2. Generating knowledge for the future
Optimizing long-term results requires generating new knowledge about all aspects of the epidemic. New technologies will be needed to prevent new infections and improve care for those who are infected, and new information is critical to tackling the underlying drivers of the epidemic. Equally important, more efficient management and utilization of knowledge for both policy and programmatic implementation are vital to a sustainable approach.
This chapter examines strategies to generate the breadth of knowledge that will be needed between now and 2031. AIDS illustrates, perhaps better than any other global issue of our time, the potential of research to transform the health and well-being of individuals, families, communities, and entire societies. Yet despite the billions of dollars poured into AIDS research and the numerous resulting advances, many additional breakthroughs are needed to sharply reduce new infections and AIDS deaths. We need to relinquish the faith in an imminent fix that would rapidly cause AIDS to disappear. The world is probably a generation or more away from a workable vaccine, and no cure appears to be available anytime soon.
Despite the sober outlook, additional breakthroughs are achievable. Some of these possible breakthroughs have the potential to revolutionize our ability to bring the epidemic under control. This chapter examines some of these potential advances and considers what needs to change to speed their emergence.
AIDS and the power of science
Scientific breakthroughs have characterized the history of AIDS. Remarkably soon after the appearance of a strange new disease in the early 1980s, investigators definitively characterized the limited means by which the disease could be transmitted.1 The discovery of the human immunodeficiency virus in 1983 was quickly followed by the development of a blood test to detect it.2 Researchers also documented some early successes in the ability of focused prevention programs to generate radical changes in sexual behavior, first in gay communities in high-income countries and then among high-risk groups in Brazil and Thailand and among heterosexuals in Uganda.3
However, early optimism about these findings quickly gave way to despair, as the epidemic exploded throughout sub-Saharan Africa and large-scale studies demonstrated that the early generation of antiretroviral therapies had failed to extend life.4 Renewed hope arose with the discovery of the need for combination therapy, and the emergence in the mid-1990s of a new class of antiretroviral drugs—protease inhibitors—which ushered in the era of highly-active antiretroviral therapy (HAART), dramatically extending lives by curtailing viral replication. The development of antiretroviral therapy represents one of the most important medical breakthroughs of the last 50 years. Today, 33 medications from six different antiretroviral classes (including some that combine multiple approved medications) have been brought to market by the pharmaceutical industry.5
Initially, it was assumed these treatment breakthroughs would almost exclusively benefit high-income countries. Not only was it expected that the high cost of antiretrovirals would make them unaffordable in low-income countries, but many also argued that it was not feasible to manage a complex chronic disease such as AIDS in countries with poorly functioning health systems. The resistance against introducing antiretroviral therapy in developing countries came from a powerful coalition of development agencies and public health experts. The combined actions of the activist movement, the UN Secretary-General, and UNAIDS, as well as competitive pressure from generic manufacturers, reduced drug costs dramatically, rendering cost-prohibitive medicines much more affordable. The massive use of antiretroviral treatment in low-income countries is an unprecedented example of the introduction of a complex new patented technology supported largely by public funding from high-income countries. By 2010, more than five million people were being treated, a figure that would have been unimaginable only a few years ago. By the end of 2008, HAART had averted 1.4 million deaths in sub-Saharan Africa alone.6
Efforts to prevent new infections have also benefited from publicly funded research. A major breakthrough was the demonstration in 1999 that administering a short course of antiretrovirals to pregnant women and their newborns sharply lowered the risk that the infant would become HIV-positive.7 These findings prompted donors and countries to collaborate in establishing and expanding HIV-prevention programs in antenatal settings; as of December 2008, those programs had averted at least 200,000 new infections among newborns.8 More recently, three studies in Africa found that male circumcision lowered the risk of men becoming infected during heterosexual intercourse by 60%,9 leading 13 countries in Africa to begin launching programs to encourage men to be circumcised.10
The limits of science to date
Although such research endeavors have saved millions of lives, scientific tools alone will not ensure a favorable future for the pandemic. Proving that condoms are effective in preventing HIV transmission has not automatically ensured that people will use them. And preventing HIV-positive people from dying may have resulted in an increase in some new infections by rendering the disease less serious in the public mind and inadvertently encouraging an increase in sexual risk-taking.11
Researchers have sometimes declared victory prematurely. For example, research findings demonstrating the efficacy of a feasible drug regimen to prevent mother-to-child HIV transmission in low-income settings were rightly hailed as a historic breakthrough. However, in the immediate aftermath of the study’s release, significantly less attention focused on the barriers to implementation of this approach in countries with weak antenatal health systems. A decade after the study results were released, most HIV-positive women worldwide still lacked access to regimens to prevent transmission to a newborn child, although in recent years substantial strides have been made in closing the access gap.12
A similar approach has been evident with respect to antiretroviral treatments. Existing regimens are a lifelong commitment, and they work well if used with high levels of adherence. Yet a remarkably meager body of research has focused on strategies to maximize adherence to these lifelong regimens, especially in resource-limited settings. Even in high-income countries, estimates suggest that a substantial proportion of people living with HIV do not have access to these medications.13
The scientific approach to AIDS demonstrates an unwavering faith in biomedical solutions but also sometimes displays a remarkable blindness about investigating human behavior or the underlying social and cultural factors that affect behavior. Research into structural interventions to reduce vulnerability to HIV has remained almost non-existent. Even as the effects of stigma, social marginalization, gender inequality, and other factors have frustrated AIDS programmers, research has continued to be almost exclusively aimed at biomedicine.
