Chapter 4
Transformations and Chemical Processes in Biomass Energy Systems
As with solar energy, biomass energy has been utilized by humans since the beginning of time for various applications, including heating and cooking. Such traditional use, through direct combustion, continues unabated today over much of the world, particularly in the developing countries. Direct burning of biomass is typically incomplete and inefficient and results in the release of particulates, greenhouse gases, and other pollutants, invariably in confined spaces causing incalculable harm to human health. Indiscriminate harvesting of biomass (firewood) also denudes the soils of nutrients and has other detrimental impacts on ecosystems, including loss of biodiversity, soil erosion, and so on. However, as mentioned in Chapter 2, Renewable Energy Sources, biomass energy has the potential to satisfy the global primary energy demand on a continual basis. Renewable biomass feedstocks include food crops, nonfood material such as corn stover, herbaceous and woody energy crops, forest product residues, algae, and municipal solid waste. The sustainable use of biomass and expanding its applications beyond direct heating require upgrading the utilization techniques through transformation of this biomass to electricity and chemical energy carriers. It should be noted that the use of food crops (corn, sugarcane, beet root, etc.) for energy and other nonfood uses is fraught with ethical considerations. On one hand, transformations of food crops are technically simpler and economically attractive. On the other hand, diversion of food crops out of the food chain has a devastating impact by lowering food security for a large fraction of an already vulnerable population. Future bioenergy and bioproduct systems must be based on nonfood biomass resources that can be converted into biopower (electricity), biofuels (primarily transportation fuels), and bioproducts (substitutes for petrochemicals).
The biomass transformation processes can be classified into two primary types: thermochemical processes and biochemical processes. Thermochemical processing includes direct combustion, pyrolysis, or gasification of biomass, whereas biochemical processing involves anaerobic digestion and fermentation. In addition to these two, some types of biomass can also be subjected to extractive transformations typically for synthesizing biodiesel.
Fundamental chemical processes and separations underlying these transformations are discussed in this chapter. It is necessary to understand the basic chemical structure of the biomass in order to understand the theoretical basis for the transformations, and the next section takes a closer look at the chemical structure of the biomass. That is followed by a discussion of the various pretreatment techniques used to condition the biomass for increasing the effectiveness of subsequent processing steps, and then the discussion of the thermochemical, biochemical, and extraction processes for bioenergy applications.
4.1 Biomass Characteristics
Biomass, as can be expected, is highly heterogeneous in nature, and its composition varies not only from plant species to species but also within the species. Further, within a multicellular organism, functional differentiation leads to different tissue types with dramatically different characteristics that also vary with the age. Additional environmental and genetic factors result in a wide variation in the biomass with respect to the number of constituents and their concentrations [1].
It is instructive to get a sense of the energy content of the biomass before looking at the chemical and physical structure of the biomass. Table 4.1 lists the energy content of various types of biomass in terms of their higher and lower heating values, the difference in the two being the enthalpy of vaporization of product water vapor [2].
Table 4.1 Energy Content of Biomass and Comparison with Other Fuels
Fuel Type |
Higher Heating Value, MJ/kg |
Lower Heating Value, MJ/kg |
|---|---|---|
Wood, dry |
21 |
19.7 |
Grass, dry |
18.5 |
17.4 |
Dairy manure, dry |
20.5 |
19.3 |
Coal, bituminous |
28 |
26 |
Natural gas |
42.5 |
38.1 |
Fuel oil |
45.9 |
43 |
Gasoline |
47.9 |
43.8 |
Ethanol |
29.8 |
26.9 |
Source: Ciolkosz, D., “How Much Heat Does BioFuel Have?” 2019, https://farm-energy.extension.org/how-much-heat-does-biofuel-have/#Lower_Heating_Value_.28LHV.29, 2019, accessed September 6, 2020.
It can be seen that the heating value of the biomass is nearly half that of other liquids and gaseous fossil fuels and three-fourths that of the coal. It should also be realized that unlike other fuels, the biomass usually contains a substantial quantity of moisture that is present along with the combustible content. The amount of water is highly variable across the different biomass types, and even for a particular biomass type, it depends upon factors such as at what point of the biomass life cycle it is harvested, how long it is stored, and so on. The moisture content has a significant impact on the efficiency of the energy conversion process: first, moisture content reduces the energy density of the biomass; second, energy is consumed in vaporizing the water to dry the biomass before it can be combusted or processed otherwise; and third, the water vapor generated from the moisture imposes an additional load on process equipment and piping. Figure 4.1 explains the detrimental effect of moisture on the energy content of the biomass.

Figure 4.1 Effect of moisture on the energy content of the biomass.
Source: Ciolkosz, D., “How Much Heat Does BioFuel Have?,” 2019, https://farm-energy.extension.org/how-much-heat-does-biofuel-have/#Lower_Heating_Value_.28LHV.29, accessed September 6, 2020.
The approximate chemical formula of biomass, or more appropriately, the combustible portion of biomass, is CH1.44O0.66, and its combustion can be represented by the reaction scheme R4.1 [3].
The heating values of various types of biomass listed in Table 4.1 correspond approximately to the enthalpy change in the above reaction.
Unfortunately, the actual chemical structure of the biomass hardly conforms to the simplified chemical formula stated earlier. The complexity of biomass can be understood by taking a look at its chemical constituents.
4.1.1 Chemical Structure of Biomass
The basic building blocks of biomass are carbohydrates, lignin, protein, lipids, and ash. In addition, most types of biomass will have some type of extractives associated with them [4]. Within these classes of compounds, there are several subclasses of constituents that have common functional groups, giving them similar characteristics and susceptibility to similar processing techniques. The relative proportions of these constituents vary greatly in the different biomass types, conferring them their unique characteristics. These constituents are discussed below.
4.1.1.1 Carbohydrates
Carbohydrates are polysaccharides, that is, polymers of monosaccharides—sugars that are essentially polyhydroxy ketones or aldehydes. The chemical formula of monosaccharides is Cn(H2O)m, for example, that of glucose is C6(H2O)6 or C6H12O6. The carbonyl functional group in glucose is an aldehyde, whereas that in fructose—another sugar containing six carbon atoms and having the same molecular formula—is a ketone. Both glucose and fructose are hexoses—sugars or monosaccharides containing six carbon atoms. The molecular formula of monosaccharides may indicate a straight chain compound; however, the existence of polar groups in the molecule results in the formation of ring structures—hemiacetals, hemiketals, furanoses (five-member rings), and pyranoses (six-member rings) [4]. Monosaccharides can also oligomerize and polymerize through the formation of glycosidic bonds between the carbon atom of one molecule and the oxygen in the hydroxyl group of the second molecule in a condensation reaction. The nature of bonding and molecules formed shows a great variety depending upon the structural complexity and stereochemistry. The resulting carbohydrates have strikingly different physical and chemical properties, even though the basic units are essentially the same.
Carbohydrates in biomass are classified into four main categories: starch, cellulose, hemicellulose, and pectins [1].
Starch: The base monomer unit of starch is α-D-glucopyranose or D-glucose, which depending upon the type of the glycosidic bonds, forms two different types of polymers—amyloses and amylopectins [5, 6]. Typically, amylose chains are much longer than the amylopectin chains, and amylopectins are highly branched structures having a higher molecular weight than amyloses [7]. The amylopectin content of starches is generally greater than the amylose content [4, 6]. Plants store starches in the tubers, bulbs, seeds, and rhizomes, that is, parts consumed as food items.
Cellulose: The molecular formula for cellulose is represented by (C6H10O5)n. The base monomer unit of cellulose is β-D-glucopyranose. The glycosidic bonds in cellulose are different from those in starch. Cellulose is a homopolymer with a highly linear structure, and the polymer chain length is two to three times that in the amyloses in the starches. The individual polymer chains are highly crosslinked through hydrogen bonds. A high level of intramolecular hydrogen bonding also exists within the individual polymer chains. These polymer chains aggregate to form microfibrils, and then fibrils, and finally cellulose fibers. The crosslinking imparts to the cellulose fiber a highly ordered crystalline structure that makes it insoluble in many solvents and resistant to chemical and biological treatments [6, 8]. Cellulose is located mostly in the plant cell walls functioning to support the plant. Cellulose accounts for 30%–50% of the dry weight of the plant. Figure 4.2 shows the chemical structures of α-D-glucopyranose and β-D-glucopyranose, the base units of starch and cellulose, respectively.
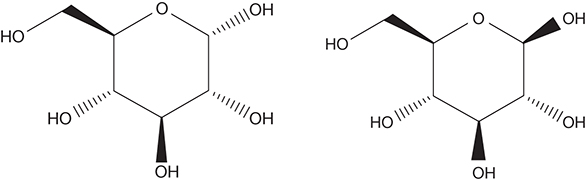
Figure 4.2 Structure of α-D-glucopyranose (left) and β-D-glucopyranose (right).
Hemicellulose: Noncellulose and nonpectin polysaccharides present in plant cell walls are termed hemicellulose that has the molecular formula of (C5H10O5)m. Unlike starch and cellulose, hemicellulose is a branched heteropolymer with base units consisting mainly of pentoses (five-carbon sugars, e.g., xylose and arabinose) with some hexoses (six-carbon sugars, e.g., glucose, galactose), higher carbon sugars (fructose, rhamnose), and sugar acids (D-galacturonic acid, D-glucuronic acid, etc.). The degree of polymerization is substantially lower in hemicellulose, giving it a more amorphous structure and lower thermal stability as compared to cellulose. Hemicellulose serves as a binding agent and may account for up to 30% of the dry weight of the biomass [4, 6, 7]. The nature of hemicellulose varies greatly in the plants, depending upon the relative amounts of the base units. Figure 4.3 shows the chemical structures of some of the monomer units of hemicellulose.
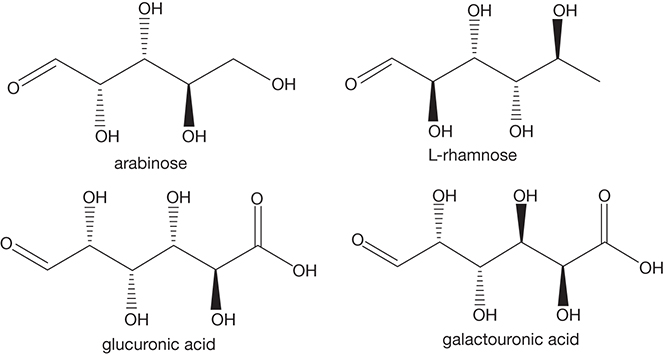
Figure 4.3 Examples of monomers constituting hemicellulose.
Pectins: The dominant monomer unit in pectins is galacturonic acid, shown in Figure 4.3. The linear homopolymer homogalacturonan dominates the composition of pectins. More complex heteropolymers consisting of rhamnogalacturonans, xylogalacturonans, and similar units make up the remainder of the pectin. These heteropolymers have crosslinked side chains of various sugars such as L-rhamnose, xylose, arabinose, and so on. [4]. Pectins are involved in the maintenance of the cell wall structure, cell adhesion, and are most abundant in fruits and young plant tissues [1].
4.1.1.2 Lignin
Lignin is a complex heteropolymer that provides mechanical strength to the plants. It is found in the secondary cell walls formed after the cell growth is complete and serves to transport water and nutrients as well as protect the plant from pathogens [9]. Lignin is composed of aromatic phenylpropane monomer units that form highly crosslinked polymers. The structures of the major base units—the monolignols p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol—are shown in Figure 4.4.
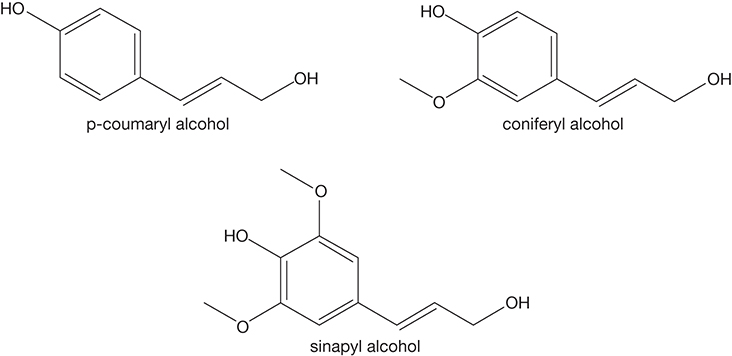
Figure 4.4 Monolignol subunits of lignin.
Phenylpropanoid radical subunits syringyl (S), guaiacyl (G), and β-hydroxyphenyl (H), derived from the three monolignols sinapyl alcohol, coniferyl alcohol, and p-coumaryl alcohol, respectively, enter into various covalent bonds, forming lignin. The core of softwoods is composed primarily of G units, while the core of hardwoods consists of G and S units. The chemical formula of lignin is (C10H11O3.5)m, indicating its significantly higher carbon content as compared to the carbohydrates.
