Introduction and Adhesion Theories
An adhesive is a material that is applied to the surfaces of articles to join them permanently by an adhesive bonding process. An adhesive is a substance capable of forming bonds to each of the two parts when the final object consists of two sections that are bonded together. A feature of adhesives is the relatively small quantities that are required compared to the weight of the final objects.
Keywords
Adhesive; adhesion; bonding; superglues; sealing
1.1 Definition of Adhesives and Adhesive Bonding
An adhesive is a material that is applied to the surfaces of articles to join them permanently by an adhesive bonding process. An adhesive is a substance capable of forming bonds to each of the two parts when the final object consists of two sections that are bonded together [1]. A feature of adhesives is the relatively small quantities that are required compared to the weight of the final objects.
Adhesion is difficult to define, and an entirely satisfactory definition has not been found. The following definition has been proposed by Wu [2].
Adhesion refers to the state in which two dissimilar bodies are held together by intimate interfacial contact such that mechanical force or work can be transferred across the interface. The interfacial forces holding the two phases together may arise from van der Waals forces, chemical bonding, or electrostatic attraction. Mechanical strength of the system is determined not only by the interfacial forces, but also by the mechanical properties of the interfacial zone and the two bulk phases.
There are two principal types of adhesive bonding: structural and nonstructural. Structural adhesive bonding is bonding for applications in which the adherends (the objects being bonded) may experience large stresses up to their yield point. Structural adhesive bonds must be capable of transmitting stress without loss of integrity within design limits [3]. Bonds must also be durable throughout the useful service life of a part, which may be years. A structural bond has been defined as having a shear strength >7 MPa in addition to significant resistance to aging. Nonstructural adhesives are not required to support substantial loads but merely hold lightweight materials in place. This type of adhesive is sometimes called a “holding adhesive.” Pressure-sensitive tapes and packaging adhesives are examples of nonstructural adhesives.
The distinction between structural and nonstructural bonds is not always clear. For example, is a hot melt adhesive used in retaining a fabric’s plies structural or nonstructural? One could argue that such an adhesive may be placed in either classification. However, the superglues (cyanoacrylates) are classified as structural adhesives even though they have poor resistance to moisture and heat.
1.2 Functions of Adhesives
The primary function of adhesives is to join parts together. Adhesives accomplish this goal by transmitting stresses from one member to another in a manner that distributes the stresses much more uniformly than can be achieved with mechanical fasteners. Adhesive bonding often provides structures that are mechanically equivalent to or stronger than conventional assemblies at lower cost and weight. In mechanical fastening, the strength of the structure is limited to that of the areas of the members in contact with the fasteners [4]. It is not unusual to obtain adhesive bonds that are of a strength greater than the strength of adherends.
Smooth surfaces are an inherent advantage of adhesively joined structures and products. Exposed surfaces are not defaced and contours are not disturbed, as happens with mechanical fastening systems. This feature is important in function and appearance. Aerospace structures, including helicopter rotor blades, require smooth exteriors to minimize drag and to keep temperatures as low as possible. Lighter weight materials can often be used with adhesive bonding in contrast to conventional fastening because the uniform stress distribution in the joint permits full utilization of the strength and rigidity of the adherends [4]. Adhesive bonding provides much larger areas for stress transfer throughout the part, thus decreasing stress concentration in small areas.
Dissimilar materials, including plastics, are readily joined by many adhesives, provided that proper surface treatments are used. Adhesives can be used to join metals, plastics, ceramics, cork, rubber, and combinations of materials. Adhesives can also be formulated to be conductive. The focus of this book is on adhesives for bonding plastics, thermosets, elastomers, and metals.
Where temperature variations are encountered during the service of an item containing dissimilar materials, adhesives perform another useful function. Flexible adhesives are able to accommodate differences in the thermal expansion coefficients of the adherends and therefore prevent damage that might occur if stiff fastening systems were used.
Sealing is another important function of adhesive joining. The continuous bond seals out liquids or gases that do not attack the adhesive (or sealant). Adhesives/sealants are often used in place of solid or cellular gaskets. Mechanical damping can be imparted to a structure through the use of adhesives formulated for that purpose. A related characteristic, fatigue resistance, can be improved by the ability of such adhesives to withstand cyclic strains and shock loads without cracking. In a properly designed joint, the adherends generally fail in fatigue before the adhesive fails. Thin or fragile parts can also be adhesive bonded. Adhesive joints do not usually impose heavy loads on the adherends, as in riveting, or localized heating, as in welding. The adherends are also relatively free from heat-induced distortion [4].