Especially noteworthy is the focus on controlled studies to demonstrate the efficacy of new technologies and public health strategies, to the neglect of research on effectiveness to inform the application of research advances in the field. As a result, early optimism about research advances has almost invariably been followed by frustration at the slow pace of programmatic implementation. Furthermore, inadequate investments in evaluation studies have often limited the ability of program implementers to integrate new learning to improve their efforts over time.
The remainder of this chapter addresses how generating knowledge for AIDS needs to change to maximize impact over the long term.
Generating global public goods
The Holy Grail of AIDS research would be a vaccine to prevent infection and a cure for the disease. Such products would represent historic advances, and research efforts to pursue their development merit high priority. In the meantime, however, other new tools are urgently needed to prevent HIV transmission and treat those living with HIV.
When the concept of public goods is mentioned, tangible products such as medicines, diagnostic devices, and other health commodities usually come to mind. But knowledge itself is an essential public good.14 Making further progress in reducing both deaths and new infections over the next generation depends as much on our knowledge of how to most effectively use the tools we have as on the development of new tools. Moving forward, substantially greater emphasis should focus on translational, or operational, research that will enable available technologies and strategies to achieve maximum impact.
Prospects for a cure
If a cure for AIDS were found, it would overcome the problems of lifelong adherence to antiretroviral treatment. It would also restore people living with HIV to full health, a critical need in light of emerging evidence that HIV-associated harm continues to occur even among patients who have achieved the best possible response to treatment. And a cure would interrupt transmission.
One of the key recommendations of the aids2031 Science and Technology Working Group is to “develop better products to treat HIV, with the eventual aim of finding a cure.”15 While an ideal cure would completely clear the body of all traces of the virus, most experts question whether complete eradication in an infected individual is possible, given the ability of the virus to hide in organs and tissues that have remained beyond the reach of existing therapies.16 Yet it may be possible to find a “functional cure”—that is, one that achieves durable control of the virus in the absence of ongoing antiretroviral therapy.
New diagnostic technologies
Until a cure is available, the world needs to ensure that antiretroviral therapy is as effective as possible. In high-income countries, HIV clinical practice is characterized by a proliferating array of diagnostic tools that gauge key virologic and immunologic markers. Resistance tests help physicians know which antiretroviral regimens are likely to be most effective for individual patients, and routine monitoring of each patient’s viral load and immune function enables clinicians to know when to change drugs to sustain viral suppression and to avert a preventable deterioration of the body’s immune system.
Table 2.1. Point-of-care and low-cost diagnostic development needs in different patient groups17

Most of these cutting-edge diagnostic tools are unavailable in most low-income settings18 The sophisticated equipment needed for diagnosis, such as tests for CD4 counts and resistance to drugs, are designed for well-equipped laboratories and highly trained staff. They are not suitable for the field conditions in which so many people with HIV receive treatment in the developing world. While the U.S. government and other donors have worked to expand access to these diagnostic tools in low-income countries, it is unlikely to be feasible to provide all these technologies to the countless thousands of clinical settings on which people living with HIV depend.
New, simpler, more affordable diagnostic technologies are needed, such as simple tests for use where a patient is being treated that can be delivered on a large scale, at low cost, and used by primary health-care workers with minimal training. Several new point-of-care CD4 counting technologies are currently being evaluated, including the rapid, instrument-free CD4 count from the CD4 Initiative and the PIMA CD4 instrument from Inverness Medical. These tests are low-cost and designed to be used in peripheral health centers with limited laboratory services. Field trial results are expected shortly.
Point-of-care viral load tests present a much more difficult technological challenge and are some years away from introduction, as are point-of-care tests for TB diagnosis and drug sensitivity. However, the development of these tests should be encouraged as simple-to-use point-of-care tools would greatly aid medical decision-making. Even qualitative tools that could identify whether a patient’s viral load is suppressed would represent a major step forward in the treatment toolkit for HIV. Such a qualitative test would have an enormous impact on early infant diagnosis. Recent experience with rapid HIV testing technologies indicates that developing countries will put appropriately designed diagnostic tools to use if and when they are made available.
Recent studies demonstrate that excellent clinical results are achievable even when state-of-the-art diagnostic tools are not available, especially if there is intensive clinical monitoring.19 However, robust, affordable diagnostic assays remain an urgent research priority, to enable clinicians to determine when to switch regimens and to minimize the transmission of drug-resistant virus.
Another key recommendation of the aids2031 Science and Technology Working Group is to launch a global initiative to coordinate the development of diagnostic and monitoring tools for HIV, which they feel is an urgent need to bridge what they call the “diagnostic divide.”20
New prevention tools
Whereas greatly expanded coverage of current HIV prevention interventions may further reduce HIV incidence by 50% (see discussion in Chapter 1, “The future of AIDS: a still-unfolding global challenge”), new tools will be necessary to virtually halt the spread of HIV in the most affected populations such as in Southern Africa or in concentrated populations such as among men who have sex with men. Such tools would include a microbicide, pre-exposure prophylaxis (PrEP), a vaccine, a cure, and possibly high coverage with early antiretroviral therapy.
In the quest to develop new prevention tools, researchers have identified several potentially promising approaches that involve the use of antiretrovirals to prevent transmission:21
• Pre-exposure prophylaxis (PrEP) and microbicides—Evidence from animal studies suggests that it may be possible to reduce the risk of HIV transmission by taking antiretrovirals before a possible exposure to the virus. Eight human trials are currently underway to study the pre-exposure use of antiretrovirals to reduce the risk of HIV transmission. Microbicides are compounds that can be formulated in gels, films, rings, or sponges. Most microbicide candidates are intended for vaginal application, although some are also being studied to protect against transmission during anal intercourse.
