9
The neuroscience of problem solving
As we have seen, cognitive theories of how information is processed are at a level of abstraction (Marr’s [1982/2010] algorithmic level) that not only seeks to explain behaviour but allows us to model such behaviour on other information processing systems such as a computer (see Chapters 2 and 5). Such theories are theories of how the mind works, but minds are implemented by brains (Markman, 2012, p. 36). If there is no neurological mechanism that allows the kinds of processing postulated in a particular theory of problem solving, then there is something wrong with the theory. Problem solving involves a variety of cognitive processes depending on the problem type such as interpreting information, planning, reading, focussing attention, calculation, accessing semantic information, retrieving episodic memories, relating concepts one to another and so on. There are other influences affecting our ability to reason and perform tasks such as those governing motivation, attention, control, mood and so on. To ascertain whether the theories covering these processes are valid we have to assume that there are brain regions that support them. In other words, different regions of the brain presumably perform different functions or work together to perform a function. For example, arithmetic problems involve calculation which involves retrieving information from long-term memory and integrating the information retrieved. Problems such as the Tower of Hanoi involve planning and looking ahead and hence some means–ends analysis as well as visual analysis and motor control.
Neuroscientific methods of examining problem solving seek to identify those areas of the brain and the connections between them that are active during the various stages of problem solving. The aim is to have a more refined understanding of problem solving, reasoning and learning, one potential practical benefit of which would be to inform educational practice.
Methods used in studying brain functions
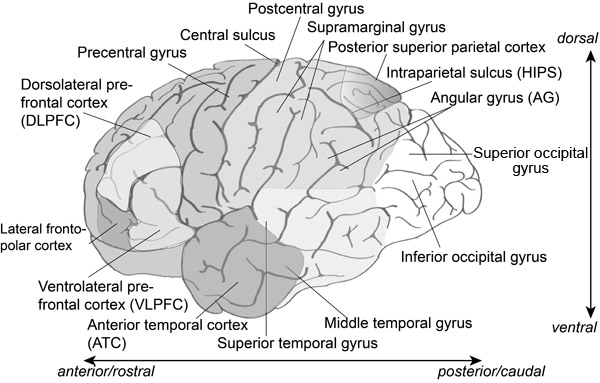
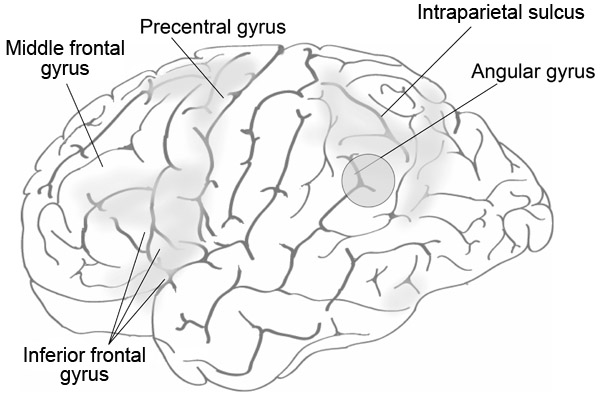
One method of determining the functions of different brain areas is cognitive neuropsychology – a technique with a long history. By looking at areas of the brain that are damaged through injury or some form of developmental disability, we can try to identify if there are specific “deficits” in people’s ability to perform certain tasks. Although it may seem paradoxical, by looking at the effects of such damage on behaviour and on neural function we can get an understanding of how the undamaged brain works. Figure 9.1 shows many of the brain areas discussed in this chapter.
Another set of methods that can be used on healthy participants as well as patients uses neuroimaging. Various types of brain imaging such as functional magnetic resonance imaging (fMRI) and magnetoencephalography (MEG) involving large expensive machines, and the simpler electroencephalography (EEG) involving placing electrodes on the scalp. These techniques not only indicate which areas of the brain are active through fMRI and through other imaging techniques but also the time course of this activity, particularly through EEG measurements. Here is a brief overview of some of the main techniques:
EEG uses electrodes on the scalp that allow us to see patterns of activity in “cell assemblies”. These are neuronal networks that appear to relate to mental states and processes such as accessing information from long-term memory or solving reasoning problems.
Transcranial magnetic stimulation (TMS) can be used to stimulate areas of the brain. Depending on the area of the brain stimulated, the magnetic field can enhance or disrupt processing leading to changes in performance on tasks such as arithmetic calculations or analogical reasoning.
Magnetoencephalography (MEG) is used to pick up minute magnetic fields produced by variations in electrical activity in the brain. MEG can create a map of the brain allowing us to detect possible pathology or to identify potential functions of particular brain areas.
The fMRI is a non-invasive technique that generates a picture or map of the active areas of the brain. It has excellent spatial resolution and allows researchers and clinicians to look at different slices of the brain. fMRI measures are dependent on blood flow and rely on blood oxygen level-dependent (BOLD) responses. After each necessarily simple task the BOLD response must go back to a baseline level before the next task can be performed, so EEG measures can be used along with fMRI to improve temporal resolution.
A technique using fMRI, plus a number of statistical transformations, that allows regions in an individual’s brain to be compared to an “average” brain region is voxel-based morphometry (VBM). The technique is described in Information Box 9.1.
Information Box 9.1 Voxel-based morphometry (VBM)
There are individual differences in brain size and thickness and in the proportions of grey and white matter in the brain. In order to find out if a specific area of an individual’s brain is affected by an external or internal influence we need a way of comparing that brain area with what you might call an “average” brain. For example, some areas of the brain can increase in volume due to learning particular skills such as sight reading of music, foreign language learning, juggling and so on. Equally, brain areas can be affected by strokes or neurodegenerative diseases. However, it is hard to tell by looking at an individual’s brain if a particular region is affected in this way. One method for assessing whether a brain region differs from what one might expect is voxel-based morphometry. Morphometry refers to the measurement of the shape and size of brain structures. You are probably familiar with a pixel which is a picture element in a 2D display whose position can be defined by its x- and y-coordinates. A voxel is a “volume pixel” that adds a z coordinate to denote its position in a 3D shape allowing the same point (essentially a small cuboid) in a 3D shape such a brain structure to be examined from any angle using brain imaging. Voxel dimensions vary from less than 1 × 1 × 1 mm to 5 × 5 × 5 mm typically, depending on the thickness of the slice of brain being examined, and don’t have to be exact cubes. The cubes in the game Minecraft (if you are familiar with it) are voxels.
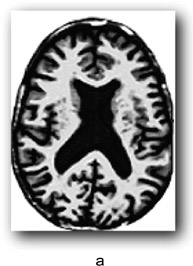
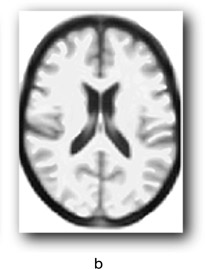
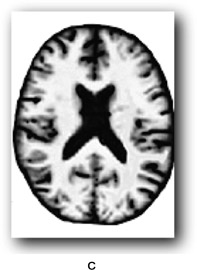
VBM can be used to identify differences in the local composition of brain tissue while ignoring large-scale differences in brain anatomy. The first step is to take images of the brain while employing a procedure for enhancing the contrast between the brain tissue we are interested in and other types of tissue (this is known as T1 weighting). This allows us to see more clearly regions of interest (ROIs) such as individual structures or areas affected by some form of pathology. Cerebrospinal fluid and dense bone appear dark, and myelinated white matter (the fatty sheath covering neuronal axons) appear bright. The next step is to normalise the image to an averaged group brain image or group template by stretching and compressing local areas to produce a deformation field. The voxels on the original image are thereby moved to a corresponding point on the template image. In Figure 9.2, a represents an input image, b is the group template and c the outcome of applying the spatial normalisation and deformation field.
A useful finding in brain research that can be found either through forms of neurological deficits or through neuroimaging techniques is a double dissociation. If patient A can perform a task such as, say, multiplication, but has difficulty doing subtraction, and patient B can manage subtraction but has difficulty with multiplication, then one can infer that different brain regions are responsible for, or at least implicated in, the two tasks (see e.g., Cohen, Dehaene, Chochon, Lehericy, & Naccache, 2000; Dehaene & Cohen, 1997)
Arithmetic in the brain
Early studies of brain function in relation to arithmetic tended to look at damage to brain regions that were strongly associated with acalculia, an inability to perform arithmetic calculations or to determine which of two numbers is the larger. Studies have also looked at dyscalculia, which is a developmental condition, so many of the studies into this condition have involved children. These conditions have led to more recent studies of healthy individuals using fMRI in particular to examine these regions of the brain involved in different aspects of arithmetic calculation in detail.
The number line
Lesions to areas in the parietal cortex, particularly the angular gyrus (AG), are associated with acalculia (Figure 9.1) including a loss of number meaning and magnitude: Is 12 greater than 3? What does 12 mean anyway? The AG has a role related to a number of cognitive functions, particularly aspects of memory (retrieval of numbers and visuospatial facts in calculations, episodic and autobiographical memory, semantic processing, and processing abstract concepts, orienting the attentional system to relevant information). Of particular relevance to this topic is the role of the AG in manipulating mental representations and conceptual knowledge. In relation to arithmetic problem solving the AG appears to be involved in the retrieval of arithmetic facts from verbal memory rather than in calculation (Dehaene, Piazza, Pinel, & Cohen, 2003; Grabner et al., 2009).