1.3 Classification of Adhesives
Adhesives as materials can be classified in a number of ways such as chemical structure or functionality. In this book, adhesives have been classified into two main classes: natural and synthetic. The natural group includes animal glue, casein- and protein-based adhesives, and natural rubber adhesives. The synthetic group has been further divided into two main groups: industrial and special compounds. Industrial compounds include acrylics, epoxies, silicones, etc. An example of the specialty group is pressure-sensitive adhesives.
1.4 Advantages and Disadvantages of Joining Using Adhesives
The previous discussion highlighted a number of advantages of adhesive bonding. This section will cover both advantages and disadvantages, recognizing that some of the points have already been mentioned.
1.4.1 Advantages [5,6]
• Uniform distribution of stress and larger stress-bearing area
• Join thin or thick materials of any shape
• Join similar or dissimilar materials
• Minimize or prevent electrochemical (galvanic) corrosion between dissimilar materials
• Resist fatigue and cyclic loads
• Provide joints with smooth contours
• Seal joints against a variety of environments
• Insulate against heat transfer and electrical conductance (in some cases adhesives are designed to provide such conductance)
• The heat required to set the joint is usually too low to reduce the strength of the metal parts
• Dampen vibration and absorb shock
1.4.2 Disadvantages [5–7]
• The bond does not permit visual examination of the bond area (unless the adherends are transparent)
• Careful surface preparation is required to obtain durable bonds, often with corrosive chemicals
• Long cure times may be needed, particularly where high cure temperatures are not used
• Holding fixtures, presses, ovens, and autoclaves, not usually required for other fastening methods, are necessities for adhesive bonding
• Upper service temperatures are limited to approximately 177°C in most cases, but special adhesives, usually more expensive, are available for limited use up to 371°C
• Rigid process control, including emphasis on cleanliness, is required for most adhesives
• The useful life of the adhesive joint depends on the environment to which it is exposed
• Natural or vegetable-origin adhesives are subject to attack by bacteria, mold, rodents, or vermin
• Exposure to solvents used in cleaning or solvent cementing may present health problems.
1.5 Requirements of a Good Bond
The basic requirements for a good adhesive bond are [6]:
• Wetting of surfaces that are to be bonded together
• Proper adhesive bonding process (solidification and cure).
1.5.1 Proper Choice of Adhesive
There are numerous adhesives available for bonding materials. Selection of the adhesive type and form depends on the nature of adherends, performance requirements of the end use, and the adhesive bonding process.
1.5.2 Good Joint Design
It is possible to impart strength to a joint by design [8]. A carefully designed joint can yield a stronger bond by combining the advantages of the mechanical design with adhesive bond strength to meet the end use requirements of the bonded part.
1.5.3 Cleanliness
To obtain a good adhesive bond, it is important to start with a clean adherend surface. Foreign materials, such as dirt, oil, moisture, and weak oxide layers, must be removed, else the adhesive will bond to these weak boundary layers rather than to the substrate. There are various surface treatments that may remove or strengthen the weak boundary layers. These treatments generally involve physical or chemical processes, or a combination of both [9].
1.5.4 Wetting
Wetting is the displacement of air (or other gases) present on the surface of adherends by a liquid phase. The result of good wetting is greater contact area between the adherends and the adhesive over which the forces of adhesion may act [10].
1.5.5 Adhesive Bonding Process
Successful bonding of parts requires an appropriate process. The adhesive must not only be applied to the surfaces of the adherends but the bond should also be subjected to the proper temperature, pressure, and hold time. The liquid or film adhesive, once applied, must be capable of being converted into a solid in any one of three ways. The method by which solidification occurs depends on the choice of adhesive.
The ways in which liquid adhesives are converted to solids are [6]:
• Chemical reaction by any combination of heat, pressure, and curing agents
The requirements to form a good adhesive bond, processes for bonding, analytic techniques, and quality control procedures are discussed in this book.
1.6 Introduction to Theories of Adhesion
There is no universal agreement about the causes (or theories) of adhesion. Nor is there an agreement about the mechanisms involved. Various theories have emerged to explain adhesion. Clearly, complete agreement on these causes/theories will be enormously helpful to those who plan to work with adhesives.
Historically, mechanical interlocking or anchoring, electrostatic, diffusion, and adsorption/surface reaction theories have been postulated to describe mechanisms of adhesion. More recently, other theories on the causative actions involved have been put forward to explain adhesive bonding mechanisms. Figure 1.1 and Table 1.1 show examples of proposed causes of adhesion, which have some commonality and some differences. It is often difficult to fully ascribe adhesive bonding to an individual mechanism. A combination of different mechanisms is most probably responsible for bonding within a given adhesive system. The extent of the role of each mechanism could vary for different adhesive bonding systems.
