Chapter 8
General Science
IN THIS CHAPTER
Figuring out the scientific method
Grasping measurements
Studying scientific disciplines
Using scientific strategies to improve your score
As you study for this subtest, you may feel overwhelmed by facts and figures. General Science requires a lot of straight-up memorization. You’re presented with questions about facts you probably learned in high school in various science classes, such as health, Earth science, biology, and chemistry. If you don’t know that Earth is the third planet from the sun, then all the other science knowledge you have won’t help you one bit when the question asks, “What is the third planet from the sun?”
You have 11 minutes to answer 25 questions on the paper version of the General Science subtest, or you have 8 minutes to answer 16 General Science questions on the CAT-ASVAB. That comes out to about 26 or 30 seconds per question, so there’s no time to dilly-dally. For the most part, you either know the answer or you don’t. If you don’t know the answer, you can always guess (check out Chapter 3 for tips on guessing on the ASVAB).
You can relax this time around … well, just a little. The General Science subtest has no bearing on your Armed Forces Qualification Test (AFQT) score. On the other hand, your score on this subtest is used to calculate some of the military composite scores that are used for job qualification purposes (see Appendix A for more information).
Take some time to review the facts in this chapter as a mini science lesson. If the job you want requires a good score on this subtest, dedicate yourself to the information in this chapter to boost your General Science score. You may also want to seek additional study time in these references to boost your science knowledge: Chemistry For Dummies by John T. Moore, Biology For Dummies by Rene Fester Kratz and Donna Rae Siegfried, Astronomy For Dummies by Stephen P. Maran, Weather For Dummies by John D. Cox, and Physics I For Dummies and Physics II For Dummies by Steven Holzner, PhD (all from Wiley).
There’s a Scientific Method to the Madness
Scientists are pretty skeptical. They don’t necessarily believe anything said by anyone else unless it’s been shown to be true (time after time after time) using a process called the scientific method. Scientists know that personal and cultural biases may influence perceptions and interpretations of data, so they’ve derived a standard set of procedures and criteria to minimize those influences when developing a theory. Because the scientific method is prevalent in all fields of science, you can expect to see a few questions about the process on the General Science subtest.
Here are the usual steps to solving a problem using the scientific method:
- Observe some aspect of the universe.
- Develop an explanation (theory) about why this is happening.
- Make a prediction (hypothesis) based on the theory.
- Experiment and observe to test the hypothesis.
- If the results don’t match the hypothesis, modify the theory and create a new hypothesis.
- Keep repeating Steps 3, 4, and 5 until the hypothesis and experiment match.
When developing and testing a theory, scientists are guided by a principle known as Occam’s razor. This rule states, “When given two equally valid explanations for a phenomenon, one should embrace the less complicated formulation.” In other words, the simplest theory that explains the facts is usually the best one. If a theory holds up to repeated testing, scientists gain confidence in it, and a hypothesis that’s supported consistently over time eventually comes to be considered as a law, fact, or principle.
Understanding Forms of Measurement
Because science is based on developing objective facts — evidence and results that are measurable and experiments that can be reproduced — measurements are an important part of science. And because this subtest is all about science, you can expect to run into a few questions about measuring scientifically on the ASVAB.
Doing the metric thing
The metric system (or SI, the International System of Units) is based on a decimal system of multiples (and fractions) of ten. Scientists almost always use the metric system for precise measurement. No, they don’t use it just to make the ASVAB harder for you; they use this system so a standard exists among scientists around the world. In fact, the majority of countries around the globe use the metric system — the United States is in its own world when it comes to the Imperial (non-metric) system.
- The meter (m) is the unit of length.
- The liter (L) is the unit of volume.
- The gram (g) is the unit of mass (similar to weight).
You can attach prefixes to these base units to indicate units that are larger or smaller. Check out Table 8-1 for metric prefixes and Table 8-2 for some abbreviations of common metric measurements.
Table 8-1 Metric Prefixes
Prefix |
Symbol |
What It Means |
milli- |
m |
One-thousandth (0.001) |
centi- |
c |
One-hundredth (0.01) |
deci- |
d |
One-tenth (0.1) |
deca- |
da |
10 |
hecto- |
h |
100 |
kilo- |
k |
1,000 |
mega- |
M |
1,000,000 |
Table 8-2 Common Metric Units and Their Abbreviations
Length |
Liquid Volume |
Mass |
millimeter (mm) |
milliliter (mL) |
milligram (mg) |
centimeter (cm) |
centiliter (cL) |
centigram (cg) |
meter (m) |
liter (L) |
gram (g) |
kilometer (km) |
kiloliter (kL) |
kilogram (kg) |
Figuring temperature conversions
When you think of temperature, you may think of the Fahrenheit and Celsius scales, which measure temperatures in degrees. Scientists actually use three different scales to report temperature:
- Fahrenheit
 : This scale is more common in the United States. On the Fahrenheit scale, water freezes at
: This scale is more common in the United States. On the Fahrenheit scale, water freezes at  and boils at
and boils at  .
. - Celsius or Centigrade
 : This scale is the metric standard worldwide. On the Celsius scale, the freezing point for water is
: This scale is the metric standard worldwide. On the Celsius scale, the freezing point for water is  , and the boiling point for water is
, and the boiling point for water is  .
. -
Kelvin (K): Scientists have theorized that the coldest anything can get is –
 . They believe that at this temperature, molecular motion would stop. That’s pretty darn cold! This temperature, often called absolute zero, is assigned to be 0 on the Kelvin scale (with the units the same size as degrees on the Celsius scale). On this scale, the freezing point of water is 273.15 K, and the boiling point is 373.15 K.