4.1.1.3 Protein
All biomass contains proteins that are essential constituents of enzymes and photosynthetic systems. Proteins serve many functions including governing cell expansion and cell wall transport [1]. Proteins are the source of N content of the biomass, which can vary from <1 wt% to >10 wt% for food crops such as Jatropha, soybean, mustard, olive, some algae, and so on [6, 10]. It should be noted that while the N content of lignocellulosic biomass is relatively low, that of municipal waste and sludges can be quite high.
4.1.1.4 Lipids
Lipids in plants are composed of vegetable oils and fats—high-value products that are consumed by human beings and used for medicinal/cosmetic purposes, animal feed, and so on. These oils and fats consist of saturated and unsaturated fatty acids and triglycerides—esters of fatty acids with glycerol. Figure 4.5 shows the structures of linoleic acid, oleic acid, and palmitic acid, which are the dominant fatty acids in many oilseeds from soybean to sunflower and peanut [4].

Figure 4.5 Structures of selected fatty acids.
Various microalgae (unicellular eukaryotes), such as Chlorella and Scenedesmus species, and cyanobacteria (blue–green algae) such as Spriulina and Nostoc species, also contain up to 20 wt% of oil on a dry basis. These algae also have a very high protein content, as much as 70 wt% on a dry basis, and can be valuable resources for many bioproducts [11].
4.1.1.5 Ash
The inorganics present in the biomass constitute its ash content. The amount of ash remaining after complete incineration of the biomass may range from ~0.5% in hardwoods and softwoods, to as much as 20% in agricultural crops [6]. Silica is a major component of ash, with metals such as Ca, Mg, and K accounting for the rest.
4.1.1.6 Extractives
Extractives are not the structural components of the biomass, but extracellular products secreted or produced by the cells. As these chemicals are extracellular, they can be easily extracted using water or organic solvents. Extractives serve as an energy reserve, metabolic intermediates, or a defense against microbes and other pests [6]. Extractives impart distinct fragrance and color to the lignocellulosic biomass and provide resistance to decay. Extractives may amount to as much as 10% of the weight of the harvested biomass and can be removed from the biomass rather readily by extraction. Some of the extractives, such as turpentine and tall oil, are economically valuable products as well. Figure 4.6 shows some of the extractive compounds.
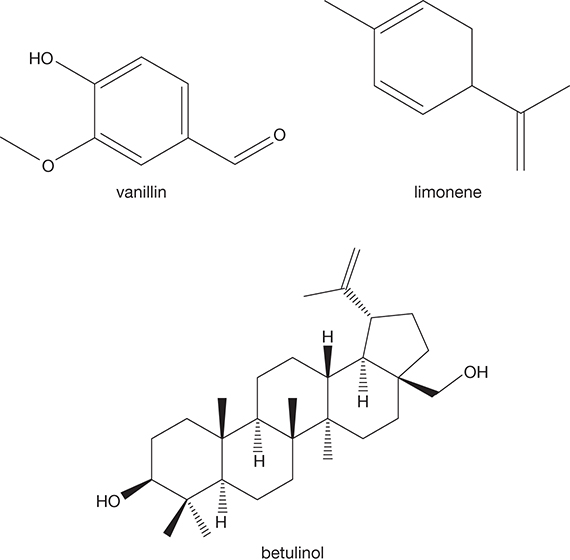
Figure 4.6 Examples of extractives in biomass.
4.1.2 Physical Structure of Biomass
Although the basic chemical structure of the building blocks of all types of biomass is identical, it is manifested in infinitely varied forms across different biomass species. These forms can be broadly classified into two classes: lignocellulosic biomass and algal biomass, based on the differences in the physical structure.
Lignocellulosic biomass is highly heterogeneous, with the nature and form of the biomass varying greatly not only across the species but also even across different parts of a harvested specimen. Overall, lignocellulosic biomass typically consists of 40%–50% cellulose, 25%–30% hemicellulose, and 15%–20% lignin, with the balance made up of pectins, ash, and other components mentioned earlier [12]. The basic structure of the cell wall in the lignocellulosic plants is shown in Figure 4.7. The primary wall of the cell is composed mostly of cellulose and also contains xyloglucans, pectin, and protein. The crystallinity of cellulose imparts it a rigid structure. It encloses a secondary wall made of cellulose, xylan, glucomannan, and lignin. Plant cells are connected to each other by a thin, pectin-rich layer called the middle lamella [13].
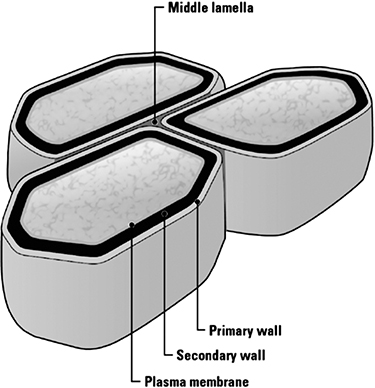
Figure 4.7 Structure of plant cell wall.
Source: Wendt, L. M., and Zhao, H., “Review on Bioenergy Storage Systems for Preserving and Improving Feedstock Value,” Frontiers in Bioengineering and Biotechnology, Vol. 8, 2020, pp. 370–384.
As mentioned earlier, cellulose polymers aggregate into microfibrils, and hemicellulose fibers surround it, forming hydrogen bonds with it. Pectin, the third type of carbohydrate, forms covalent bonds with it, with both hemicellulose and pectin bonds serving to increase the strength of the cell wall, as well as protecting the cellulose from enzymatic attack [13]. Figure 4.8 shows a simplified schematic of the interaction between cellulose, hemicellulose, and pectin in the plant cell wall.
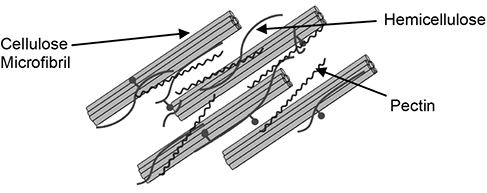
Figure 4.8 Macrostructural organization of plant cell wall constituents.
Source: Wendt, L. M., and Zhao, H., “Review on Bioenergy Storage Systems for Preserving and Improving Feedstock Value,” Frontiers in Bioengineering and Biotechnology, Vol. 8, 2020, pp. 370–384.
The microalgae structure differs from that of larger, multicellular plants and other lignocellulosic biomass. There is no differentiation of these unicellular organisms into distinct parts such as leaves, stems, and roots. Microalgae differ from other biomass compositionally as well, in lacking lignin and having significantly higher proportions of both proteins and lipids. The protein content may be as high as 70%, whereas the maximum lipid levels are close to 25% by weight. This high lipid content makes microalgae extremely attractive for biodiesel production [12].
4.1.3 Implications of Biomass Composition and Structure on Processing for Bioenergy Applications
It can be seen that the biomass structure is characterized by the complexity of its basic building blocks as well as its macrostructural construction. The energy content of biomass is lower, with the heating values of biomass being one-half to three-fourths of those of fossil hydrocarbon fuels. Further, significant quantities of unbound moisture are associated with biomass, which impose energy requirements for drying before the biomass can be converted into energy or other bioenergy products. These factors translate into higher mass requirements for the biomass than fossil fuels for the same amount of energy. The unbound moisture also creates challenges in the handling of process streams.
Apart from electricity, biomass can also be processed thermochemically and biochemically to obtain bioenergy products, bioethanol and biodiesel being the main ones. The simplicity of the molecular structures of these two compounds is in marked contrast to the complexity of the biomass structure. Bioethanol, ethanol derived from bioresources, is a simple C2 alcohol, whereas biodiesel is mostly methyl esters of fatty acids. Thermochemical and biochemical processing of biomass requires degradation of complex biopolymers that constitute the biomass, followed by chemical or enzymatic processing of resulting intermediates into these simpler molecules.
Recalcitrance is a significant challenge in these processes, as the cellulose microfibril is not easily accessible to the reagents until it is decoupled from the hemicellulose and lignin matrix. Cellulose itself is not readily amenable to degradation or depolymerization due to multitude bonding within its structure, including inter- and intramolecular hydrogen bonds. Lignin not only shields other constituents from chemical and biochemical reagents but also offers resistance in the mechanical processing steps that include shearing, size reduction, and so on.
The simplest biomass energy feedstocks are sugar crops such as sugarcane and sugar beet that yield sugar with minimal processing for further fermentative conversion to bioethanol. Biofuels synthesized from these and other food crops are termed the first generation of biofuels. However, diverting the food crops and grains for energy applications has impacts on the global food security, exacerbating the problem for those most at risk. The second generation of biomass energy systems and biofuels are based on lignocellulosic biomass from nonfood crops and plant resources [8, 14].
Recalcitrance of the lignocellulosic biomass to transformations stems from its physical and chemical structure, and an effective pretreatment is essential in the overall process of converting it to useful bioenergy and bioproducts. Pretreatment of lignocellulosic biomass decreases its crystallinity and improves accessibility to chemical feedstocks, making it more amenable to subsequent thermochemical and biochemical transformations [15].
4.2 Biomass Pretreatment
As with most other renewable energy systems, the biomass energy systems also face an intermittency challenge, although on a much different time scale and form than the other systems. The availability of the biomass feedstock depends upon the harvesting schedule. Food crops (corn, sugarcane, etc.) are harvested annually, or after each growing season if multiple harvests are possible in a year. Agricultural residues—corn stover, wheat straw, and so on—also become available at the same time. Woody trees such as poplar, willow, pine, and so on may be harvested every 2–5 years. Energy crops such as switchgrass and miscanthus are perennials and can provide biomass for the energy system on a more regular basis. Similarly, other biomass sources such as waste streams with high organic content and forest residues may be available on a continual basis.
The storage of biomass harvested for energy systems presents another set of logistical challenges: the moisture present in the biomass may lead to fermentations and other undesired microbial processes consuming the resource before it reaches the processing stage; dry biomass may pose a fire hazard; and wet storage increases transportation costs while also imposing an energy cost of vaporization of water during processing. Most of the energy crops are generally stored under dry conditions, with a few exceptions such as sugarcane or cane bagasse that have a very initial high moisture content and are processed biochemically [13].
Logistics of harvesting, storage, and ensuring a steady supply of biomass of consistent quality to the bioenergy system is a nontrivial exercise, and bioresource management upstream of the pretreatment and processing steps has a significant impact on the economics of the process. A detailed discussion of these factors is beyond the scope of this book, which is focused on the biomass transformations to biopower, biofuels, and bioproducts.
The raw biomass needs to be subjected to pretreatment before it can be fed to the transformation processes. These pretreatment steps include moisture removal, size reduction, delignification, and so on. The physical and chemical characteristics of biomass are altered so that it becomes more susceptible to the downstream transformations, in particular, increasing the availability of cellulose and hemicellulose. Separation of lignin from the other constituents also enables its recovery for additional energy or bioproduct generation [8, 12, 16].
Biomass pretreatment options can be classified into four categories: physical, chemical, physicochemical, and biological [14]. Several alternative pretreatment options are available under each of these main categories, as shown in Figure 4.9. Each of these treatment options is briefly discussed next.
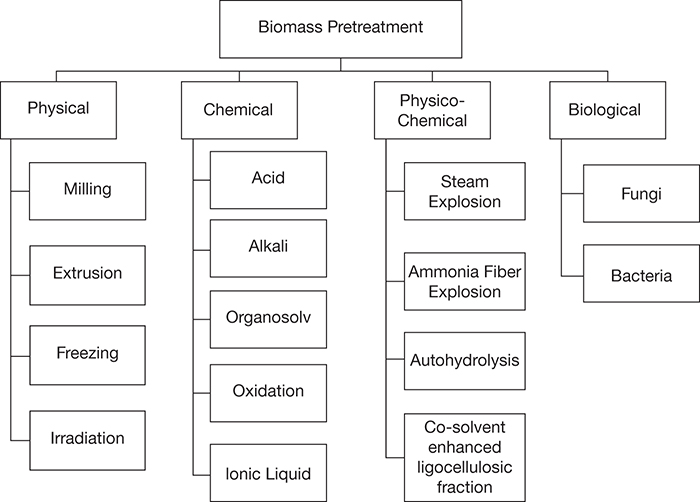
Figure 4.9 Biomass pretreatment alternatives.
4.2.1 Physical Pretreatment
The techniques used in the physical pretreatment—milling, grinding, extrusion, and others—accomplish a reduction in the size of fibers and crystallinity through physical breakdown of the material. The shearing and mixing actions in extruder screws and ball mills reduce the fiber length and the size of the particle to less than 1 mm [8]. The reduction in particle size results in an increased surface area for subsequent reactions.
Although the extrusion process operates at higher temperatures (100°C–300°C) and employs external shear, the pretreatment process based on freezing takes advantage of the volumetric expansion of water upon freezing. This volumetric expansion leads to breakdown of cell walls, making its contents, specifically cellulose and hemicellulose, more accessible to chemical and biological agents.
The disruption of cell walls, delignification, and reduction of crystallinity can also be accomplished by irradiating the biomass using microwaves, γ-rays, electron beams, or ultrasonic waves. The energy absorbed by the cells results in the cleavage of chemical bonds [14]. Irradiation techniques can be quite effective, as the energy can be focused where it is needed the most, raising temperatures and pressures only locally while the bulk of the material remains at a lower temperature and pressure.