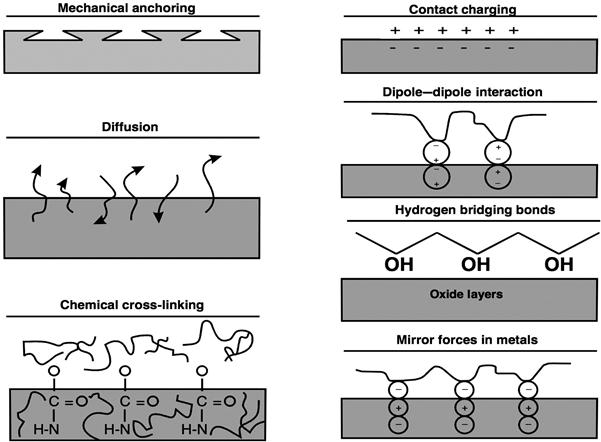
Table 1.1
| Traditional | Recent | Scale of Action |
| Mechanical interlocking | Mechanical interlocking | Microscopic |
| Electrostatic | Electrostatic | Macroscopic |
| Diffusion | Diffusion | Molecular |
| Adsorption/surface reaction | Wettability | Molecular |
| Chemical bonding | Atomic | |
| Acid–base | Molecular | |
| Weak boundary layer | Molecular |
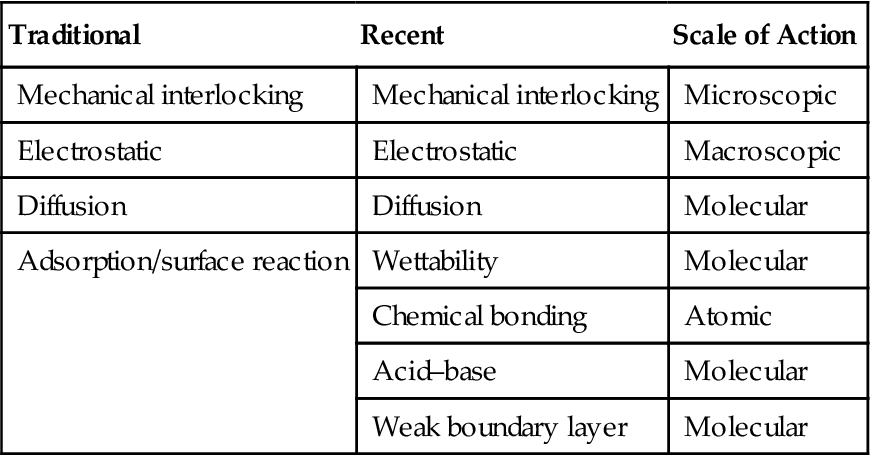
An important aspect of adhesion bonding is the scale of the proposed action by which the adhesive and adherend interact. Table 1.1 shows a scale of action for each cause, which is intended to aid in the understanding of these mechanisms. Of course, adhesive–adherend interactions always take place at atomic and molecular level, which have not been fully understood. Aside from scale complications, it is difficult to study adhesion-bonding mechanisms because they occur at the interface of materials.
The microscopic parameter of interest in mechanical interlocking or anchoring is the contact surface of the adhesive and the adherend. Specific surface area (SSA, total surface area per unit mass) of the adherend is an example of a measure of SSA. Surface roughness is the means by which interlocking is believed to work and can be detected by optical or electron microscopy. In the electrostatic or contact charging mechanism, the surface charge is the macroscopic factor of interest. The charge in question is similar to that produced in a glass rod after rubbing it several times with a woollen cloth. Diffusion involves molecular and atomic scale interactions, respectively.
Readers who wish to gain an in-depth understanding of the interaction forces, adhesion mechanism, and thermodynamics of adhesion can consult a number of references [12–14] including Fundamentals of Adhesion, edited by Lieng-Huang Lee [15]. This reference provides a qualitative and quantitative treatment of adhesion, complete with derivation of force interaction equations.
1.6.1 Mechanical Theory
According to this theory, adhesion occurs by the penetration of adhesives into pores, cavities, and other surface irregularities on the surface of the substrate. The adhesive displaces the trapped air at the interface. Therefore, it is concluded that an adhesive penetrating into the surface roughness of two adherends can bond them. A positive contribution to the adhesive bond strength results from the “mechanical interlocking” of the adhesive and the adherends. Adhesives frequently form stronger bonds to porous abraded surfaces than they do to smooth surfaces. However, this theory is not universally applicable, since good adhesion also takes place between smooth surfaces.