. They believe that at this temperature, molecular motion would stop. That’s pretty darn cold! This temperature, often called absolute zero, is assigned to be 0 on the Kelvin scale (with the units the same size as degrees on the Celsius scale). On this scale, the freezing point of water is 273.15 K, and the boiling point is 373.15 K. The word degrees isn’t used when stating temperature in kelvins. Scientists who work with thermodynamics, such as physicists and astronomers, measure temperature using kelvins. For instance, the surface temperature of planets is always stated in kelvins.
The word degrees isn’t used when stating temperature in kelvins. Scientists who work with thermodynamics, such as physicists and astronomers, measure temperature using kelvins. For instance, the surface temperature of planets is always stated in kelvins.
- To convert from Celsius to Fahrenheit, use this formula:

- To convert from Fahrenheit to Celsius, use the following formula:

- To get temperatures in the Kelvin scale, add 273.15 degrees to the Celsius temperature:

To go from kelvins to degrees Celsius, do the opposite: Subtract 273.15 from the kelvin temperature. Then you can convert the Celsius temperature to Fahrenheit if you like.
- Add 40 to the temperature you want to convert.
- Multiply this sum by
 if converting from Fahrenheit to Celsius or
if converting from Fahrenheit to Celsius or  if converting from Celsius to Fahrenheit.
if converting from Celsius to Fahrenheit. - Subtract the 40 you added at the beginning to yield the result.
Another Day, Another Science: Scientific Disciplines You Should Know
Science is divided into areas of study called disciplines, and most of these disciplines have subdisciplines. When you take the ASVAB, the General Science subtest may ask you some definitions of these disciplines. I couldn’t possibly list all the scientific disciplines, but here’s a handy list for you to look over.
First, here are some popular Earth and space sciences (see the later sections “Where Few Have Gone Before: Astronomy” and “Down to Earth: Rocking Out with Geology and Meteorology” for more info on these disciplines):
- Astronomy: Astronomers (not to be confused with astrologists) study outer space. They get their jollies examining the existence, locations, orbits, energy, and compositions of planets and other celestial matter.
- Geology: Is it a real diamond or just a piece of glass? A geologist can tell you. These scientists study the dynamics and physical history of the Earth; the rocks of which it’s composed; and the physical, chemical, and biological changes that the Earth has undergone or is undergoing.
- Meteorology: You know that person who gets on the TV each day and tells you whether your planned outing to the beach is going to be ruined by rain? Meteorologists study the weather and attempt to predict it.
- Paleontology: Paleontologists study prehistoric life, including dinosaurs. How cool is that? This science involves the examination of fossils, including those of plants, animals, and other organisms.
Biologists love everything to do with living organisms and life sciences. There are more subdisciplines of biology than you can shake a stick at. And yes, some biologists study sticks. Other biologists specialize in fish, trees, snakes, insects … you get the picture. Here are some subdisciplines of biology (for further info, check out the next section):
- Agriculture: An agriculturalist studies farming. This discipline includes studying methods of cultivating soil, producing crops, and managing livestock.
- Botany: A botanist studies plant life. This includes everything from flowers to the moss that grows on the north side of trees.
- Ecology: Ecologists do more than just warn people that they’re destroying the ozone layer. They study all aspects of the environment and how organisms (such as people) interact with it.
- Entomology: Entomologists like bugs. Specifically, they like insects (bugs with six legs). This position isn’t to be confused with an arachnologist, who studies spiders and other critters with eight legs.
- Genetics: Geneticists study heredity, especially the aspect that deals with inherited characteristics, such as eye color. (For details, see the later section “Swimming in the gene pool: Genetics.”)
- Ichthyology: This discipline is the branch of zoology (the study of animals) dealing with fish.
Here are a couple of social sciences:
- Archaeology: For an archaeologist, the older, the better. Archaeologists study past human life and culture. The job requires recovery and examination of material evidence, such as graves, tools, pottery, and buildings.
- Genealogy: If you want to find out where your great-great-great-great-great-grandfather was born and what he did for a living, ask a genealogist. These specialists study ancestry and family history.
Another large discipline is chemistry, in which people mix things together to see what happens. These scientists study the structure, properties, composition, and reactions of matter. I discuss chemistry later in “Chemistry: Not Blowing Up the Lab.”
Lastly, don’t forget physics. Physics involves the study of matter and its movement. This includes concepts such as energy, force, and motion. In short, physics is concerned with the study of the universe’s behavior and, in general, how things work in nature. Mechanics, which plays a big role in the ASVAB’s Mechanical Comprehension subtest (see Chapter 10), is a major topic in physics.
If the ASVAB only asked questions like “What does a chemist do?” the test would be a piece of organic matter (cake). Unfortunately, it’s not that easy. The ASVAB writers expect you to know a little more than just the definitions of various scientific disciplines. The following sections detail a few of the main branches of science you see on the ASVAB.
Uncovering Biology, from Big to Small
It would be impossible to cover all the areas of biology in this book, and I’m not going to try. Luckily, the General Science subtest of the ASVAB measures your knowledge of scientific disciplines at the average high school level. You remember studying about the Animal Kingdom and the human body and cell structures in high school, right? If not, the following sections can serve as a short refresher course.
Relating to your world through ecology
Ecology is the study of the environment — more specifically, the relationship between organisms and the world around them. All plants and animals are part of an ecosystem (a community including living things and their environment). Like the economy, an ecosystem includes producers (which make their own food) and consumers (which eat other things). An ecosystem also has decomposers, such as bacteria, which break down dead plants, animals, and the waste of all organisms.
- Carnivores eat only meat. A few examples include lions, tigers, polar bears, snakes, crocodiles, hawks, and eagles.
- Herbivores eat only plants. Cows, moose, giraffes, and elk are herbivores.
