Chapter 23
MYCOBACTERIA
Gregg M. Stave*
MYCOBACTERIUM TUBERCULOSIS (M. tb.)
Common names for disease: Tuberculosis, consumption
Occupational setting
Tuberculosis (TB) exposure may occur in healthcare facilities, including hospitals, dental clinics and nursing homes, and in clinical or research laboratories processing tuberculosis cultures or infected specimens. Exposure may occur in funeral homes and is a significant risk in drug treatment centers, in correctional institutions, and in facilities for the homeless, alcoholics, or persons with AIDS. Animal caretakers can contract tuberculosis from primates even though the animal may not appear ill. Maintenance and construction workers may be exposed while manipulating ventilation systems for patient care isolation rooms or for biologic safety cabinets in which infectious samples are handled.
Exposure (route)
Tuberculosis is contracted after inhalational exposure via droplet nuclei that are 1–5 µm in size and thus remain airborne for long periods of time. TB is not contracted by skin contact with surfaces such as hospital room furniture, equipment, or walls. Infection by gastrointestinal exposure is not a significant risk.
Pathobiology
There are more than 30 members of the genus Mycobacterium, many of which are saprophytes that cause no human disease. Mycobacterium tuberculosis is the organism that causes tuberculosis. The surface lipids of mycobacteria cause them to be resistant to decolorization by acid alcohol during staining procedures. This property gives rise to the name acid-fast bacilli. Mycobacteria will not grow in common culture media but require techniques and reagents found in specialized laboratories.
TB may be insidious in onset, causing symptoms that the affected individual may ignore. These nonspecific symptoms include anorexia, weight loss, low-grade fever, fatigue, and cough. Pulmonary and pleural TB is the most common acute manifestation, presenting with pleuritic chest pain, cough productive of bloody sputum, high fever, and profuse sweating.
Multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB, also referred to as extremely drug-resistant TB) have high case-fatality rates.
Tuberculosis can affect organs other than the lungs. Tuberculous infection in the genitourinary system can cause ureteral obstruction or irregular menses. Lymphatic infection can cause the swelling of the lymph nodes known as scrofula. If vertebral bodies are affected, pain and compression fractures may occur. Meningeal TB is associated with abnormal behavior, headaches, or seizures. Tuberculous peritonitis causes abdominal pain and ascites. Tuberculosis can affect the pericardium, causing heart failure, and the larynx, causing persistent hoarseness. Adrenal involvement may cause Addison’s disease, and tuberculus skin infiltration has been reported. If infection is overwhelming and disseminated (miliary tuberculosis), the presentation can mimic acute leukemia.1,2
Eliminating occupational exposure to tuberculosis has become crucial because large numbers of strains have become resistant to several of the commonly used antituberculous agents (MDR-TB, XDR-TB). Intermittent compliance in taking antituberculous drugs increases the risk that initially susceptible strains will develop resistance. After a century of decline, the incidence of tuberculosis in the United States began rising in the 1980s, especially in inner cities and certain states.3 Although the incidence has declined, several states continue to have above-average rates of infection (Figure 23.1). The majority of cases occur in foreign-born persons (Figure 23.2). The resurgence of tuberculosis has been attributed to economic deprivation, homelessness, alcoholism, drug use, and the rising incidence of AIDS. Public health workers and clinicians subsequently increased their efforts to trace contacts and ensure completion of therapy among individuals with tuberculosis. After reaching a peak of >26 000 confirmed cases of tuberculosis reported per year in the United States, incidence rates fell by 2012 to under 10 000 cases per year. In 2014, there were 9 412 reported cases (Figure 23.2).3,4

FIGURE 23.1 Incidence of tuberculosis (TB) cases, by state and national average, during 2014. The national TB incidence in 2014 was 3.0 cases per 100 000 persons, ranging by state from 0.3 in Vermont to 9.6 in Hawaii (median = 2.0).
Source: MMWR 2014; 64(10):267.