Following several failed attempts with other microbicides, investigators from Durban, South Africa, demonstrated in the so-called CAPRISA trial that a vaginal gel with 1% tenofovir (an antiretroviral drug used for treating people with HIV) reduces the risk for women of acquiring HIV during sex by 39%, and about 54% in those who used the gel consistently.22 This study provides a first proof-of-concept for two prevention approaches: the use of antiretrovirals to prevent sexual transmission of HIV (as already used for the prevention of mother-to-child transmission) and the use of a topical gel for the same.
Figure 2.1. Past PrEP trials and estimated end-dates for ongoing trials. Note: Trial end-dates are estimates. Due to the nature of clinical trials, the actual dates may change.
Source: AIDS Vaccine Advocacy Group (AVAC) May 2010

These results need to be confirmed in a large trial and further studies are needed to determine optimal regimens. This will take several years, while other oral PrEP regimens are being evaluated in diverse populations. While highly encouraging, the CAPRISA trial should also remind us of the complexity, cost, and time needed for such trials.
• Treatment as prevention—By suppressing the HIV virus in people who are infected, antiretroviral therapy may dramatically reduce infectivity and slow the rate of new infections. A number of studies support this hypothesis, including one that found that HIV transmission in serodiscordant couples—in which one partner is HIV positive and the other is not—was reduced when the infected partner was receiving antiretroviral therapy.23 In late 2008, a team of WHO scientists released the results of a modeling exercise that suggested that providing annual HIV testing combined with free antiretroviral therapy to all who test HIV positive could sharply lower annual HIV incidence within a decade and lay the foundation for the eventual elimination of HIV.24 Modeling suggests that, under these assumptions, initiating antiretroviral therapy earlier in the course of infection would significantly lower the number of incident infections, as would significant increases in treatment coverage. Faster scaling-up of treatment would accelerate the assumed prevention gains associated with antiretrovirals. Numerous studies are now underway to test various aspects of this so-called “test and treat” approach, although initiation of large-scale efficacy or effectiveness trials is at least several years away and would entail formidable implementation challenges.
Each of these research avenues holds promise, but well-designed studies are required to support their widespread use. Numerous experimental HIV prevention options have been grounded in strong evidence of plausibility, only to have studies yield disappointing results. Based on evidence of women’s physiological vulnerability to HIV, it was once thought that a physical cover for the cervix could reduce the risk of transmission, but studies of female diaphragms did not find the approach efficacious for HIV prevention.25 Similarly, although epidemiological studies have consistently found a strong association between HIV infection and herpes simplex virus type 2 (HSV-2 ),26 evaluations of acyclovir suppressive therapy for HSV-2 have failed to demonstrate an HIV prevention benefit.27 Regardless of the eventual outcome for the current leading candidates for HIV prevention, it is imperative that researchers continue to innovate and push the field forward.
In doing so, researchers should ensure that trials have sufficient power to obtain answers regarding the efficacy of experimental prevention methods. Many trials of experimental prevention technologies have failed to yield definitive results.
Proof of efficacy is, of course, an essential step in the development of new prevention tools. However, additional knowledge will be needed to guide the use of new tools. For new prevention technologies, which will inevitably confer only partial protection, new social norms and patterns of behavior may emerge that could increase or decrease new infections. For instance, the risk exists that sexually active people will erroneously view any new tool as a foolproof shield against infection, rendering superfluous the need to use a condom or reduce the number of sex partners. Alternatively, new prevention tools might encourage greater attention to risk reduction. Community education programs will continue to be needed as new tools are rolled out, and longer-term monitoring will be needed to detect unforeseen consequences of new prevention strategies.
A preventive vaccine
When Margaret Heckler, then the U.S. Secretary of Health and Human Services, reported on the discovery of HIV in 1983, she boldly predicted that a preventive vaccine would be developed within two years. Despite this early optimism, progress towards a vaccine has been slow.
After extensive scientific experimentation, it is widely accepted that the development of an HIV vaccine is one of the most difficult challenges confronting biomedical research. An ideal vaccine would be feasible for widespread use in low-income settings, confer lifelong immunity, protect against all routes of HIV transmission, and work against diverse viral strains and subtypes.
In 2009, a trial in Thailand was the first large human vaccine efficacy trial to show promise, with the candidate, a prime-boost regimen of two genetically engineered vaccine components, demonstrating modest protection against infection.28 What seems most likely is that future progress will be incremental; more effective products will supersede earlier vaccines over a considerable period of time. The limited efficacy of early HIV vaccines means they would most likely have to be used as complementary tools in combination with existing prevention strategies. Funders and policymakers must understand that several generations of vaccine development are needed before a product emerges that is capable of ending the epidemic. Product development, animal testing, and human efficacy trials are all expensive, but the health of future generations demands robust and sustained investments. Modeling indicates, for instance, that a vaccine with 70% efficacy provided to 40% of the population beginning in 2015 could reduce the annual number of infections by 81% by 2030.29
The urgent need for an effective vaccine, combined with the painfully slow progress achieved to date, suggests that standard approaches to biomedical research may need to be supplemented by a more focused and strategic approach. Traditionally, public sector agencies fund basic and applied medical research, typically awarding grants to independent scientific teams that compete among themselves to come forward with the most promising ideas. With the possibility of financial rewards for future patents, intellectual property is carefully guarded, with independent research teams competing to show the world that their approach is superior to all others. This traditional approach has repeatedly paid dividends, generating an array of antiretroviral drugs that have saved the lives of millions. But it has yet to produce tangible progress toward an AIDS vaccine.
The Global HIV Vaccine Enterprise, a multistakeholder partnership uniting key players in the AIDS vaccine field, has worked to enhance collaboration to solve key scientific challenges. Although this approach has facilitated dialogue among researchers in the field, it has not been able to overcome the turf-consciousness of many leading players.
