Chapter 20
The Rise and Rise of Digital Pharma
It has never been easier to find health information online. Be that via patient-oriented websites, NHS Online sources, user-generated forum content or a range of health, disease awareness and fitness applications available for mobile devices.
Healthcare professionals are increasingly using platforms such as doctors.net to communicate with each other and receive intelligence and share information about disease awareness and medicines in a more social and collaborative manner.
2012 saw the first mobile health app registered and regulated as a medical device in the UK, providing a strong indication that digital health tools are growing up. So what can healthcare organizations do to incorporate social platforms and digital tools into their campaign armoury and truly begin to benefit patient health outcomes?
The global healthcare landscape
Worldwide, governments aim to deliver effective, safe and affordable healthcare to their people with increasingly effective outcomes. And the budgets this sector attracts are not small change either from a political spend perspective or a PR perspective. In 2011169 the World Health Organization (WHO) expected a global annual spend on healthcare to reach US$6 trillion and over the last 50 years, healthcare spend has outpaced GDP growth by about 2% a year. There are, says McKinsey, few signs that this trend will slow.
New approaches such as digital and mobile health, remote medicine delivery, remote diagnosis, patient-led monitoring and physician digital knowledge sharing are cited as key ways in which costs can be lowered and access increased between and across the developing and developed world. As these innovations become more commonplace and filter down from healthcare practitioners to pharma companies and patients, technology becomes more than the delivery mechanism.
How patients approach digital health
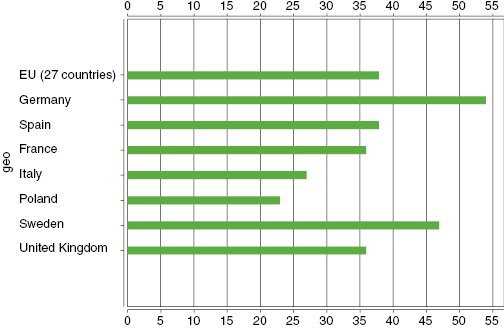
According to the most recent research from Eurostat (see Figure 20.1), in the UK alone, 80% of us have access to broadband internet as of 1 January 2012 and over 36% search for health information online.
Figure 20.1: Eurostat Information Statistics 2012170
The annual survey by Experian Hitwise and Private Healthcare UK171 outlines which sites are most visited and which issues UK citizens are most concerned about. The results show fat is more than a feminist issue with the British. Health seekers are more concerned with excess weight and how to lose it than any other health area.
In terms of where we go, the top five most visited healthcare websites in 2012 were:
Perhaps surprisingly, the trusted voice of the NHS online – NHS Direct – ranks 16th, putting it below commercial brand sites such as Holland & Barrett, BUPA and Specsavers.
Alongside an increase in high-speed broadband access in the UK and internet usage across all areas, the increase in smartphone penetration is a key driver in the digital health arena. In 2013, mobile phones will overtake PCs as the most common way to access the web172 and analysts predict that the smartphone application market for mobile healthcare will reach US$1.3 billion in 2012 with 247 million users downloading an app worldwide (see Figure 20.2).
Figure 20.2: mHealth: Consumer Attitudes Survey of 2,000 consumers (Ruder Finn/ YouGov June 2012)
Reproduced by permission of Ruder Finn UK Ltd

Healthcare professionals and the increased adoption of digital health
Healthcare professional (HCP) sources can be split into two key streams of “open” and “HCP only” social media. Both fulfil different roles and both are growing. Open channels tend to be used for research, information gathering and product searches whilst “HCP only” enables HCPs to connect with colleagues and share both perspectives and experiences. From a PR perspective, opportunities exist to engage HCPs in both streams of social media.
In Europe alone, the HCP-only community is fragmented. The large US-founded community WebMD recently entered Germany, where there were already half a dozen small networks. Similarly, a number of networks are also joining a partnership with doctors.net.uk in the UK. The site claims to have 40,000 daily users,173 ranking it as Europe's biggest network. A recent survey174 amongst European HCPs, showed those in the UK and Germany were most likely to use an online medical community.
As the time pressures on HCPs increase, they are less able to attend medical congresses and see pharmaceutical reps face-to-face – 52% of GPs did not see any pharmaceutical sales representatives in a typical week according to research from doctors.net.uk. As such, digital channels become an increasingly crucial way of sourcing independent product information. Nearly a quarter of them said they preferred to obtain their own product information via independent online resources. Only 3% of doctors think that online pharma resources are credible and 42% never visit pharmaceutical websites, meaning the role of PR is increasingly important in ensuring that credible and validated information appears across a range of trusted sources.