The AG and the surrounding areas are associated with visuospatial attention. The relationship between arithmetic and a brain area responsible for visuospatial attention gives weight to the idea of a mental “number line” where numbers are represented mentally, usually from left to right, with small numbers on the left and larger numbers on the right. One result of this mental organisation of numbers is the so-called SNARC effect (spatial-numerical association of response codes; Dehaene, Bossini, & Giraux, 1993), which is a long-winded name for a simple effect, namely when asked to respond to the presentation of numbers, people are faster at responding to small numbers with the left hand than the right and faster at responding to large numbers with the right hand than the left.
Göbel, Walsh and Rushworth (2001) investigated the role of the parietal cortex in number representation, specifically the mental number line, using repetitive transcranial magnetic stimulation (rTMS). Magnetically stimulating brain areas using TMS can temporarily disrupt normal neuronal processing. Their participants were presented with double-digit numbers (31–99) on a computer screen and then asked to indicate if the number was smaller or bigger than 65 (the reference number). The index finger of the right hand was used to indicate greater than and the index finger of the left hand to indicate less than. Based on previous findings one expected result was that numbers close to the reference number took longer to classify than numbers further away from the reference number. Applying TMS to the left and right AG slowed down performance on both a visuospatial search task and the number comparison task. The conclusion was that the representation of number is spatial in nature, as one would expect of a mental number line, and that it was localised in the left AG rather than the right AG.
A study using the bisection of a number line in a similar way to what is known as the “visual line bisection task” was implemented by Cattaneo, Silvanto, Pascual-Leone and Battelli (2009). In the visual line bisection task patients with a form of visual neglect are asked to identify the midpoint of a line. If the patient has a right parietal lesion, for example, then the midpoint estimation will be shifted to the right as there is a degree of neglect of the left visual field. They presented a series of lines to unimpaired participants and asked some to assess which side of the line was shorter and some which side was longer in relation to a fixation point. In one condition they presented small number “primes” from 16 to 24 before presenting a fixation point followed by the lines; in another they presented large primes from 76 to 84. In some conditions TMS was used to one or the other side of the brain in line with the AG.
In conditions when no TMS was administered, the small primes had the effect of biasing the responses so that the left side of the line was seen as longer than the right. Applying TMS to the right AG abolished this effect but TMS applied to the left side did not. For conditions involving large numbers, administering TMS at either side of the brain removed the priming effect. The authors suggest that this is because the right hemisphere processes attentional information covering both visual fields, whereas the left hemisphere covers the right visual field only. In fact, applying TMS to the right AG seemed to boost the priming effect rather than reducing it. There appears therefore to be a degree of hemispheric asymmetry in representations of the number line, which the authors put down to an asymmetry in the allocation of visuospatial attention in which the AG plays a critical role.
How the brain performs calculations
Based on a several studies of arithmetic representations in the brain, Dehaene developed a triple-code theory (Dehaene, 2011; Dehaene et al., 2003) that identified three main circuits that were involved in representations of number (see Figure 9.1). These are:
- 1 Horizontal intraparietal sulcus (HIPS):
- Plays a role in processing numerical quantity;
- Is a nonverbal semantic representation of the size and distance relations between numbers;
- Is active in mental arithmetic involving calculation but not so much in merely reading numerical symbols.
- 2 Left angular gyrus (AG):
- Plays a role in the verbal processing of numbers;
- • Lexical is involved in phonological and syntactic representations of numbers;
- Arithmetic is involved in fact retrieval.
- 3 Posterior superior parietal system:
- Plays a role in spatial and nonspatial attention;
- Visual is involved in the processing of numbers encoded as strings of Arabic numerals.
Solving arithmetic problems involves not only arithmetic fact retrieval, but also procedural strategies. There is no need to calculate 9 × 5 if you already know the answer and can retrieve it from long-term memory. Grabner et al. (2009) related fMRI data to self-reports of participants solving arithmetic problems. They found that that the AG was involved in retrieving arithmetic facts whereas the reported procedural strategies were related to widespread activation in the fronto-parietal region. Previous research had found evidence of an effect of task difficulty and individual differences relating to the AG, but the Grabner et al. study provided strong evidence for a general role of the AG in arithmetic fact retrieval (see Figure 9.3). Dehaene et al. (2003) had pointed out that the AG was linked to the language processing system and involved in the verbal coding of numbers.
It would appear, therefore, that the AG is implicated in a range of cortical functions. Seghier (2013) discussed the importance of this structure and has pointed out that, due to its position in the lower part of the parietal lobe next to the occipital and temporal lobes, its connectivity and its functions, the AG is an important “cross-modal hub”. There is evidence of readily measurable structural changes in the AG in adults as a result of learning, for example learning to juggle, showing “phenomenal structural plasticity in the AG when subjects are learning new skills that tap on spatial coordination, verbal storage, and creativity” (Seghier, 2013, p. 45). That said, Seghier also points out that it is unwise to associate the AG with particular cognitive functions without taking into account the many other areas that can also be involved in particular tasks.
Development of neural representations of arithmetic
Chang, Rosenberg-Lee, Metcalfe, Chen and Menon (2015) were interested in how numerical problems (addition and subtraction) were represented neurally and how representations of abstract problems (such as manipulating numbers using arithmetic operators) developed over time. Children learn addition before subtraction. At first, addition requires simple counting (Dehaene, 2011; Dehaene et al., 2003), and Chang et al. argue that children use a range of relatively effortful and varied strategies to add and subtract such as counting with fingers, verbal counting and some fact retrieval. After episodes of repeated counting, some of the results are stored as facts. For example, to calculate 5 + 3 a child may at first hold up five fingers, hold up three more and then count from 5 to 8. Eventually the child learns that 5 + 3 = 8 and can retrieve this as a fact. Subtraction is more cognitively demanding as it involves counting backwards while at the same time keeping track of the number of backward steps (Baroody, 1984). Again, eventually some subtraction facts will become learned and can be retrieved from semantic memory. Thus with age the strategies they use shift to automatic fact retrieval for both addition and subtraction, hence the adults in their study showed more consistent and similar cognitive processes and common representations to deal with the two arithmetic operations.
Chang et al. used what they refer to as multivoxel representational similarity (MRS) to demonstrate the common coding between addition and subtraction problems. There was activation in a range of brain areas in adults (areas that have been consistently associated with arithmetic problem solving) where addition and subtraction are both represented neurally in the same way, that is they were represented similarly in terms of the density of voxels. Their results showed MRS in adults between arithmetic and subtraction problems in the posterior parietal cortex (PPC), ventral temporal–occipital cortex (VTOC), prefrontal cortex (PFC) and anterior temporal cortex (ATC) (see Figure 9.1). In children’s brains no such MRS areas were found. Chang et al. claim that they are the first to show common neural representations across the two arithmetic operations due to the development of arithmetic problem solving skills.
Stages in problem solving
In addition to looking at those brain areas that are activated during a problem solving task, you can also examine what happens over time when people are engaged in trying to solve an arithmetic problem. One example is a study by Anderson, Lee and Fincham (2014) described in Information Box 9.2.
Information Box 9.2 The time course of arithmetic problem solving
Anderson et al. (2014) looked at the time course of solving arithmetic problems using a form of algebraic problem solving on a computer. They used data from both fMRI scans and computer mouse movements to divide the problem solving into distinct phases and states within those phases. They identified five problem solving phases that called upon a number of brain regions. Although several processes are active during each phase including perceptual, motor (“mousing behaviour”), representational, memory and control processes, an individual phase refers to a period of time when the amounts of the different processes appear constant. The phases are:
- 1 Define phase: During this phase, solvers are defining the problem based on the novel nature of the task. The brain regions involved are related to visual search and orienting attention and gaze. This phase also involves the “default mode network”, which is a network of brain regions that are active during mentation, when people are thinking about things (planning, daydreaming) and not engaged with the outside world, as it were.
- 2 Encode phase: In this phase, visual areas of the brain (the fusiform gyrus: see Figure 9.5) are active, particularly those associated with fine visual detail. Anderson et al. believe that this phase involves the encoding of the numbers and operators that are needed to generate an answer. Chang et al. (2015) also assume that this area is “tuned to represent visual number form”. The area has also been associated with difficulties in subtraction (Borst, Taatgen, Stocco, & van Rijn, 2010).
- 3 Compute phase: this phase sees the activation of the parietal and prefrontal regions including the HIPS. These regions are known to be active in calculation in arithmetic and algebra, with the retrieval of semantic information (left prefrontal region) and in the representation of quantity. The PPC has been found to correlate with executive control in working memory along with the HIPS, and with transformations of mental representations.
- 4 Transform phase: As the name suggests, in this phase participants are “performing structural transformations” of their answer. No distinctive region is active other than those involved in other phases, particularly phase 3, but with less activation in regions involving calculation and more in regions involved in motor control.
- 5 Respond phase: Since the study involves manipulating a computer mouse while problem solving, there is activation in left hemisphere regions associated with control of the right hand.