Recent history suggests that other approaches are possible when it comes to mission-driven research undertakings. International collaboration resulted in a remarkably swift mapping of the human genome, and CERN’s multicountry collaborative efforts aim to answer key questions of physics. For an enterprise as urgent as the search for a preventive AIDS vaccine, a similar mission-driven approach is needed. Research funding should be provided on condition that all research findings are shared. Efforts should be made to avoid the duplication of work and the lack of communication among different players. This strong commitment to vaccine research should investigate multiple approaches simultaneously, to avoid the risk that the failure of a single vaccination theory could cause the entire research edifice to come crashing down.
Building the evidence base for community-level and structural prevention approaches
The continuing focus on developing new prevention technologies reflects both recognition of the need for a combination of approaches and some disappointment and skepticism regarding the long-term effectiveness of strategies to change sexual behaviors. Behavior change has been central to every major HIV prevention success ever recorded. But changing behaviors is a difficult, complex, lifelong endeavor. Furthermore, the success of approaches that have proven efficacious in controlled clinical trials has often been difficult to replicate in the real world.
Existing models for behavioral HIV-prevention programs are grounded in a rather narrow spectrum of cognitive behavioral theories. These strategies focus on the individual as the fulcrum for prevention success. Even when these behavioral strategies work, their effectiveness is often short lived. And to the extent that behavioral strategies are effective, their lessons have to be heeded one individual at a time, one sexual episode at a time.
By contrast, when entire societies change and adopt new social norms, these norms tend to be self-perpetuating and self-policing. Social norms are backed by formal and informal social sanctions, and groups develop ways to support one another in adhering to these norms.30 With a mindset demanding immediate results, the AIDS movement has often adopted a reductionist approach that focuses on individual behaviors, regarding social change as a “luxury” item that requires too much time to achieve impact. As the aids2031 Working Group on Social Drivers concluded, a long-term focus on sustainability recognizes social forces as fundamental to success against the epidemic.31 As part of this approach, it is more useful to think in terms of “practices” than “behaviors,” as the former conveys the social dimensions of unprotected intercourse, sexual concurrency, sharing of syringes, or human reproduction.32
Notwithstanding the centrality of social forces to the continued perpetuation of the pandemic, remarkably little research has focused on strategies to marshal social forces to prevent new infections. The overwhelming majority of behavioral intervention studies have evaluated individual-level or small group interventions, with scant attention paid to societal-level interventions.
Nor is the evidence base well-developed for structural or public policy interventions to reduce vulnerability.33 For example, the effects of gender inequality on women’s risk and vulnerability are plain in many countries, yet, to date, only one structural intervention that combines women’s empowerment with HIV prevention has been rigorously evaluated to assess its impact on the rate of new HIV infections.34 Similarly, although participatory research methods have yielded overwhelming consensus that HIV stigma is a primary impediment to an effective AIDS response, the evidence base for antistigma programming remains weak.
For every strategy that seeks to promote HIV prevention, reduced HIV incidence is the desired endpoint. But structural interventions and social change processes may require a decade or more to generate actual reductions in new infections. Their longer-lasting and self-reinforcing qualities make them critical to a long-term approach to AIDS, but building the evidence base for action demands knowledge-generation approaches that differ from the time-limited clinical trials on which the AIDS field has traditionally focused.
Articulation and testing of the anticipated causal pathway for individual epidemic drivers will help guide research on key social factors. By generating evidence relevant to particular settings at key steps along a causal pathway, it is possible to steer programs to better contribute to the ultimate goal: a reduction in new HIV infections.
Innovative financing and mission-oriented research and development
Strong, sustained funding from the public sector is needed to help generate the knowledge required to develop new tools and technologies. An analysis commissioned by the Bill & Melinda Gates Foundation concluded that, in 2007, global research and development funding related to AIDS in resource-limited settings amounted to US$ 1.083 billion (of which 63% was devoted to vaccines, 18% to microbicides, and 1.2% to diagnostics). In the United States, the research funding has flattened, and Europe has an even greater lack of public funding. Clearly, these trends must be reversed if scientists are to have the resources they need to generate new approaches to fighting AIDS.
Whereas the public sector specializes in supporting research to generate basic science advances, product development expertise generally lies with private industry. As long as a robust market for antiretroviral drugs exists in high-income countries, the private sector has a strong financial incentive to invest in research and development on new therapeutics. However, industry’s incentive is less clear when it comes to simple, low-cost tools for primary use in resource-limited settings.
When market dynamics do not align with international needs, innovative approaches are required. Two common approaches to the problem involve the use of so-called “push” and “pull” mechanisms. Push mechanisms provide a direct nudge to private companies, offering them up-front financial support to pursue promising research avenues. Pull mechanisms seek to affect the private sector’s calculus regarding likely future profits associated with new products or health tools, effectively “pulling” them to invest in research approaches that might not otherwise seem promising.
AIDS has helped give rise to a common push mechanism: the public-private product development partnership that seeks to combine the public sector’s commitment to international public goods with the private sector’s business approach to product development. Public-private partnerships in the AIDS field include the International AIDS Vaccine Initiative (IAVI) and the International Partnership for Microbicides (IPM). IAVI and IPM provide direct grants or enter into license agreements with private companies to encourage their engagement in AIDS research. Although it is too soon to know whether either IAVI or IPM has actually accelerated progress toward their respective product goals, both have mobilized new resources for AIDS research, increased awareness of the need for new health tools, and engaged pharmaceutical companies that were previously uninvolved in AIDS work.
An important question is whether a similar public-private partnership will be needed in the future for antiretroviral products. To date, little evidence of market failure for antiretrovirals exists. However, even with dramatic declines in the prices of antiretrovirals, these drugs remain far more expensive than therapeutic agents normally used in resource-limited settings. Sustaining treatment access in developing countries will require new, less expensive approaches to HIV treatment, such as the development of easy-to-administer compounds that need to be taken only periodically rather than daily.