Enhanced adhesion after abrading the surface of an adherend may be due to (i) mechanical interlocking, (ii) formation of a clean surface, (iii) formation of a highly reactive surface, and (iv) an increase in contact surface area. It is believed that changes in physical and chemical properties of the adherend surface produce an increase in adhesive strength [16]. It can be debated whether mechanical interlocking is responsible for strong bonds or an increase in the adhesive contact surface enhances other mechanisms. More thorough wetting and more extensive chemical bonding are expected consequences of increased contact surface area.
There is supportive data in the literature that relate joint strength and bond durability to increased surface roughness. There are also contrary observations indicating that increased roughness can lower joint strength [17].
1.6.2 Electrostatic (Contact Charging) Theory
This theory proposes that adhesion takes place due to electrostatic effects between the adhesive and the adherend [18–21]. Electron transfer is supposed to take place between the adhesive and the adherend as a result of unlike electronic band structures. Electrostatic forces in the form of an electrical double layer are thus formed at the adhesive–adherend interface. These forces account for the resistance to separation. This theory gains support from the fact that electrical discharges have been noticed when an adhesive is peeled from a substrate [16].
The electrostatic mechanism is a plausible explanation for polymer–metal adhesion bonds. The contribution of the electronic mechanism in nonmetallic systems to adhesion has been calculated and found to be small when compared with that of chemical bonding [22,23].
1.6.3 Diffusion Theory
This theory suggests that adhesion is developed through the interdiffusion of molecules in between the adhesive and the adherend. The diffusion theory is primarily applicable when both the adhesive and the adherend are polymers with relatively long chain molecules capable of movement. The nature of materials and bonding conditions will influence whether and to what extent diffusion takes place. The diffuse interfacial (interphase) layer typically has a thickness in the range of 10–1000 Å (1–100 nm). Solvent cementing or heat welding of thermoplastics is considered to be due to diffusion of molecules [16].
No stress concentration is present at the interface because no discontinuity exists in the physical properties. Cohesive energy density (CED, Eq. (1.1)) can be used to interpret diffusion bonding, as defined by Eq. (1.2). Bond strength is maximized when solubility parameters are matched between the adhesive and the adherend.
(1.1)
(1.2)
Ecoh is the amount of energy required to separate the molecules to an infinite distance, V is the molar volume, and δ is the solubility parameter.
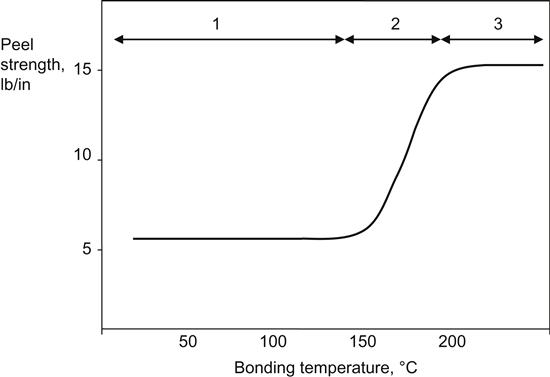
A relevant example is the adhesion of polyethylene and polypropylene to a butyl rubber. The adhesive bond is weak when two polymers are bonded at temperatures below the melting point of the polyolefin. Bond strength increases sharply when the adhesion process takes place above the melting temperature of polyethylene (135°C) and polypropylene (175°C). Figure 1.2 illustrates the bond strength (peel strength) as a function of bonding temperature. An inference can be made that at elevated temperatures, the interdiffusion of polyolefins and butyl rubber increases, thus leading to higher bond strength.
1.6.4 Wetting Theory
This theory proposes that adhesion results from molecular contact between two materials and the surface forces that develop. The first step in bond formation is to develop interfacial forces between the adhesive and the substrates. The process of establishing continuous contact between the adhesive and the adherend is called wetting. For an adhesive to wet a solid surface, the adhesive should have a lower surface tension than the critical surface tension of the solid. This is precisely the reason for surface treatment of plastics, which increases their surface energy and polarity.
Van der Waals forces are extremely sensitive to the distance (r) between molecules, decreasing by the inverse of the seventh power (1/r7) of the distance between two molecules and the cubic power of the distance between two adherends. These forces are too small to account for the adhesive bond strength in most cases.