Physical treatment techniques do not introduce any external chemical reagents and generally have a positive impact on downstream processes, increasing the rates and yields of desired products. However, these techniques are also expensive and energy intensive, possibly requiring energy input greater than the energy content of the biomass itself [8].
4.2.2 Chemical Pretreatment
The most commonly applied pretreatment techniques involve digestion of biomass using mineral acids, alkalines, organic acids and solvents, and ionic liquids (ILs). Acid pretreatment utilizes sulfuric, nitric, phosphoric, or hydrochloric acid to effect hydrolysis of hemicellulose and cellulose. The acids can be used in concentrated or diluted form. Lower processing temperatures (20°C–50°C) are needed if concentrated acids are used, while much higher temperatures (up to 200°C) are needed if dilute acids (<10%) are used [8, 16]. Corrosivity of solutions is a concern when acid pretreatment is employed, which also results in the formation of intermediates such as hydroxymethylfurfural (HMF) and acetic acid that act as inhibitors in the subsequent biochemical processing steps. Acidic solutions also impose a downstream alkali requirement for pH control.
Alkalies such as NaOH and KOH are effective in separating lignin from other constituents and breaking down the crystallinity of cellulose. Ammonia and lime treatment also have the same effect, with lime treatment being most attractive due to Ca(OH)2 being less expensive and less hazardous than other chemicals [17]. The processing can be conducted at mild (atmospheric temperatures, 25°C–65°C) or severe conditions (7–20 atm, temperatures up to 200°C). The time needed to achieve a desired level of lignin removal varies inversely with the severity of the conditions employed, and processing times range from a low of 1 hour to a high of 8 weeks.
Biomass, despite its fundamental organic nature, is not readily attacked by organic compounds to an appreciable degree at ambient conditions. However, delignification and solubilization of hemicellulose can be accomplished in the organosolv process at temperatures in the range of 100°C—250°C by a mixture of organic solvents such as methanol, ethanol, acetone, ethylene glycol with organic acids like oxalic, salicylic, and acetylsalicylic acids; and so on, in aqueous solutions [8]. This pretreatment, while effective, is also costly due to the solvent requirements. Further, additional downstream processing steps are needed for the recovery and recycling of excess solvent.
Oxidative pretreatments are also effective in delignification and separation of hemicellulose from cellulose. A wide variety of reagents are used for effecting these oxidations, including ozone, hydrogen peroxide, peracetic acid, oxygen, air, LiCl/N,N-dimethylacetamide (LiCl/DMAc), NaOH/urea, cadoxen (cadmium ethylenediamine solvent), and carboxy-methylcelluloses (CMCs). Hydrogen peroxide is an attractive oxidant due to its cost advantages over other reagents. The pretreatment is carried out at 90°C, and its efficiency increases with peroxide concentration (1%–5%) and treatment time (30–90 minutes) [16]. Weaker oxidative agents, such as hydrogen peroxide and peracetic acids, are able to achieve delignification without affecting cellulose; however, their efficiency is not very high. Ozone is a much stronger oxidant due to the formation of highly reactive hydroxyl (OH+) radicals [12]. Unfortunately, the stronger oxidizing agent also lacks selectivity and attacks cellulose and hemicellulose as well. Ozonolysis is an energy-intensive and expensive pretreatment option that also may result in wastage of biomass resources, besides presenting challenges in generation and safe handling of ozone gas.
ILs are organic salts, which are typically liquid at room temperatures, that have several attractive properties, including negligible vapor pressure, high thermal and chemical stability, nonflammability, and noncorrosivity. Most common ILs are typically alkyl substituted imidazolium ([(C3N2)Xn]+) or pyridinium ([(C5N)Xn]+) halides, though practically unlimited combinations of other cations (substituted ammonium, pyrrolidinium, etc.) and anions (nitrates, sulfates, tetrafluoroborates, etc.) are also possible. Figure 4.10 shows the structures of 1-butyl-3-methylimidazolium chloride ([BMIM][Cl]) and 1-ethyl-3-methyl imidazolium acetate ([EMIM] [AC]), two of the ILs used in the biomass pretreatment.
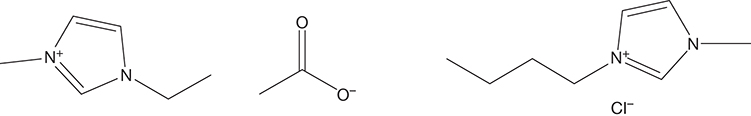
Figure 4.10 Ionic liquids [EMIM] [AC] (left) and [BMIM][Cl] (right).
The applicability of ILs in biomass pretreatment stems from their excellent solvating ability. The entire biomass, including cellulose and lignin, can be dissolved in it. Typical temperatures for dissolution range from 50°C to 100°C, and the solubility of the biomass may be as high as 75 wt% [16]. Various biomass fractions dissolved in the IL can be recovered by contacting the solution with solvents that preferentially extract/salt out particular fractions, as shown in Figure 4.11. The cellulose recovered from such process, while having the same degree of polymerization as the untreated biomass, has lower crystallinity and increased porosity, increasing its susceptibility to transformations in the subsequent processing steps [15].

Figure 4.11 Pretreatment of biomass using ionic liquid.
Source: Usmani, Z., et al., “Ionic Liquid Based Pretreatment of Lignocellulosic Biomass for Enhanced Bioconversion,” Bioresource Technology, Vol. 304, 2020, #123303 (13 pp.).
(DMF, dimethyl formamide; DMSO, dimethyl sulfoxide)
Complete dissolution of the biomass, the ability to recover its important fractions separately, and minimum chemical changes while enhancing the reactivity of fractions in subsequent processing steps are some of the attractive features of the IL pretreatment. The wide variety of combinations of the cations, anions, and the substituted alkyl groups offers a possibility to tune the IL for specific applications. However, the high cost of ILs is a significant drawback, as are properties such as high viscosity, which make the handling of ILs energy intensive. Further, the recovery of ILs for recycling is challenging, as the nonvolatility of ILs precludes the use of distillation that is the staple of chemical separations. ILs are excellent solvents not only for biomass but also for other polar compounds and other species that may be present in the system. In the absence of effective purification and recycling techniques, these contaminants accumulate in the ILs, affecting the process. The effects of ILs that may get carried to the downstream processing steps with cellulose/hemicellulose fractions, including their toxicity on microorganisms used in biochemical processing, are not known.
4.2.3 Physicochemical Pretreatment
Physical pretreatment techniques are aimed at conditioning and preparing the biomass for subsequent processing through mechanical impacts. Chemical pretreatment techniques use chemical agents to achieve a similar effect, though the characteristics of biomass after the pretreatment are different in the two techniques. Physicochemical pretreatment combines both chemical and physical effects in a single step by employing chemical agents to achieve a mechanical impact. Steam explosion involves subjecting the biomass to a high-pressure, high-temperature steam (160°C–270°C, 20–50 bar) for few minutes. Rapid release of pressure1 results in an explosive decompression, separating the fibers. Hydrolysis of hemicellulose and lignin also takes place, with resulting acids further catalyzing the hemicellulose hydrolysis [12, 14]. The method is reportedly a low-cost, rapid alternative that requires substantially lower energy than the physical treatment options.
1. The technology is also referred to as DIC—Détente Instantanee Controlee (instant controlled pressure drop).
Ammonia fiber explosion (AFEX) operates in a similar manner, with ammonia applied in 1:1 to 2:1 ratio to dry biomass at high pressure and temperatures (60°C–120°C, 30 bar). Sufficient residence time (15–30 minutes) is provided for the ammonia to percolate into the biomass. Rapid decompression, as in the case of the steam explosion technique, results in the cleavage of C–O–C bonds in lignin and linkage. The pretreatment, while effective for some types of biomass lignin–carbohydrate complexes, is costly due to the cost of ammonia, particularly if applied at higher ratios. Further, additional operations and equipment are needed to recover and recycle ammonia [8].
Autohydrolysis a hydrothermal technique, wherein acids formed by hydrolysis of biomass under hot aqueous conditions (160°C–240°C) further catalyze the hydrolysis of hemicellulose. Autohydrolysis helps reduce cellulose crystalline and lignin recalcitrance [12, 18].
Co-solvent-enhanced lignocellulosic fractionation (CELF) is an organic extraction process similar to the organosolv process, geared toward the recovery of various lignin fractions. It utilizes a tetrahydrofuran (THF) water solution containing dilute acid (H2SO4) or acid catalyst (FeCl3) to dissolve lignin from the biomass. Dissolved lignin can be recovered by boiling off the THF and drying the precipitated mass. Fractionation of this mass involves first separating the lignin fractions based on their solubility in ethanol (or other solvent), redissolving the solvent-soluble fraction in THF, and effecting step changes in the THF concentration by adding water to the solution. At each stage in the cascade wherein the THF concentration is reduced, lignin is separated into soluble and insoluble fractions [19, 20]. Insoluble fractions are recovered by filtration, and these fractions, with their characteristic compositions and properties, can find specialized applications, improving the economics of biomass utilization [19]. Other solvents such as γ-valeroacetone and tetrahydro-2-furanmethanol can also be employed for fractionation, effecting lignin fraction recovery as well as improving the digestibility of cellulose [20].
4.2.4 Biological Pretreatment
Biological pretreatment generally involves enzymatic ligninolysis and cellulose/hemicellulose hydrolysis mediated by microorganisms such as fungi and bacteria. White-rot, brown-rot, and soft-rot fungi are known to have the ability to degrade lignin and hemicellulose, and to a lesser extent, cellulose as well. White-rot fungi are the most effective lignin degraders, whereas brown-rot fungi depolymerize lignin and attack cellulose and hemicellulose [8]. Several bacteria, particularly those belonging to the species Streptomycetes, Actinomycetes, Nocardia, and Eubacteria, also possess the ability to metabolize lignin. Bacteria tend to have a higher rate of metabolism than fungi, which translates into a faster rate of transformation of lignin, making them more attractive than fungi [12]. Microorganism consortia can catalyze multiple simultaneous and sequential processes that can result in the formation of biofuels (hydrogen, methane, etc.) and bioproducts (volatile fatty acids, etc.).
Many macroorganisms, such as earthworms, insects, or other worms, also have the ability to break down lignocellulosic biomass. Larger animals, such as ruminants, have microorganisms in their digestive systems that can also decompose/transform lignocellulosic biomass. This ability of these microorganisms can be utilized for biological pretreatment.
Biological pretreatment is highly attractive, as little chemical or energy input is needed. The processing conditions are mild, and natural biota is employed in the transformations that have the lowest impact on the environment among all the pretreatment options. However, the rate of transformation is much lower than other pretreatment options, and appropriate conditions—pH, temperature, moisture, and so on—need to be maintained for optimum operation. The mixed consortia can consume cellulose and hemicellulose in the pretreatment step itself, reducing biofuel and bioproduct formation.
4.2.5 Pretreatment of Algal Biomass
The same techniques used for the pretreatment of lignocellulosic biomass harvested from land plants—physical, chemical, physicochemical, and biological—are also used for the pretreatment of aquatic algal biomass [11, 21]. However, there are certain differences in the applications of these techniques, stemming from the differences in the compositions of the two (lignocellulosic and algal) biomasses. As mentioned earlier, the proportions of lipids and proteins are higher in the algal biomass. Further, microalgae lack lignin. While macroalgae—brown, red, and green seaweeds—do contain lignin, it is typically at much lower levels than in lignocellulosic biomass. The lignin content of green algae (Chlorophyta) and red algae (Rhodophyceae) is <5%, while it can range from 10% to 20% in brown algae (Phaeophyceae) [22]. The most noticeable difference is the application of chemical pretreatment—the alkali pretreatment is rarely used for algal biomass due to its low lignin content [23].
Most other techniques, such as milling, shredding, microwave, and ultrasound in the physical pretreatment option, or acid and hydrothermal chemical pretreatment options, have broadly similar applicability for algal systems. Enzymatic deconstruction of cell wall components using mixtures of bacterial and fungal enzymes, particularly involving white-rot fungi, has also been employed for biological pretreatment of algal biomass [21, 22].
As with lignocellulosic biomass, harvesting and handling of the algal biomass until the pretreatment stage also present logistical challenges. Dewatering of algae is a major challenge, as the algal culture is very dilute (0.02%–0.05%) and needs to be concentrated to 15%–20% for reducing the processing volumes in subsequent steps [24]. Drying of algae imposes another significant energy cost on the process. Spray drying and freeze drying are superior to conventional drum drying as they tend to result in minimal structural changes to the algal biomass, although at a higher cost. Even dried algal biomass has some residual water, and the effect of residual water on subsequent processing steps needs to be understood when designing the drying system [25].