- Omnivores eat both plants and other animals. People are omnivores, and so are pigs, mice, raccoons, chickens, crows, and foxes.
Conditions in the world either encourage or prevent the establishment of individual ecosystems. For plants (producers) to grow, adequate sunlight, good soil, moderate temperatures, and water must be part of the environment. If plants aren’t around, plant-eating consumers can’t be sustained, which means predators (who eat other animals) can’t be sustained, either. For consumers, mates are as essential as a food supply. Diseases and enemies can prevent an animal from establishing itself in an ecosystem.
Human actions, such as wasting natural resources and polluting the air, water, or soil, can disrupt an entire ecosystem.
Categorizing Mother Nature
A long time ago, scientists looked at the world, noticed the hundreds of thousands of plants and animals around them, and decided that all these organisms (living things) needed to be labeled and grouped. To effectively study and discuss plants, animals, and other living creatures, all scientists needed to use the same names. Thus, a system of scientific classification was developed.
The most common classification system was created by Swedish botanist Carl Linnaeus, who published ten editions of his works from 1753 to 1758. Scientists often refer to this system as taxonomy. Not only does taxonomy provide official names for every plant and animal, but it also helps scientists understand how living creatures are related to one another. Modern-day taxonomy has its roots in the Linnaean taxonomic system.
Counting down the classification system
The scientific classification system notes the relationships and similarities among organisms. It consists of seven main levels:
- Kingdom: A kingdom is the broadest level, so it contains the most kinds of organisms. The relationship between organisms in a kingdom is extremely loose, so members share only a few key characteristics.
- Phylum: Phylum (plural phyla) is the next major taxonomic group. Within the kingdoms, organisms are divided into phyla by general characteristics. For example, in the Animal kingdom, animals with backbones (vertebrates) are placed in a separate phylum from animals without backbones.
- Class: Organisms in a phylum are divided into classes. In the Animal kingdom, for example, birds, mammals, and fish all go in their own classes. Among plants, all flowering plants comprise the Angiosperm class, and all conifers, such as pines and spruces, comprise the Conifer class.
- Order: Scientific groupings don’t follow hard and fast rules, so when you get to the order of a living thing, there’s disagreement about where it belongs. You may find that different scientific organizations group creatures in different orders or families.
- Family: Families further divide organisms of the same class by similar characteristics. Sometimes not all scientific organizations agree about the exact family an organism should be classified in.
- Genus: Two or more species that share unique body structures or other characteristics are closely related enough to be placed in a single genus. A genus may include only a single species if no other organism has characteristics similar enough for it to be considered the same genus.
- Species: A species is the most specific level, so it contains the fewest types of organisms. Organisms of the same species have very similar characteristics.
- Kingdom Animalia: This kingdom includes all animals.
- Phylum Chordata: All vertebrate animals belong to the phylum Chordata.
- Class Mammalia: All mammals belong to this class.
- Order Carnivora: All mammals that eat meat belong to the order Carnivora.
- Family Felidae: The family Felidae includes all cats.
- Genus Panthera: This genus includes all the roaring cats, such as lions, tigers, jaguars, and leopards.
- Species leo: This is just a lion.
Visiting the kingdoms
Not every scientist agrees (scientists rarely agree on any subject), but in general, most lab-coated individuals settle on five as the number of kingdoms. Check out the kinds of organisms that comprise the five kingdoms:
- Animals: This is one of the two largest kingdoms, and it includes many-celled organisms that, unlike plants, don’t have cell walls, chlorophyll, or the capacity to use light to make energy (photosynthesis). Members of this kingdom can move. The Animal kingdom includes more than one million species.
- Plants: Plants are also one of the two largest kingdoms. This kingdom includes organisms that can’t move, don’t have obvious nervous or sensory systems (the Venus flytrap is one exception), and possess cell walls made of cellulose. More than 250,000 species belong to the Plant kingdom.
- Monerans: This kingdom includes bacteria and cyanobacteria (blue-green algae) — one-celled organisms that don’t have a nucleus (see the later section “Thinking small: A look at cells”). More than 10,000 species have been discovered and classified in the Monera kingdom.
- Protists: Protists include one-celled organisms that do have a nucleus, such as the protozoan, which you may remember from biology class. This kingdom consists of more than 250,000 species.
- Fungi: Examples of common fungi are mushrooms and yeast. Fungi don’t photosynthesize (use light to create energy) like plants, but they do have cell walls made of a carbohydrate called chitin. More than 100,000 species belong to the Fungi kingdom.
Just name it: Showing off your genius about the species
Each organism is given a scientific name that consists of two words (usually derived from Latin) — the genus and the species of the organism. The genus is the first word, and the species is the second. Thus, Homo sapiens refers to humans. Canis familiaris is the family dog, and Canis lupus is the family wolf. Because wolves and dogs share many similarities, they share the same genus (no, no, not the same genes, the same genus).
When writing a scientific name, the genus name is capitalized, and the species name is all lowercase. Both names are italicized.
Perusing the human body systems
Your body consists of major systems that work together to keep you alive. (And staying alive is a good thing, so be sure to thank your circulatory system and all the rest!) These systems include the ones listed in Table 8-3.