The earliest phase of problem solving normally involves making sense of the instructions in the Define Phase. Once you have interpreted the instructions you can start carrying them out. Interpreting instructions means translating an abstract representation of a task into specific actions. Later, faced with the same problem with whose instructions you are now familiar, there is no need to re-interpret the instructions. Instead you can rely on long-term memory to tell you what to do. Cole, Bagic, Kass and Schneider (2010) used fMRI and MEG to compare the effects of encountering novel instructions with ones that had been practiced before. They used a paradigm called Permuted Rule Operations, which allows a set of simple rules to be combined to create a large number of complex and novel tasks. They found that when a novel task was presented there was a bottom-up hierarchical process where the task instructions involved lower-level rule representations in the dorsolateral prefrontal cortex (DLPFC) suggesting a rehearsal of instructions in working memory. This process led to the development of a goal (higher-level task set) – an integrated, relational representation of the various components of the task – within the anterior prefrontal cortex (aPFC) which supported the coordination of subsequent task performance. For practiced tasks the opposite pattern was found such that when instructions were presented, it was the aPFC that was activated first. It loaded a goal representation from long-term memory which in turn activated individual rules in the DLPFC – representations of the various terms and actions to be performed. There therefore appears to be two ways humans can “reconfigure their minds” via instruction. One is a mechanism for interpreting novel tasks in terms of possible actions in order to define a goal and carry it out. The other is triggering a pre-established goal and accessing the actions and rules that allow a task to be carried out.
Importantly, the novel task preparation process begins to explain how we are able to rapidly learn a virtually infinite variety of possible tasks […], allowing our species to efficiently adapt to the many unique situations and new technologies of an ever-changing world.
(Cole et al., 2010, p. 14253)
Stocco, Lebiere, O’Reilly and Anderson (2012) also examined how instructions were interpreted and executed. Stocco et al. referred to the representation developed with practice of how instructions should be carried out as a “mental template”. They also pointed out that, although there was a reversal in the order in which brain regions were activated in novel compared with practiced tasks, the same LPFC areas that were involved in encoding these tasks were also involved in executing them. Nevertheless, they identified 18 regions that were more active when encoding than when executing instructions.
They hypothesised and found that a network of regions (striatum, rostral LPFC, and PPC) were involved in interpreting task instructions (they used different combinations of three arithmetic operators such as “add 1 to x, divide y by 2, and sum the results.” The parietal cortex was responsible for maintaining the “mental template” and there was a two-way link between it and the DLPFC (working memory). The caudate nucleus was more active during execution of novel instructions than during encoding in both novel and practiced tasks. Stocco et al. therefore see its role as a coordinating one transmitting information between areas when there are no previously established pathways.
Neurological processes in analogical reasoning
Since just before the end of the last century, much research on analogical reasoning seems to have shifted to neurological studies of relational reasoning. As we have seen, analogical reasoning involves an often complex relational structure and the need, therefore, to integrate multiple relations. The ability to map relational roles from a target to a source when the elements are dissimilar is an important aspect of several models of analogical reasoning. Only relatively recently have there been studies to find out if there is neurological evidence for the theories underpinning the models. The role of the PFC in reasoning and problem solving discussed earlier has been known for some time, but more recently researchers have been looking at some of the finer detail including the role of different parts of the PFC in integrating multiple relational representations. For example, the lateral frontopolar region seems to play a special role in relational integration (Cho et al., 2010; Christoff et al., 2001; Knowlton & Holyoak, 2009; Kroger et al., 2002). At the same time some cortical regions appear to be involved in controlling interference from irrelevant stimuli. Furthermore, verbal analogical reasoning in particular requires accessing semantic information (Bunge, Wendelken, Badre, & Wagner, 2005; Cho et al., 2010; Krawczyk, 2012). Recent theories of working memory suggest that the limited capacity to integrate information – the binding of concepts (Oberauer, 2005) – requires the inhibition of irrelevant information and explains much of the difficulty people have with analogical reasoning.
Many studies in this area used four-term analogy tasks to study the effects of relational reasoning (e.g., Cho et al., 2010; Halford, Wilson, & Phillips, 1998; Morrison et al., 2004). These tasks are of the form we have already encountered, A:B::C:D, and include alternatives to the D term, one of which is a distractor. An example might be play:game::give:? (1) party or (2) take (Morrison et al., 2004). Morrison et al., for example, tested patients with frontal or temporal lobe damage using four-term analogies such as the one just mentioned. These analogies tend to require a single relation and in some cases the non-analogical distractor was semantically associated with the C term, as is the case with give and take, although take does not work as an analogical relation. In order to determine which of party or take is correct the strongly associated take needs to be inhibited.
Morrison et al. also used pictorial analogies where there was a choice of a relational match or a featural match. They used pairs of pictures of scenes involving, for example, a man, a dog and a cat (Figure 9.4). In the top image the dog breaks the lead being held by the man and chases the cat; in the other the dog is tied to a tree and breaks the lead and chases the man while ignoring the cat. The relational match has the man in the second (target) picture mapping across to the cat in the source image. The man, dog, cat and tree are features that appear in both images. Morrison et al. found that patients with frontal lobe deficits (FTLD) made fewer relational matches and more featural or object matches showing that intact frontal and temporal lobes are needed to support relational matches.
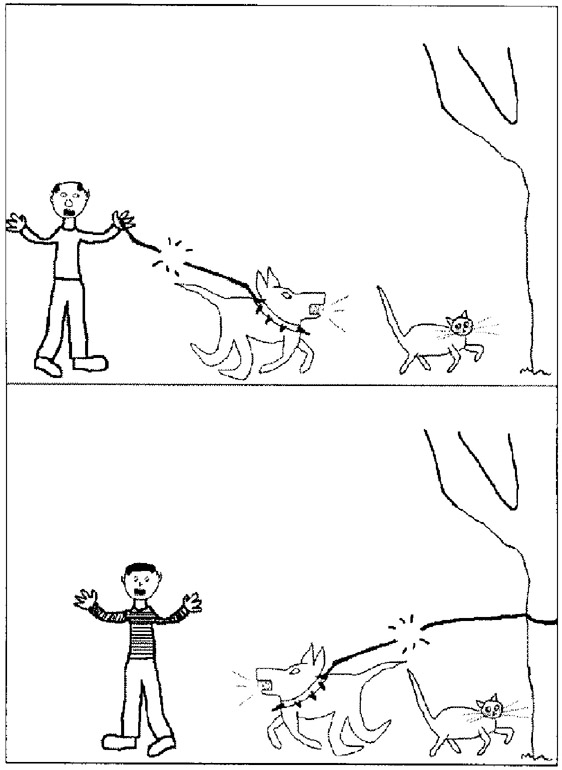
Tohill, J. M., & Holyoak, K. J. (2000). The impact of anxiety on analogical reasoning. Thinking & Reasoning, 6(1), 27–40. Figure reprinted by permission of the publisher (Taylor & Francis Ltd, http://www.tandfonline.com).
In a follow up investigation, Krawczyk et al. (2008) also used imagery-based analogy tasks. In one experiment participants were presented with images in the form sandwich:lunchbox::hammer:? followed by an appropriate relation-based response (toolbox) and a semantic distractor (nail). Previous studies had shown that frontal-variant frontotemporal lobar degeneration (fvFTLD) patients had difficulty manipulating and integrating multiple relations. Their study compared fvFTLD patients with temporal-variant FTLD (tvFTLD) patients and healthy controls. The fvFTLD patients made more incorrect answers than correct analogical answers and were significantly more likely to make such errors than controls. They also made significantly more perceptual distractor choices than controls.
Relational complexity |
||
One relation |
increasing size of object |
|
One relation |
changing colour of clothes |
|
Two relations |
increasing size and changing colour of clothes |
|
Example of semantic distraction theme: |
||
Consistent |
relation: colour change |
Man with fishing net, man with fishing line, man with fish |
Inconsistent |
relation: colour change |
Man with fishing net, man with fishing line, man with axe |
A second experiment used relational patterns of varying complexity. The stimuli included images of three people that varied along one or two dimensions (size and/or colour) and some included a “theme” intended to be semantically distracting (see Table 9.1).
The second experiment showed a significant performance deficit for fvFTLD patients who produced fewer relationally correct answers than the tvFTLD patients and controls. They also showed a selective deficit for the perceptual and semantic distraction problems over non-distraction problems implying that they had difficulty inhibiting interference from irrelevant information. It appears, then, that controlling this kind of interference to focus on a goal is an important function of the PFC. In the case of analogical reasoning the PFC is critical in maintaining a focus on the relational structure of an analogy. “Theories of relational reasoning should thus include interference control as a central cognitive operation required for successful problem solving” (Krawczyk et al., 2008, p. 2029).
Individual differences in analogical reasoning
Another approach to understanding brain functioning during problem solving is to examine how individual differences impact on brain activation. There are many studies of individual differences, often in areas such as intelligence or creativity, that are often explained in terms of the group or population from which the subjects are taken or as a result of some form of intervention. Preusse, van der Meer, Deshpande, Krueger and Wartenburger (2011) looked for the cerebral correlates of geometric analogical reasoning by comparing people with high versus average fluid intelligence using scores on Raven’s Matrices as a proxy measure of fluid intelligence. The analogies were of the type A:B::C:D. Solving geometric analogies involves mentally manipulating geometric figures to identify the relation between A and B (for example, B may be a mirror image of A) and applying this relation to the C:D pair. The task was to decide if the relation between C and D was the same as that between A and B or not. Preusse et al. believed that this kind of task did not rely greatly on verbal, semantic or contextual processing and was therefore a useful measure of fluid intelligence.
The difficulty of the tasks could be increased by manipulating the axes of the mirroring task, so image B might be a rotated mirror image of A. As expected, the high fluid intelligence (hiFluIQ) group performed the task consistently more quickly (although the differences were not statistically significant) and with significantly fewer errors than the average fluid intelligence (aveFluIQ) group. Based on behavioural measures such as these, we can see that there are differences between the two groups, but what is the source of these differences? Why does the aveFluIQ group make more errors? It is in answering questions such as these that studies of brain function can be very useful.