Chemical constituents of biomass that can be significant resources for energy, fuels, and products are present in highly stable forms inside the complex physical and chemical structure of the biomass. Pretreatment of biomass disrupts this complex structure and improves the accessibility of the resource for processing it into useful forms. Effective pretreatment enhances yields of sugars that are the raw materials for downstream processes, while enabling lignin recovery for offsetting energy costs, as well as conversion to valuable products. The overall impact of the pretreatment on biomass can be understood from Figure 4.12. The highly ordered structure of biomass feed is shown on the left, and the loose aggregate of disorderly components resulting from the pretreatment shown on the right. The pretreatment successfully deconstructs the lignin sheath and cleaves the bonds between cellulose and other carbohydrates in the biomass. Hydrolysis of some of the constituents of the biomass may occur yielding various chemical products.
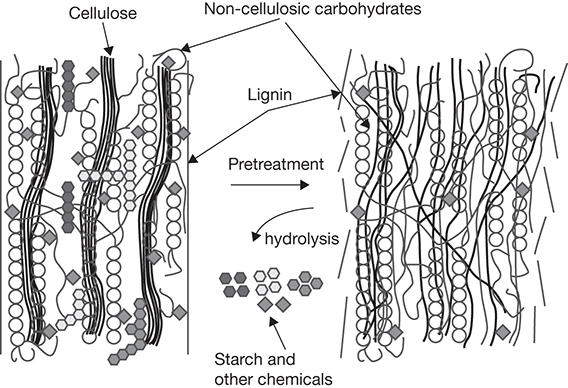
Figure 4.12 Effect of pretreatment on biomass.
Source: Weldemhret, T. G., et al., “Current Advances in Ionic Liquid-based Pre-treatment and Depolymerization of Macroalgal Biomass,” Renewable Energy, Vol. 152, 2020, pp. 283–299.
The type of pretreatment employed for the biomass depends upon the nature of biomass (chemical and physical characteristics), as well as the kind of transformation it will undergo. Physical pretreatment steps involving size reduction and drying may be sufficient for biomass that will be subjected to thermochemical transformations, while more complex pretreatments that result in improved access to cellulose and hemicellulose are needed if the subsequent transformations involve biochemical processing. The various biomass transformation techniques are discussed in the next section.
4.3 Biomass Transformations
Biomass transformations to power, fuels, and products can be classified broadly into three categories: thermochemical, biochemical, and extractive processes, as stated in Chapter 2, Renewable Energy Sources [26]. The thermochemical conversion processes can be further classified as combustion, gasification, or pyrolysis processes, whereas biochemical conversions involve either anaerobic digestion or fermentation. The categorization was shown in Figure 2.13, which is represented here again in Figure 4.13, followed by a detailed discussion of the different transformations and chemical changes involved in these transformations.
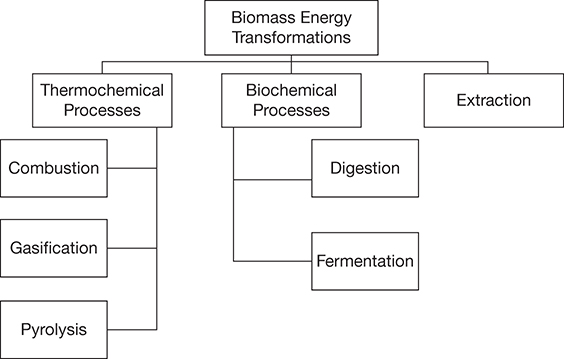
Figure 4.13 Biomass energy conversion processes.
4.3.1 Thermochemical Processing of Biomass
The subcategorization of thermochemical processes shown above is essentially based on the severity of conditions and the type of environment employed for the transformations. Combustion processes take place in an oxygen-rich environment, usually with an excess of air, at temperatures that may range from 2000°C to 3000°C. Gasification is conducted at lower temperatures (300°C–1200°C) with less than stoichiometric quantities of oxidants, while pyrolysis is conducted in an anoxic or very low oxygen environment. The temperatures employed for pyrolysis are similar to those in gasification, sometimes as low as 200°C [27]. The three processes also differ in terms of the end product of the transformations: combustion processes yield electricity and heat; gasification yields primarily syngas, which is then further processed into transportation fuels and electricity; and pyrolysis processes convert biomass primarily to liquid fuels and bioproducts [26]. Figure 4.14 provides an overview of the classification of the thermochemical processes with respect to the temperatures and the oxygen levels employed in the processes [28].
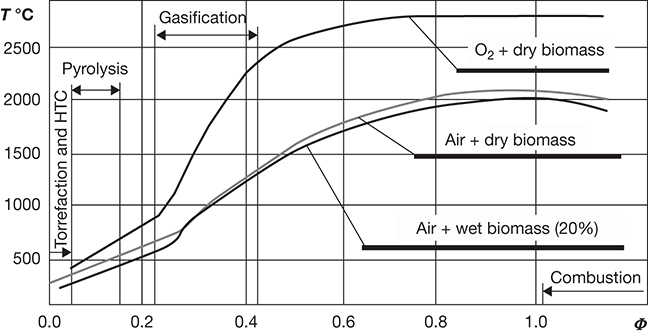
Figure 4.14 Classification of thermochemical processes with respect to temperature and oxygen/biomass ratio (Φ).
Source: Lewandowski, W. M., Michał Ryms M., and Kosakowski, W., “Thermal Biomass Conversion: A Review,” Processes, Vol. 8, 2020, #516 (45 pp.).
4.3.1.1 Combustion
Direct combustion of biomass for power generation is the simplest transformation technique and an extension of the traditional biomass burning used for heating application. Biomass, having a heating value of ~20 MJ/kg, can be combusted in dedicated furnaces similar to those in thermal power plants operating with solid fuels to generate electricity. Practically any type of biomass, from energy crops to municipal waste and agricultural and forest residues, can be transformed into power (and heat) using combustion. This technique can also process biomass with the least amount of pretreatment; however, for efficient combustion and ease of fuel handling, the biomass is generally converted into pelletized form. Typical energy efficiencies of dedicated biomass furnaces are ~60% [29].
There are several challenges in biomass in dedicated furnaces: the biomass composition can vary greatly, particularly with respect to the moisture content; depending on the composition, several pollutants such as gaseous acid anhydrides, nonmetal oxides, and halogens (chlorine and hydrogen chloride), in addition to CO and NOx, may be emitted into the atmosphere, and a significant quantity of ash may be generated. Halogen emissions may be controlled by adding amendments such as alkali and alkaline earth carbonates, or by installing postcombustion equipment. Both of these options impose additional costs on the process. The ash consisting of silica, alkaline chloride and sulfate salts, oxides, and hydroxides presents disposal problems, and its accumulation in the furnace results in lowering the combustion efficiency.
Combustion of biomass in dedicated furnaces may have a niche in microgenerator systems (10–100 kW thermal, ~10 kW electric) for small-scale combined heat and power applications, such as on small farms; for larger systems, co-firing of biomass with coal is a more promising alternative for biomass combustion [30, 31]. Co-firing involves adding biomass to the coal in the coal thermal plant and has several benefits. First, it substitutes coal, the worst fossil fuel for its greenhouse gas emissions/climate change impact, with the carbon-neutral biomass, making it extremely attractive from the environmental point of view. Second, the severity of challenges inherent in the combustion of biomass, as described earlier, is lessened. Depending upon the configuration of the co-firing system, little or no modification may be needed in the existing coal power plants.
Direct co-firing involves mixing the biomass with coal upstream of any size reduction and feed-conditioning operations on the raw coal and feeding the combined mixture into the furnace. Alternately, biomass size reduction and feed-conditioning operations may be conducted separately from those on coal, and the two streams blended together before being fed into the furnace. The third option for direct co-firing involves using dedicated biomass burners in the coal combustion furnace. The first two direct co-firing options do not require any modification of the furnace. The characteristics of biomass determine which alternative will be suitable for direct co-firing. Separating the size reduction and fuel conditioning systems protects the coal handling system from any disturbances in the biomass handling system. Similarly, using distinct burners will help mitigate disturbances in the biomass combustion and increase the stability of the system. Direct co-firing does result in common emissions, including a single solid reside/ash stream. Direct co-firing systems can usually accommodate up to 10% of biomass in the fuel without a major impact on the operation.
Parallel co-firing involves separating the combustions of coal and biomass and then blending the boiler steams for power generation. The biomass is combusted in a dedicated furnace, and the system is faced with the challenges mentioned earlier. However, benefits are realized through upgrading of the low-quality biomass steam and the use of the existing balance of a coal thermal plant. Further, the volume of the troublesome ash stream from the biomass furnace is smaller and can be handled separately from that of the coal furnace. As the biomass combustion takes place in its dedicated furnaces, the coal combustion process is shielded from any disturbances in biomass combustion.
Indirect/gasification co-firing involves subjecting the biomass to gasification (discussed in the next section) in different process equipment and then feeding the generated gas into the coal furnace for combustion. Depending on the biomass composition, a cleanup system may be needed for sequestering problematic constituents, such as chlorine and alkali metals, from the flue gas before it is fed into the coal furnace [31]. As with parallel co-firing, the ash streams are managed separately. The disturbances in biomass gasification are damped to some extent as relatively clean gas stream enters the coal furnace.
4.3.1.2 Gasification
Biomass combustion takes place in excess of air or oxygen. Gasification is an autothermal process conducted at high temperatures with substoichiometric quantities of oxygen to convert the biomass to syngas—a mixture of CO and H2. Any carbonaceous material can be converted to syngas via gasification, making it possible to apply it to any type of biomass [29, 32]. The process also generates a solid residue, which differs from the ash in the combustion process in that it contains some carbon and hydrogen and is called biochar.
The fundamental reaction in syngas formation is that of reforming carbonaceous material by steam. The reaction is endothermic, and the high temperature (~1000°C) needed for the reaction is obtained through partial oxidation of the material itself. The basic reactions involved in gasification are shown in reactions R4.2 (partial oxidation) and R4.3 (stream reforming).
The challenges in the transformation of biomass by gasification stem from low heating value, low density, and high moisture content of the biomass. Co-gasification of biomass with coal provides a possible solution for improving the efficiency of gasification, as well as providing a more consistent process by minimizing the variation in feed quality [33]. The overall reaction in the process can be represented by reaction R4.4.
The syngas formed can be combusted for power generation or synthesis of hydrocarbon fuels using the Fischer–Tropsch process, or methanol and other chemicals.
The chemical composition of biomass gives rise to many contaminants in the syngas. These contaminants include tar (heavy hydrocarbons), particulates; nitrogen, sulfur, and chlorine compounds; and alkali metals [34, 35]. The levels of these contaminants must be reduced to prevent their emissions into the environment as well as adverse impacts on downstream processes and equipment, which may include corrosion, fouling, catalyst deactivation, and so on. Similarly, any ash generated in the gasification process must be managed to prevent its environmental impact. Particulates can be captured using bag filters, cyclones, or electrostatic precipitators. Cooling of the gas can result in the condensation of tars and other volatiles. Acid gases can be scrubbed from the gas using alkaline solutions. Tars can also be subjected to catalytic and thermal cracking to convert them to lighter gases and possibly increase the yield of syngas [36].
Gasification of biomass, particularly with very high moisture content (algae, kelp, sewage, etc.), can be conducted in a supercritical water medium at temperatures ranging from 500°C to 750°C and pressures from 250 to 300 bar, with the critical point of water being 374°C and 220 bar. This process offers several advantages including obviating the need for drying pretreatment and ease of storage. The process itself is characterized by high efficiency, low tar formation, retention of impurities in the aqueous medium, and a cleaner product gas having a higher H2:CO ratio that is available at higher pressures [28]. Such hydrothermal gasification, that is, gasification in the presence of water, can also be effected under subcritical conditions. Hydrothermal gasification conducted at temperatures ranging from 250°C to 550°C and pressures below the critical pressure requires the use of catalysts—Ni and Ru being the most promising. Operating at temperatures higher than 550°C obviates the need for a catalyst [37, 38].
4.3.1.3 Pyrolysis
Pyrolysis is the thermal decomposition of materials in the absence of oxygen. As seen from Figure 4.13, pyrolysis of biomass takes place at temperatures ranging from 200°C to 800°C, in very low oxygen environments. Pyrolysis can be further classified as mild pyrolysis, slow pyrolysis, and fast pyrolysis, though in some classifications intermediate pyrolysis and gasification are also included as two additional categories [35].
Mild pyrolysis, variously referred to as low-temperature pyrolysis, thermal rectification, or torrefaction, is conducted at temperatures ranging from 200°C to 320°C. Depending upon the temperature in the process, it can be further classified as light (200°C–235°C), mild (235°C–275°C), or severe (275°C–320°C) [36]. Torrefaction results in the breakdown of the structures of lignin, hemicellulose, and cellulose, releasing volatile matter and leaving behind biochar and ash. The reaction in dry torrefaction can be represented by R4.5 [28].
Approximately 70% of the biomass is converted to biochar, with the remaining 30% getting volatilized.