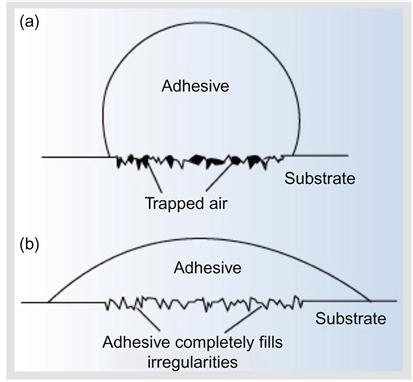
Figure 1.3 illustrates complete and incomplete wetting of an adhesive spreading over a surface. Good wetting results when the adhesive flows into the valleys and crevices on the substrate surface. Poor wetting results when the adhesive bridges over the valley and results in a reduction of the actual contact area between the adhesive and the adherend, resulting in a lower overall joint strength [16]. Incomplete wetting generates interfacial defects, thereby reducing the adhesive bond strength. Complete wetting achieves the highest bond strength.
Most organic adhesives readily wet metal adherends. On the other hand, many solid organic substrates have surface tensions lower than those of common adhesives. The criteria for good wetting requires the adhesives to have a lower surface tension than the substrate, which explains, in part, why organic adhesives such as epoxies have excellent adhesion to metals but offer weak adhesion on untreated polymeric substrates such as polyethylene, polypropylene, and fluoroplastics [16]. The surface energy of plastic substrates can be increased by various treatment techniques to allow wetting.
1.6.5 Chemical Bonding
This mechanism attributes the formation of an adhesion bond to surface chemical forces. Hydrogen, covalent, and ionic bonds formed between the adhesive and the adherends are stronger than the dispersion attractive forces. Table 1.2 lists examples of these forces and their magnitudes. In general, there are four types of interactions that take place during chemical bonding: covalent bonds, hydrogen bonds, Lifshitz–van der Waals forces, and acid–base interactions. The exact nature of the interactions for a given adhesive bond depends on the chemical composition of the interface.
Table 1.2
Examples of Energies of Lifshitz–van der Waals Interactions and Chemical Bonds
| Type | Example | E (kJ/mol) |
| Covalent | C |
350 |
| Ion–Ion | Na+ … Cl− | 450 |
| Ion–dipole | Na+ … CF3H | 33 |
| Dipole–dipole | CF3H … CF3H | 2 |
| London dispersion | CF4 … CF4 | 2 |
| Hydrogen bonding | H2O … H2O | 24 |
Covalent and ionic bonds (Table 1.2) are examples of chemical bonding that provide much higher adhesion values than that provided by secondary forces. Secondary valence bonding is based on the weaker physical forces exemplified by hydrogen bonds. These forces are more prevalent in materials that contain polar groups such as carboxylic acid groups than in nonpolar materials such as polyolefins. The interactions that hold the adhesive and the adherends together may also receive contributions from mechanical interlocking, diffusion, or electrostatic mechanisms.
The definitions of intermolecular interactions are listed below:
Dipole (polar molecule): A molecule whose charge distribution can be represented by a center of positive charge and a center of negative charge, which do not coincide.
Dipole–dipole forces: Intermolecular forces resulting from the tendency of polar molecules to align themselves such that the positive end of one molecule is near the negative end of another.
Hydrogen bonding: A special type of dipole–dipole interaction that occurs when a hydrogen atom that is bonded to a small, highly electronegative atom (most commonly F, O, N, or S) is attracted to the lone electron pairs of another molecule.
London dispersion forces (dispersion forces): Intermolecular forces resulting from the small, instantaneous dipoles (induced dipoles) that occur because of the varying positions of the electrons during their motion about the nuclei.
Polarizability is defined as the ease with which the electron cloud of an atom or molecule is distorted. In general, polarizability increases with the size of an atom and the number of electrons on an atom. The importance of London dispersion forces increases with the atom size and number of electrons.
Covalent chemical bonds can form across the interface and are likely to occur in cross-linked adhesives and thermoset coatings. This type of bond is usually the strongest and most durable. However, they require that mutually reactive chemical groups should exist. Some surfaces, such as previously coated surfaces, wood, composites, and some plastics, contain various functional groups that under appropriate conditions can produce chemical bonds with the adhesive material. There are ways to intentionally generate these conditions, such as by surface treatment of plastics with techniques like corona or flame treatment.
Organosilanes are widely used as primers on glass fibers to promote the adhesion between the resin and the glass in fiberglass-reinforced plastics. They are also used as primers or integral blends to promote adhesion of resins to minerals, metals, and plastics. Essentially, during application, silanol groups are produced, which then react with the silanol groups on the glass surface or possibly with other metal oxide groups to form strong ether linkages. Coatings containing reactive functional groups such as hydroxyl or carboxyl moieties tend to adhere more tenaciously to substrates containing similar groups. Chemical bonding may also occur when a substrate contains reactive hydroxyl groups, which may react with the isocyanate groups from the incoming coating in thermoset polyurethane coatings.