Pull mechanisms seek to create confidence that a profitable market for a new product will exist. One innovative pull mechanism is the advance market commitment, whereby donors make an advance commitment to buy a certain quantity of a product at an agreed price once the product is available for use. The first advance market commitment focused on a new pneumococcal vaccine. Experience with the pneumococcal vaccine will indicate whether the advance market commitment approach might work for other tools, such as AIDS vaccines, microbicides, or point-of-care diagnostics.
Innovative funding mechanisms, such as the Global Fund to Fight AIDS, Tuberculosis and Malaria, and the Global Alliance for Vaccines and Immunization, also function as “pull” mechanisms, in that their existence demonstrates global resolve to bring international public goods to those who need them. The potential impact of these mechanisms on industry’s thinking about investment decisions is yet another important reason to ensure their survival and success.
Regardless of the method of financing used to spur greater research investments, it is apparent that the overwhelming reliance on research centers in high-income countries to conduct AIDS studies in developing countries makes little sense for the long term. In reality, a number of emerging economies—including Brazil, China, India, South Africa, and Thailand—have rapidly growing domestic research capacities. International donors, developing country governments, foundation funders of medical research, and international technical agencies should collaborate to establish research centers of excellence in low- and middle-income countries. This approach is in widespread use with agricultural research.
A new approach to programmatic research
The AIDS response has generated a remarkable array of validated prevention and treatment strategies, yet almost invariably, results in the real world have fallen short of expectations based on the results of controlled clinical trials. Virtual elimination of mother-to-child HIV transmission may indeed be feasible with existing tools, yet in 2009, an estimated 370,000 children became infected.35 Based on efficacy studies, modeling suggests that available strategies could prevent two out of three new HIV infections—but in 2009, 2.6 million people became newly infected with HIV.36 Similarly, even though antiretroviral therapies have the capacity to prevent or delay death for the vast majority of people who receive them, 1.8 million people died of AIDS in 2009.37
Limited access to services is undoubtedly a major impediment to realizing the potential of existing tools, yet it is hardly the only obstacle. The AIDS field has adopted a remarkably narrow approach to research, focusing almost exclusively on the ideal results that may be achieved in controlled settings. Until AIDS research focuses as much energy on the real world as it does on clinical trials, we will inevitably be disappointed with the results.
From efficacy to effectiveness
Studies have documented the efficacy of a broad range of strategies to encourage individuals to adopt safer sexual behaviors.38 But these effects have been difficult to replicate in community settings. In part, this reflects the difference between the carefully controlled environment of a clinical trial and the real world. In clinical trials, participants are selected based on rigorous criteria, but actual programs are typically offered to a much broader range of individuals. With an eye toward isolating the specific benefit of a particular programmatic intervention, clinical studies tend to study single-dimension interventions, yet actual HIV-prevention programs typically combine multiple elements, such as counseling, social marketing, HIV testing promotion, and sexually transmitted infection (STI) screening and treatment.
Efficacy studies are also time-limited. Few HIV-prevention studies follow participants longer than 12 months, with many basing their conclusions on effects reported immediately after receipt of the intervention. One study found that a 10-week individualized counseling program for men who have sex with men resulted in significant positive changes in self-reported sexual behaviors and short-term reductions in HIV incidence. Concerned that most prevention trials followed study participants only for short periods, study investigators followed up more than three years later to assess the results. What they found was startling. The favorable behavior changes detected soon after the end of the 10-week program appeared to dissipate 12 to 18 months later. More than three years after the program ended, there was no difference in HIV infection rates between recipients of the intervention and the study control group that had not received the intervention.39
With few exceptions, behavioral intervention trials rely on participants’ self-reported knowledge, behaviors, and future intentions.40 Although methods have been developed to increase the reliability of self-reported behaviors, self-reports are inevitably susceptible to biases and poor recall.
Observational studies in different settings have identified declines in HIV incidence that exceed what would be expected from natural saturation of infection,41 suggesting that programmatic approaches may be having an effect. But the lack of well-planned effectiveness studies makes it impossible to draw definitive conclusions or to attribute trends to particular approaches. Substantially greater attention must be directed toward rigorous effectiveness studies.
For both prevention and treatment programs, greater investments are needed in operational or translational research to guide program implementers. Chapter 3, “Using knowledge for a better future,” addresses this priority in greater detail. Another related topic that Chapter 3 addresses is the need for comprehensive monitoring of program inputs, outputs, and costs to promote efficient and effective service delivery, and the need to evaluate the impact of interventions and combinations of interventions to measure (and improve) their effectiveness.
Generating knowledge to sustain HIV treatment
Not all antiretroviral regimens are created equal. According to national treatment monitoring in the United Kingdom, the duration of treatment success varies up to threefold, depending on which first-line regimen is administered.44 It is important to select the longest-lasting first-line regimen, especially for low-income countries where second- and third-line regimens are likely to be unaffordable and unavailable. Effective first-line regimens delay or avert the much-higher costs associated with second-line drugs and, in settings where second-line therapy is not available, they can keep patients alive and healthy until future research breakthroughs can deliver more affordable options.
Unfortunately, clear evidence does not exist regarding the optimal, longest-lasting regimen suitable for use in resource-limited settings. This is a critical research priority to enable program planners in low-income countries to use limited resources to maximize individual health and longevity.
