Generic Goals and Generic Practices
Overview
This section describes in detail all the generic goals and generic practices of CMMI—model components that directly address process institutionalization. As you address each process area, refer to this section for the details of all generic practices.
Tip
The generic goals and practices apply to every process area; however, they are listed only once in the model document. You will need to refer to this section often for the application of generic goals and generic practice areas to each process area.
Generic practice elaborations appear after generic practices to provide guidance on how the generic practice can be applied uniquely to process areas.
Process Institutionalization
Institutionalization is an important concept in process improvement. When mentioned in the generic goal and generic practice descriptions, institutionalization implies that the process is ingrained in the way the work is performed and there is commitment and consistency to performing (i.e., executing) the process.
An institutionalized process is more likely to be retained during times of stress. When the requirements and objectives for the process change, however, the implementation of the process may also need to change to ensure that it remains effective. The generic practices describe activities that address these aspects of institutionalization.
Hint
Consider institutionalization mismatches when working on process issues that span multiple organizations. An acquirer that has institutionalized a “defined” requirements process, when working with a supplier that has institutionalized a “managed” requirements process, may need to pay attention to negotiating constraints imposed by the acquisition organization across the supplier–acquirer team.
The degree of institutionalization is embodied in the generic goals and expressed in the names of the processes associated with each goal as indicated in Table 7.1.
Table 7.1 Generic Goals and Process Names
The progression of process institutionalization is characterized in the following descriptions of each process.
Performed Process
A performed process is a process that accomplishes the work necessary to satisfy the specific goals of a process area.
Managed Process
A managed process is a performed process that is planned and executed in accordance with policy; employs skilled people having adequate resources to produce controlled outputs; involves relevant stakeholders; is monitored, controlled, and reviewed; and is evaluated for adherence to its process description.
The process can be instantiated by a project, group, or organizational function. Management of the process is concerned with institutionalization and the achievement of other specific objectives established for the process, such as cost, schedule, and quality objectives. The control provided by a managed process helps to ensure that the established process is retained during times of stress.
The requirements and objectives for the process are established by the organization. The status of the work products and services are visible to management at defined points (e.g., at major milestones, on completion of major tasks). Commitments are established among those who perform the work and the relevant stakeholders and are revised as necessary. Work products are reviewed with relevant stakeholders and are controlled. The work products and services satisfy their specified requirements.
A critical distinction between a performed process and a managed process is the extent to which the process is managed. A managed process is planned (the plan can be part of a more encompassing plan) and the execution of the process is managed against the plan. Corrective actions are taken when the actual results and execution deviate significantly from the plan. A managed process achieves the objectives of the plan and is institutionalized for consistent execution.
Defined Process
A defined process is a managed process that is tailored from the organization’s set of standard processes according to the organization’s tailoring guidelines; has a maintained process description; and contributes process related experiences to the organizational process assets.
Organizational process assets are artifacts that relate to describing, implementing, and improving processes. These artifacts are assets because they are developed or acquired to meet the business objectives of the organization and they represent investments by the organization that are expected to provide current and future business value.
The organization’s set of standard processes, which are the basis of the defined process, are established and improved over time. Standard processes describe the fundamental process elements that are expected in the defined processes. Standard processes also describe the relationships (e.g., the ordering, the interfaces) among these process elements. The organization-level infrastructure to support current and future use of the organization’s set of standard processes is established and improved over time. (See the definition of “standard process” in the glossary.)
A project’s defined process provides a basis for planning, performing, and improving the project’s tasks and activities. A project can have more than one defined process (e.g., one for developing the product and another for testing the product).
A defined process clearly states the following:
• Purpose
• Inputs
• Entry criteria
• Activities
• Roles
• Measures
• Verification steps
• Outputs
• Exit criteria
A critical distinction between a managed process and a defined process is the scope of application of the process descriptions, standards, and procedures. For a managed process, the process descriptions, standards, and procedures are applicable to a particular project, group, or organizational function. As a result, the managed processes of two projects in one organization can be different.