Torrefaction of biomass containing large quantities of water, such as manure, sewage, and municipal waste, is conducted under wet conditions, and the process is called wet torrefaction or hydrothermal carbonization (HTC). HTC of biomass is conducted by raising the temperature of its aqueous suspension to 180°C–260°C under an autogenous pressure of 20–60 bar [39]. The processing time may range from 5 minutes to 4 hours. HTC proceeds via depolymerization of lignin, hemicellulose, and cellulose into the oligomers of the building blocks and then into derivatives of monomers, as described in Section 4.1. Finally, an aromatic structured solid that has similar carbon content as lignite is formed [39]. The solid product of HTC is termed hydrochar to distinguish it from biochar, the product of dry torrefaction. Hydrochar is generally more energy dense and has lower levels of metallic impurities and ash [36]. The components released from the biomass are contained in the aqueous medium either as dissolved organics or gas.
Biochar and hydrochar, the torrefaction products, can be used for power generation or subjected to gasification for conversion to biofuels. Partial substitution of coal by biochar/hydrochar will have a positive environmental impact due to the reduction in greenhouse gas emissions.
Slow pyrolysis is conducted at temperatures of 300°C–500°C, higher than those employed in mild pyrolysis. The end product of slow pyrolysis is also the same as mild pyrolysis, that is, biochar. In essence, slow pyrolysis is a carbonization process, as shown in R4.6.
Slow pyrolysis is conducted at lower heating rates and temperatures with a longer residence time (5–30 minutes). Fast pyrolysis employs higher temperatures (500°C–800°C), greater heating rates, and shorter residence times (0.5–5 seconds) yielding bio-oil as the main product [28, 34, 40]. The transformation is represented in reaction R4.7.
Nearly 60–75 wt% of the biomass is converted to bio-oil, with 10%–20% converted to char. The remaining fraction is converted to gases, which do not have the opportunity to react with the char due to low residence times employed in the process. Bio-oil formed in the fast pyrolysis is acidic (pH <4), and contains up to 20–25 wt% of water. The organic fraction of bio-oil is a complex mixture of varied chemical compounds, mostly oxygenates (aldehydes, phenols, ketones, and organic acids). These compounds are unstable and corrosive in nature, and the bio-oil needs to be deoxygenated and upgraded for it to be useful [41, 42]. The upgrading and deoxygenated are generally accomplished through catalytic hydrodeoxygenation (HDO), which involves the reaction of bio-oil with hydrogen, supplied externally or generated from the biomass itself through reforming. Oxygen is extracted from the bio-oil constituents and removed as water. Various catalysts are used for HDO including alumina-supported Ni–Mo, Co–Mo, noble metal catalysts (Pt, Ru, and Rh), and zeolite-supported Pt, as well as metal sulfides, nitrides, and phosphides [42, 43]. Catalytic pyrolysis is another alternative to obtain deoxygenated bio-oil products, where the catalyst is either embedded with the biomass during the pyrolysis reaction (in situ) or deoxygenates the pyrolysis vapors prior to their condensation into the bio-oil product.
Bio-oil with low oxygen content can be produced from biomass through hydrothermal liquefaction (HTL), which involves effecting deconstruction of biomass constituents at high temperature and pressure in an aqueous environment. The temperatures and pressures range from 280°C to 380°C and 70 to 300 bar, respectively. The reaction time ranges from 10 to 60 minutes, and a reducing environment may be created through provision of CO and H2. The processing may be catalyzed using homogeneous alkaline catalysts (e.g., Na/K hydroxides or carbonates) or heterogeneous transition metals (Fe, Co, Ni, etc.). The conversion and product distribution are dependent on several factors including the type of biomass, pressure, temperature, catalyst employed, processing time, and so on. Heating rate also has a significant impact on the product yield—high heating rates result in the formation of gaseous products, whereas lower heating rates lead to char formation. Moderate heating rates are indicated for the formation of liquid products [37]. The liquid products formed in HTL are characterized by higher C content and much lower water contents (5%) as compared to the fast pyrolysis bio-oil [44]. The operating pressures and temperatures for various hydrothermal processing technologies are shown in Figure 4.15.
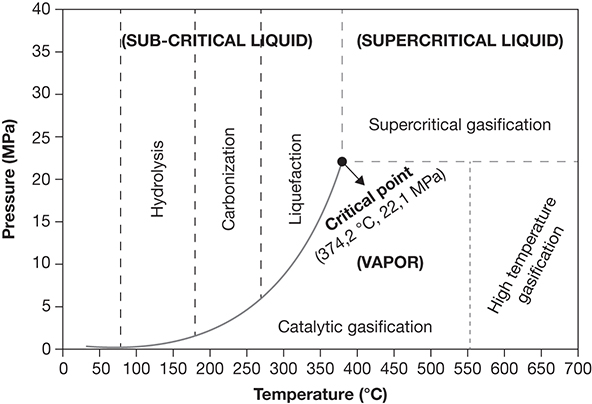
Figure 4.15 Categorization of hydrothermal processing of biomass with respect to the operating condition.
Source: Alper, K., et al., “Sustainable Energy and Fuels from Biomass: A Review Focusing on Hydrothermal Biomass Processing,” Sustainable Energy & Fuels, Vol. 4, 2020, pp. 4390–4414.
4.3.1.4 Hydrotreating
Hydrotreating is also essentially a thermochemical process; however, it differs from the other thermochemical processes described earlier, in that it is rarely applied for direct processing of the raw biomass. The feedstock for hydrotreating consists of lipids (triglycerides), which are typically obtained from virgin vegetable oils, used cooking oils, animal fat from the food industry, waste vegetable oils, and so on. Hydrotreating transforms these oils into renewable gasoline and diesel (green gasoline and diesel) or sustainable aviation fuel (SAF). Hydrotreating involves catalytic hydrogenation at high pressures and moderate temperatures using supported metal catalysts [45].
The unsaturated bonds in triglycerides are first saturated through hydrogenation in the process, and this is followed by hydrogenolysis forming fatty acids and liberating propane. The fatty acids undergo HDO yielding a saturated hydrocarbon (alkane) containing the same number of carbons as the fatty acid. Alternately, the fatty acid can also undergo hydrodecarbonylation/decarbonylation (HDC) forming an alkane containing one less carbon atom. Various reactions that constitute hydrotreating are shown in Figure 4.16 [45].
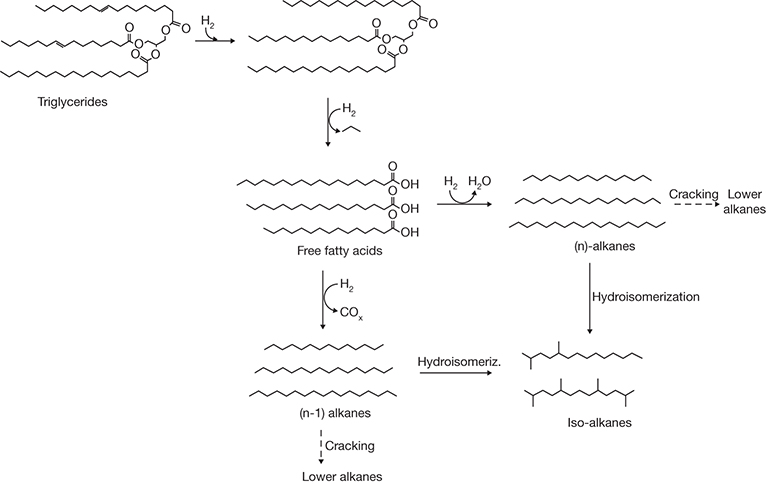
Figure 4.16 Reactions occurring in hydrotreating.
Source: Serrano-Ruiz, J. C., Ramos-Fernández, E. A., and Sepúlveda-Escribano, A., “From Biodiesel and Bioethanol to Liquid Hydrocarbon Fuels: New Hydrotreating and Advanced Microbial Technologies,” Energy & Environmental Science, Vol. 5, 2012, pp. 5638–5652.
Hydrotreating is typically conducted at temperatures ranging from 300°C to 350°C and pressures of 20 to 70 bar. The initial product of hydrotreating is typically a normal alkane, which will invariably undergo, to some extent, isomerization to iso-alkane, as well as cracking to a lower alkane. The isomerization is generally desired for improved performance as a transportation fuel, whereas cracking has the opposite effect and is not desired. Further, cracking may also cause catalyst deactivation [45]. As mentioned earlier, the catalysts used for HDO are typically supported noble metals, with Pt and Pd reported to be the most effective ones. Metal sulfides are also commonly used due to their cost advantage and superior resistance to impurities in the feedstock. However, they are also more sensitive to the presence of water and susceptible to losing sulfur, which can affect alkane product quality. It can be seen from Figure 4.16 that the hydrotreating process requires large quantities of hydrogen, with requirements ranging from 7 to 16 moles per mole of the triglyceride. Some of this hydrogen can be recovered from the biomass itself; however, an external supply is invariably needed. Non-fossil-derived hydrogen is expensive and has an adverse impact on the economics of the process, while using fossil-derived hydrogen does not help in averting greenhouse gas emissions nor make the resulting fuel truly renewable.
Thermochemical processing of biomass involves either its direct oxidation (complete, as in combustion or partial, as in gasification,) or conversion to reduced forms of carbon (biochar, bio-oil, or hydrocarbons through the syngas route) via pyrolysis for subsequent oxidation. Some fraction of biomass may also be converted to useful bioproducts, particularly from lignin or using syngas as a platform for chemicals, to improve biomass valorization; however, thermochemical transformations primarily involve conversion of biomass energy into other energy forms and carriers.
4.3.2 Biochemical Processing of Biomass
Biochemical processing of biomass differs from thermochemical processing in two primary aspects: first, the conversion is mediated by microorganisms rather than thermal energy, and second, the primary end product is an organic chemical containing a partially reduced form of carbon. In addition, biochemical processing offers a much wider platform for the synthesis of organic chemicals than thermochemical processing.
As mentioned earlier, biomass is inherently recalcitrant by nature, and natural microbial transformation (degradation) processes occur at extremely low rates. The pretreatment is a crucially important step in biochemical processing, as an effective pretreatment will deconstruct the biomass, making its individual constituents—starch, cellulose, hemicellulose, lignin, and so on—accessible and available to the microorganisms to effect further conversions, as shown in Figure 4.12. The first step in the biochemical processes is broadly characterized as hydrolysis, which depolymerizes enzymatically the constituents into oligomers and then monomers. For example, polysaccharides are broken down into oligosaccharides and then monosaccharides. Subsequently, these monosaccharides can be further processed via fermentation to alcohols, or in another pathway through acidogenesis (forming carboxylic acid) to methane. These three biochemical transformations are described below in detail.
4.3.2.1 Enzymatic Hydrolysis of Biomass
Polysaccharides in the biomass can be converted into sugar monomers by abiotic acid-catalyzed hydrolysis at temperatures ranging from 100°C to 240°C. Some fraction of the biomass does get hydrolyzed in this manner in the acid pretreatment. However, acid-catalyzed saccharification (sugar formation) may also result in the formation of compounds that can act as inhibitors in the subsequent steps, and hence enzymatic hydrolysis that is conducted at much milder conditions (50°C–60°C with pH 4.8–5.0) is preferable due to higher recovery of sugar with minimal formation of inhibitors [14].
The various enzymes that act on different constituents of the biomass are classified according to the substrate metabolized by them [6]:
Cellulases are the class of enzymes that hydrolyze cellulose. Specific enzymes within the cellulases perform specific functions.
Hemicellulases are the enzymes that affect the deconstruction of hemicellulose.
Amylases are the class of enzymes that metabolize carbohydrates that are present as starch. Human saliva contains amylases that catalyze the digestion of starch in food.
Pectinases are enzymes that depolymerize pectins.
Laccases are the ligninolytic enzymes that depolymerize lignin, converting it to phenolic compounds.
Lipases are the enzymes that catalyze the breakdown of lipids into fatty acids and glycerides.
Proteases are enzymes that catalyze proteolysis, that is, the breakdown of proteins into peptide chains and amino acids.
Starch in food crops and cellulose/hemicellulose in lignocellulose biomass dominate the carbohydrate content of the biomass and are the most important resources for obtaining monosaccharides for further conversion to biofuels, specifically bioethanol.
As mentioned earlier, starch is hydrolyzed by amylases, which are complex molecules containing hundreds of amino acids. There are several different types of amylases that attack different sites in the starch polymer, cleaving the bonds. Endoamylases or α-amylases randomly attack the polysaccharide chains, breaking the glycosidic bond and generating oligosaccharides of various lengths. Exoamylase (β-amylase), pullanases, and glycosidases are some of the other amylases that target different sites on the polysaccharide chain/oligosaccharide fragments. Finally, γ-amylase is the enzyme whose action results in the formation of glucose.
Biomass energy systems are transitioning away from the first-generation, starch-based system. The second generation of the systems utilizes lignocellulosic biomass, and the important cellulases that mediate the hydrolysis of cellulose are endoglucanase that has a random cleaving action on the glycosidic bonds resulting in the production of cellobiose and cellotriose units and exoglucanase that generates cellobiose units much the same way endo- and exo-amylases depolymerize starch. Finally, β-glucosidase metabolizes the cellobiose units to form glucose [14]. Figure 4.17 shows the formulas of cellobiose and cellotriose units.