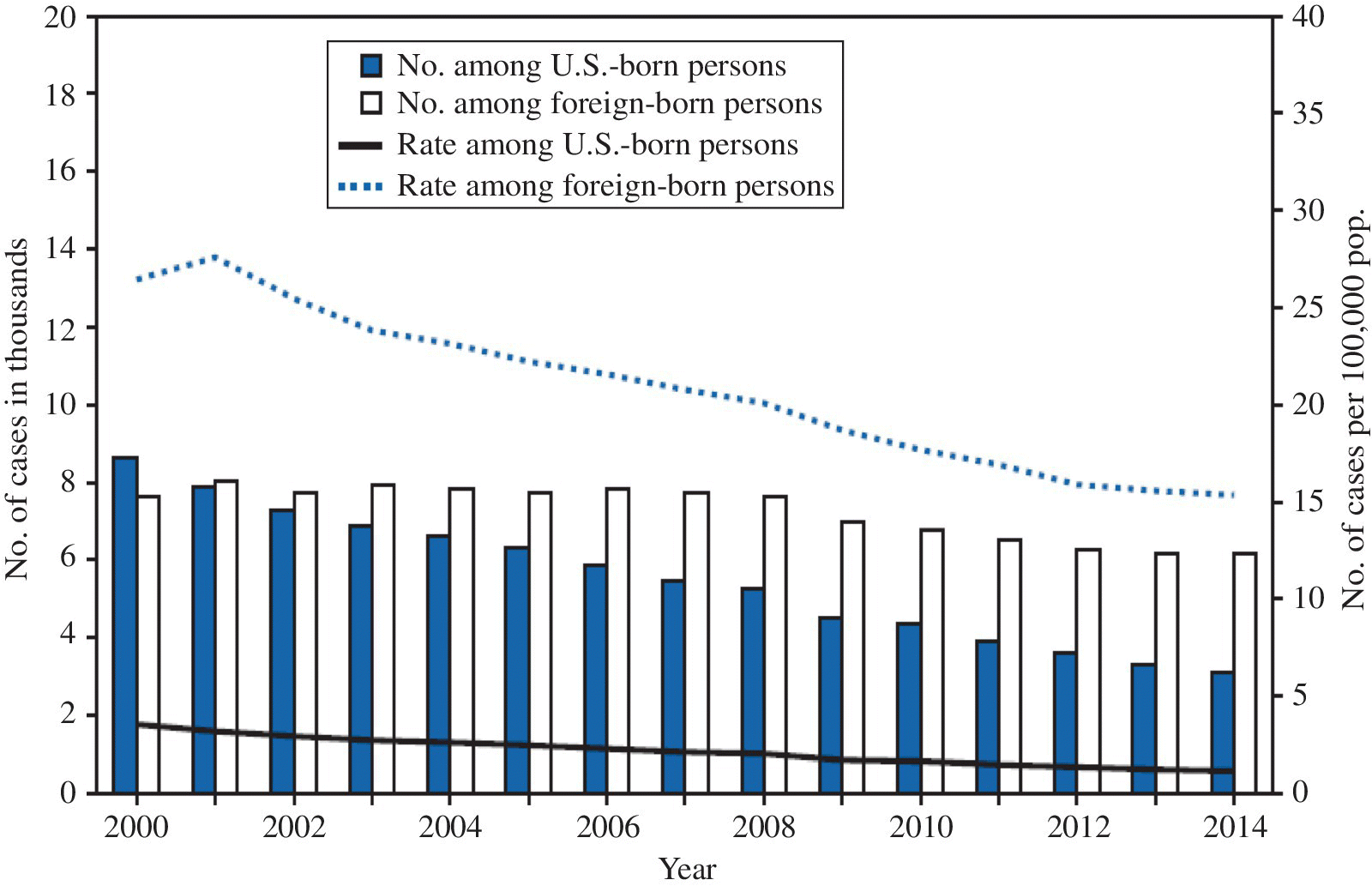
FIGURE 23.2 Number and rate per 100 000 of tuberculosis (TB) cases among U.S.-born and foreign-born persons, by year reported — United States, 2000–2014.
Source: MMWR 2014; 64(10):267.