Another critical distinction is that a defined process is described in more detail and is performed more rigorously than a managed process. This distinction means that improvement information is easier to understand, analyze, and use. Finally, management of the defined process is based on the additional insight provided by an understanding of the interrelationships of the process activities and detailed measures of the process, its work products, and its services.
Relationships Among Processes
The generic goals evolve so that each goal provides a foundation for the next. Therefore, the following conclusions can be made:
• A managed process is a performed process.
• A defined process is a managed process.
Thus, applied sequentially and in order, the generic goals describe a process that is increasingly institutionalized from a performed process to a defined process.
Achieving GG 1 for a process area is equivalent to saying you achieve the specific goals of the process area.
Achieving GG 2 for a process area is equivalent to saying you manage the execution of processes associated with the process area. There is a policy that indicates you will perform the process. There is a plan for performing it. There are resources provided, responsibilities assigned, training on how to perform it, selected work products from performing the process are controlled, and so on. In other words, the process is planned and monitored just like any project or support activity.
Achieving GG 3 for a process area is equivalent to saying that an organizational standard process exists that can be tailored to result in the process you will use. Tailoring might result in making no changes to the standard process. In other words, the process used and the standard process can be identical. Using the standard process “as is” is tailoring because the choice is made that no modification is required.
Each process area describes multiple activities, some of which are repeatedly performed. You may need to tailor the way one of these activities is performed to account for new capabilities or circumstances. For example, you may have a standard for developing or obtaining organizational training that does not consider web-based training. When preparing to develop or obtain a web-based course, you may need to tailor the standard process to account for the particular challenges and benefits of web-based training.
Generic Goals and Generic Practices
This section describes all of the generic goals and generic practices, as well as their associated subpractices, notes, examples, and references. The generic goals are organized in numerical order, GG 1 through GG 3. The generic practices are also organized in numerical order under the generic goal they support.
GG 1 Achieve Specific Goals
The specific goals of the process area are supported by the process by transforming identifiable input work products into identifiable output work products.
GP 1.1 Perform Specific Practices
Perform the specific practices of the process area to develop work products and provide services to achieve the specific goals of the process area.
The purpose of this generic practice is to produce the work products and deliver the services that are expected by performing (i.e., executing) the process. These practices can be done informally without following a documented process description or plan. The rigor with which these practices are performed depends on the individuals managing and performing the work and can vary considerably.
GG 2 Institutionalize a Managed Process
The process is institutionalized as a managed process.
GP 2.1 Establish an Organizational Policy
Establish and maintain an organizational policy for planning and performing the process.
The purpose of this generic practice is to define the organizational expectations for the process and make these expectations visible to those members of the organization who are affected. In general, senior management is responsible for establishing and communicating guiding principles, direction, and expectations for the organization.
Tip
Policy direction may come from multiple levels above the project. For example, in the Department of Defense (DoD), policy is established by legislation, the Pentagon, senior acquisition executives, product center management, and others.
Not all direction from senior management will bear the label “policy.” The existence of appropriate organizational direction is the expectation of this generic practice, regardless of what it is called or how it is imparted.
Elaboration for all PAs
This policy establishes organizational expectations for planning and performing the process, including not only the elements of the process addressed directly by the acquirer but also the interactions between the acquirer and suppliers.
GP 2.2 Plan the Process
Establish and maintain the plan for performing the process.
The purpose of this generic practice is to determine what is needed to perform the process and to achieve the established objectives, to prepare a plan for performing the process, to prepare a process description, and to get agreement on the plan from relevant stakeholders.
The practical implications of applying a generic practice vary for each process area.
Therefore, this generic practice can either reinforce expectations set elsewhere in CMMI or set new expectations that should be addressed.
Refer to the Project Planning process area for more information about establishing and maintaining plans that define project activities.
Establishing a plan includes documenting the plan and a process description. Maintaining the plan includes updating it to reflect corrective actions or changes in requirements or objectives.