Parietal, frontal and occipito-temporal areas were activated during the analogical reasoning task and this activation was modulated by the relative difficulty of the task. Of particular interest in this case is that the left dorsal anterior cingulate cortex (ACC; see Figure 9.5 to see where this region is located) is more activated in the aveFluIQ group than in the hiFluIQ group and seems to be especially involved when cognitive effort is needed. The role of the ACC is to allocate control (Botvinick & Cohen, 2014). There is more activation in this area when someone is engaged in an unfamiliar task or is trying to integrate information. There are costs involved in this in terms of cognitive effort, and this has been referred to as expected value of control (EVC: Shenhav, Botvinick, & Cohen, 2013). (The EVC account suggests that the dorsal ACC integrates information about the costs in terms of cognitive effort, the likely payoff and the degree of control needed to achieve that payoff.)
It appears that the aveFluIQ group needed to exert greater executive monitoring and control to perform the analogy task than the hiFluIQ group. Preusse et al. argue that the latter group were able to allocate cerebral resources more flexibly than the average group and inter-hemispheric connectivity seemed to be more efficient: “stronger effective connectivity within the frontal brain regions for the hiFluIQ with simultaneously lower BOLD signal changes in these regions may indicate that efficient communication between these regions was possible without using too many resources” (Preusse et al., 2011, p. 11).
Wendelken, Nakhabenko, Donohue, Carter and Bunge (2008) conducted an fMRI analysis of people solving four-term verbal analogies, again of the form A:B::C:D. Participants were presented with a cue which was either a relation (e.g., wear) or pair of related items (boots:foot). This was presented for one second and followed by a probe which remained until the participant had responded. There were four conditions (Table 9.2), two of which involved comparing the probe with the cue and two in which the participants had to complete the analogy and respond once they had done so.
In the compare conditions the subject has to respond yes or no if the final term is appropriate. In the complete conditions the subject has to generate an appropriate final term and respond with yes if they had found an answer and no if they failed to do so.
Wendelken at al. found that the two comparison conditions produced activation in the rostrolateral PFC (RLPFC) but the two completion conditions did not. They interpreted these results as showing that the RLFPC involved processes of integration supporting the comparison of relational representations rather than relation completion. Furthermore, the verbal analogy task showed more activation in the ventral subregion of the RLPFC whereas the dorsal RLPFC showed some deactivation. They conclude that the RLFPC is therefore involved in important aspects of high-level cognition particularly in relational integration (Bunge et al., 2005; Cho et al., 2010).
Time course of analogical reasoning
Bunge et al. (2005) examined the role the PFC plays in analogical reasoning, particularly the processes of retrieval of semantic information from long-term memory and relational integration. They found that the left anterior inferior PFC had a role in semantic retrieval, the left frontopolar cortex had a role in integrating the semantic information retrieved, and the right DLPFC played a role in response selection such as rejecting an invalid analogy. Thus there is a time course for analogical comparisons starting with retrieval, then integration and finally response selection. There is also evidence that the right RLPFC is increasingly involved as processing demands increase (Crone et al., 2009; Krawczyk, 2012).
Condition |
Cue |
Probe |
Response type |
|---|---|---|---|
Term compare |
uses:: |
writer:pen |
yes/no |
Term complete |
uses:: |
writer: ? |
infer final term |
Example compare |
painter:brush:: |
writer:pen |
yes/no |
Example complete |
painter:brush:: |
writer:? |
infer final term |
Maguire, McClelland, Donovan, Tihiman and Krawczyk (2012) employed three conditions (analogical, semantic and perceptual) to ascertain the time course of the succession of phases involved in analogical reasoning. They used event-related potentials (ERPs), tiny voltages generated by the brain in response to various stimuli, to identify the neural timing of the analogical encoding, mapping and response phases. Using EEG measurements, they were looking for the kinds of typical responses in a particular band of electrical activity known as N300/N400. These are waveforms reflecting a negative going voltage in the brain that peak at around 300ms for the N300 and 400ms for the N400 waveforms after the onset of a semantic or perceptual stimulus – particularly an incongruous stimulus such as “He shaved his moustache and ears.” Maguire et al. therefore predicted that the same pattern of response would be elicited by analogical as well as semantic or perceptual incongruities.
Stimuli were presented to participants in different phases: encoding, mapping and response. In the encoding phase, participants were presented with an analogical stimulus (hockey stick:hockey puck), a semantic stimulus (e.g., pictures of a doe and a buck), or a perceptual stimulus similar to the semantic stimuli except that the task was to judge if the final term looked visually similar. In the mapping phase, an item such as a tennis racket for the analogical condition or an elephant in the semantic condition was presented. In the response phase for the analogical conditions, an item was presented that was either a good analogical match, such as a tennis ball in the example, or an incongruous item such as an aeroplane. Similarly, relevant or incongruous items were presented in this phase for the semantic and perceptual conditions. As predicted, analogical incongruity produced the N300/N400 signature as did the semantic and perceptual incongruity items. This suggests that some of the processes involved in relational reasoning are the same as those used in many other forms of reasoning.
Metaphor comprehension
One aspect of metaphor comprehension discussed in Chapter 3 is that some metaphors are processed as established idioms, essentially as part of normal language use, whereas other types of metaphor involve analogical comparison. This is Bowdle and Gentner’s (2005) “career of metaphor” model. A study by Chettih, Durgin and Grodner (2012) found neurological evidence for this distinction. Their results showed that conventional metaphors are processed in the left hemisphere which is involved in semantic and structural aspects of language, whereas the right hemisphere is involved in constructing meanings of novel metaphors and alternative structural alignments to fit with the particular contexts in which the metaphor is situated. The study therefore provided evidence for the distinction Bowdle and Gentner made between the processing pathways for different types of metaphor.
Neurocomputational models
Learning and inference with schemas and analogies
Both relational reasoning and control of interference are built into a neurocomputational model developed by Hummel and Holyoak (2003, 2005) called LISA (Learning and Inference with Schemas and Analogies). This was probably the first to model both theories of analogising combined with neuropsychological data. The aim was to develop an architecture that allowed both retrieval of a source and consequent structure mapping without losing flexibility (see the discussion on flexibility in Chapter 6). Models of structure mapping have mostly used a symbolic architecture such as a production system, but these tend to be rather inflexible in the sense that they do not cope well with missing information and are hard to adapt. Connectionist systems can cope with degraded information and can spontaneously generalise but are not so good at representing hierarchical propositional structures. LISA uses a symbolic connectionist architecture hopefully combining the best of both worlds. Some of the details of this neurocomputational model can be found in Information Box 9.3.
Information Box 9.3 Learning and Inference with Schemas and Analogies (LISA)
LISA codes relational structures within a neural network where patterns of activation among nodes represent semantic features. Hummel and Holyoak (2005) give the example of the object John to which the features human, adult, male are attached. Similarly, Sally is represented by the features human, adult, female. There are also roles that these objects can play such as the lover role and the beloved role. These roles in turn also have attached features such as emotion, positive, strong in the case of lover and emotion-object, positive representing the beloved role.
These objects and roles are represented as patterns of activation across units and the mechanism for binding John to the role lover, for example, is achieved through the synchronous firing of units representing these objects. The units for Sally and beloved also fire synchronously but not at the same time as the John and lover units. In fact, all active role bindings are mutually desynchronised.
LISA can represent a hierarchical system of semantic features, objects, roles, role bindings and sets of role bindings forming a proposition (Hummel & Holyoak, 2003). Hummel and Holyoak (2005) give the example of the high-level proposition “Sally knows that John loves Sally.” This can be represented in working memory or long-term memory as knows{Sally, [loves(John, Sally)]} which includes the proposition loves(John, Sally), which contains the objects John and Sally linked due to the loves relation to lover and beloved, which constitute a further sub-proposition. Propositions are stored in the system’s long-term memory as a four-tier, tree-like hierarchy. Having several layers in the hierarchy means that each level can be treated as independent when it comes to mapping to an analogue and generating inferences.
LISA has what Hummel and Holyoak refer to as an active memory, which is the set of currently active structures and semantic units somewhat similar to the long-term working memory proposed by Ericsson and Kintsch (1995; see Chapter 6). Within this active memory a small number or set of hierarchical role-bindings can take place within a phase set – “the set of active, mutually desynchronised role-filler bindings representing one or more propositions” (Hummel & Holyoak, 2005, p. 155), with each individual period of synchronised firing constituting a phase corresponding to the smallest unit of working memory.
When a novel target problem is input it acts as a “driver” with up to three propositions firing at the same time. Activation spreads down the branches of the four-tier hierarchical tree eventually activating patterns of semantic units in working memory. The patterns activated in this way then activate propositions in the system’s long-term memory, thereby cuing the retrieval of a relevant source analogue and thus allowing for analogical inference and schema induction. For example, if the system has the information Ann hates Bill, Bill hates Cathy and Ann likes Cathy (the source), and new information is input (a target) (Desmond hates Edward and Edward hates Frances), LISA can generate the inference that Desmond likes Frances on the basis of the shared roles, i.e., hates(person_1, person_2).
Hummel and Holyoak claim that the processes involved in analogical inference are the same as those that can generate rule or schema based inferences so the same algorithm employed to generate analogical inferences is itself augmented by a “self-supervised learning algorithm” that can create general rules of the type
- IF hates(person_1, person_2) and hates(person_2, person_3)
- THEN likes(person_1, person_3).