Another area where better knowledge is needed centers on simplifying and decentralizing the administration of antiretroviral treatment to the greatest extent that is consistent with sound clinical outcomes. In a recent randomized trial in Uganda, researchers found that home-based care delivered by community workers was at least as effective as clinic-based programs.45
Sustaining treatment gains also requires building the knowledge base for promoting strong treatment adherence. The relatively few studies that have assessed HIV treatment adherence have followed patients for short periods of time. A recent, longer study in London found that patients in the U.K. are often able to maintain high adherence levels a decade or more after initiating treatment.46 Additional studies that use robust research methods are needed to build the evidence base for interventions to support treatment adherence in low- and middle-income countries.
Strengthening local knowledge
AIDS epidemics can vary considerably from country to country, as well as within countries, underscoring the need for locally specific and relevant data.
Timely and accurate information about the epidemic is the starting point for sound planning of HIV strategies and programs. Knowing who is becoming infected and at what rate, as well as what practices and factors are driving the epidemic, is important. To plan treatment programs, it is important to understand where and at what rate HIV infections are occurring, the size and distribution of gaps in testing services, the number of people who immediately need therapy, the likely number of people who will require treatment in future years, the relative need for services among populations or geographic districts, the success of different clinical settings in retaining patients in care, the rate of treatment failure, and details about the emergence of viral strains that are resistant to standard antiretroviral regimens.
Researchers have developed a range of strategies to use limited data from public health surveillance to estimate new HIV infections in different settings.47 One of the most promising is the “estimating HIV incidence by modes of transmission” model, which uses available data to develop a single-year estimate of the number and distribution of new HIV infections in a given country.48 In 2008–2009, with support from UNAIDS, epidemiological syntheses by modes of transmission were conducted in 26 countries.49 UNAIDS plans to extend this approach to at least 30 additional countries in 2010–2011.
These recent epidemiological assessments have provided critical insights to inform programmatic priorities. A comparison of epidemiological trends in Ghana and Swaziland is instructive (see Figure 2.2). Both are African countries, yet the factors driving their respective epidemics are notably different. HIV transmission during sex work accounts for a considerably larger share of new infections in Ghana than in Swaziland, where the proportion of incident infections among individuals in stable heterosexual relationships is substantially greater. Clearly, Ghana and Swaziland need to pursue national AIDS responses that differ markedly from one another if they are to address their respective national priorities and maximize long-term impact.
Figure 2.2. Distribution of new infections by mode of exposure in Ghana and Swaziland, 2008. (Note: Sensitivity analysis for Swaziland used different data sources.)
Source: WHO/UNAIDS AIDS epidemic update 2009.
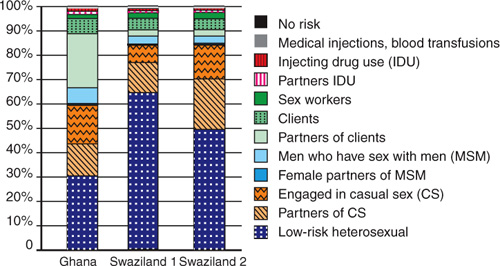
Periodic assessments of new infections by modes of transmission must become standard operating procedure for AIDS planning. Additional efforts are needed to improve and standardize the methodology for such reviews, to allow for comparisons across different geographic locations and over time.
Epidemiological assessments are essential to, but not sufficient in, informing national and subnational decision-making on AIDS. The AIDS response has badly neglected social science assessments. If countries are to address the drivers of their epidemics, they need to supplement assessments of the modes of transmission for incident infections with routine sociological assessments, to identify and explore the dimensions of social context that increase risk and vulnerability.50, 51
The lack of country-specific sociological assessments often leads to assumptions about the drivers of national epidemics that are not supported by evidence. For example, it is frequently said that poverty is a major cause of AIDS. A correlation may exist between household poverty and vulnerability to HIV in some settings, such as Burkina Faso, but evidence from numerous countries in sub-Saharan Africa fails to identify a strong association between wealth and HIV prevalence (see Figure 2.3). Likewise, it is frequently asserted as a universal proposition that gender inequality increases women’s vulnerability to HIV; although strong evidence for this proposition arises in many African countries, in many settings worldwide, women have few rights but HIV prevalence is significantly higher among men. Only rigorous setting-specific assessments can help ensure that national strategies are based on sound evidence rather than received wisdom.
Figure 2.3. HIV prevalence in men by wealth status. Quintiles 1–5 represent the poorest 20% of population (1) through to the richest 20% (5), increasing in 20% increments.
Source: Report on the Global AIDS Epidemic, UNAIDS 2008.

An extraordinary body of knowledge already exists on what approaches work best both for prevention of new infections and effective treatment for people who are living with HIV. But, as discussed in this chapter, while the use of existing tools can be improved significantly, new tools are needed to achieve radical reductions in new infections and AIDS deaths by 2031.
The AIDS field has made some of the most important scientific advances of the last 30 years. But only new ways of thinking and operating can generate the knowledge that will be needed to drive innovation and achieve results over the next generation.
Ultimately, knowledge is meaningful only to the degree that it is put to effective use. The next chapter explores how best to translate knowledge into effective, sustainable action.
Endnotes
1 Curran, J. W., W. M. Morgan, A. M. Hardy, H. W. Jaffe, W. W. Darrow, and W. R. Dowdle, “The Epidemiology of AIDS: Current Status and Future Prospects,” Science 229, no. 4,720 (1985): 1,352–1,357.
2 Merson, M. H., J. O’Malley, D. Serwadda, and C. Apisuk, “HIV Prevention 1: The History and Challenge of HIV Prevention,” The Lancet 372, no. 9,637 (2008): 475–488.
3 Global HIV Prevention Working Group, “Behavior Change and HIV Prevention: (Re)considerations for the 21st Century,” 2008. Accessed on 16 June 2010 at www.globalhivprevention.org/reports.html.