Hint
This practice does not require a separate plan for each process area. Consider incorporating process area planning requirements into existing project plans (e.g., systems engineering plan, program management plan).
Subpractices
1. Define and document the plan for performing the process.
This plan can be a stand-alone document, embedded in a more comprehensive document, or distributed among multiple documents. In the case of the plan being distributed among multiple documents, ensure that a coherent picture of who does what is preserved. Documents can be hardcopy or softcopy.
2. Define and document the process description.
The process description, which includes relevant standards and procedures, can be included as part of the plan for performing the process or can be included in the plan by reference.
3. Review the plan with relevant stakeholders and get their agreement.
This review of the plan includes reviewing that the planned process satisfies the applicable policies, plans, requirements, and standards to provide assurance to relevant stakeholders.
4. Revise the plan as necessary.
GP 2.3 Provide Resources
Provide adequate resources for performing the process, developing the work products, and providing the services of the process.
Hint
In addition to a skilled staff, consider other resource needs of the project team and suppliers throughout the lifecycle. Examples include collaboration environments for “systems of systems” acquisition, tools for engineering analysis and decision making, test beds or simulators, access to operational data or environments, benchmark data, and access to targeted “experts” in areas of high risk.
The purpose of this generic practice is to ensure that the resources necessary to perform the process as defined by the plan are available when they are needed. Resources include adequate funding, appropriate physical facilities, skilled people, and appropriate tools.
The interpretation of the term “adequate” depends on many factors and can change over time. Inadequate resources may be addressed by increasing resources or by removing requirements, constraints, and commitments.
GP 2.4 Assign Responsibility
Assign responsibility and authority for performing the process, developing the work products, and providing the services of the process.
The purpose of this generic practice is to ensure that there is accountability for performing the process and achieving the specified results throughout the life of the process. The people assigned must have the appropriate authority to perform the assigned responsibilities.
Hint
Don’t pass the buck to your suppliers. Acquirers must assume responsibility for executing important processes such as managing risk, analyzing measurement indicators, making important trade-study decisions, and managing customer requirements and expectations.
Responsibility can be assigned using detailed job descriptions or in living documents, such as the plan for performing the process. Dynamic assignment of responsibility is another legitimate way to implement this generic practice, as long as the assignment and acceptance of responsibility are ensured throughout the life of the process.
Subpractices
1. Assign overall responsibility and authority for performing the process.
2. Assign responsibility and authority for performing the specific tasks of the process.
3. Confirm that the people assigned to the responsibilities and authorities understand and accept them.
GP 2.5 Train People
Train the people performing or supporting the process as needed.
The purpose of this generic practice is to ensure that people have the necessary skills and expertise to perform or support the process.
Hint
Consider sharing training opportunities for project-specific needs across the acquirer, supplier, and end-user teams.
Appropriate training is provided to those who will be performing the work. Overview training is provided to orient people who interact with those who perform the work.
Training supports the successful execution of the process by establishing a common understanding of the process and by imparting the skills and knowledge needed to perform the process.
The organization should conduct a training needs analysis to understand its process training needs at both the organization and project levels. Then, appropriate training vehicles can be identified and provided to minimize process execution related risks.
Refer to the Organizational Training process area for more information about developing skills and knowledge of people so they can perform their roles effectively and efficiently.
Determining appropriate skills for people performing roles in the acquisition process can serve as a basis for identifying relevant training for each role.
GP 2.6 Control Work Products
Place selected work products of the process under appropriate levels of control.
The purpose of this generic practice is to establish and maintain the integrity of the selected work products of the process (or their descriptions) throughout their useful life.
The selected work products are specifically identified in the plan for performing the process, along with a specification of the appropriate level of control.
Different levels of control are appropriate for different work products and for different points in time. For some work products, it may be sufficient to maintain version control so that the version of the work product in use at a given time, past or present, is known and changes are incorporated in a controlled manner. Version control is usually under the sole control of the work product owner (which can be an individual, group, or team).