Table 8-3 Five Major Human Body Systems
System |
Components |
What the System Does |
Central nervous system |
Brain, spinal cord, and nerves |
Receives, processes, and responds to all physical stimuli; for example, if you burn your hand on the stove, this system prompts you to remove your hand from the stove |
Circulatory system |
Heart, blood, and blood vessels |
Delivers oxygenated blood from the heart to the rest of the body and returns the blood to the heart to be oxygenated again |
Digestive system |
Mouth, esophagus, stomach, small and large intestines, rectum, and anus |
Breaks down food into smaller substances that the body can absorb and process into energy and eliminates the resulting waste |
Musculoskeletal system |
Bones, joints, voluntary and involuntary muscles |
Bones support the body’s muscles and organs; joints allow bones to move; voluntary muscles work in pairs to move joints; involuntary muscles, which you can’t control, are found in organs such as the heart |
Respiratory system |
Nose, nasal cavity, trachea, lungs, and blood |
Inhales air, uses the oxygen in the air to release energy, and exhales the carbon dioxide that results from this process |
Thinking small: A look at cells
Living things are made up of cells that share certain characteristics. Cells come in different sizes and shapes, depending on what they do. In the human body, a muscle cell looks very different from a brain cell. (Has all this talk of cells caused your brain cells to hurt yet?) Cells combine to create tissues, which form structures like bones and skin.
Looking at cell structure
A cell has three main parts — the nucleus, the cytoplasm, and the cell membrane:
-
Nucleus: The nucleus controls cellular activity. It’s like the brains behind the cell, and it holds the cell’s genetic material, such as DNA.
 Bacteria are prokaryotes, which means their cells don’t have nuclei. Their genetic material floats in the cytoplasm instead of being held inside a membrane (nuclear envelope).
Bacteria are prokaryotes, which means their cells don’t have nuclei. Their genetic material floats in the cytoplasm instead of being held inside a membrane (nuclear envelope). - Cytoplasm: The cytoplasm is a gel-like substance, composed mostly of water, that’s inside the cell membrane and outside the nucleus. Cytoplasm contains many chemicals that carry out the life processes in the cell.
- Cell membrane (plasma membrane): This thin membrane holds the cell together, protecting the nucleus and cytoplasm.
See Figure 8-1 for a description of other cell structures.
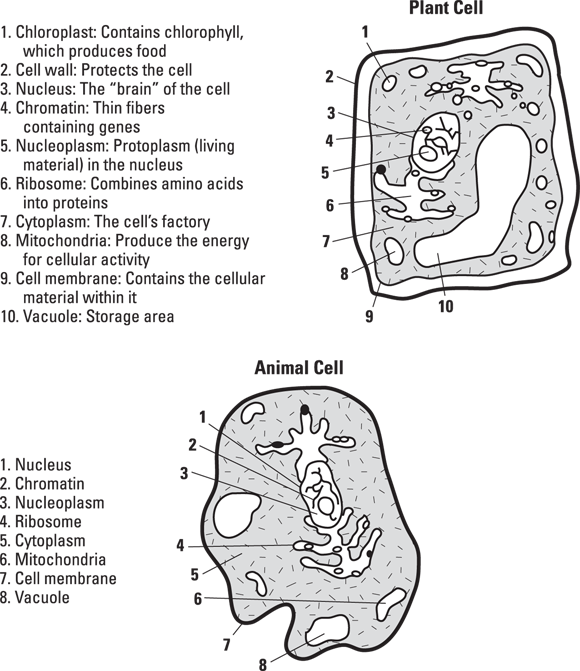
© John Wiley & Sons, Inc.
FIGURE 8-1: Basic structures of plant and animal cells.
- Plant cells have a firm cell wall that supports and protects the cell. Animal cells don’t have such a structure.
- Plant cells have larger vacuoles (storage areas) than those found in animal cells.
- Unlike animal cells, many plant cells contain chloroplasts, which contain chlorophyll, a chemical that helps plants create food with the help of sunlight.
- Animal cells contain centrioles (cylindrical structures involved in cell division). Most plant and fungus cells don’t.
- Animal cells have lysosomes (sacs of enzymes), which aren’t found in plant cells.
Profiting from cell processes
Cells perform various processes to function at an optimum level. Here are a few of these processes:
- Metabolism: Chemical processes within a cell that are necessary for life to be maintained
- Osmosis: Movement of water through the cell membrane
- Phagocytosis: Acquisition of particles of material from outside the cell; it’s accomplished by surrounding the particles and passing them through the cell membrane
- Photosynthesis: Conversion of carbon dioxide and water into glucose and oxygen (in plants); in other words, sunlight is used to create energy
- Cellular respiration: Process in which food is broken down, producing energy
Swimming in the gene pool: Genetics
Someday you’re going to find yourself acting like your mother or father. Whether you like it or not, it happens because parents pass their traits on to their offspring. Understanding genetics — how traits are physically passed from parents to offspring and what happens when the process goes wrong — helps scientists pinpoint the causes of diseases and disorders and can help them develop treatments and cures.
Copying genes
When body cells multiply to produce tissues and organs (and eventually a complete living thing), they reproduce their genetic material. Most cells reproduce by mitosis, in which the nucleus of a cell divides, forming two cells and two identical sets of chromosomes.
However, sex cells (eggs and sperm) reproduce differently. Through meiosis, each cell divides into four cells, each containing only half the number of chromosomes as a nonsex cell. This process takes place so the sex cells of one person (with 23 chromosomes) can hook up with the sex cells of another person (with 23 chromosomes) to produce 46 chromosomes, or 23 pairs. Otherwise, way too many chromosomes would be floating around.
Sometimes cells don’t copy themselves and divide perfectly, and a genetic mistake is made. This frequently results in a fetus who doesn’t live or in a fetus with a genetic disease or disorder. For example, Down syndrome is the result of a fetus’s having 47 instead of 46 chromosomes.
Determining your gender with two little letters
The genes on one pair of chromosomes, called the sex chromosomes, determine whether a child will be male or female. In females, the two sex chromosomes are alike, and they’re labeled XX. In males, the chromosomes are different and are labeled XY.
Knowing which genes get passed down the family line
Many characteristics that you possess (from the way your nose turns up at the end to the color of your eyes) are determined by a pair of genes (or multiple pairs of genes). These two genes may be alike, or they may not.
