin, A.H. Distribution of carbon-14 labeled C60 ([14c60] in the pregnant and in the lactating dam and the effect of C60 exposure on the biochemical profile of urine. Journal of Applied Toxicology. 2010; 30:354–360.
Taylor, M.R. Assuring the Safety ofNanomaterials in Food Packaging. Washington: Woodrow Wilson International Center for Scholars; 2008.
Wang, J., Zhou, G., Chen, C., Yu, H., Wang, T., Ma, Y., Jia, G., Gao, Y., Li, B., Sun, J., Li, Y., Jiao, F., Zhao, Y., Chai, Z. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicology Letters. 2007; 168:176–185.
Xia, X.R., Monteiro-Riviere, N.A., Riviere, J.E. Intrinsic biological properties of colloidal fullerene nanoparticles (nC60): lack of lethality after high dose exposure to human epidermal and bacterial cells. Toxicology Letters. 2010; 197:128–134.
Xia, X.R., Monteiro-Riviere, N.A., Riviere, J.E. An index for characterization of nanomaterials in biological systems. Nature Nanotechnology. 2010; 5:671–675.
Yamago, S., Tokuyama, H., Nakamura, E., Kikuchi, K., Kananishi, S., Sueki, K., Nakahara, H., Enomoto, S., Ambe, F. In vivo biological behavior ofa water-miscible fullerene: 14c labeling, absorption, distribution, excretion and acute toxicity. Chemistry & Biology. 1995; 2:385–389.
Zhang, L.W., Monteiro-Riviere, N.A. Mechanism ofquantum dot nanoparticle cellular uptake. Toxicological Sciences. 2009; 110:138–155.