The system can also generate increasingly abstract, decontextualised schemas when additional examples are provided.
Hummel and Holyoak (2005) argue that LISA, as a neurocomputational model, can account for findings in cognitive neuropsychology and from fMRI studies. For example, Lisa can provide a model of the effects of types of frontal lobe degeneration by damaging its ability to learn mapping connections between analogues and reducing inhibitory control. The mechanism for relational role binding involves synchronous firing of units and this form of temporal synchrony is a “fundamental property of neural circuits” (Morrison & Knowlton, 2012). LISA provides an effective model of the functions of the prefrontal cortex in both learning and inhibitory control.
ACT-R as a neurocomputational model
As with LISA, the recent versions of ACT-R have developed into a neurocomputational model. The architecture of ACT-R includes a procedural memory, declarative memory, working memory, condition–action bonds, chunking and a mechanism for the development of skill and the concomitant forgetting of the declarative knowledge that originally supported it. Neurological evidence for a distinction between declarative and procedural knowledge has existed for some time (see e.g., Squire, Knowlton, & Musen, 1993).
Graybell (1998) found that the striatum (basal ganglia – see Figure 9.5) is involved in recoding information in the cortex causing sequences of actions to become automated. This comes about by the “chunking” of motor and cognitive action sequences. This would appear to give a neurological correlate of the kinds of stimulus–response or condition–action learning that ACT-R incorporates. One of the results of this kind of recoding and chunking is that a sequence of actions that was once conscious and slow becomes automated and fast.
The lack of conscious awareness in S-R learning may also be an advantageous property for a chunking mechanism in that the action chunks are treated as units (not, for example, as response chains). We do not want “supervisory attention” (Shallice, 1988) … or conscious manipulation to intervene inside the macro unit itself. Chunks take their advantage from being manipulable as entities, and the intervention of consciousness or attention might actually disrupt their smooth implementation.
(Graybell, 1998, p. 131)
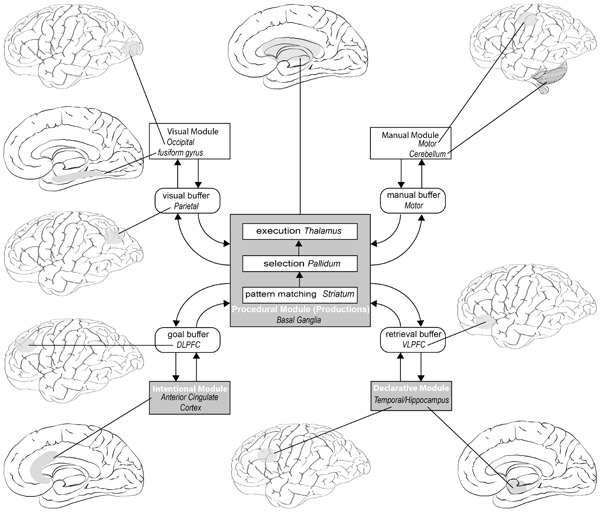
The basal ganglia and its functions are an important central feature of the ACT-R architecture, as can be seen from Figure 9.5.
Raichle (1998) also sought to identify those brain systems involved in skill learning. What brain regions are active when a verbal task is novel and effortful and how do they compare with those brain systems active when the task becomes routine and automated? Studies of the type that Raichle carried out involved imaging the brain using positron emission tomography (PET) scans and fMRI.
Five brain states of the participants were examined. The participants:
- 1 Were alert, eyes closed, performing no task;
- 2 Maintained visual fixation on TV monitor containing only a fixation point;
- 3 Maintained visual fixation on TV monitor while common English nouns are presented just below the point of fixation;
- 4 Read aloud names of objects (nouns) presented at the rate of 40 per minute;
- 5 Spoke aloud an appropriate use or verb for each noun as it is presented.
Computers were used to subtract the PET-generated digital image of one stage from another. For example, subtracting the “illuminated” parts of the brain in state 1 from state 2 shows what areas of the brain are involved in visually fixating a point, without it being confused with the areas of the brain active when resting. Practice on a verbal task led to a reduction in voice onset latency; that is, the more often a noun was presented the quicker participants responded – usually with a stereotyped answer, giving the same verb each time the noun appeared. The learning task revealed dramatic changes over time in the brain regions that were active during the performance of the task. Large areas of brain were involved in early learning but the number of areas active dropped dramatically with practice. Furthermore, previously inactive centres become active with practice.
As we have seen, early learning requires a lot of brain activity, particularly in those regions involved in monitoring and planning behaviour such as the ACC, the left PFC and the left temporal cortex. The wide distribution of areas of the brain active at the beginning seems to correspond to the supervisory attention system (consciousness, in effect). When these brain areas are no longer active and the task becomes automated, it is often no longer accessible to consciousness.
Back in 1990, Anderson pointed out that behavioural data on cognitive tasks can be explained by many different theories and models. He predicted that physiological data would be needed to link the more abstract algorithmic level to the implementation level. Over the last 20 years such data have become available, and some of the studies that Anderson and his colleagues have undertaken are discussed throughout this chapter. He has argued (Anderson, 2007) that a consequence of the brain’s structural and functional constraints is that it needs to be organised hierarchically and has a “modular” structure (Fodor, 1983). One of the outcomes of studies examining those brain regions involved in particular functions is the linking of the modules in recent versions of ACT-R with specific brain regions. Figure 9.5 shows the modules proposed in ACT-R and the brain regions that are most activated when people are engaged in different forms of activity. Most of the time several regions will be activated at the same time. If a motorist is driving along a busy road with many hazards and trying to remember where a building is situated, then all of the modules will be activated (vision, motor control, goal, retrieval, execution of procedures, etc.).
An important feature of the model is the role of the basal ganglia, which performs a coordinating role by facilitating communication between the different modules. This brain area represents the procedural module which has two-way links with each buffer. However, it is assumed that the production system can execute only one production at a time and that a production takes 50 ms to fire, so this system represents a central information processing bottleneck. In ACT-R the rationale is that you cannot have multiple rules firing simultaneously and therefore making conflicting demands on the system. Given the apparent competing demands in the scenario where the motorist is doing many things at once, Salvucci (2006) has developed a model of driving behaviour in ACT-R that shows how control and monitoring of driver behaviour can be understood.
The central element of ACT-R from its inception has been the idea of a production system. The basal ganglia therefore provide a plausible neuroanatomical correlate of the ACT-R production system. The evolution of the ACT-R architecture over the decades driven by the outcomes of experimental studies is set to continue driven by the outcomes of neuroimaging and other studies of what goes on in the brain when people perform tasks.
Designing instruction – what can studies of the brain tell us?
To solve a problem one can use examples and/or rely on explanations of how to solve it (instructions). We have seen how the nature of the instructions can make a problem more difficult or easier (cf. the Monster problems, manipulating extraneous load, using multimedia, etc.). We have seen how concrete examples can instantiate a rule or concept. It would be useful to know what neurological differences there are between using an example and interpreting instructions. A study by Lee, Fincham, Betts and Anderson (2014) showed that there were students who failed to learn from examples but were able to learn successfully from verbal instructions. Lee, Fincham and Anderson (2015) followed up on this study to examine more closely what effect different instructional conditions had on problem solving. They expected different brain regions to be active when reading about how to solve a mathematical problem compared with trying to follow an example to solve it. Mathematical calculation is not required while studying verbal instructions but would be involved in all other conditions. During the solution phase, however – the actual calculation –the same brain areas would be involved in both conditions.
On average their participants were better able to solve problems following verbal instructions than following examples, although the advantage was small. Studying an example increased activation in the left inferior PFC and the HIPS, suggesting numerical processing was required while studying the example. Processing written instructions in the verbal instructions condition necessarily involved greater activity in parts of the visual system (the fusiform gyrus) than the example condition when participants were studying the problem (the study phase). The AG region was active in both conditions during the study phase but less than anticipated during the solution phase. In the solution phase there was no real difference between the two groups in terms of the neural processing involved.
Based on their findings, they conclude that processing examples directs students to the procedural knowledge required more effectively than processing verbal instructions does. There are educational implications arising from this and other studies of the neural processing involved in problem solving, as they are starting to clarify what exactly is going on under different instructional conditions.
“Neuroeducation”
When it comes to using what we have learned about the role of different brain areas to inform how we teach, many researchers have referred to a “gap” or “boundary” between neuroscience and educational practice (Edelenbosch, Kupper, Krabbendam, & Broerse, 2015). Where this gap has been bridged it has often been by commercial enterprises selling “brain-based” products, usually with an emphasis on “brain training”. In the United States this is a multimillion-dollar industry. However, there is a strong view that the neuromarketing involved in selling such products is based on “neuromyths”. For example, it was claimed that the Mozart Effect (Rauscher, Shaw, & Ky, 1995), playing the first movement of the Sonata for Two Pianos in D Major (KV 448), enhances children’s spatial reasoning, presumably by influencing processes in the right cerebral hemisphere. Immordino-Yang (2011) stated that this finding has been misapplied, and Pietschnig, Voracek and Formann (2010) did a meta-analysis of research into the effect and found very little evidence for an effect at all.