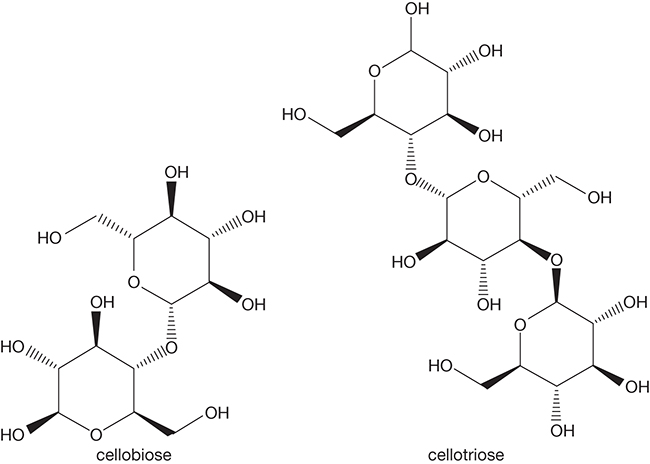
Figure 4.17 Cellobiose and cellotriose structures.
While cellulose is a hexose (six-carbon sugar)-based polysaccharide, hemicellulose, the second most abundant carbohydrate in the lignocellulosic biomass, is a pentose (five-carbon sugar)-based polysaccharide. Similar to starch and cellulose hydrolyzing enzymes, hemicellulases consist of several different enzymes that attack specific sites. Xylanases randomly attack the xylan backbone of the hemicellulose, generating xylooligosaccharide chains of varying lengths. The xylooligosaccharide metabolism is mediated by β-D-xylosidases resulting in the formation of pentoses, primarily xylose [6]. Xylose has a limited utility for microbial conversion to ethanol, as it is not readily fermented to ethanol by the common yeast that ferments glucose. Many bacteria and some fungi do have the ability to convert the xylose to ethanol and other chemicals; however, activity of these microorganisms is inevitably suppressed in the presence of glucose [46]. Xylose, and by extension hemicellulose, is more valuable as a raw material for producing furfural, which can function as a commodity chemical for a variety of other chemical products. Furfural can be converted to other useful compounds. Furfuryl alcohol, methyltetrahydrofuran, furoic acid, tetrahydrofurfuryl alcohol, piperidine furoic esters, and tetrahydrofuroic acid are just some of the chemicals that can be produced from furfural [47].
Furfural is obtained from xylose by dehydration mediated by a homogeneous mineral (hydrochloric, sulfuric) or heterogeneous acid (sulfonic acid) catalysts. Furfural can also be obtained from hexoses, the synthesis route consisting of glucose isomerization to fructose, which upon acid-catalyzed triple dehydration yields 5-HMF. Decarboxylaion of HMF results in the formation of furfural. HMF itself is an important intermediate product and building block for secondary chemical products, including C9–C15 alkanes, γ-valerolactone (a green fuel), 2,5-furandicarboxylic acid, levulinic acid, formic acid, and so on. Obviously, many of these products can be utilized as fuels; however, they likely are more useful for conversion to value-added products including polyesters, pharmaceuticals, and many others. The structures of xylose, furfural, and HMF are shown in Figure 4.18.
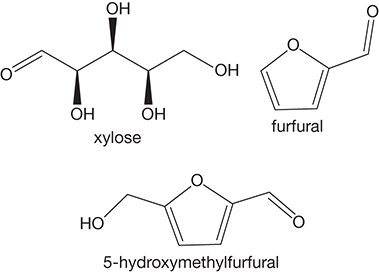
Figure 4.18 Xylose, furfural, and HMF structures.
The optimum conditions for enzymatic processes depend not only upon the type of enzyme but also the microorganism expressing the enzyme. As can be seen from the above discussion, a large number of enzymes act in an interconnected sequence of reactions to bring about the conversion of polysaccharides into monomeric sugars. The optimum pH and temperature for various microbes and enzymes may range from 4 to 8, and 40°C to 70°C, respectively [48]. Typically, the process is conducted at around pH 5 in the temperature range 45°C–50°C to achieve an optimum balance. Separation of various components may allow a multistep process that can take advantage of different optimum conditions for different microorganisms. For example, hemicellulose-degrading thermophilic bacteria Caldanaerobius polysaccharolyticus and Caldicellulosiruptor bescii have their optimum temperatures at 70°C and 80°C, respectively [49]. Separating hemicellulose from the pretreated biomass will permit its hydrolysis to be conducted in a separate step using these bacteria that have faster kinetics at elevated temperatures.
Removal of lignin or delignification of biomass is essential for effective hydrolysis of the polysaccharides, as lignin inhibits the activity of many enzymes that act on cellulose and hemicellulose [6]. Laccases are multicopper oxidases that can oxidize the lignin fragments, converting them to phenolic compounds [50]. Conversion of lignin to these compounds that can then be processed into valuable chemicals is important for improving the economics of the process. However, this is best accomplished by separating lignin and its derivative compounds before subjecting the pretreated biomass to hydrolysis, as many of the compounds, as well as laccases, are inhibitory to enzymes affecting the hydrolysis of polysaccharides.
The lipid content of lignocellulosic biomass is quite low, and so lipase’s action is not generally of consequence in the processing of this biomass. However, lipase’s action is important for biomass that has high lipid content, such as algal biomass or biomass from waste streams from animal processing facilities or food industries. This biomass can be a source of biodiesel, as discussed in a later section.
4.3.2.2 Fermentation of Sugars
Fermentation of sugar is one of the oldest biochemical processes known to humankind and continues to be practiced on a micro- to megascale all over the world, albeit for producing ethanol for human consumption. Bioethanol for energy applications is also produced through the same basic process using the same principles. The chemical reaction involved can be represented by R4.8.
As can be seen by the equation, a fraction of glucose (an acetal, i.e., a carbonyl compound) is reduced to an alcohol, while the other fraction is oxidized to carbon dioxide. The fermentation takes place at ~30°C–35°C and is mediated by baker’s yeast—Saccharomyces cerevisiae. S. cerevisiae is the microorganism of choice, though there are other microorganisms that are capable of fermenting sugar, because of its fast growth and high ethanol yield, as well as robustness in response to environmental stress factors, such as ethanol, low pH, and low oxygen [51].
The hydrolysis and fermentation steps can be spatially separated, or more commonly conducted in the same process vessel simultaneously, the technology commonly being referred to as simultaneous saccharification and fermentation (SSF). Saccharification product inhibition in fermentation is the main drawback of the configuration where the two steps are spatially separated. Further, it has increased capital and operating costs due to the use of two processing units. SSF has a low investment cost, and as glucose produced is consumed immediately by the fermenters, product inhibition is averted, leading to higher ethanol yields. However, optimal temperatures for the two steps are different—hydrolysis requiring temperatures of 45°C–50°C, while fermentation is best carried out at 30°C–35°C [14].
Distillation is the preferred mechanism for the recovery of the product ethanol from the reaction mass. However, the ethanol–water mixture forms an azeotrope at 95.6 wt% of ethanol, and extractive distillation techniques using dissolved salts are needed to break the azeotrope and obtain pure, anhydrous ethanol for use as fuel.
4.3.2.3 Anaerobic Digestion
Anaerobic digestion of biomass results in the formation of biogas that consists primarily of methane (approximately two-thirds of the biogas) and carbon dioxide. Other gases such as hydrogen sulfide, ammonia, and hydrogen may also be present. The exact composition of the biogas depends on the biomass fed to the process and the processing conditions [52]. Practically, any type of biomass, including manure, sewage, and food waste, as well as lignocellulosic and algal biomasses, can be subjected to anaerobic digestion for conversion to biogas. The biogas can be used for electricity generation and heat applications. The various microbial processes occurring in the anaerobic digestion of the solution resulting from the hydrolysis of the pretreated biomass are shown in Figure 4.19 [53].
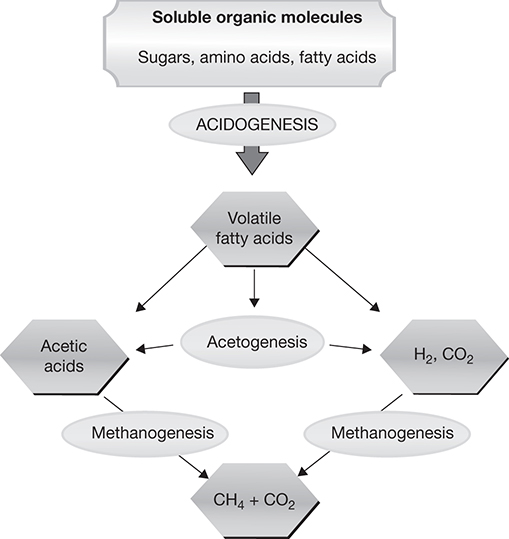
Figure 4.19 Processes occurring in anaerobic digestion.
Source: Koniuszewska, I., et al., “Intensification of Biogas Production Using Various Technologies: A Review,” International Journal of Energy Research, Vol. 44, 2020, pp. 6240–6258.
The first stage of the process involves the conversion of sugars and other organics into low-carbon carboxylic acids such as formic, acetic, propionate, butyric, lactic acid, and so on. This conversion, which leads to the formation of acids, is called acidogenesis and is mediated by various species of bacteria including Streptococcus, Lactobacillus, Bacillus, Escherichia coli, and Salmonella.
The acetogenesis stage of the process involves the conversion of these acids into acetic acid and acetates. Syntrophomonas and Syntrophobacter affect the conversion of compounds to acetate, whereas Methanobacterium may first convert the larger compounds to propionic acid before other bacteria process it further to acetic acid.
The final stage of the process is mediated by methanogens—bacteria belonging to the genera Methanosarcina, Methanothrix, Methanobacterium, Methanococcus, and many others—that convert the acetic acid to methane.
The overall reaction can be represented by reaction R4.9.
Part of the carbon present in glucose is oxidized to CO2, while the other part is reduced to CH4. Figure 4.16 shows the anaerobic digestion process starting with hydrolyzed biomass. In a general case, when the biomass fed to the process may not be preprocessed, hydrolysis described in Section 4.3.2.1 is the first step of the anaerobic digestion process. The intermediate acetogenesis reactions are shown by reactions R4.10a and R4.10b, while the two methanogenic reactions are shown by R4.11a and R4.11b [27].
Approximately 70% of the methane produced is due to the reaction R4.11a, which transforms acetic acid; the bacteria effecting the conversion are called acetotrophs. Reaction R4.11b accounts for the balance of methane generation and is mediated by hydrogenotrophs.
It can be seen that anaerobic digestion is a complicated process that depends on synergistic actions of a large microbial consortia that perform different functions. These microorganisms or consortia that perform a specific step have their own optimum operating conditions of temperature, pH, substrate concentration, and a delicate balance is achieved among them for biogas generation at reasonable efficiency and costs. The process, conducted in batch mode, starts under acidic conditions, and the pH increases slowly as the process enters the latter stages. The optimum pH for methanogens is 6.8–7.2. Depending on the temperature of conversion, the digestion process is termed either mesophilic (operating at temperatures <45°C, usually at 30°C–38°C) or thermophilic (50°C–60°C, up to 70°C). Thermophilic processes, though not necessarily yielding higher yields of methane, are much faster, offering the benefits of higher throughput/lower equipment volume [53].
Algal biomass, particularly macroalgal biomass that has high carbohydrate content, is quite attractive for biogas generation. However, effective pretreatment and improved cost-effective saccharification technologies are needed before it can be economically viable in a stand-alone biogas generation system. Current algal biogas systems are under development and exist as coupled systems with wastewater treatment plants [22, 54].
At a fundamental level, the fermentation to bioethanol and anaerobic digestion to biogas, discussed in Sections 4.2.3.2 and 4.2.3.3, respectively, are both based on glucose as the starting material. The origin of this glucose is in the cellulose in the lignocellulosic biomass, which contains hemicellulose and lignin as well. Hydrolysis of the biomass deconstructs both of these components; however, the presence of degradation products of these two is undesirable in fermentation and anaerobic digestion. Co-fermentation processes containing enzymes that can ferment pentoses as well as hexoses can possibly accommodate the presence of hemicellulose in bioethanol formation; however, such processes are still under development and it is desirable to separate cellulose from the other two components of lignocellulosic biomass before subjecting it to subsequent conversion processes for energy fuels.
It is possible to separate the fractions through appropriate pretreatment technologies. Hydrothermal processing or alkaline extraction methods are effective in solubilizing hemicellulose and removing it from the biomass [55, 56]. The organosolv process, described earlier, can remove lignin and recover it in distinct fractions, improving the economics of the process. Lignin can also be separated from cellulose by catalytic hydrogenolysis using Raney Ni catalyst to depolymerize it into phenolic compounds [57]. Cellulose, stripped off the two (hemicellulose and lignin), can then be processed effectively as described earlier.