Most likely, chemical bonding accounts for the strong adhesion between an epoxy coating and a substrate with a cellulose interface. The epoxy groups of an epoxy resin react with the hydroxyl groups of cellulose at the interface.
1.6.5.1 Acid–Base Theory
A special type of interaction, the acid–base interaction, is a fairly recent discovery. It is based on the chemical concept of a Lewis acid and base, which is briefly described. The acid/base definition was proposed separately by J.N. Bronsted and G.N. Lewis. Restatement of these definitions by Lewis in 1938 led to their popularity and acceptance. The Lewis definitions are “an acid is a substance which can accept an electron pair from a base; a base is a substance which can donate an electron pair” [24]. By this definition, every cation is an acid in addition to chemical compounds such as BF3 and SiO2. Conversely, anions and compounds like NH3, PH3, and C6H5CH2NH2 are bases. According to the acid–base theory, adhesion results from the polar attraction of Lewis acids and bases (i.e., electron-poor and electron-rich elements) at the interface. This theory is attributed to the work by Fowkes and colleagues [25–28], Gutmann [29], and Bolger and Michaels [30].
In BF3, the higher electronegativity of fluorine atoms preferentially displaces the shared electrons away from the boron atom. Thus, a bipolar molecule is created that has positive charge on the boron side and negative charge on the fluorine side. On the other hand, NH3, by a similar analogy, has a negative nitrogen end that renders it a Lewis base. Naturally, the positive boron end of BH3 and negative nitrogen end of NH3 interact.
A special case of acid–base interaction is hydrogen bonding such as among water molecules that exhibit both acidic and basic tendencies. Table 1.2 shows that the hydrogen bond strength, while substantially less than ionic and covalent bond energies, is one of the most significant among the secondary interactions. The reader can refer to inorganic chemistry texts [31,32] to learn about Lewis acids and bases and their chemical reactions. In summary, the interactions between compounds capable of electron donation and acceptance form the foundation of the acid–base theory of adhesion.
1.6.6 Weak Boundary Layer Theory
This theory was first described by Bikerman. It states that bond failure at the interface is caused by either a cohesive break or a weak boundary layer [33]. Weak boundary layers can originate from the adhesive, the adherend, the environment, or a combination of any of these three factors. Weak boundary layers can occur in the adhesive or adherend if an impurity concentrates near the bonding surface and forms a weak attachment to the substrate. When failure takes place, it is the weak boundary layer that fails, although failure appears to take place at the adhesive–adherend interface.
Polyethylene and metal oxides are examples of two materials that may inherently contain weak boundary layers. Polyethylene has a weak, low-molecular-weight constituent that is evenly distributed throughout the polymer. This weak boundary layer is present at the interface and contributes to low failing stress when polyethylene is used as an adhesive or an adherend. Some metal oxides are weakly attached to their base metals. Failure of adhesive joints made with these materials occurs cohesively within the oxide. Certain oxides, such as aluminum oxide, are very strong and do not significantly impair joint strength. Weak boundary layers, such as those found in polyethylene and metal oxides, can be removed or strengthened by various surface treatments. Weak boundary layers formed from the bonding environment, generally air, are very common. When the adhesive does not wet the substrate, as shown in Figure 1.3, a weak boundary layer (air) is trapped at the interface, causing a reduction in joint strength [16,34].
1.7 Definition of Failure Modes
A hypothetical adhesion bond is shown in Figure 1.4. Assume that the bond is tested in the tensile mode in which the two adherends are pulled apart in a direction perpendicular to the bond. There are different possibilities for the occurrence of failure. The surfaces involved in bond failure are called the locus of failure.
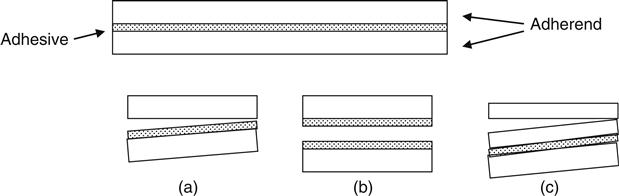
If the bond failure occurs between the adhesive layer and one of the adherends, it is called adhesive failure (Figure 1.4a). A failure in which the separation occurs in such a manner that both adherend surfaces remain covered with the adhesive is called cohesive failure in the adhesive layer (Figure 1.4b). Sometimes the adhesive bond is so strong that the failure occurs in one of the adherends away from the bond. This is called a cohesive failure in the adherend (Figure 1.4c). Bond failures often involve more than one failure mode and are ascribed as a percentage to cohesive or adhesive failure. This percentage is calculated based on the fraction of the area of the contact surface that has failed cohesively or adhesively.