Lindell and Kidd (2011) are scathing of attempts to use “pseudoscience”, particularly the right-brain/left-brain neuromyth to inform educationalists about how to teach: “there is no evidence to suggest (1) that traditional teaching neglects the right hemisphere, (2) that people favor one side of the brain, or (3) that any educational tool or strategy can selectively activate one hemisphere” (p. 121). Goswami (2006) also has criticised the right-brain/left-brain myths prevalent in education as well as the “synaptogenesis” myth concerning critical periods when certain skills should be taught during optimal “synaptic density” before this presumed window of opportunity closes.
Looking at the effectiveness of how brain-based educational programmes are marketed, Lindell and Kidd (2013) presented participants with adverts for an educational programme referring to Right Start Training or Right Brain Training with or without a MRI brain image in one corner. There was a very strong effect of language with the “Right Brain” wording generating much more interest, and this effect was enhanced by the inclusion of an image of the brain. Linking a putative educational programme with the brain gave the programme a scientific legitimacy in the minds of most of those who took part.
Edelenbosch et al. (2015) refer to brain-based learning as a “boundary object” because educational professionals and neuroscientists view it from different perspectives. There are “bridges being built” to span the gap between neuroscience and practice but there is little transdisciplinary research or discussion. There are also bridges to be built between the cognitive, affective and social neurosciences. Much of the foregoing discussion in this chapter has been focussed on the cognitive neuroscience of problem solving, reasoning and learning. Some researchers believe that we cannot develop theories and models of “brain-based” learning and education without including genetics and the cultural, social and affective neurosciences (Chiao & Immordino-Yang, 2013; Fischer, 2009; Fischer, Goswami, & Geake, 2010; Immordino-Yang, 2011; Immordino-Yang & Damasio, 2007) as well as metacognition (Guy & Byrne, 2013) and self-efficacy (Byrnes & Fox, 1998). Immordino-Yang (2011), for example, has argued that we cannot have a full understanding of the neurological basis of how people solve problems unless emotion, social processing and the self are taken into account. She argues that these cannot be dissociated from cognitive aspects of problem solving or from each other.
Emotions, such as anger, fear, happiness and sadness, are cognitive and physiological processes that involve both the body and mind (Damasio et al., 2000). As such, they utilize brain systems for body regulation (e.g. for blood pressure, heart rate, respiration, digestion) and sensation (e.g. for physical pain or pleasure, for stomach ache). They also influence brain systems for cognition, changing thought in characteristic ways.
(Immordino-Yang, 2011, p. 99)
While there are neuroscientific studies of affect, self and social cognition, there is relatively little that combines those with problem solving and learning. That said, Posner, Rothbart and colleagues (e.g., Posner, Rothbart, Sheese, & Tang, 2007; Posner, Rothbart, & Tang, 2013; Tang, Rothbart, & Posner, 2012) have been examining ways that certain forms of training can impact on how well children learn. By combining neuroimaging techniques with different forms of training, Posner and Rothbart (2005) and Posner et al. (2013) discuss ways in which neural networks can be altered through training with particular foci on attentional systems and self-regulatory systems. Improving executive attention through training can have consequences on children’s literacy and numeracy as well as other areas. Improvements in self-regulation can have an impact on how well children can control their thoughts, actions and emotions.
Posner et al. (2013) summarise ways in which network training and brain state training can improve self regulation and brain state training to reduce stress and induce a “quiet alert state”. The network training “(1) tunes the neurons in each node to fit more completely with the mental operation being performed and (2) strengthens the connection between nodes” (Posner et al., 2013, p. 108). Various tasks involving working memory and executive function can be used to train the relevant networks. Brain state training uses meditation and aerobic exercise. Tang, Yang, Leve and Harold (2012) describe randomised control trials examining the impact of such brain state training. They found that Integrative Body-Mind Training (IBMT) – a form of meditation – induces rapid changes in brain state, particularly in the parasympathetic nervous system, and appears to have positive effects on executive function in children including attentional control, stress reduction as measured by cortisol levels and in neuroimaging, and regulation of emotion. Those children in the IBMT group showed evidence of increased connectivity between ACC and striatum. Posner et al. (2013) point out that beneficial effects of some forms of training do not always last but self-regulation seems to be maintained.
Over the past few years there has been an increasing interest in brain-based education. The European Association for Research on Learning and Instruction (EARLI) have a number of special interest groups, one of which is Neuroscience and Education. It had the first of its bicentennial conferences in 2010. It is likely that this area of study will develop greatly over the next few years as researchers try to find useful ways to integrate brain, behaviour and education.
Neurological aspects of insight and creativity
Recent research into insight and creativity has focussed on what kinds of information are processed and how. In particular, if creative insights differ from “normal” problem solving processes then we ought to be able to identify differences in brain activity when those processes are active. Studies of the neuroscience of creativity and insight have taken place mainly since the year 2000 with a small number of exceptions, so this method of studying creativity is largely in its infancy.
A major difficulty in studying creativity from a neuroscience perspective is that it is hard to examine artistic or scientific creation when the person you are studying is inside an fMRI scanner. Instead researchers need to be creative in the way they devise tasks that can be performed relatively easily and that hopefully correlate with creativity. In many cases the tasks used are derived from the various psychometric tests such as the remote associates test (three words to which a third can be added), or the alternative uses task (how many uses can you think of for a brick), the Nine-Dot problem, and so on. These are typical tests of divergent thinking and insight, however some researchers devise their own tests, potentially creating problems of validity and reliability. Relatively simple tests such as these are reasonably tractable and can be done within a short time frame and inside an fMRI scanner, for example. There have also been attempts at investigating the neural correlates of musical and artistic creativity using tasks that reflect what goes on in real life (composing a tune, thinking of a poem).
An example of a neurological study is that of Jung-Beeman et al. (2004). They got participants to try to solve both non-insight and insight problems and to state whether they had an “Aha!” experience. The latter was used to categorise a solution as one involving insight for that individual. Using a remote associates test, they found that the right hemisphere anterior superior temporal gyrus (see Figure 9.1) showed increased activity for insight problem solutions compared with non-insight problem solutions.
Several studies have shown that activation in the right hemisphere correlates with an increase in participants’ ability to solve insight problems. Chi and Snyder (2011) used transcranial direct current stimulation, on both the right and left hemispheres to examine its effects on reducing mental set and thereby enhancing insight problem solving. Decreasing the excitability of the left anterior temporal lobe (ATL) and increasing on the right ATL resulted in three times more successful solutions to matchstick arithmetic problems than in a control condition.
An unfortunate outcome in trying to determine the neuroanatomy of creativity is that when some studies identify a brain region that appears to play an important role in creativity, another set of studies comes along to show the opposite. For example, based on several studies, the PFC would appear to be involved in the creative process (see e.g., Jung, Mead, Carrasco, & Flores, 2013; Wiggins & Joydeep, 2014). Therefore, if there is damage to the PFC through neurodegenerative disease, for example, one would expect a reduction in creativity. However, de Souza et al. (2014) among others have found that, in some cases, artistic production increased during the course of a PFC disease; in de Souza et al.’s study it was someone suffering from fronto-temporal dementia. Where several studies have suggested that the right hemisphere is important for creativity, Andreasen and Ramchandran (2012) found that for a small number of highly creative “Big-C individuals” the predominant hemisphere was the left for both artists and scientists and not the right.
According to Dietrich and Kanso (2010) there is an assumption in many studies that divergent thinking and insight are suitable proxies for the concept of creativity. They conducted a review of the studies on the neuroscience of creativity and insight mostly in the decade from 2000 to 2010. They argue that much of the research has examined the view that creativity is a function of the right hemisphere of the brain, low cortical arousal as measured by alpha waves based on EEG data, and defocussed attention, and that divergent thinking is a useful measure of creativity in such studies.
Divergent thinking, however, is not a single process so different studies have found different areas of the brain to be involved in whatever measure of divergent thinking the researchers chose to use. Furthermore, when a particular brain area is implicated results can be conflicting. Some studies of artistic creativity show an activation of the PFC and others show a deactivation. “The most sensible conclusion from these data is that divergent thinking is not neuroanatomically detectable as a stand-alone, independent entity” (Dietrich & Kanso, 2010, p. 833). A summary of Dietrich and Kanso’s conclusions is shown in Information Box 9.4.
Information Box 9.4 Some conclusions from the review by Dietrich and Kanso (2010)
- The prefrontal cortices appear to play an important role in divergent thinking but the data from EEG and neuroimaging do not allow a more specific conclusion.
- EEG and brain imaging do not support a particular brain laterality for divergent thinking.
- Data do not show a particular anatomical region in the brain being linked to divergent thinking other than the prefrontal cortices.
- EEG, neuroimaging and pharmacological studies show no effect of arousal on creativity.
- Divergent thinking and the psychometric tests underpinning it are not useful in the search for a neuroanatomical basis for creativity.
- Creativity (given that it is poorly operationally defined overall) does not seem to reside in a particular brain area.
- Creativity does not appear to be correlated with a specific neurocognitive process, although the superior temporal gyrus and the anterior cingulate cortex seem to be involved in successful insight problem solutions.
Since Dietrich and Kanso’s review, there have been a number of useful reviews of the neurological literature on creativity (Abraham, 2013; de Souza et al., 2014; Kounios & Beeman, 2014) all pointing out the variety of ways in which creativity and insight have been operationalised and the variety of methods used to study these phenomena. Overall they provide a useful basis for the re-examination of some of the processes proposed in insight and creativity and some possible future directions.