Hint
Pay special attention to the configuration management of program documentation and products when moving from one phase of the program to another, especially if system development or maintenance responsibility is handed off from supplier to supplier or to an internal maintenance group.
Sometimes, it can be critical that work products be placed under formal or baseline configuration management. This type of control includes defining and establishing baselines at predetermined points. These baselines are formally reviewed and approved, and serve as the basis for further development of the designated work products.
Refer to the Configuration Management process area for more information about establishing and maintaining the integrity of work products using configuration identification, configuration control, configuration status accounting, and configuration audits.
Additional levels of control between version control and formal configuration management are possible. An identified work product can be under various levels of control at different points in time.
Elaboration for all PAs
The acquirer is responsible for establishing and maintaining baselines and ensuring designated acquirer work products and supplier deliverables are placed under appropriate levels of control.
GP 2.7 Identify and Involve Relevant Stakeholders
Identify and involve the relevant stakeholders of the process as planned.
The purpose of this generic practice is to establish and maintain the expected involvement of relevant stakeholders during the execution of the process.
Involve relevant stakeholders as described in an appropriate plan for stakeholder involvement. Involve stakeholders appropriately in activities such as the following:
• Planning
• Decisions
• Commitments
• Communications
• Coordination
• Reviews
• Appraisals
• Requirements definitions
• Resolution of problems and issues
Hint
Use surrogates when the relevant stakeholder group is too large. For example, stakeholders for review and acceptance of an environmental impact statement may include the entire population of a given area, so a representative subgroup may need to be selected for practical reasons.
Refer to the Project Planning process area for more information about planning stakeholder involvement.
The objective of planning stakeholder involvement is to ensure that interactions necessary to the process are accomplished, while not allowing excessive numbers of affected groups and individuals to impede process execution.
Subpractices
1. Identify stakeholders relevant to this process and their appropriate involvement.
Relevant stakeholders are identified among the suppliers of inputs to, the users of outputs from, and the performers of the activities in the process. Once the relevant stakeholders are identified, the appropriate level of their involvement in process activities is planned.
2. Share these identifications with project planners or other planners as appropriate.
3. Involve relevant stakeholders as planned.
GP 2.8 Monitor and Control the Process
Monitor and control the process against the plan for performing the process and take appropriate corrective action.
The purpose of this generic practice is to perform the direct day-to-day monitoring and controlling of the process. Appropriate visibility into the process is maintained so that appropriate corrective action can be taken when necessary. Monitoring and controlling the process can involve measuring appropriate attributes of the process or work products produced by the process.
Hint
Use this practice to determine the effectiveness of your processes. A SCAMPI appraisal team may not be able to assess process effectiveness, but it will check whether you can judge effectiveness based on your monitoring activities and whether you are making adjustments accordingly.
Refer to the Measurement and Analysis process area for more information about developing and sustaining a measurement capability used to support management information needs.
Refer to the Project Monitoring and Control process area for more information about providing an understanding of the project’s progress so that appropriate corrective actions can be taken when the project’s performance deviates significantly from the plan.
Subpractices
1. Evaluate actual progress and performance against the plan for performing the process.
The evaluations are of the process, its work products, and its services.
2. Review accomplishments and results of the process against the plan for performing the process.
3. Review activities, status, and results of the process with the immediate level of management responsible for the process and identify issues.
These reviews are intended to provide the immediate level of management with appropriate visibility into the process based on the day-to-day monitoring and controlling of the process, and are supplemented by periodic and event-driven reviews with higher level management as described in GP 2.10.
4. Identify and evaluate the effects of significant deviations from the plan for performing the process.
5. Identify problems in the plan for performing the process and in the execution of the process.
6. Take corrective action when requirements and objectives are not being satisfied, when issues are identified, or when progress differs significantly from the plan for performing the process.
Inherent risks should be considered before any corrective action is taken.
7. Track corrective action to closure.
Elaboration for all PAs
The project collects and analyzes measurements from the acquirer and from the supplier to effectively monitor and control the project.