Nosocomial spread of tuberculosis has been documented; some outbreaks have been associated with tuberculin skin test conversions in >50% of healthcare workers in a single year. These significant occupational exposures have been associated with failure to comply with basic personal protective practices, delayed diagnosis of infected patients, and inadequate hospital room ventilation.5–7 Healthcare facilities have made improvements in the availability of isolation rooms for potentially infectious patients, in use of proper respiratory protective devices, and in training healthcare workers; even so, optimal practices are not always followed.3,8–11 Along with the patient care setting, the risk for occupational infection is also present in the autopsy room and the clinical laboratory.12–14
Diagnosis
Skin testing coupled with clinical evaluation serves to distinguish between infection with the tubercle bacillus and disease caused by the infection. When first exposure results in the entry of tubercle bacilli into the body, bacilli that are not immediately cleared can reside in lymph nodes and other tissues for long periods. A cell-mediated immune response develops that will cause a tuberculin skin test to register positive in ~2–10 weeks. Most individuals never develop disease manifestations. An estimated 5% of newly infected individuals develop clinical illness within 1 year. Later in life, particularly during advanced age, another 5% of infected people develop “reactivation” tuberculosis. In these cases, dormant tubercle bacilli begin multiplying and clinical TB develops, often in the lungs.1–3
When symptoms are present, organ system evaluations coupled with cultures for acid-fast bacilli are needed to confirm the diagnosis. Tuberculous lung infection typically causes lesions in the upper lung fields, such as granulomatous infiltrates. Radiographs may also show a diffuse miliary pattern or sterile pleural effusions. Urinary system tuberculosis can cause white blood cells in the urine with negative cultures for routine urinary pathogens. Skin tests can be used to help diagnose tuberculosis, but individuals with extensive TB or other immunosuppressing conditions may have false-negative skin tests.1–3
Sputum, urine, or other tissues can be evaluated rapidly by Ziehl-Nielsen (acid-fast) staining for tubercle bacilli. Biopsy of infected tissue may show caseating granulomas as well as acid-fast organisms. Acid-fast organisms can be detected and further classified by culture. A proficient and experienced microbiology laboratory should be used. Specimens must be prepared correctly and cultures may require 1 or more months to grow. New techniques, such as DNA polymerase reactions and immunoassays, are under development to allow more rapid identification of mycobacterium species and to determine drug sensitivity.1,15 DNA fingerprinting of tuberculosis isolates using IS6110 RFLP and spoligotyping has been used to study how tuberculosis is transmitted through a population.16 The technique was able to determine that contact with a patient who had tuberculosis was not the source of a healthcare worker’s infection in many cases, and to confirm in other cases that unsuspected transmission in occupational, nosocomial, and school settings had occurred.
Active TB is a reportable condition. Local health departments can provide information about this procedure and will assist with tracing contacts that might also be infected. The Centers for Disease Control and Prevention (CDC) has published Guidelines for the Investigation of Contacts of Persons with Infectious Tuberculosis to assist with these investigations.17
Screening for latent tuberculosis infection
CDC guidelines and the Joint Commission require screening of healthcare workers and those at risk of exposure in healthcare facilities. Two approaches are available for screening: tuberculosis skin testing (TST) and blood testing.
Skin testing by the Mantoux method using purified protein derivative (PPD) is preferred over other skin test methods because of its greater sensitivity and reproducibility. Contact with nonpathogenic mycobacteria can cause small cross reactions to tuberculin skin tests, so minimum skin induration diameters have been established to define a tuberculin skin test as positive (Table 23.1). Using the Mantoux method, 5 tuberculin units (5 TU) are injected intradermally by a syringe according to the manufacturer’s instructions. The 1 TU and the 250 TU products have limited clinical applications. Improper administration, such as failure to administer the skin injection soon after drawing the solution from the vial, can lead to false-negative results. Other causes of false-negative PPD results are immunosuppression, including AIDS, hematogenous malignancy, concurrent serious infection, recent live virus vaccination (measles, mumps, polio), and improper storage or denaturation of the reagent. One can differentiate between general anergy and a truly negative PPD by simultaneously skin testing for reaction to other common exposures, such as Candida albicans.1,2 Diagnostic methods and other information about treatment and prevention of tuberculosis can be found on the CDC website (www.cdc.gov/tb).