4.3.3 Biomass Lipid Extraction and Conversion to Biodiesel
The first and second generations of biomass energy systems are aimed at utilizing carbohydrate and lignin fractions of the biomass that typically has a very low lipid content. However, there are several biomass streams that are rich in lipid content. These lipids can be recovered and converted to biodiesel, which is a chemically alkyl ester of fatty acids. The processing of this lipid-rich biomass differs from thermochemical and biochemical processes described in Sections 4.3.1 and 4.3.2, respectively, and involves the extraction of lipids followed by the esterification/transesterification using an alcohol, mostly methanol. Figure 4.20 shows a simplified schematic of the biodiesel synthesis process [58].
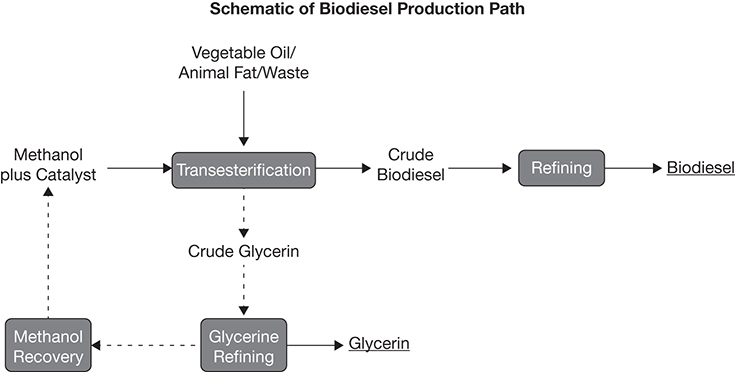
Figure 4.20 Biodiesel process flow sheet.
Source: U.S. Department of Energy, Energy Efficiency and Renewable Energy (EERE), “Alternative Fuels Data Center,” https://afdc.energy.gov/fuels/biodiesel_production.html.
The vegetable oil needed for biodiesel was extracted from edible sources through mechanical extraction for the first generation of biodiesels. As with the transition away from starch to lignocellulosic biomass, the second generation of biodiesel technologies seek to use nonedible vegetable oil, waste oil, and animal fat sources for biodiesel synthesis. The third generation of biodiesel and overall biomass energy will be based on algal biomass [59].
Some of the second-generation sources include Jatropha curcas, papaya seed oil, rubber seed, desert date, fish oil, jojoba, neem, apricot seed, waste coffee grounds, chicken fat, pork lard, beef tallow, and waste cooking oil. The simplest technique to extract oil from these sources involves mechanical pressing. Typically, a screw press is used to apply pressure and squeeze the oil out of the source in a continuous process. Alternatively, solvent extraction, typically using hexane as the solvent, is also employed for extracting oil from the source of oily biomass [59, 60]. Enzymatic extraction, though possible, is generally not employed due to cost considerations.
Extraction of oil from microalgae that contains significant lipid content is challenging due to the moisture content. For mechanical pressing extraction, the algae need to be dry, which imposes significant energy costs on the process. Wet extraction is an attractive alternative for averting the costs associated with the drying of algae [61].
The oils, essentially the triglycerides of fatty acids, are subjected to transesterification or alcoholysis using methanol or ethanol to form fatty acid alkyl esters. Methanol is the preferred alcohol, as the transesterification (methanolysis) is faster and the reaction temperatures are lower (~60°C). The reaction is conducted using either a homogeneous catalyst such as NaOH, KOH, or sulfuric acid, or a heterogeneous catalyst such as sulfonic acid- or alkaline earth-based catalysts or ion exchange resins [62, 63]. The reaction is typically conducted under mild to moderate temperature (60°C).
Methanol required for the transesterification can be obtained from biomass in one of two ways: (1) biomass gasification yields syngas, which is a mixture of CO and H2. Methanol synthesis from syngas is a well-known process involving an exothermic reaction catalyzed by alumina-supported transition metal oxide catalysts at high pressure (~300 bar) and at temperature of 200°C–400°C. (2) Methanotrophic (methane-utilizing) bacteria convert methane to carbon dioxide through a sequence of enzymatic reactions. The first step in this conversion is catalyzation by the methane monooxygenase enzyme and involves conversion of methane to methanol [52].
4.3.4 Generation 4 of Biomass Energy Systems—Emerging Technologies
The gradual evolution of biomass energy systems has seen the biomass feedstock shift from food crops (sugarcane, corn, etc.) in the first-generation systems through lignocellulosic biomass of the second generation and algal biomass of the third generation. It should be noted that only the first-generation systems are established and operating commercially. The second- and third-generation systems are still developing and gradually entering the market.
The logical progression of the shift in the biomass feedstock will culminate into biomass energy systems of the future, which rely upon redesigning and genetically engineering natural biological systems to harness solar energy solely for the purpose of producing biofuels. The fourth-generation biomass energy systems are aimed at manipulating algae, cyanobacteria, and other microorganisms for the production of photobiological solar fuels and electro-biofuels by combining photovoltaics with microbial fuel production [64].
These systems seek to produce primarily hydrogen as opposed to the ethanol and hydrocarbon fuels of prior generations. Biological hydrogen production can be accomplished through several different routes [65]:
Direct photobiolysis involves splitting of water by photoautotrophic biomass, primarily algae. Oxygen and hydrogen evolutions are affected by two different photosystems (complex biomolecules performing electron transfers) harnessing the solar energy. These photosystems are spatially separated, preventing a recombination of the two gases.
Indirect photobiolysis overcomes some of the energy barriers in direct photolysis by first producing glucose through photosynthesis, followed by its fermentation to hydrogen. This process is mediated by some cyanobacteria and has the advantage of needing sunlight only for the photosynthesis step, not the fermentation step.
Photofermentation of carbohydrates (glucose) to hydrogen is affected by purple and green sulfur bacteria belonging to the genera Rhodobacter, Chromatium, Chlorobium, and so on in the presence of sunlight.
Dark fermentations involve the fermentation of glucose to hydrogen, rather than methane. As described in Section 4.3.2.3 and shown in Figure 4.19, the process of anaerobic digestion involves several steps, the final one being methanogenesis. Inhibition of methanogenesis through techniques such as heat shock (boiling or freezing), sudden pH change, sonication, mechanical shock, or chemical agents will interrupt the process at a stage where acids and hydrogen are the products. The process does not require sunlight, and hence is termed dark fermentation to contrast with photofermentation. The two important dark fermentation reactions yielding hydrogen are shown in R4.12 and R4.13 [16].
The first reaction involves complete fermentation to hydrogen, indicating that the maximum possible hydrogen yield is 12 molecules per molecule of glucose. The practical yield is four molecules of hydrogen per molecule of glucose as indicated by the R4.13. Examination of the Gibbs energy change (ΔG°) for the two reactions reveals that the acetate formation with the evolution of four hydrogen molecules is far more favorable, with a ΔG° of −215 kJ/mol, as compared to −26 kJ/mol for complete oxidation of carbon in glucose to carbon dioxide with the evolution of 12 hydrogen molecules per molecule of glucose [66]. However, both these values are substantially more positive than the Gibbs energy change for the anaerobic digestion of glucose to methane, which has a ΔG° of approximately −420 kJ/mol. Methane formation is thermodynamically favorable, particularly since increasing partial pressure of hydrogen formed through the fermentation actually makes the reactions more unfavorable by increasing the Gibbs energy change, that is, making it more positive.
Of course, the biomass in generation 4 of biomass energy systems can be subjected to thermochemical processing for conversion to electricity and chemicals as described earlier. Evolving biomass energy systems present a fertile area for research and development, with continual progress advancing the role of renewable energy in energy systems.
4.4 Summary
Biomass is structurally and compositionally a highly complex material that can be a valuable resource for producing electricity (biopower), fuels (biofuels), and value-added chemicals (bioproducts). Several pretreatment alternatives—physical, chemical, physicochemical, and biological—are available to alter the physical and chemical properties of biomass, reducing its recalcitrance for the subsequent transformations. Biomass transformation to biopower can take place via direct combustion or through gasification and pyrolysis, transforming it to gaseous (syngas), liquid (bio-oil), and solid (biochar/hydrochar) biofuels. Various chemical bioproducts can also be synthesized from syngas and bio-oils, as can hydrocarbon transportation fuels through well-known technologies such as Fischer–Tropsch synthesis. Instead of thermochemical transformations, biomass can also be subjected to biochemical transformations to form various chemicals, primarily ethanol (bioethanol) and methane (biogas) that can be used for transportation, heating applications, and power generation. These transformations target carbohydrates present in the biomass, specifically starch or cellulose. The complex transformations of these polysaccharides proceed through hydrolysis to sugars, followed by fermentation to ethanol or methane. Effective pretreatment of biomass is critical for making the starch and cellulosic fractions accessible and available for microbial action. The transformations of lipid-rich biomass differ from those that target the carbohydrate fraction of the biomass and involve transesterification/alcoholysis for producing biodiesel, fatty acid alkyl esters.
The first generation of biomass energy systems are based on conversions of sugars and starch sourced from food crops. The diversion of food crops for energy applications can have severe consequences on the food security of populations, and the second-generation systems seek to harness lignocellulosic biomass for energy and biofuels, while algal biomass is the resource for the third generation of biomass energy systems. Algal biomass is particularly attractive for biodiesel generation due to the high lipid content of many microalgae. Finally, the future biomass energy systems—the fourth-generation systems—are envisioned to be based on biomass engineered specifically for energy applications, potentially converting solar energy directly to hydrogen through biochemical processes mediated by specialty microorganisms. At the current time, only the first-generation systems are operating on a large scale, with the second- and third-generation systems starting to gain importance. All three of the later generations of biomass energy systems provide fertile areas for research and development in energy systems.
References
1. Hames, B. R., “Biomass Compositional Analysis for Energy Applications,” Biofuels: Methods and Protocols, edited by J. R. Mielenz, Chapter 11, Humana Press, New York, 2009.
2. Ciolkosz, D., “How Much Heat Does BioFuel Have?,” 2019, https://farm-energy.extension.org/how-much-heat-does-biofuel-have/#Lower_Heating_Value_.28LHV.29, accessed September 6, 2020.
3. Ciolkosz, D., “Introduction to Biomass Combustion,” 2019, https://farm-energy.extension.org/how-much-heat-does-biofuel-have/#Lower_Heating_Value_.28LHV.29, accessed September 6, 2020.
4. Keshwani, D. R., “Biomass Chemistry,” Biomass to Renewable Energy Processes, edited by J. Cheng, Second Edition, Chapter 2, CRC Press, Boca Raton, Florida, 2018.
5. Damager, I., et al., “First Principles Insight into the r-Glucan Structures of Starch: Their Synthesis, Conformation, and Hydration,” Chemical Reviews, Vol. 110, 2010, pp. 2049–2080.
6. Kumar, B., and Verma, P., “Enzyme Mediated Multi-Product Process: A Concept of Bio-based Refinery,” Industrial Crops & Products, Vol. 154, 2020, #1112607 (26 pages).
7. Imre, B., et al., “Reactive Compatibilization of Plant Polysaccharides and Biobased Polymers: Review on Current Strategies, Expectations and Reality,” Carbohydrate Polymers, Vol. 209, 2019, pp. 20–37.
8. Cheah, W. Y., et al., “Pretreatment Methods for Lignocellulosic Biofuels Production: Current Advances, Challenges and Future Prospects,” Biofuel Research Journal, Vol. 25, 2020, pp. 1115–1127.
9. Rawal, T. B., et al., “The Relation Between Lignin Sequence and Its 3D Structure,” Biochimica et Biophysica Acta—General Subjects, Vol. 1864, 2020, #129547 (7 pages).
10. Leng, L., et al., “A Review on Pyrolysis of Protein-rich Biomass: Nitrogen Transformation,” Bioresource Technology, Vol. 315, 2020, #123801 (16 pages).
11. Bhatia, L., et al., “Third-generation Biorefineries: A Sustainable Platform for Food, Clean Energy, and Nutraceuticals Production,” Biomass Conversion and Biorefinery, 2020, https://doi.org/10.1007/s13399-020-00843-6.
12. Sankaran, R., et al., “Recent Advances in the Pretreatment of Microalgal and Lignocellulosic Biomass: A Comprehensive Review,” Bioresource Technology, Vol. 298, 2020, #122476 (13 pages).
13. Wendt, L. M., and Zhao, H., “Review on Bioenergy Storage Systems for Preserving and Improving Feedstock Value,” Frontiers in Bioengineering and Biotechnology, Vol. 8, 2020, pp. 370–384.
14. Sharma, B., Larroche, C., and Dussap, C.-G., “Comprehensive Assessment of 2G Bioethanol Production,” Bioresource Technology, Vol. 313, 2020, #123630 (9 pages).
15. Usmani, Z., et al., “Ionic Liquid Based Pretreatment of Lignocellulosic Biomass for Enhanced Bioconversion,” Bioresource Technology, Vol. 304, 2020, #123303 (13 pages).
16. Solowski, G., Konkol, I., and Cenian, A., “Production of Hydrogen and Methane from Lignocellulose Waste by Fermentation. A Review of Chemical Pretreatment for Enhancing the Efficiency of the Digestion Process,” Journal of Cleaner Production, Vol. 267, 2020, #121721 (21 pages).