Many HCPs' roles are inherently mobile – GPs on home visits, specialists moving around hospitals or clinics, midwives attending home births or paramedics on duty – and this increases the interest and relevance of mobile devices. Doctors, in particular, have embraced smartphones and tablets wholeheartedly and 26% of European doctors now have and use an iPad professionally, spending a quarter of their online time using the device.175
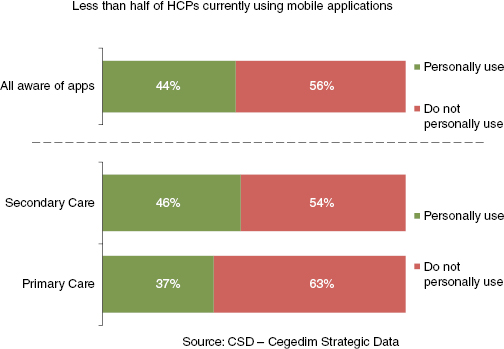
In terms of application usage, less than half176 of HCPs were personally using mobile applications for professional purposes. Of those that did, a higher proportion were in secondary care and more likely to be physicians than nurses (see Figure 20.3).
Figure 20.3: What percentage of HCPs use mobile applications? – Cegedim Strategic Data 2012
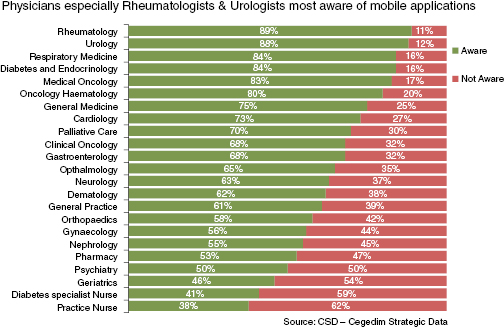
In terms of disease areas, there was greater use amongst clinical oncology, haematology and cardiology, with nursing, geriatrics and psychiatry rated the lowest users of mobile apps (see Figure 20.4).
Figure 20.4: Physicians most aware of mobile applications – Cegedim Strategic Data 2012
Avoiding the code breakers – how to navigate regulatory responsibilities
The regulatory environment differs hugely from market to market and must be carefully considered at the outset of any global or pan-regional campaign. In terms of regulatory environment in the UK, the control of medical marketing is based on a long-established system of self-regulation supported by the Medicines and Healthcare Products Regulatory Agency (MHRA). The MHRA administers legal regulation on behalf of UK Health Ministers.
The Association of the British Pharmaceutical Industry (ABPI) Code of Practice for the Pharmaceutical Industry, administered by the PMCPA, is the self-regulatory system covering prescription medicines. The ABPI Code extends beyond UK law and provides guidance on marketing, digital and social media activity.
The ABPI Code does not cover the advertising or promotion of medicines that are available without prescription or “over the counter” (OTC). The Proprietary Association of Great Britain177 (PAGB) regulates OTC medicines and food supplements. OTC products are far less restricted in terms of how they can be promoted than prescription only medicines (POMs).
In accordance with the ABPI Code,178 the advertising and promotion of (POMs) to the public is prohibited. Therefore, any promotional material about POMs directed to a UK audience which is provided on the internet either in an owned site or on a social network must comply with all relevant requirements of the Code.
Therapeutic area/disease awareness websites can be sponsored by companies provided that all the material available at the site complies with the Code. However, according to the MHRA,179 the primary purpose of a disease awareness campaign must be to increase awareness of a disease or diseases and to provide health educational information on that disease, not to promote the use of a particular pharmaceutical product or products. Websites for HCPs are possible as long as they have a clear firewall and require the viewer to confirm their HCP status.
A disease awareness campaign may make reference to the availability of treatment options but not specific medicines. And when there is only one treatment for a condition (i.e. no competitive drugs) disease awareness campaigns will naturally point to the only available drug on the market. In these circumstances, a campaign should only focus on general disease education with details of where to get appropriate advice and not on treatment options.
Control and adversity
A large proportion of healthcare campaigns in the social media or digital areas are ultimately funded by pharmaceutical companies, either directly or through charity funding or grants. Either way, their involvement must be clearly declared and any assets created are liable for the same levels of scrutiny and responsibility as the pharma company's own website. As such, this often causes the more risk-averse pharma companies to back away from two-way dialogue and to run campaigns with closed walls and comments disabled. This not only affects the overall success of many campaigns but also throws questions over whether they are actually social at all.
The reason for this risk aversion is primarily due to a lack of control over the dialogue that happens on social media and a fear of being seen to be conducting promotional activity to the public.