Summary
In looking for the neurological correlates of problem solving processes, we can be reasonably confident that particular part of the cortex performs a particular function in relation to a particular task when that task is carefully circumscribed. For example, researchers generally have a shared understanding about what subtraction is, or analogical comparison, or relational integration. Things get murkier when we consider concepts such as creativity or divergent thinking or insight – hence the critique by Dietrich and Kanso (2010). While it is true that a full understanding of the processes involved when a child tries to solve a problem should include something about motivation, affect, self-regulation and so on, these are irrelevant if we are trying to ascertain where, say, relational integration takes place. For the moment we really need to take one step at a time before we can start to integrate different neurological systems.
In relation to any connection between neuroscience and pedagogy, Goswami (2008) warns against reading too much into the results of neuroimaging studies since, despite being physiological measures, they are essentially correlational. In many cases one can find a correlation without establishing a cause. It is not for nothing that neuropsychologists refer to the neural correlates of behaviour rather than causes.
- 1 Studies of arithmetic have looked at the “number line” revealing the important role played by the anterior gyrus and visual spatial areas. The AG is also involved in aspects of skill learning, retrieving number facts, and the verbal processing of numbers. The horizontal intraparietal sulcus (HIPS) is involved in calculation and processing quantity.
- 2 Anderson et al. (2014) have identified the neural correlates (in brackets) of problem solving phases:
- Default phase (default mode network)
- Encode phase (fusiform gyrus)
- Compute phase (HIPS)
- Transform phase (no distinct region)
- Respond phase (motor cortex).
- 3 Relational reasoning indicates the involvement of the fronto-polar region of the brain but importantly requires the inhibition of irrelevant concepts.
- 4 Individual differences appear to be due to differential activation of different brain regions due to differential resource allocation.
- 5 Different hemispheres are involved in processing conventional metaphors as opposed to normal ones.
- 6 Some computational models have developed into neurocomputational ones:
- LISA, concerned with such processes as analogical problem solving and schema induction;
- ACT-R, a general cognitive architecture.
- 7 There is evidence that the functional elements ACT-R can link to specific neuroanatomical structures.
- 8 Some forms of training seem to have a beneficial effect on student learning and self-regulation, but not much has happened to integrate cognitive, social and affective neuroscience to inform educational practice, and it is often prey to neuromyths.
- 9 Neurological studies of insight and creativity suggest that there needs to be a fine-grained analysis of the tasks involved, as lumping several disparate tasks together as “divergent thinking” is unhelpful.
- 10 Some proxy measures of creativity are not useful in identifying the neuroanatomy of creativity as creativity is not a monolithic concept and not localisable.
References
Abraham, A. (2013). The promises and perils of the neuroscience of creativity. Frontiers in Human Neuroscience, 7. Retrieved from doi:10.3389/fnhum.2013.00246
Anderson, J. R. (2007). How can the Human Mind Occur in the Physical Universe? Oxford: Oxford University Press.
Anderson, J. R., Lee, H. S., & Fincham, J. M. (2014). Discovering the structure of mathematical problem solving. Neuroimage, 97, 163–177. doi:10.1016/j.neuroimage.2014.04.031
Andreasen, N. C., & Ramchandran, K. (2012). Creativity in art and science: Are there two cultures? Dialogues in Clinical Neuroscience, 14(1), 49–54.
Baroody, A. J. (1984). Children’s difficulties in subtraction: Some causes and questions. Journal for Research in Mathematics Education, 15(3), 203–213. doi:10.2307/748349
Borst, J. P., Taatgen, N. A., Stocco, A., & van Rijn, H. (2010). The neural correlates of problem states: Testing FMRI predictions of a computational model of multitasking. PLOS ONE, 5(9), e12966–e12966. doi:10.1371/journal.pone.0012966
Botvinick, M. M., & Cohen, J. D. (2014). The computational and neural basis of cognitive control: Charted territory and new frontiers. Cognitive Science, 38(6), 1249–1285. doi:10.1111/cogs.12126
Bowdle, B. F., & Gentner, D. (2005). The career of metaphor. Psychological Review, 112(1), 193–216. doi:10.1037/0033-295X.112.1.193
Bunge, S. A., Wendelken, C., Badre, D., & Wagner, A. D. (2005). Analogical reasoning and prefrontal cortex: Evidence for separable retrieval and integration mechanisms. Cerebral Cortex, 15(3), 239–249.
Byrnes, J. P., & Fox, N. A. (1998). The educational relevance of research in cognitive neuroscience. Educational Psychology Review, 10(3), 297–342.
Cattaneo, Z., Silvanto, J., Pascual-Leone, A., & Battelli, L. (2009). The role of the angular gyrus in the modulation of visuospatial attention by the mental number line. Neuroimage, 44(2), 563–568. doi:10.1016/j.neuroimage.2008.09.003
Chang, T.-T., Rosenberg-Lee, M., Metcalfe, A.W.S., Chen, T., & Menon, V. (2015). Development of common neural representations for distinct numerical problems. Neuropsychologia, 75, 481–495. doi:10.1016/j.neuropsychologia.2015.07.005
Chettih, S., Durgin, F. H., & Grodner, D. J. (2012). Mixing metaphors in the cerebral hemispheres: What happens when careers collide? Journal of Experimental Psychology: Learning, Memory, and Cognition, 38(2), 295–311. doi:10.1037/a0025862
Chi, R. P., & Snyder, A. W. (2011). Facilitate insight by non-invasive brain stimulation. PLOS ONE, 6(2), 1–7. doi:10.1371/journal.pone.0016655
Chiao, J. Y., & Immordino-Yang, M. H. (2013). Modularity and the cultural mind: Contributions of cultural neuroscience to cognitive theory. Perspectives on Psychological Science (Sage Publications Inc.), 8(1), 56–61. doi:10.1177/1745691612469032
Cho, S., Moody, T. D., Fernandino, L., Mumford, J. A., Poldrack, R. A., Cannon, T. D., … Holyoak, K. J. (2010). Common and dissociable prefrontal loci associated with component mechanisms of analogical reasoning. Cerebral Cortex, 20(3), 524–533. doi:10.1093/cercor/bhp121
Christoff, K., Prabhakaran, V., Dorfman, J., Zhao, Z., Kroger, J. K., Holyoak, K. J., … Gabrieli, J.D.E. (2001). Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage, 14(5), 1136–1149.
Cohen, L., Dehaene, S., Chochon, F., Lehericy, S., & Naccache, L. (2000). Language and calculation within the parietal lobe: A combined cognitive, anatomical and fMRI study. Neuropsychologia, 38(10), 1426–1440.
Cole, M. W., Bagic, A., Kass, R., & Schneider, W. (2010). Prefrontal dynamics underlying rapid instructed task learning reverse with practice. Journal Of Neuroscience: The Official Journal of the Society for Neuroscience, 30(42), 14245–14254. doi:10.1523/JNEUROSCI.1662-10.2010
Crone, E. A., Wendelken, C., van Leijenhorst, L., Honomichl, R. D., Christoff, K., & Bunge, S. A. (2009). Neurocognitive development of relational reasoning. Developmental Science, 12(1), 55–66.
Damasio, A. R., Grabowski, T. J., Bechara, A., Damasio, H., Ponto, L.L.B., Parvizi, J., & Hichwa, R. D. (2000). Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience, 3(10), 1049–1056.
de Souza, L. C., Guimarães, H. C., Teixeira, A.N.L., Caramelli, P., Levy, R., Dubois, B., … Volle, E. (2014). Frontal lobe neurology and the creative mind. Frontiers in Psychology, 5. Retrieved from doi:10.3389/fpsyg.2014.00761
Dehaene, S. (2011). The Number Sense: How the Mind Creates Mathematics (2nd ed.). Oxford: Oxford University Press.
Dehaene, S., Bossini, S., & Giraux, P. (1993). The mental representation of parity and number magnitude. Journal of Experimental Psychology: General, 122(3), 371–396. doi:10.1037/0096-3445.122.3.371
Dehaene, S., & Cohen, L. (1997). Cerebral pathways for calculation: Double dissociation between rote verbal and quantitative knowledge of arithmetic. Cortex (Science Direct), 33(2), 219–250.
Dehaene, S., Piazza, M., Pinel, P., & Cohen, L. (2003). Three parietal circuits for number processing. Cognitive Neuropsychology, 20(3–6), 487–506.
Dietrich, A., & Kanso, R. (2010). A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychological Bulletin, 136(5), 822–848. doi:10.1037/a0019749
Edelenbosch, R., Kupper, F., Krabbendam, L., & Broerse, J.E.W. (2015). Brain-based learning and educational neuroscience: Boundary work. Mind, Brain, and Education, 9(1), 40–49.
Ericsson, K. A., & Kintsch, W. (1995). Long-term working memory. Psychological Review, 102, 211–245.
Fischer, K. W. (2009). Mind, brain, and education: Building a scientific groundwork for learning and teaching1. Mind, Brain & Education, 3(1), 3–16. doi:10.1111/j.1751-228X.2008.01048.x
Fischer, K. W., Goswami, U., & Geake, J. (2010). The future of educational neuroscience. Mind, Brain & Education, 4(2), 68–80. doi:10.1111/j.1751-228X.2010.01086.x
Fodor, J. A. (1983). The Modularity of Mind. Cambridge, MA: MIT Press/Bradford Books.
Göbel, S., Walsh, V., & Rushworth, M. F. (2001). The mental number line and the human angular gyrus. Neuroimage, 14(6), 1278–1289.
Goswami, U. (2006). Neuroscience and education: From research to practice? Nature Reviews Neuroscience, 7(5), 406–413.