Some genes are dominant, and some genes are recessive. If you have two unlike genes, the characteristic that they produce comes from the dominant gene; the gene that doesn’t overshadow the other is called the recessive gene. If each parent has two unalike genes, both parents will have the dominant trait, but they can have a child with the recessive trait — because each parent contributes a gene to the offspring, each parent may contribute a recessive gene to the child. Whew!
Chemistry: Not Blowing Up the Lab
Chemists study matter, and everything that has mass and takes up space — including your old Chevy that’s up on blocks and the mosquito buzzing around the room — is matter. All matter is made up of basic substances (building blocks) called elements.
Those mad scientists in the movies always seem to be chemists, but chemistry shouldn’t drive you crazy. Here’s a straightforward review of the chemistry you need to know for the General Science subtest.
Understanding the elements, my dear Watson
The atom is the smallest part of an element that still retains the characteristics of that element. Every atom has particles — pieces of matter that are very, very small. Electrons are negatively charged particles that float around the atom’s nucleus, or core, which is made up of neutrons (particles with no charge) and protons (positively charged particles).
Atoms can combine with each other to form molecules. If those atoms are of two or more different elements, the molecule is called a compound. A compound can have very different properties from the elements that make it up. For example, table salt, which is mostly harmless, consists of two lethal elements — sodium and chlorine. But when combined, these elements make a compound that people ingest every day, salt.
Sitting down at the periodic table
The periodic table (also known as the table of elements) classifies all elements, because scientists love to classify things. Elements are listed according to their atomic numbers (number of protons) and are arranged into families of similar elements.
The periodic table lists the atomic number, the abbreviation for each element, and its atomic weight, which is the average mass of one atom of the element. Looking at Figure 8-2, you can see that copper (Cu, atomic number 29) has an atomic weight of 63.546, which means that copper is much, much heavier than helium (He, atomic number 2), which has an atomic weight of 4.0026.

© John Wiley & Sons, Inc.
FIGURE 8-2: The periodic table.
You don’t have to memorize these charts to do well on the ASVAB, but you should know the atomic numbers for common elements such as hydrogen (1), helium (2), carbon (6), nitrogen (7), oxygen (8), sodium (11), iron (26), copper (29), gold (79), mercury (80), lead (82), uranium (92), and plutonium (94).
Getting physical: Changing states
Particles of matter are always in motion. How much kinetic energy (motion energy) a particle has determines whether the matter is a solid, liquid, or gas in its normal state. Gas particles move around very quickly, liquid particles move more slowly, and solid particles move much more slowly than either of the other two.
When heat or cold is applied to matter, the kinetic energy of the matter changes; therefore, the nature of the substance can change. Heat applied to water changes the water from a liquid to a gas (steam), and cold applied to water changes it from a liquid to a solid (ice). When physical changes occur, the molecule itself remains the same. For example, water is still made of hydrogen and oxygen, no matter which state it’s in.
Causing a chemical reaction
Unlike physical changes, chemical reactions create new molecules. For example, when iron rusts, a chemical change occurs. The rust isn’t the same molecule as the iron.
In a chemical reaction, two kinds of substances are present:
- Reactants: The elements or molecules involved in the reaction
- Products: The elements or molecules that result from the chemical reaction
Where Few Have Gone Before: Astronomy
Earth’s solar system consists of the sun and a number of smaller bodies (such as planets, the planets’ moons, and asteroids) that the sun’s mass holds in orbit. The sun’s mass creates gravity, and this gravity controls the movements of the smaller bodies.
Taking a quick glimpse at the sun
The sun is the largest and most important object in the solar system. It contains 99.8 percent of the solar system’s mass (quantity of matter). The sun provides most of the heat, light, and other energy that makes life possible.
The sun’s outer layers are hot and stormy. The hot gases and electrically charged particles in those layers continually stream into space and often burst out in solar eruptions. This flow of gases and particles forms the solar wind, which bathes everything in the solar system.
The sun is much larger than Earth. The distance from the sun’s center to its surface (the sun’s radius) is about 109 times the radius of Earth. Some of the streams of gas rising from the solar surface are even larger than the Earth’s diameter.
Knowing the planets
A planet is a nonluminous celestial body larger than an asteroid or comet, illuminated by light from a star that the planet revolves around. The solar system consists of eight known planets. In order from closest to the sun to farthest from the sun, they are Mercury, Venus, Earth, Mars, Jupiter, Saturn, Uranus, and Neptune. Pluto is no longer classified as a planet by most scientists. (See the sidebar “Is Pluto really a planet?” for details.)
The Earth revolves around the sun in an oval-shaped pattern called an ellipse. Every ![]() days, the Earth completes its orbit around the sun and starts again. The Earth rotates (spins) on its axis, completing a rotation every 24 hours, but because of the tilt of the Earth, hours of daylight and darkness aren’t equal, except for on two days a year.
days, the Earth completes its orbit around the sun and starts again. The Earth rotates (spins) on its axis, completing a rotation every 24 hours, but because of the tilt of the Earth, hours of daylight and darkness aren’t equal, except for on two days a year.
The inner four planets consist chiefly of iron and rock. They’re known as the terrestrial (earthlike) planets because they’re somewhat similar in size and composition. The outer planets are giant worlds with thick, gaseous outer layers. Almost all of their mass consists of hydrogen and helium, giving them compositions more like that of the sun than of Earth. Beneath their outer layers, the giant planets have no known solid surfaces. The pressure of their thick atmospheres turns their insides liquid, though they may have rocky cores.
Rings of dust, rock, and ice chunks encircle all the giant planets. Saturn’s rings are the most familiar, but thin rings also surround Jupiter, Uranus, and Neptune.