4 Aboulker, J. P. and A. M. Swart, “Preliminary Analysis of the Concorde Trial,” The Lancet 341, no. 8,849 (1993): 889–890.
5 U.S. Food and Drug Administration, “Antiretroviral Drugs Used in the Treatment of HIV Infection,” 2010. Accessed 10 May 2010 at www.fda.gov/ForConsumers/byAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm118915.htm. Alcorn, S. HIV/AIDS Technologies: A Review of Progress to Date and Current Prospects, working paper, aids2031 Science and Technology Working Group, Seattle, 2008.
6 UNAIDS and WHO, AIDS Epidemic Update (Geneva: UNAIDS and WHO, 2009).
7 Guay, L. A., P. Musoke, T. Fleming, D. Bagenda, et al., “Intrapartum and Neonatal Single-Dose Nevirapine Compared with Ziduovudine for Prevention of Mother-to-Child Transmission of HIV-1 in Kampala, Uganda: HIVNET 012 Randomized Trial,” The Lancet 354, no. 9,181 (1999): 795–802.
8 UNAIDS and WHO. 2009. Op cit.
9 Gray, R. H., G. Kigozi, D. Serwadda, F. Makumbi, et al., “Male Circumcision for HIV Prevention in Men in Rakai, Uganda: A Randomized Trial,” The Lancet 369, no. 9,562 (2007): 657–666; Bailey, R. C., S. Moses, C. B. Parker, K. Agot, et al., “Male Circumcision for HIV Prevention in Young Men in Kisumu, Kenya: A Randomized Controlled Trial,” The Lancet 369, no. 9,562 (2007): 643–656; Auvert, B., D. Taljaard, E. Lagarde, J. Sobngwi-Tambekou, R. Sitta, and A. Puren, “Randomized, Controlled Intervention Trial of Male Circumcision for Reduction of HIV Infection Risk: The ANRS 1265 Trial,” PLoS Medicine 2, no. 11 (2005): e298.
10 WHO, UNICEF, and UNAIDS, Towards Universal Access: Scaling Up Priority HIV/AIDS Interventions in the Health Sector, progress report (Geneva: World Health Organization, 2009).
11 Ostrow, D. E., K. J. Fox, and J. S. Chmiel, “Attitudes Towards Highly Active Antiretroviral Therapy Are Associated with Sexual Risk Taking Among HIV-Infected and Uninfected Homosexual Men,” AIDS 16, no. 5 (2002): 775–780.
12 WHO, UNICEF, and UNAIDS, Towards Universal Access: Scaling Up Priority HIV/AIDS Interventions in the Health Sector, progress report (Geneva: World Health Organization, 2009).
13 Fleming, P., R. H. Byers, P. A. Sweeney, D. Daniels, J. M. Karon, and R. S. Janssen, “HIV Prevalence in the United States,” Ninth Conference on Retroviruses and Opportunistic Infections, 2002, Seattle, WA.
14 Stiglitz, J. E., “Knowledge As a Global Public Good,” in Global Public Goods: International Cooperation in the 21st Century, edited by I. Kaul, I. Grunberg, and M. Stern (New York: Oxford University Press, 1999).
15 Aids2031 Science and Technology Working Group Report. Advancing science and technology to change the future of the AIDS pandemic. Seattle: Program for Appropriate Technology (PATH) and Duke University, 2010. http://www.aids2031.org/working-groups/science-and-technology (Accessed August 6, 2010).
16 Deeks, S. G., “HIV Eradication: Is It Feasible?” working paper, aids2031 Science and Technology Working Group, Seattle, Wash., 2008.
17 Aids2031 Science and Technology Working Group Report: Advancing Science and Technology to Change the Future of the AIDS Pandemic (Seattle: Program for Appropriate Technology [PATH] and Duke University, 2010).
18 Gerlach, J., D. Boyle, G. Domingo, B. Weigl, and M. Free, “Increased Access to Diagnostic Tests for HIV Case Management,” working paper, aids2031 Science and Technology Working Group, Seattle, Wash., 2008.
19 DART Trial Team, “Routine Versus Clinically Driven Laboratory Monitoring of HIV Antiretroviral Therapy in Africa (DART): A Randomized Non-inferiority Trial,” The Lancet 375, no. 9,709: 123–131.
20 Aids2031 Science and Technology Working Group Report. Advancing science and technology to change the future of the AIDS pandemic. Seattle: Program for Appropriate Technology (PATH) and Duke University, 2010. http://www.aids2031.org/working-groups/science-and-technology (Accessed August 6, 2010).
21 Mastro, T. D., W. Cates, and M. S. Cohen, “Antiretroviral Products for HIV Prevention: Looking Toward 2031,” working paper, aids2031 Science and Technology Working Group, Seattle, 2008.
22 Karim Q.A., et al, “Effectiveness and Safety of Tenofovir Gel, an Antiretroviral Microbicide, for the Prevention of HIV Infection in Women,” Sciencexpress 20 (July 2010).
23 Donnell, D., J. M. Baeten, J. Kiarie, K. K. Thomas, et al., “Heterosexual HIV-1 Transmission After Initiation of Antiretroviral Therapy: A Prospective Cohort Analysis,” The Lancet 375, no. 9,731: 2,092–2,098.
24 Granich, R. M., C. F. Gilks, C. Dye, K. M. De Cock, and B. G. Williams, “Universal Voluntary HIV Testing with Immediate Antiretroviral Therapy As a Strategy for Elimination of HIV Transmission: A Mathematical Model,” The Lancet 373, no. 9,657: 48–57.
25 Padian, N. S., A. van der Straten, G. Ramjee, T. Chipato, et al., “Diaphragm and Lubricant Gel for Prevention of HIV Acquisition in Southern African Women: A Randomized Controlled Trial,” The Lancet 370, no. 9,583: 251–261.