Goswami, U. (2008). Principles of learning, implications for teaching: A cognitive neuroscience perspective. Journal of Philosophy of Education, 42(3–4), 381–399.
Grabner, R. H., Ansari, D., Koschutnig, K., Reishofer, G., Ebner, F., & Neuper, C. (2009). To retrieve or to calculate? Left angular gyrus mediates the retrieval of arithmetic facts during problem solving. Neuropsychologia, 47(2), 604–608. doi:10.1016/j.neuropsychologia.2008.10.013
Graybell, A. M. (1998). The basal ganglia and chunking of action repertoires. Neurobiology of Learning and Memory, 70, 119–136.
Guy, R., & Byrne, B. (2013). Neuroscience and learning: Implications for teaching practice. Journal of Experimental Neuroscience, 7, 39–42. doi:10.4137/JEN.S10965
Halford, G. S., Wilson, W. H., & Phillips, S. (1998). Processing capacity defined by relational complexity: Implications for comparative, developmental, and cognitive psychology. Behavioral and Brain Sciences, 21, 803–831.
Hummel, J. E., & Holyoak, K. J. (2003). A symbolic-connectionist theory of relational inference and generalization. Psychological Review, 110(2), 220–264. doi:10.1037/0033-295X.110.2.220
Hummel, J. E., & Holyoak, K. J. (2005). Relational reasoning in a neurally plausible cognitive architecture: An overview of the LISA project. Current Directions in Psychological Science, 14(3), 153–157.
Immordino-Yang, M. H. (2011). Implications of affective and social neuroscience for educational theory. Educational Philosophy and Theory, 43, pp. 98–103.
Immordino-Yang, M. H., & Damasio, A. (2007). We feel, therefore we learn: The relevance of affective and social neuroscience to education. Mind, Brain & Education, 1(1), 3–10. doi:10.1111/j.1751-228X.2007.00004.x
Jung, R. E., Mead, B. S., Carrasco, J., & Flores, R. A. (2013). The structure of creative cognition in the human brain. Frontiers in Human Neuroscience. Retrieved from doi:10.3389/fnhum.2013.00330
Jung-Beeman, M., Bowden, E. M., Haberman, J., Frymiare, J. L., Arambel-Liu, S., Greenblatt, R., … Kounios, J. (2004). Neural activity when people solve verbal problems with insight. Public Library of Science Biology, 2(4), 1–23.
Knowlton, B. J., & Holyoak, K. J. (2009). Prefrontal substrate of human relational reasoning. In M. S. Gazzaniga (Ed.), The Cognitive Neurosciences (4th ed., pp. 1005–1017). Cambridge, MA: MIT Press.
Kounios, J., & Beeman, M. (2014). The cognitive neuroscience of insight. Annual Review of Psychology, 65(1), 71–93. doi:10.1146/annurev-psych-010213-115154
Krawczyk, D. C. (2012). The cognition and neuroscience of relational reasoning. Brain Research, 1428, 13–23. doi:10.1016/j.brainres.2010.11.080
Krawczyk, D. C., Morrison, R. G., Viskontas, I., Holyoak, K. J., Chow, T. W., Mendez, M. F., … Knowlton, B. J. (2008). Distraction during relational reasoning: The role of prefrontal cortex in interference control. Neuropsychologia, 46, 2020–2032. doi:10.1016/j.neuropsychologia.2008.02.001
Kroger, J. K., Sabb, F. W., Fales, C. L., Bookheimer, S. Y., Cohen, M. S., & Holyoak, K. J. (2002). Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: A parametric study of relational complexity. Cerebral Cortex, 12(5), 477–485.
Lee, H. S., Fincham, J. M., & Anderson, J. R. (2015). Learning from examples versus verbal directions in mathematical problem solving. Mind, Brain, and Education, 9(4), 232–245.
Lee, H. S., Fincham, J. M., Betts, S., & Anderson, J. R. (2014). An fMRI investigation of instructional guidance in mathematical problem solving. Trends in Neuroscience and Education, 3(2), 50–62.
Lindell, A. K., & Kidd, E. (2011). Why right-brain teaching is half-witted: A critique of the misapplication of neuroscience to education. Mind, Brain & Education, 5(3), 121–127. doi:10.1111/j.1751-228X.2011.01120.x
Lindell, A. K., & Kidd, E. (2013). Consumers favor “right brain” training: The dangerous lure of neuromarketing. Mind, Brain & Education, 7(1), 35–39. doi:10.1111/mbe.12005
Maguire, M. J., McClelland, M. M., Donovan, C. M., Tihiman, G. D., & Krawczyk, D. C. (2012). Tracking cognitive phases in analogical reasoning with event-related potentials. Journal of Experimental Psychology. Learning, Memory & Cognition, 38(2), 273–281. doi:10.1037/a0025485
Markman, A. B. (2012). Knowledge representation. In K. J. Holyoak & R. G. Morrison (Eds.), The Oxford Handbook of Thinking and Reasoning (pp. 36–51). New York, NY: Oxford University Press.
Marr, D. (1982; 2010). Vision. New York: Freeman.
Morrison, R. G., & Knowlton, B. J. (2012). Neurocognitive methods in higher cognition. In K. J. Holyoak & R. G. Morrison (Eds.), The Oxford Handbook of Thinking and Reasoning (pp. 67–89). Oxford: Oxford University Press.
Morrison, R. G., Krawczyk, D. C., Holyoak, K. J., Hummel, J. E., Chow, T. W., Miller, B. L., … Knowlton, B. J. (2004). A neurocomputational model of analogical reasoning and its breakdown in frontotemporal lobar degeneration. Journal of Cognitive Neuroscience, 16(2), 260–271.
Oberauer, K. (2005). Binding and inhibition in working memory: Individual and age differences in short-term recognition. Journal of Experimental Psychology: General, 134(3), 368–387. doi:10.1037/0096-3445.134.3.368
Pietschnig, J., Voracek, M., & Formann, A. K. (2010). Mozart effect-shmozart effect: A meta-analysis. Intelligence, 38(3), 314–323.
Posner, M. I., & Rothbart, M. K. (2005). Influencing brain networks: Implications for education. Trends in Cognitive Sciences, 9(3), 99–103. doi:10.1016/j.tics.2005.01.007
Posner, M. I., Rothbart, M. K., Sheese, B. E., & Tang, Y. (2007). The anterior cingulate gyrus and the mechanism of self-regulation. Cognitive, Affective & Behavioral Neuroscience, 7(4), 391–395. doi:10.3758/CABN.7.4.391
Posner, M. I., Rothbart, M. K., & Tang, Y. (2013). Developing self-regulation in early childhood. Trends in Neuroscience and Education, 2(3–4), 107–110.
Preusse, F., van der Meer, E., Deshpande, G., Krueger, F., & Wartenburger, I. (2011). Fluid intelligence allows flexible recruitment of the parieto-frontal network in analogical reasoning. Frontiers in Human Neuroscience, 5. Retrieved from doi:10.3389/fnhum.2011.00022
Raichle, M. E. (1998). The neural correlates of consciousness: An analysis of cognitive skill learning. Philosophical Transactions of the Royal Society, London B: Biological Sciences, 353(1377), 1889–1901.
Rauscher, F. H., Shaw, G. L., & Ky, K. N. (1995). Listening to Mozart enhances spatial-temporal reasoning: Towards a neurophysiological basis. Neuroscience Letters, 185(1), 44–47.
Salvucci, D. D. (2006). Modeling driver behavior in a cognitive architecture. Human Factors, 48(2), 362–380.
Seghier, M. (2013). The angular Gyrus. Neuroscientist, 19(1), 43–61.
Shallice, T. (1988). Information-processing models of consciousness: possibilities and problems. In A. J. Marcel & E. Bisiach (Eds.), Consciousness in Contemporary Science (pp. 305–333). Oxford: Oxford Science.
Shenhav, A., Botvinick, Matthew M., & Cohen, Jonathan D. (2013). The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron (Science Direct), 79(2), 217–240.
Squire, L. R., Knowlton, B., & Musen, G. (1993). The structure and organisation of memory. Annual Review of Psychology, 44, 453–495.
Stocco, A., Lebiere, C., O’Reilly, R., & Anderson, J. (2012). Distinct contributions of the caudate nucleus, rostral prefrontal cortex, and parietal cortex to the execution of instructed tasks. Cognitive, Affective, & Behavioral Neuroscience, 12(4), 611–628.
Tang, Y.-Y., Rothbart, M. K., & Posner, M. I. (2012). Neural correlates of establishing, maintaining, and switching brain states. Trends in Cognitive Sciences, 16(6), 330–337. doi:10.1016/j.tics.2012.05.001
Tang, Y. Y., Yang, L., Leve, L. D., & Harold, G. T. (2012). Improving executive function and its neurobiological mechanisms through a mindfulness-based intervention: Advances within the field of developmental neuroscience. Child Development Perspectives, 6(4), 361–366.
Wendelken, C., Nakhabenko, D., Donohue, S. E., Carter, C. S., & Bunge, S. A. (2008). ““Brain Is to thought as stomach is to ??””: Investigating the role of rostrolateral prefrontal cortex in relational reasoning. Journal of Cognitive Neuroscience, 20(4), 682–693.
Wiggins, G. A., & Joydeep, B. (2014). Mind the gap: An attempt to bridge computational and neuroscientific approaches to study creativity. Frontiers in Human Neuroscience, 8. Retrieved from doi:10.3389/fnhum.2014.00540