It is important to determine the exact mode(s) of bond failure when a problem occurs. Determination of the failure mode allows action to be taken to correct the true cause and save time and money.
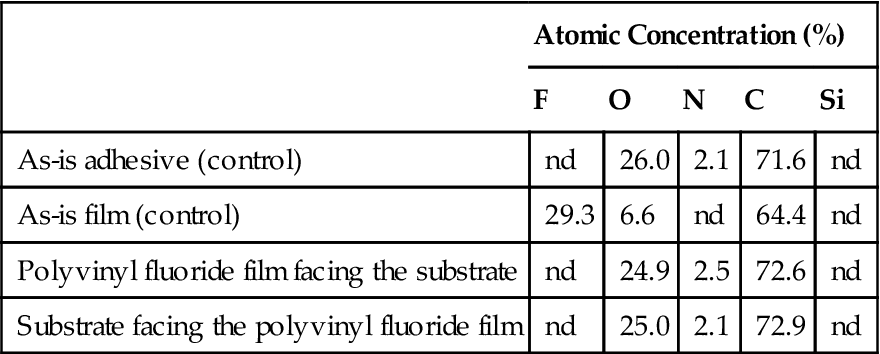
Tables 1.3–1.5 show the result of analyses of several bonds between a substrate and a polyvinyl fluoride film using an acrylic adhesive. All surfaces were analyzed by electron spectroscopy for chemical analysis (ESCA). ESCA yields chemical analysis of organic surfaces in atomic percentage, with the exclusion of hydrogen, which is undetectable by this technique. To determine the type of bond failure, ESCA results for the failed surfaces are compared with those of the adhesive and the polyvinyl fluoride film.
Table 1.3
Surface Chemical Analysis (ESCA) in a Cohesive Failure of Adhesive Bond
| Atomic Concentration (%) | |||||
| F | O | N | C | Si | |
| As-is adhesive (control) | nd | 26.0 | 2.1 | 71.6 | nd |
| As-is film (control) | 29.3 | 6.6 | nd | 64.4 | nd |
| Polyvinyl fluoride film facing the substrate | nd | 24.9 | 2.5 | 72.6 | nd |
| Substrate facing the polyvinyl fluoride film | nd | 25.0 | 2.1 | 72.9 | nd |
Data were provided by Dr. James J. Schmidt at the DuPont Company, 2003.
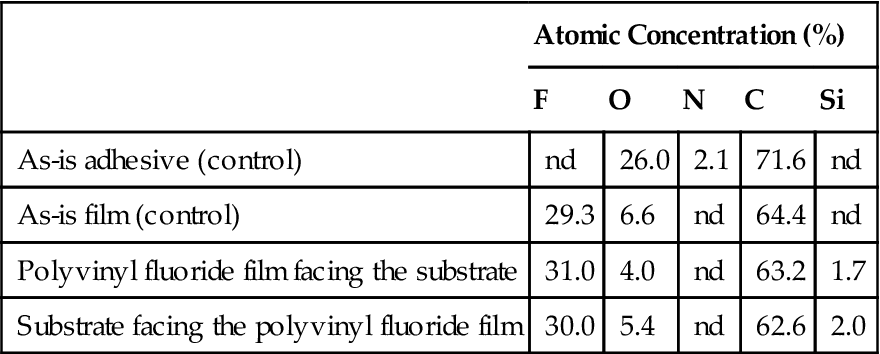
Table 1.4
Surface Chemical Analysis (ESCA) in a Cohesive Failure of Polyvinyl Fluoride
| Atomic Concentration (%) | |||||
| F | O | N | C | Si | |
| As-is adhesive (control) | nd | 26.0 | 2.1 | 71.6 | nd |
| As-is film (control) | 29.3 | 6.6 | nd | 64.4 | nd |
| Polyvinyl fluoride film facing the substrate | 31.0 | 4.0 | nd | 63.2 | 1.7 |
| Substrate facing the polyvinyl fluoride film | 30.0 | 5.4 | nd | 62.6 | 2.0 |
Data were provided by Dr. James J. Schmidt at the DuPont Company, 2003.