17. Sierra, R., Granda, C. B., and Holtzapple, M. T., “Lime Pretreatment,” Biofuels: Methods and Protocols, edited by J. R. Mielenz, Chapter 9, Humana Press, New York, 2009.
18. Sarip, H., et al., “A Review of the Thermal Pretreatment of Lignocellulosic Biomass towards Glucose Production: Autohydrolysis with DIC Technology,” BioResources, Vol. 11, No. 4, 2016, pp. 10625–10653.
19. Meng, X., et al., “Characterization of Fractional Cuts of Co-solvent Enhanced Lignocellulosic Fractionation Lignin Isolated by Sequential Precipitation,” Bioresource Technology, Vol. 272, 2019, pp. 202–208.
20. Xy, Y.-H., et al., “Tetrahydro-2-Furanmethanol Pretreatment of Eucalyptus to Enhance Cellulose Enzymatic Hydrolysis and to Produce High-Quality Lignin,” Bioresource Technology, Vol. 280, 2019, pp. 489–492.
21. Chaudhary, P., et al., “A Review of Biochemical and Thermochemical Energy Conversion Routes of Wastewater Grown Algal Biomass,” Science of the Total Environment, Vol. 726, 2020, #137961 (24 pages).
22. Thompson, T. M., Young, B. R., and Baroutian, S., “Advances in the Pretreatment of Brown Macroalgae for Biogas Production,” Fuel Processing Technology, Vol. 195, 2019, #106151 (12 pages).
23. Weldemhret, T. G., et al., “Current Advances in Ionic Liquid-Based Pre-Treatment and Depolymerization of Macroalgal Biomass,” Renewable Energy, Vol. 152, 2020, pp. 283–299.
24. Patil, P. D., et al., “Comparison of Direct Transesterification of Algal Biomass under Supercritical Methanol and Microwave Irradiation Conditions,” Fuel, Vol. 97, 2012, pp. 822–831.
25. de Carvalho, J. C., et al., “Microalgal Biomass Pretreatment for Integrated Processing into Biofuels, Food, and Feed,” Bioresource Technology, Vol. 300, 2020, #122719 (15 pages).
26. Turkenberg, W. C., “Renewable Energy Technologies,” World Energy Assessment: Energy and the Challenge of Sustainability, Chapter 7, pp. 219–272, United Nations Development Program, New York, New York, 2000.
27. Capareda, S. C., Introduction to Biomass Energy Conversions, Chapter 2, CRC Press, Boca Raton, Florida, 2013.
28. Lewandowski, W. M., Michał Ryms M., and Kosakowski, W., “Thermal Biomass Conversion: A Review,” Processes, Vol. 8, 2020, #516 (45 pages).
29. Greinert, A., et al., “The Use of Plant Biomass Pellets for Energy Production by Combustion in Dedicated Furnaces,” Energies, Vol. 13, 2020, #463 (17 pages).
30. Sornek, K., “Prototypical Biomass-Fired Micro-Cogeneration Systems—Energy and Ecological Analysis,” Energies, Vol. 13, 2020, #3903 (16 pages).
31. Karampinis, E., et al., “Co-firing of Biomass with Coal in Thermal Power Plants: Technology Schemes, Impacts, and Future Perspectives,” WIREs Energy and Environment, Vol. 3, 2014, pp. 384–399.
32. Shahbuddin, M., et al., “A Review on the Production of Renewable Aviation Fuels from the Gasification of Biomass and Residual Wastes,” Bioresource Technology, Vol. 312, 2020, #123596 (15 pages).
33. Kamble, A. D., et al., “Co-gasification of Coal and Biomass an Emerging Clean Energy Technology: Status and Prospects of Development in Indian Context,” International Journal of Mining Science and Technology, Vol. 29, 2019, pp. 171–186.
34. Jameel, H., and Keshawani, D., “Thermochemical Conversion of Biomass to Power and Fuels,” Biomass to Renewable Energy Processes, edited by J. Cheng, Chapter 10, CRC Press, Boca Raton, Florida, 2018.
35. Neves, R. C., et al., “A Vision on Biomass-to-Liquids (BTL) Thermochemical Routes in Integrated Sugarcane Biorefineries for Biojet Fuel Production,” Renewable and Sustainable Energy Reviews, Vol. 119, 2020, #109607 (16 pages).
36. Olugbade, T. O., and Ojo, O. T., “Biomass Torrefaction for the Production of High-Grade Solid Biofuels: A Review,” BioEnergy Research, Vol. 13, 2020, pp. 999–1015, https://doi.org/10.1007/s12155-020-10138-3.
37. Alper, K., et al., “Sustainable Energy and Fuels from Biomass: A Review Focusing on Hydrothermal Biomass Processing,” Sustainable Energy & Fuels, Vol. 4, 2020, pp. 4390–4414.
38. Elliott, D. C., “Catalytic Hydrothermal Gasification of Biomass,” Biofuels, Bioproducts & Biorefining, Vol. 2, 2008, pp. 254–265.
39. Shen, Y., “A Review on Hydrothermal Carbonization of Biomass and Plastic Wastes to Energy Products,” Biomass and Bioenergy, Vol. 134, 2020, #105479 (15 pages).
40. Ong, H. C., et al., “A State-of-the-Art Review on Thermochemical Conversion of Biomass for Biofuel Production: A TG-FTIR Approach,” Energy Conversion and Management, Vol. 209, 2020, #112634 (21 pages).
41. Ahmad, S. F. K., Ali, U. F. M., and Isa, M., “Compilation of Liquefaction and Pyrolysis Method used for Bio-oil Production from Various Biomass: A Review,” Environmental Engineering Research, Vol. 25, 2020, pp. 18–28.
42. Sorumnu, Y., Billen, P., and Spatari, S., “A Review of Thermochemical Upgrading of Pyrolysis Bio-oil: Techno-economic Analysis, Life Cycle Assessment, and Technology Readiness,” GCB Bioenergy, Vol. 12, 2020, pp. 4–18.
43. Saidi, M., et al., “Upgrading of Lignin-Derived Bio-Oils by Catalytic Hydrodeoxygenation,” Energy & Environmental Science, Vol. 7, 2014, pp. 103–129.
44. Peterson, A. A., et al., “Thermochemical Biofuel Production in Hydrothermal Media: A Review of Sub- and Supercritical Water Technologies,” Energy & Environmental Science, Vol. 1, 2008, pp. 32–65.
45. Serrano-Ruiz, J. C., Ramos-Fernández, E. A., and Sepúlveda-Escribano, A., “From Biodiesel and Bioethanol to Liquid Hydrocarbon Fuels: New Hydrotreating and Advanced Microbial Technologies,” Energy & Environmental Science, Vol. 5, 2012, pp. 5638–5652.
46. Zhao, Z., et al., “Biochemical Routes for Uptake and Conversion of Xylose by Microorganisms,” Biotechnology for Biofuels, Vol. 13, 2020, #21 (12 pages).
47. Dulie, N. W., et al., “An Insight into the Valorization of Hemicellulose Fraction of Biomass into Furfural: Catalytic Conversion and Product Separation,” Waste and Biomass Valorization, Vol. 12, 20121, pp. 531–552, https://doi.org/10.1007/s12649-020-00946-1.
48. Cheng, J., “Biological Process for Ethanol Production,” Biomass to Renewable Energy Processes, edited by J. Cheng, Second Edition, Chapter 7, CRC Press, Boca Raton, Florida, 2018.
49. Cann, I., et al., “Thermophilic Degradation of Hemicellulose, a Critical Feedstock in the Production of Bioenergy and Other Value-Added Products,” Applied and Environmental Microbiology, Vol. 86, 2020, #e02296-19 (17 pages).
50. Granja-Travez, R. S., et al., “Functional Genomic Analysis of Bacterial Lignin Degraders: Diversity in Mechanisms of Lignin Oxidation and Metabolism,” Applied Microbiology and Biotechnology, Vol. 104, 2020, pp. 3305–3320.
51. Ruchala, J., et al., “Construction of Advanced Producers of First- and Second-Generation Ethanol in Saccharomyces cerevisiae and Selected Species of Non-conventional Yeasts (Schefersomyces stipitis, Ogataea polymorpha),” Journal of Industrial Microbiology & Biotechnology, Vol. 47, 2020, pp. 109–132.
52. Gautam, P., et al., “Bio-methanol as a Renewable Fuel from Waste Biomass: Current Trends and Future Perspective,” Fuel, Vol. 273, 2020, #117783 (12 pages).
53. Koniuszewska, I., et al., “Intensification of Biogas Production Using Various Technologies: A Review,” International Journal of Energy Research, Vol. 44, 2020, pp. 6240–6258.
54. Milledge, J. J., et al., “A Brief Review of Anaerobic Digestion of Algae for Bioenergy,” Energies, Vol. 12, 2019, #1166 (12 pages).
55. Sprock, D., et al., “Xylan Extraction from Pretreated Sugarcane Bagasse Using Alkaline and Enzymatic Approaches,” Biotechnology for Biofuels, Vol. 10, 2017, #296 (11 pages).
56. Sasaki, K., et al., “Precipitate Obtained Following Membrane Separation of Hydrothermally Pretreated Rice Straw Liquid Revealed by 2D NMR to Have High Lignin Content,” Biotechnology for Biofuels, Vol. 8, 2015, #88 (10 pages).
57. Zhai, Q., et al., “Integrated Separation Process of C5 Sugars and Phenolics from Poplar Wood Using CO2-Assisted Hydrolysis Followed by Hydrogenolysis,” ACS Sustainable Chemistry and Engineering, Vol. 7, 2019, pp. 526–536.
58. U.S. Department of Energy, Energy Efficiency and Renewable Energy (EERE), “Alternative Fuels Data Center,” https://afdc.energy.gov/fuels/biodiesel_production.html, accessed September 15, 2020.
59. Anwar, M., et al., “The Potential of Utilising Papaya Seed Oil and Stone Fruit Kernel Oil as Non-edible Feedstock for Biodiesel Production in Australia—A Review,” Energy Reports, Vol. 5, 2019, pp. 280–297.
60. Sander, A., et al., “From Coffee to Biodiesel—Deep Eutectic Solvents for Feedstock and Biodiesel Purification,” Separations, Vol. 7, 2020, #22 (18 pages).
61. Lardon, L., et al., “Life-Cycle Assessment of Biodiesel Production from Microalgae,” Environmental Science and Technology, Vol. 43, 2009, pp. 6475–6481.
62. Osorio-Gonzalez, C. S., et al., “Production of Biodiesel from Castor Oil: A Review,” Energies, Vol. 13, 2020, #2467 (22 pages).
63. Fattah, I. M. R., et al., “State of the Art of Catalysts for Biodiesel Production,” Frontiers in Energy Research, Vol. 8, 2020, #101 (17 pages).
64. Ahorsu, R., Medina, F., and Constanti, M., “Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review,” Energies, Vol. 11, 2018, #3366 (19 pages).
65. Shahbaz, M., et al., “State of the Art Review on Biomass Processing and Conversion Technologies to Produce Hydrogen and its Recovery via Membrane Separation,” International Journal of Hydrogen Energy, Vol. 45, 2020, pp. 15166–15195.
66. Cord-Ruwisch, R., and Darwin, “Thermodynamics of Anaerobic Digestion: Mechanism of Suppression of Biogas Production During Acidogenesis,” INMATECH Agricultural Engineering, Vol. 57, 2019, pp. 287–296.
Problems
4.1 The United States consumed approximately 390 million gallons of gasoline per day in 2019. Based solely on the energy content, what are the equivalent amounts of wood, grass, or dairy manure needed to replace it? Use data from Table 4.1 for the calculations. Which of the two heating values will you use in the calculations? Why?
4.2 How will the answers to question 4.1 change if the moisture content in the biomass is taken into account? What would be the effect of incorporating efficiency of conversion of biomass to hydrocarbons in this calculation?
4.3 Describe the different chemical constituents of the biomass and their structures. Which of these constituents is the most valuable? What are the factors that enter into consideration while answering this question?
4.4 What are the significant differences between the lignocellulosic biomass and the algal biomass?
4.5 What are the objectives of biomass pretreatment? Identify the major biomass resource in your region. What pretreatment option(s) will you recommend for this biomass?
4.6 Compare the physical and physicochemical pretreatment alternatives.
4.7 What are the attractive features of the biological pretreatment? What are its drawbacks?
4.8 How does the pretreatment of algal biomass differ from that of the lignocellulosic biomass?
4.9 What are the different factors that influence the choice of the thermochemical processing alternative for biomass? As in question 4.5, frame your answer in the context of the major biomass resource in your region.
4.10 What are the advantages of hydrothermal processing? What are the biomass characteristics that impact the hydrothermal processing route? Which other factors affect the choice?
4.11 Compare and contrast the biochemical and thermochemical treatments of biomass.
4.12 What are the advantages and disadvantages of conducting the entire anaerobic digestion process in a single vessel?
4.13 How does green diesel differ from biodiesel?
4.14 Compare photofermentation with dark fermentation.