Shooting for the moons
Moons (sometimes called satellites) orbit all the planets except Mercury and Venus. The moon you refer to as the moon revolves around Earth. It makes a complete revolution every ![]() days. When the moon moves into the Earth’s shadow, a lunar eclipse results — Earth is positioned between the sun and the moon. When Earth moves into the moon’s shadow, a solar eclipse results — the moon is positioned between Earth and the sun.
days. When the moon moves into the Earth’s shadow, a lunar eclipse results — Earth is positioned between the sun and the moon. When Earth moves into the moon’s shadow, a solar eclipse results — the moon is positioned between Earth and the sun.
The inner planets have few moons. The giant planets probably have more small moons not yet discovered. See Table 8-4 for a lineup of the planets and their moons. Although Pluto is no longer officially considered a planet, you never know what those rascally ASVAB test-writers will ask, so I’ve included Pluto in the table.
Table 8-4 The Number of Moons per Planet in Earth’s Solar System
Planet |
Number of Moons |
Mercury |
0 |
Venus |
0 |
Earth |
1 |
Mars |
2 tiny satellites |
Jupiter |
63 |
Saturn |
61 |
Uranus |
27 |
Neptune |
13 |
Pluto (dwarf planet) |
3 |
Watching for meteors, comets, and asteroids
A meteor is a rock from space that hits Earth’s atmosphere and glows as it heats up, resulting in a brief streak of light. It’s often called a shooting star. When a meteor enters the Earth’s atmosphere, it usually burns up (and that’s a good thing). If a meteor actually strikes the Earth, it’s called a meteorite.
Comets are snowballs composed mainly of ice and rock. When a comet approaches the sun, some of the ice in its nucleus (center) turns into gas. The gas shoots out of the sunlit side of the comet. The solar wind then carries the gas outward, forming it into a long tail. Astronomers divide comets into two main types:
- Long-period comets, which take 200 years or more to orbit the sun.
- Short-period comets, which complete their orbits in fewer than 200 years.
Asteroids are sometimes called minor planets because they’re small bodies that orbit the sun. Some have elliptical orbits that pass inside the orbit of Earth or even that of Mercury. Others travel on a circular path among the outer planets. Most asteroids circle the sun in a region called the asteroid belt, between the orbits of Mars and Jupiter. The belt contains more than 200 asteroids larger than 60 miles (100 kilometers) in diameter. Scientists estimate that more than 750,000 asteroids with diameters larger than ![]() mile (1 kilometer) exist in the belt. There are millions of smaller asteroids, and astronomers have even found several large asteroids with smaller asteroids orbiting them.
mile (1 kilometer) exist in the belt. There are millions of smaller asteroids, and astronomers have even found several large asteroids with smaller asteroids orbiting them.
Down to Earth: Rocking Out with Geology and Meteorology
The study of the physical makeup of Earth is often called Earth science. Geology describes Earth’s physical appearance, and meteorology explains Earth’s atmosphere.
Peeling back the layers of the planet
Earth is like an onion in that it consists of several layers. The crust is Earth’s surface, and it varies in depth from a few miles to 30 miles. The mantle (including the mantle and an upper mantle) is the solid rock below the crust, and it makes up most of the mass of Earth. The core (including the inner and outer cores) is Earth’s superheated center, with a temperature inside the inner core reaching heights of 7,000 degrees Celsius (to see what that is in Fahrenheit, use the conversion equations in “Figuring temperature conversions” earlier in this chapter). The mantle accounts for about two-thirds of the Earth’s mass.
Sometimes cracks in Earth’s crust, called faults, appear. When the land shifts along these faults, earthquakes result. Molten rock trapped between the crust and the mantle is called magma. Magma collects in pockets called magma chambers and forms volcanoes. When volcanoes erupt, the magma is spewed out as lava.
Outta this world: Checking the atmosphere
The atmosphere contains many layers of air surrounding Earth’s surface. Starting with the layer closest to Earth and extending outward, Table 8-5 names those layers.
Table 8-5 Layers of Earth’s Atmosphere
Layer Name |
Location |
Details |
Troposphere |
Extends about 8 miles above the Earth |
This layer is where the jet stream is located and where almost all weather changes occur. |
Stratosphere |
Extends about 30 miles |
A major reported cause of ozone depletion is the presence of chlorofluorocarbons (CFCs) in the Earth’s stratosphere. CFCs undergo a series of chain reactions, which ultimately lead to the destruction of the ozone layer. |
Mesosphere |
Extends about 50 miles |
Millions of meteors burn up daily in the mesosphere as a result of collisions with the gas particles contained there. |
Ionosphere |
Extends about 70 miles |
This layer reflects most radio waves, making it important to communications. Note: Scientists disagree among themselves as to whether the ionosphere is a separate atmospheric layer or whether it’s part of the thermosphere. |
Thermosphere |
Extends about 350 miles |
The International Space Station has a stable orbit within the upper part of the thermosphere, between 208 and 285 miles. |
Exosphere |
Extends about 6,200 miles |
It’s only from the exosphere that atmospheric gases, atoms, and molecules can escape into outer space. No boundary exists between the exosphere and space; therefore, exosphere is sometimes used synonymously with outer space. |
Warming up to cold fronts
Temperature affects air density (how closely packed the air molecules are). When the sun shines, land and water absorb its warmth. Land warms up more quickly than water, so air over land is warmer than air over water during most of the day. At night, the air over land cools more quickly than air over water. The angle of the sun also affects air density (the sun shines directly over the equator but not the poles).
Cold air is denser than warm air. Because it’s denser, cold air has high pressure, compared to warm air’s low pressure. (A barometer measures atmospheric pressure.) Air moves from areas of high pressure to areas of low pressure, creating wind.