TABLE 23.1 Criteria for positive Mantoux test.
| Clinical situation | Minimum size of positive Mantoux test |
| No sign of illness, no risk factors for tuberculosis, no contact with tuberculosis | 15 mm |
| Employees in facilities where a person with disease would pose a hazard to large numbers of susceptible persons (i.e., healthcare facilities, schools, childcare facilities, certain food services) | 10 mm |
| Individuals with potential occupational exposure to tuberculosis including Mycobacteriology laboratory personnel | |
| Employees with silicosis | |
| People with other nonoccupational risk factors for tuberculosis (foreign-born persons from endemic area; medically underserved low-income populations; residents of long-term care facilities; persons with certain medical conditions, such as gastrectomy, severe underweight, renal failure, diabetes mellitus, immunosuppressive diseases other than HIV infection, corticosteroid, or other immunosuppressive therapy, etc.) | |
| HIV infection or unknown HIV status with risk factors for HIV infection | ≥5 mm |
| Close recent contact with infectious tuberculosis case | |
| Chest radiography consistent with old healed tuberculosis | |
| Patients with organ transplants | |
| Persons who are immunosuppressed for other reasons (e.g., taking the equivalent of >15 mg/day of prednisone for 1 month or longer, taking TNF-a antagonists) |
Prior vaccination with bacillus Calmette–Guérin (BCG) does not completely protect against TB infection and is not a likely cause of a positive skin test. Among individuals who have had infections with the tubercle bacillus, the PPD skin response may wane after age 55. To assess for this phenomenon, PPD boosting can be performed by administering two 5 TU tests 1–2 weeks apart. Immunocompetent individuals who carry the tubercle bacillus but who have experienced a wane in PPD response will react to the second PPD. This is known as “the booster phenomenon.” Repeated skin testing will not cause a positive PPD among individuals who are uninfected.1,4 Newly employed healthcare workers who will be screened with PPD testing should receive a baseline two-step TST upon hire, unless they have documentation of either a positive TST result or treatment for latent TB infection or TB disease.18 This requires a four-visit process, unless the worker screens positive after the first test.
Since screening using PPD requires at least two visits to place and read the result, as well as the availability of a trained reader, this has led to a rapid adoption of use of interferon-gamma release assays (IGRAs) for screening for infection. These blood tests use a mixture of synthetic peptides representing TB antigens that cause immune cells to release interferon-gamma when someone is infected with M. tuberculosis. IGRAs are unlikely to produce false-positive results for persons who have been vaccinated with BCG. Two tests are available: QuantiFERON®-TB Gold and T-SPOT.TB. While widely used, concerns have been raised about false positives and a lack of reproducibility with IGRAs. In a study of over 9000 healthcare workers at Stanford University Medical Center, investigators found that the conversion rate using QuantiFERON®-TB Gold was higher than the historical TST conversion rate.19 More than half of workers with a positive result who had a repeat within 60 days had a subsequent negative result, suggesting that these were false positives. The investigators determined that if they increased the cut-off level for the tests (from 0.35 to 5.3 IU/mL), this eliminated most of the false positives. While this reduced the conversion rate to historic norms, it also resulted in missing more than 50% of those with persistent positives on repeat testing.19 The use of IGRAs is an appropriate option in all circumstances in which TST can be used.20 Decisions about whether to use TST or IGRA should be based on consideration of cost, time lost from work for employees needing repeated visits, availability of trained personnel to apply and read the TST, and issues relating to false-positive and false-negative results.