26 Corey, L., “Synergistic Copathogens—HIV-1 and HSV-2,” The New England Journal of Medicine 356, no. 8: 854–856.
27 Celum, C., A. Wald, J. R. Lingappa, A. S. Magaret, et al., “Acyclovir and Transmission of HIV-1 from Persons Infected with HIV-1 and HSV-2,” The New England Journal of Medicine 362, no. 5 (2010): 427–439.
28 Rerks-Ngarm, S., P. Pitisuttihum, S. Nitayahpan, J. Kaewkungwal, et al., “Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand,” The New England Journal of Medicine 361, no. 23: 2,209–2,220.
29 Stover, J., L. Bollinger, R. Hecht, C. Williams, and E. Roca, “The Impact of an AIDS Vaccine in Developing Countries: A New Model and Initial Results,” Health Affairs 26, no. 4 (2007): 1,147–1,158.
30 Auerbach, J. D., J. O. Parkhurst, C. F. Cáceres, and K. E. Keller, “Addressing Social Drivers of HIV/AIDS: Conceptual, Methodological, and Evidentiary Considerations,” working paper, aids2031 Social Drivers Working Group. Accessed 4 October 2010 at http://www.aids2031.org/pdfs/aids2031%20social%20drivers%20paper%2024-auerbach%20et%20all.pdf.
31 aids2031 Social Drivers Working Group, “Revolutionizing the AIDS Response: Enhancing Individual Resilience and Supporting AIDS Competent Communities,” synthesis paper, aids2031 Social Drivers Working Group, Clark University, Worcester, Mass., 2010.
32 Auerbach, et al. Op cit.
33 Gupta, G. R., J. O. Parkhurst, J. A. Ogden, P. Aggelton, and A. Mahal, “HIV Prevention 4: Structural Approaches to HIV Prevention,” The Lancet 372, no. 9,640 (2008): 764–775.
34 Pronyk P. M., J. R. Hargreaves, J. C. Kim, L. A. Morison, et al., “Effect of a Structural Intervention for the Prevention of Intimate-Partner Violence and HIV in Rural South Africa: A Cluster Randomized Trial,” The Lancet 368, no. 9,551): 1,973–1,983.
35 UNAIDS, AIDS Info: 2010 UNAIDS Reference Report (Geneva: 2010).
36 Ibid.
37 Ibid.
38 Global HIV Prevention Working Group, “Behavior Change and HIV Prevention: (Re)considerations for the 21st Century,” 2008. Accessed 16 June 2010 at www.globalhivprevention.org/reports.html.
39 Ibid.
40 Lyles, C. M., L. S. Kay, N. Crepaz, J. H. Herbst, et al., “Best-Evidence Interventions: Findings from a Systematic Review of HIV Behavioral Interventions for U.S. Populations at High Risk, 2000–2004,” American Journal of Public Health 97, no. 1 (2007): 133–143
41 Gregson, S., G. P. Garnett, C. A. Nyamukapa, T. B. Hallett, et al., “HIV Decline Associated with Behavior Change in Eastern Zimbabwe,” Science 311, no. 5,761 (2006): 664–666; Stoneburner, R. L, and D. Low-Beer, “Sexual Partner Reductions Explain Human Immunodeficiency Virus Declines in Uganda: Comparative Analyses of HIV and Behavioral Data in Uganda, Kenya, Malawi, and Zambia,” International Journal of Epidemiology 33 (2004): 1–10.
42 Hall, H. I., R. Song, P. Rhodes, J. Prejean, Q. An, et al., “Estimation of HIV Incidence in the United States,” Journal of the American Medical Association 300, no. 5 (2008): 520–529.
43 Hargrove, J. W., J. H. Humphrey, K. Mutasa, P. H. Parekh, et al., “Improved HIV-1 Incidence Estimates Using the BED Capture Enzyme Immunoassay,” AIDS 22, no. 4 (2008): 511–518.
44 Beck, E. J., S. Mandalia, M. Youle, R. Brettle, et al., “Treatment Outcome and Cost-Effectiveness of Different Highly Active Antiretroviral Therapy Regimens in the U.K. (1996–2002),” International Journal of STD & AIDS 19 (2008): 297–304.
45 Jaffar, S., B. Amuron, S. Foster, J. Birungi, et al., “Rates of Virological Failure in Patients Treated in a Home-Based Versus a Facility-Based HIV-Care Model in Jinja, Southeast Uganda: A Cluster-Randomized Equivalence Trial,” The Lancet 374, no. 9,707 (2009): 2,080–2,089.
46 Cambiano, V., F. C. Lampe, A. J. Rodger, C. J. Smith, et al., “Long-Term Trends in Adherence to Antiretroviral Therapy from Start of HAART,” AIDS 24, no. 8 (2010): 1,153–1,162.
47 UNAIDS and WHO. Op cit.
48 Guows, E. P., White, J. Stover, T. Brown, et al., “Short Term Estimates of Adult HIV Incidence by Modes of Transmission: Kenya and Thailand As Examples,” Sexually Transmitted Infections 82 (2006): iii51–iii55.
49 UNAIDS, “2008–2009 Unified Budget and Workplan: Technical Supplement,” Twenty-Sixth Meeting of the UNAIDS Programme Coordinating Board, Geneva, 22–24 June 2010.
50 aids2031 Social Drivers Working Group, “Revolutionizing the AIDS Response: Enhancing Individual Resilience and Supporting AIDS Competent Communities,” synthesis paper, aids2031 Social Drivers Working Group, Worcester, Mass., 2010.
51 Global HIV Prevention Working Group, Global HIV Prevention Progress Report Card, 2010.