Air masses have certain characteristics depending on where they form:
- If an air mass forms over land, it’s dry, and if it forms over water, it’s wet.
- Air masses formed in Earth’s northern and southern regions are cold, and those formed at the equator are warm.
When two different air masses meet, they don’t mix. They form a boundary called a front. When cold air meets warm air, a cold front develops. The warm air may be pushed up to form clouds, causing heavy rain. When a warm air mass meets a cold air mass, a warm front develops. The warm air passes over the cold air, forming a different kind of cloud, which causes light rain.
Classifying clouds
Clouds are made of small droplets of water or bits of ice that are spread out from each other. Rain (or snow) falls when the drops get too big and heavy to stay in the cloud. Clouds have three main types, and the ASVAB may ask you a question or two about their characteristics, which are detailed in Table 8-6.
Table 8-6 Types of Clouds
Cloud Type |
Description |
What It Forecasts |
Cirrus |
Thin, wispy, high clouds |
Generally indicate rain or snow |
Cumulus |
White, puffy pillows, often flat-bottomed with rounded tops |
Common during fair weather, but when they gather, they cause heavy rains |
Stratus |
Broad, flat, and low-hanging (gray blanket) |
If close to the ground, they may produce drizzle |
Additionally, a prefix or suffix is frequently given to the cloud name to indicate which level of the atmosphere it’s in or whether it’s producing precipitation (rain, sleet, snow, and the like):
- Cirro- is the prefix given to high clouds (base above 20,000 feet).
- Alto- is the prefix given to midlevel clouds (base between 6,000 and 20,000 feet).
- Nimbo- added to the beginning of a cloud name or -nimbus added to the end means the cloud is producing precipitation.
Therefore, a cirrocumulus cloud is a white, puffy, flat-bottomed, rounded-topped cloud at high altitude. Altostratus clouds are gray, broad, flat clouds at mid-altitude.
Improving Your Chances on the General Science Subtest
Even if you study hard for the General Science subtest, chances are you may come across at least a couple of questions that you can’t answer. That’s the nature of this subtest — it pretty much asks you to know all there is to know about the universe. However, you can use several strategies to improve your chances of selecting the correct answer.
Using common sense to make educated guesses
If you don’t know the answer to a question right off the bat, don’t panic. You can often eliminate a few incorrect choices simply by using common sense. Even if you can’t determine the answer, keep in mind that this subtest doesn’t penalize you for guessing (unless you guess incorrectly on several questions in a row at the end of the subtest when taking the CAT-ASVAB), so guessing makes sense — you have a 25 percent chance of guessing the right answer even if you can’t eliminate any obviously wrong answers. If you can eliminate just one wrong answer, you improve your chances to 33 percent.
Try the process of elimination on the following question:
(A) pivot joint.
(B) fixed joint.
(C) ball-and-socket joint.
(D) hinge joint.
Looking at the choices, you can eliminate Choice (B), fixed joint, because your knee isn’t fixed, or not moveable (or if it is, it shouldn’t be). Your skull is an example of a fixed joint, but that’s irrelevant to this question. Is your knee a pivot joint? If you think of something that pivots, you think of it moving in a circular or at least a semi-circular manner. Your knee doesn’t do that either; therefore, you can safely eliminate Choice (A). A ball-and-socket joint is one that permits limited movement in any direction (your shoulder joint is a ball-and-socket joint). Your knee doesn’t do that, so you can strike off Choice (C) and choose Choice (D), hinge joint, as the most likely answer. Your knee moves like a door on a hinge.
Now suppose you have a question like this:
(A) oxygen.
(B) nitrogen.
(C) calcium.
(D) helium.
Eliminate Choice (C) because calcium isn’t a gas. You can also cross out Choice (D) because if helium were the most common gas, everyone would be talking in squeaky voices (you know, like after sucking helium from a balloon). Eliminating these two answers leaves you with just two choices, and if you simply guessed, you’d have a 50 percent chance of being right. Unfortunately, most people would guess that oxygen is the most common gas in Earth’s atmosphere, but they’d be wrong. Nitrogen — Choice (B) — tops the list, making up 78 percent of the atmosphere.
Getting back to your Latin roots
For example, the Latin root homo means human being, and the Greek root homo means same. So Homo sapiens refers to members of the human species, but homogeneous means “of the same kind.” So if you were to run across the word homologous on the General Science subtest, you’d know that it has something to do with humans or with things that are the same.
Take a look at the following example question:
(A) aspirator
(B) hydrophone
(C) calorimeter
(D) centrifuge
Even if you don’t have a clue about what any of these instruments do, if you know that hydro relates to water, you’ve significantly increased your chances of getting the right answer, Choice (B).
General Science Practice Questions
General science is a hard topic to study for because the field is so broad. To score well on this subtest, you pretty much have to wade through the textbooks and memorize the facts. See how well you do on the following 18 practice questions.
1. If the temperature in Fahrenheit is ![]() , the temperature in Celsius is
, the temperature in Celsius is
(A) ![]() .
.
(B) ![]() .
.
(C) ![]() .
.
(D) ![]() .
.
2. A cell nucleus is often referred to as the
(A) control center.
(B) cytoskeleton.
(C) cell membrane.
(D) chromosome.
3. The human circulatory system
(A) uses air to release energy.
(B) processes food and eliminates waste.
(C) moves oxygenated blood throughout the body.
(D) controls movement of joints.
4. Compasses work by
(A) measuring heat in the air.
(B) reacting to magnetic fields.
(C) repulsing wave currents.
(D) magic.
5. If an atom has one proton and one electron, the atomic number is
(A) 2.
(B) 10.
(C) 5.