Treatment
A physician familiar with the most up-to-date treatment recommendations should always be consulted when an employee needs antituberculous drug therapy. When therapy is initiated, it is crucial that the individual comply with therapy, both to combat his or her disease and to reduce the likelihood that multidrug resistance will develop. Initial therapy should follow established guidelines; pregnant women may also be safely treated.21,22
If an individual who has had a skin test conversion does not have evidence of active tuberculosis, prophylactic treatment should be considered. The purpose of prophylaxis is to reduce the likelihood of tuberculous infection progressing to active disease. The usual prophylaxis regimen is isoniazid 300 mg per day for 6 months; however, certain groups, such as immunosuppressed or pregnant individuals, should be treated for 9 months.6,21,22 Pyridoxine (vitamin B6) should be taken with isoniazid to prevent peripheral neuropathy, especially in individuals with poor nutrition or other diseases that may cause peripheral neuropathy.6,21–23
Isoniazid can cause hepatic toxicity, particularly among individuals over age 35 or those exposed to other hepatotoxicants. Isoniazid can rarely cause liver failure and death, so patients should receive clear instructions to report promptly malaise, nausea, abdominal pain, or jaundice and to discontinue isoniazid until cleared by a physician. Baseline liver function tests and periodic surveillance for symptoms should be performed. Surveillance should include a contact within the first month of beginning isoniazid. At baseline, providers should take a complete medication history to assess concurrent use of hepatotoxic drugs, including acetaminophen. Because alcohol abuse can increase the risk of hepatic toxicity, abstention should be recommended. Close follow-up of alcoholics is warranted.23
Active TB is treated with a combination of drugs. The most commonly used drugs are isoniazid, rifampin, pyrazinamide, ethambutol, and streptomycin. An accepted protocol must be used. Microbiologic tests for drug resistance are crucial.2,3,24 An effective duration of therapy can be as short as 6 months for low-risk, compliant individuals with no evidence of drug resistance. Each drug has potential side effects that should be monitored. To prevent peripheral neuropathy, concomitant use of pyridoxine (vitamin B6) should be considered when administering isoniazid. Persons at greatest risk include individuals with poor nutrition or other diseases, such as diabetes and alcoholism, that may cause peripheral neuropathy.6,21–23 Patients with active tuberculosis should be evaluated clinically at least monthly. They should also have sputum cultures and sensitivities performed monthly for the first 3 months. At the conclusion of therapy for pulmonary tuberculosis, sputum cultures should be repeated if sputum can be obtained and a chest radiograph performed. Monthly chest radiographs are not necessary if the patient is progressing well.
Individuals with high likelihood of poor compliance should be placed on observed therapy, that is, they should report to an outpatient healthcare facility for each dose of medication.
Any individual may be noncompliant with therapy but most at risk are homeless people, migrants, alcoholics, and drug abusers. Local health departments can assist with providing observed therapy.
TB is common among people with AIDS. A smaller skin test response is positive for people with HIV infection (Table 23.1) than for individuals without immunosuppression. Even if a skin test is negative, the index of suspicion should be high for any AIDS patient with symptoms. Treatment, which should be managed by a physician expert in AIDS, may require more aggressive drug therapy than is needed for individuals without HIV infection.
Employees with active pulmonary tuberculosis should be restricted from work until they are documented to be noninfectious. Coworkers of employees with active tuberculosis should be assessed for skin test conversion if there was a likelihood of exposure to aerosolized bacilli. Employees with skin test conversion in the absence of active pulmonary tuberculosis do not need to be restricted from work or from patient care activities (Table 23.2).
TABLE 23.2 Courses of action for typical scenarios when occupational tuberculosis surveillance is conducted.