OPF Elaboration
GP 2.9 Objectively Evaluate Adherence
Objectively evaluate adherence of the process and selected work products against the process description, standards, and procedures, and address noncompliance.
The purpose of this generic practice is to provide credible assurance that the process and selected work products are implemented as planned and adhere to the process description, standards, and procedures. (See the definition of “objectively evaluate” in the glossary.)
Refer to the Process and Product Quality Assurance process area for more information about objectively evaluating processes and work products.
Tip
Many acquirers don’t use an independent quality assurance (QA) function to objectively evaluate process and product quality. In these situations, using objective criteria to evaluate quality and allowing for escalation of issues without retribution become even more critical.
People not directly responsible for managing or performing the activities of the process typically evaluate adherence. In many cases, adherence is evaluated by people in the organization, but external to the process or project, or by people external to the organization. As a result, credible assurance of adherence can be provided even during times when the process is under stress (e.g., when the effort is behind schedule, when the effort is over budget).
GP 2.10 Review Status with Higher Level Management
Review the activities, status, and results of the process with higher level management and resolve issues.
Hint
Some acquisition projects have multiple reporting paths. Make explicit who is considered “higher level management.”
The purpose of this generic practice is to provide higher level management with the appropriate visibility into the process.
Higher level management includes those levels of management in the organization above the immediate level of management responsible for the process. In particular, higher level management can include senior management. These reviews are for managers who provide the policy and overall guidance for the process and not for those who perform the direct day-to-day monitoring and controlling of the process.
Hint
Not every process area will be included in each review with senior management. Establish a strategic rhythm for reviews that includes both examination of high-risk areas and regular health checks on process execution.
Different managers have different needs for information about the process. These reviews help ensure that informed decisions on the planning and performing of the process can be made. Therefore, these reviews are expected to be both periodic and event driven.
Proposed changes to commitments to be made external to the organization (e.g., changes to supplier agreements) are typically reviewed with higher level management to obtain their agreement with the proposed changes.
GG 3 Institutionalize a Defined Process
The process is institutionalized as a defined process.
GP 3.1 Establish a Defined Process
Establish and maintain the description of a defined process.
The purpose of this generic practice is to establish and maintain a description of the process that is tailored from the organization’s set of standard processes to address the needs of a specific instantiation. The organization should have standard processes that cover the process area, as well as have guidelines for tailoring these standard processes to meet the needs of a project or organizational function. With a defined process, variability in how the processes are performed across the organization is reduced and process assets, data, and learning can be effectively shared.
Hint
Don’t go overboard. Use lightweight descriptions that are usable by project team members. Incorporate these descriptions (directly or by reference) into program plans (e.g., systems engineering plan, program management plan).
Refer to the Integrated Project Management process area for more information about establishing the project’s defined process.
Refer to the Organizational Process Definition process area for more information about establishing standard processes and establishing tailoring criteria and guidelines.
The descriptions of the defined processes provide the basis for planning, performing, and managing the activities, work products, and services associated with the process.
Subpractices
1. Select from the organization’s set of standard processes those processes that cover the process area and best meet the needs of the project or organizational function.
2. Establish the defined process by tailoring the selected processes according to the organization’s tailoring guidelines.
3. Ensure that the organization’s process objectives are appropriately addressed in the defined process.
4. Document the defined process and the records of the tailoring.
5. Revise the description of the defined process as necessary.
GP 3.2 Collect Process Related Experiences
Collect process related experiences derived from planning and performing the process to support the future use and improvement of the organization’s processes and process assets.
The purpose of this generic practice is to collect process related experiences, including information and artifacts derived from planning and performing the process. Examples of process related experiences include work products, measures, measurement results, lessons learned, and process improvement suggestions. The information and artifacts are collected so that they can be included in the organizational process assets and made available to those who are (or who will be) planning and performing the same or similar processes. The information and artifacts are stored in the organization’s measurement repository and the organization’s process asset library.