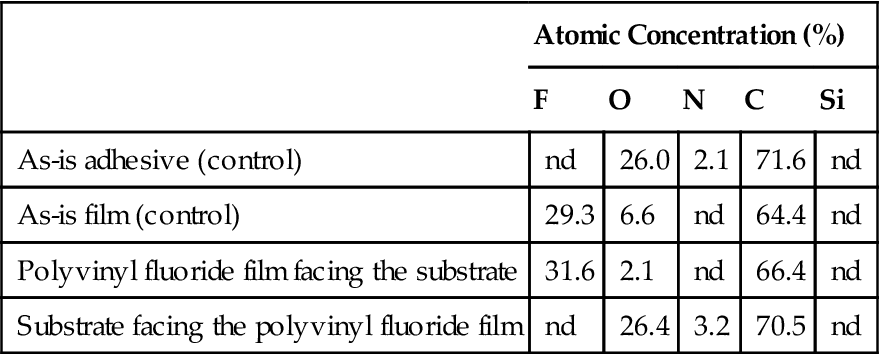
Table 1.5
Surface Chemical Analysis (ESCA) in an Adhesive Failure of Polyvinyl Fluoride
| Atomic Concentration (%) | |||||
| F | O | N | C | Si | |
| As-is adhesive (control) | nd | 26.0 | 2.1 | 71.6 | nd |
| As-is film (control) | 29.3 | 6.6 | nd | 64.4 | nd |
| Polyvinyl fluoride film facing the substrate | 31.6 | 2.1 | nd | 66.4 | nd |
| Substrate facing the polyvinyl fluoride film | nd | 26.4 | 3.2 | 70.5 | nd |
Data were provided by Dr. James J. Schmidt at the DuPont Company, 2003.
In a pure cohesive failure, the two surfaces involved should have virtually identical chemical compositions, which is nearly the case in Tables 1.3 and 1.4. In a 100% adhesive failure, one of the surfaces should have the same chemical composition as the adherend and the other the same as the adhesive. The examples presented in Tables 1.3 and 1.4 illustrate cohesive failure cases for polyvinyl fluoride (adherend) and the adhesive. Table 1.5 gives an example of an adhesive failure. One can see from the chemical composition that the adhesive and polyvinyl fluoride surfaces have been separated in a “clean” manner.
1.8 Mechanisms of Bond Failure
Adhesive joints may fail adhesively or cohesively. Adhesive failure is an interfacial bond failure between the adhesive and the adherend. Cohesive failure occurs when a fracture allows a layer of adhesive to remain on both surfaces. When the adherend fails before the adhesive, it is known as a cohesive failure of the substrate. Various modes of failure are shown in Figure 1.4. Cohesive failure within the adhesive or one of the adherends is the ideal type of failure because with this type of failure the maximum strength of the materials in the joint has been reached. In analyzing an adhesive joint that has been tested to destruction, the mode of failure is often expressed as a percentage cohesive or adhesive failure, as shown in Figure 1.4. The ideal failure is a 100% cohesive failure in the adhesion layer.
The failure mode should not be used as the only criterion for a useful joint [3]. Some adhesive–adherend combinations may fail adhesively, but exhibit greater strength than a similar joint bonded with a weaker adhesive that fails cohesively. The ultimate strength of a joint is a more important criterion than the mode of joint failure. An analysis of failure mode, nevertheless, can be an extremely useful tool in determining whether the failure was due to a weak boundary layer or due to improper surface preparation.
The exact cause of premature adhesive failure is very difficult to determine. If the adhesive does not wet the surface of the substrate completely, the bond strength is certain to be less than maximal. Internal stresses occur in adhesive joints because of a natural tendency of the adhesive to shrink during setting and because of differences in physical properties of adhesive and substrate. The coefficient of thermal expansion of adhesive and adherend should be as close as possible to minimize the stresses that may develop during thermal cycling or after cooling from an elevated temperature cure. Fillers are often used to modify the thermal expansion characteristics of adhesives and limit internal stresses. Another way to accommodate these stresses is to use relatively elastic adhesives.
The types of stress acting on completed bonds, their orientation to the adhesive, and the rates at which it is applied are important factors in determining the durability of the bond. Sustained loads can cause premature failure in service, even though similar unloaded joints may exhibit adequate strength when tested after aging. Some adhesives break down rapidly under dead load, especially after exposure to heat or moisture. Most adhesives have poor resistance to peel or cleavage loads. A number of adhesives are sensitive to the rate at which the joint is stressed. Rigid, brittle adhesives sometimes have excellent tensile or shear strength but have very poor impact strength. Operating environmental factors are capable of degrading an adhesive joint in various ways. If more than one environmental factor (e.g., heat and moisture) is acting on the sample, their combined effect can be expected to produce a synergistic result of reducing adhesive strength. Whenever possible, candidate adhesive joints should be evaluated under simulated operating loads in the actual environment the joint is supposed to encounter.