Whilst disease awareness campaigns are permitted, pharma companies cannot ensure the conversation remains about disease. In extreme cases, campaigns that are deemed direct to consumer and promotional in nature can result in imprisonment for medical directors, so the stakes are high!
However, another large factor in using social tools for pharmaceutical marketing is adverse event (AE) reporting. An adverse event is where a consumer discusses ill effects that they had during the time that they were taking a medication. Under current regulations from the ABPI, any adverse event must be reported and acted upon immediately as soon as it is discovered.180
The ABPI181 insists that monitoring should be frequent enough to ensure regulatory obligations can be met and daily monitoring of a campaign should be considered (it is important to note that the guideline does not specify round the clock monitoring).
If an adverse event is observed, there should be a robust procedure in place between the social media administrator, the pharmaceutical company's pharmacovigilance team and the AE reporting expert in order to handle this, according to the usual regulations.
Getting creative with digital health communications
2012 saw health organizations embrace digital campaigns that used social media like never before.
What's next?
Last year, the forward-looking predictions covered by media and bloggers alike featured digital health prominently on the agenda. This is great news for communications experts as the future for digital health is both red hot and right now!
How do you kick things off?
Assuming you've got your risk analysis and AE reporting sorted, you understand your demographics and have a clear idea of what you want your campaign to achieve, the healthcare campaign world really is your creative oyster. There are many trends that can provide great creative fodder to your brainstorms. Here's some brain food to get you started:
- Big Data – looking at a disease awareness programme at a global level and allowing HCPs and media to work together to interpret the story from the data.
- Mini data – making the story of me. What does my digital health footprint reveal about me and how will it impact me in the future?
- 3D printing – how can the new phenomenon in printer hardware impact healthcare professionals? How about printing models for plastic surgery or prosthetics? Or printing out a model of a tumour so the surgeon can hold it life size in his hands before he operates?
- Quantified self applications – look at the impact for consumers on mobile health – beyond exercise bragging rights and weight loss, how does this new trend to personal tracking impact everything from our health footprint to our insurance quotes? Can I finally get a complete personal health dashboard?
- Gaming data – how can gaming change negative health behaviour in the long term?
- Creative events and storytelling – using data and insights to inform your campaign, how can we start with finding the patient before we start to decide the tactics? Finally put a stop to starting with a website or an application … how can we put our data to work for us?
- Visual campaigns – how can platforms such as Pinterest, Vine or Instagram help with health conditions and redefine beauty for people with skin disease for example?
- Original content – How can pharmaceutical companies drive the development of original media and programming? An early example of this is Lilly's collaboration with Disney for a series of diabetes books.
Biography
Becky McMichael (@bmcmichael) is head of strategy and innovation at communications consultancy and digital studio, Ruder Finn UK. She has over 15 years of experience spanning many industries, including pharma, having worked on digital campaigns for organizations such as Abbott, Pfizer and Novartis. Becky has a real passion for technology and social tools, is a blogger and runs a community for working mums in marketing. She splits her time between London and Cornwall where she spends her free time walking at toddler speed to the beach.
Notes
169mHealth; McKinsey; 2012: http://cipr.co/WBz2MM
170Individuals using the Internet for seeking health-related information; Eurostat; 2012: http://cipr.co/YchZPC
1712012 Webwatch report; Private Healthcare UK; 2012: http://cipr.co/YFNJ1V
172Top 10 technology trends for 2013; Gartner; 2012: http://cipr.co/UDAX6E
173Doctors.net own web data; 2012
174Cegedim Strategic Data; 2012; Two-thirds of UK physicians aware of mobile applications for professional use: http://cipr.co/XRk0C4
175D. Tryer, Twenty six percent of European doctors own an iPad. 2012: http://cipr.co/Ycil8X
176Cegedim Strategic Data 2012; Two-thirds of UK physicians aware of mobile applications for professional use: http://cipr.co/XRk0C4
177Proprietary Association of Great Britain; Regulations and Guidance; 2012: http://cipr.co/VFLyc9
178Prescription Medicine Code of Practice Authority; The ABPI Code of Practice for the Pharmaceutical Industry; 2012: http://cipr.co/Ww6tCl
179Medicines and Healthcare products Regulatory Agency; The ABPI Code of Practice for the Pharmaceutical Industry; 2012: http://cipr.co/12vuJI0
180Association of the British Pharmaceutical Industry; 2012; Guidance notes on the management of adverse events and product complaints from pharmaceutical company sponsored websites: http://cipr.co/WBAQW2
181Association of the British Pharmaceutical Industry; Management of adverse events and product complaints from pharmaceutical company sponsored websites (section 7), 2011