Hint
Periodically review whether the information provided to the organization is still relevant and useful, and adjust your collection practices accordingly. Often, metrics used and measurements gathered will change as the organization’s proficiency in quantitative management increases.
Refer to the Integrated Project Management process area for more information about contributing to organizational process assets.
Refer to the Organizational Process Definition process area for more information about establishing organizational process assets.
Subpractices
1. Store process and product measures in the organization’s measurement repository.
The process and product measures are primarily those measures that are defined in the common set of measures for the organization’s set of standard processes.
2. Submit documentation for inclusion in the organization’s process asset library.
3. Document lessons learned from the process for inclusion in the organization’s process asset library.
4. Propose improvements to the organizational process assets.
Applying Generic Practices
Generic practices are components that can be applied to all process areas. Think of generic practices as reminders. They serve the purpose of reminding you to do things right and are expected model components.
For example, consider the generic practice, “Establish and maintain the plan for performing the process” (GP 2.2). When applied to the Project Planning process area, this generic practice reminds you to plan the activities involved in creating the plan for the project. When applied to the Organizational Training process area, this same generic practice reminds you to plan the activities involved in developing the skills and knowledge of people in the organization.
Process Areas that Support Generic Practices
While generic goals and generic practices are the model components that directly address the institutionalization of a process across the organization, many process areas likewise address institutionalization by supporting the implementation of the generic practices. Knowing these relationships will help you effectively implement the generic practices.
Such process areas contain one or more specific practices that when implemented can also fully implement a generic practice or generate a work product that is used in the implementation of a generic practice.
An example is the Configuration Management process area and GP 2.6, “Place selected work products of the process under appropriate levels of control.” To implement the generic practice for one or more process areas, you might choose to implement the Configuration Management process area, all or in part, to implement the generic practice.
Another example is the Organizational Process Definition process area and GP 3.1, “Establish and maintain the description of a defined process.” To implement this generic practice for one or more process areas, you should first implement the Organizational Process Definition process area, all or in part, to establish the organizational process assets that are needed to implement the generic practice.
Table 7.2 describes (1) the process areas that support the implementation of generic practices and (2) the recursive relationships between generic practices and their closely related process areas. Both types of relationships are important to remember during process improvement to take advantage of the natural synergies that exist between the generic practices and their related process areas.
Table 7.2 Generic Practice and Process Area Relationships
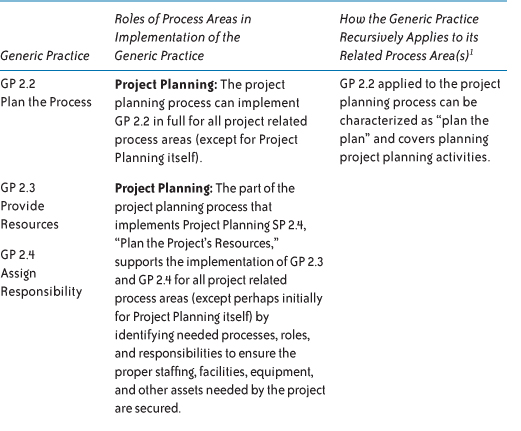



Given the dependencies that generic practices have on these process areas, and given the more holistic view that many of these process areas provide, these process areas are often implemented early, in whole or in part, before or concurrent with implementing the associated generic practices.
There are also a few situations where the result of applying a generic practice to a particular process area would seem to make a whole process area redundant, but, in fact, it does not. It can be natural to think that applying GP 3.1, “Establish a Defined Process,” to the Project Planning and Project Monitoring and Control process areas gives the same effect as the first specific goal of Integrated Project Management, “Use the Project’s Defined Process.”
Although it is true that there is some overlap, the application of the generic practice to these two process areas provides defined processes covering project planning and project monitoring and control activities. These defined processes do not necessarily cover support activities (e.g., configuration management), other project management processes (e.g., integrated project management), or other processes. In contrast, the project’s defined process, provided by the Integrated Project Management process area, covers all appropriate processes.