(D) 1.
6. The element with the lowest atomic number is
(A) hydrogen.
(B) helium.
(C) lithium.
(D) uranium.
7. Absolute zero is equivalent to
(A) 0 degrees Kelvin.
(B) 0 kelvins.
(C) –273.15 degrees Kelvin.
(D) –273.15 kelvins.
8. A comet’s tail is visible when
(A) the metal alloys react to the atmospheric change.
(B) the ice and rock collide.
(C) the comet is close enough to the sun.
(D) the comet passes the Kuiper Belt.
9. What job would you apply for if you wanted to study the life and culture of the past?
(A) genealogist
(B) archeologist
(C) ecologist
(D) ichthyologist
10. Which of the following is true about the classification system of biology?
(A) Every animal is categorized according to the proper definition.
(B) Many disagreements occur among scientists about where an organism belongs.
(C) Every organism belongs to the mammalian classification.
(D) Every organism belongs to the felidae classification.
11. What is the term for an element or molecule that results from a chemical reaction?
(A) reactant
(B) product
(C) molecule
(D) chemical
12. What causes a blue tail to trail behind a comet?
(A) Vapors from its nucleus are blown by solar winds.
(B) Rock in the comet heats up when it gets closer to the sun.
(C) A comet is made of fire.
(D) Comets do not have blue tails.
13. What part of the Earth forms volcanoes?
(A) tectonic plates
(B) lava pockets
(C) mantle cracks
(D) magma faults
14. What are fast-flowing, narrow air currents located in the Earth’s atmosphere called?
(A) density
(B) air mass
(C) jet streams
(D) air chambers
15. Why are regions of the Earth unequal in daylight and darkness?
(A) because the Earth rotates on a tilted axis
(B) because the sun changes direction halfway through the year
(C) because the Earth’s orbit is slower during different seasons
(D) because the Earth’s axis moves around during rotation
16. What is the term for a chemical substance that is broken down into its simplest form?
(A) weight
(B) element
(C) mass
(D) gene
17. What is the term for the process in which a cell converts nutrients into energy?
(A) metabolism
(B) cellular respiration
(C) photosynthesis
(D) osmosis
18. Which term refers to the collection of veins that join together to form a large vessel that collects blood from the heart muscle?
(A) right ventricle
(B) left atrium
(C) coronary sulcus
(D) coronary sinus
Answers and Explanations
Use this answer key to score the General Science practice questions.
- C. Measured in Celsius, the boiling point of water is
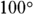 . If you don’t have this memorized, you can calculate it. To convert from Fahrenheit to Celsius, use the formula
. If you don’t have this memorized, you can calculate it. To convert from Fahrenheit to Celsius, use the formula 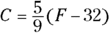 . The correct answer is Choice (C).
. The correct answer is Choice (C). - A. The nucleus contains most of the cell’s genetic material and is often referred to as the control center of the cell, so the correct answer is Choice (A).
- C. The respiratory system uses air to release energy, the digestive system processes food and eliminates waste, and the musculoskeletal system controls the movement of joints. The correct answer is Choice (C).
- B. A compass is a device that takes advantage of the Earth’s magnetic field. Choice (B) is the correct answer.
- D. The atomic number refers to the number of protons an atom has in its nucleus. Choice (D) is the correct answer.
- A. Hydrogen has an atomic number of 1. The atomic numbers for the other elements listed are 2 (helium), 3 (lithium), and 92 (uranium). The correct answer is Choice (A).
- B. Absolute zero is –273.15 degrees Celsius, which is equivalent to 0 kelvins. Temperatures stated in the Kelvin scale are measured by using units of kelvins, not degrees. The correct answer is Choice (B).
- C. Comets are balls composed mainly of ice and rock. The comet’s tail is formed when the ice turns into gas from the heat of the sun; therefore, the comet must be closer to the sun for the tail to be visible. The correct answer is Choice (C).
- B. An archeologist studies past human life and cultures by recovering evidence and examining materials left in a given area.
- B. Scientific groupings don’t follow specific rules enough to completely shut out debate, so you find some experts who are yay and some who are nay about the classification groups.
- B. A product is a new element or molecule that results from a chemical reaction.
- A. Even though a comet is made mostly of ice and rock, the ice in its center turns into gas as it approaches the sun and forms a cloud around it. Solar winds push the cloud back, forming what looks like a tail.
- A. As tectonic plates shift, magma can force its way through the cracks left behind. Magma can push through the weakened or cracked crust, causing a volcanic eruption.
- C. Jet streams are narrow air currents that flow in between the troposphere and the stratosphere.
- A. Except for two days per year (known as the vernal and autumnal equinoxes), the hours of daylight and darkness are unequal because the Earth spins on its axis on a tilt (think about your friends in Alaska).
- B. Elements are the most basic form of chemical substances. The elements known to man are categorized as metals, nonmetals, and metalloids on the periodic table.
- B. Cellular respiration is the process during which a cell produces energy by breaking down nutrients within the cell. Cellular respiration is more correct than Choice (A) because metabolism involves not only breaking down nutrients but also building molecules. Choice (B) is more specific.
- D. The coronary sinus is a collection of veins that forms a large vessel to collect blood from the heart muscle.

 Instead of trying to remember nine million individual facts, spend some time reviewing the general principles behind the facts. Think about how the facts relate to each other. Looking at the big picture is an effective learning technique.
Instead of trying to remember nine million individual facts, spend some time reviewing the general principles behind the facts. Think about how the facts relate to each other. Looking at the big picture is an effective learning technique. Here are some units of measurement you need to know for the General Science subtest of the ASVAB:
Here are some units of measurement you need to know for the General Science subtest of the ASVAB: The knee joint is known as a
The knee joint is known as a