| Surveillance scenario | Course of action |
| Previously had negative skin or blood test; now test is also negative | No action unless false-negative reaction is suspected. |
| False-negative skin test is suspected | If aged 55 or over, repeat 5 TU PPD in 2 weeks. |
| If immunosuppressive condition is suspected, refer to experienced personal physician for evaluation. | |
| Restrict from work pending evaluation only if active pulmonary tuberculosis) is suspected regardless of skin test results. | |
| Previously had negative skin test; now has positive skin test | Consider booster phenomenon (false-positive result). Refer to experienced personal physician to evaluate for active tuberculosis |
| Consider prescribing prophylactic therapy with isoniazid if active tuberculosis is ruled out (consider need for pyridoxine with isoniazid). | |
| Restrict from work pending evaluation only if active pulmonary tuberculosis is suspected. | |
| If skin test conversion is likely to have been caused by work, check infection control practices and improve them where needed. | |
| Untoward reaction to PPD (large, ulcerated reaction, lymphangitis, regional adenopathy, fever) | Refer to experienced personal physician to evaluate for active tuberculosis; restrict from work pending this evaluation if index of suspicion for active pulmonary tuberculosis is high. |
| Exclude from further routine PPD skin testing, can use blood test. | |
| History of vaccination with bacillus Calmette–Guérin (BCG) | OK to skin test or blood test. If positive test, evaluate for active tuberculosis |
| If active tuberculosis excluded with positive skin test, consider prophylaxis with isoniazid. | |
| Signs of possible tuberculosis | Refer to experienced personal physician. |
| Exclude from work pending this evaluation if index of suspicion is high. | |
| Active pulmonary tuberculosis | Refer to experienced physician for treatment and follow-up. |
| May not return to work until acid-fast bacteria have been cleared from the sputum; this usually requires 2 or more weeks of antituberculous therapy and follow-up sputum examination by Ziehl-Nielsen staining for confirmation. | |
| If high likelihood of noncompliance with drug therapy, observed treatment may be warranted to prevent relapse and multidrug-resistant tuberculosis | |
| Evaluate coworkers for skin test conversion if history of exposure to aerosolized bacilli from the index case is likely. | |
| If tuberculosis is likely to have been caused by work, check infection control practices and improve them where needed | |
| Active extrapulmonary tuberculosis | Experienced personal physician should treat and follow up. |
| If well enough to tolerate working, may work unless there a draining skin lesion. | |
| If high likelihood of noncompliance with drug therapy, observed treatment may be warranted to prevent relapse and multidrug-resistant tuberculosis. | |
| Recent significant exposure to infectious tubercle bacilli from a person, specimen, culture, primate, or other source | Skin test immediately to establish baseline reactivity status. Retest in 6–12 weeks to determine if PPD converts to positive. In the interim, if any significant new symptoms develop, refer to personal physician for evaluation. |
| Check infection control practices and improve them where needed. |
Medical surveillance
Medical surveillance should be conducted among selected employee groups at risk of exposure. Skin testing using the Mantoux technique and blood testing using interferon-gamma release assays can be used as a surveillance test. Chest radiographs should not be used for routine surveillance, but they should be performed if skin test conversions are documented or if symptoms suspicious of tuberculosis develop.
Workers who have direct contact with patients, clients, animals, or tissues known to have a high risk of infectiousness require surveillance. These workers should have skin testing annually. The frequency of skin testing can be increased based on the worksite’s recent past history of skin test conversions. For workers at very high risk, skin testing every 6 months has been recommended. Some individuals who work in healthcare facilities may not require tuberculosis surveillance because they do not have patient contact or risk of contracting TB. Guidelines from CDC can help determine which worker groups should be included in surveillance.18
The size of skin reaction considered to be a positive response among populations that have various pretest probabilities of tuberculous infection is shown in Table 23.1. Many clinicians, however, feel uncomfortable with reading as negative an 11–14 mm PPD, even if the patient is not at increased risk for tuberculosis. When skin test surveillance is indicated in occupational settings, employees by definition have some increased risk of tuberculosis exposure or transmission, so a 10-mm reaction is the correct definition of positive. In addition, employees with skin test reactions of 5–9 mm should be considered positive if they are immunosuppressed or have had close recent contact with an infectious tuberculosis case. Courses of action for typical scenarios when tuberculosis surveillance is conducted are listed in Table 23.2.
Prevention
Methods to prevent tuberculosis transmission in occupational settings focus on using a combination of tactics involving exposure control. Reducing individual susceptibility by means of vaccination with BCG has not been recommended in the United States as a general public health measure. Nor has BCG vaccination been recommended as a general form of worker protection, although its use could be considered in settings where the likelihood is high that transmission and subsequent infection with M. tuberculosis strains resistant to isoniazid and rifampin may occur.25,26 BCG vaccination should not be used, however, in lieu of infection control precautions.
The Occupational Safety and Health Administration (OSHA) is considering a new infectious disease standard that would include coverage of tuberculosis. OSHA developed and subsequently withdrew a tuberculosis standard in 2003 (29 CFR 1910.1035).27 A potential OSHA Infectious Disease standard could incorporate coverage of tuberculosis. In 2008, OSHA issued a memorandum that it will enforce respiratory protection requirements for tuberculosis under the Respiratory Protection standard, including the requirement for medical evaluation, fit testing, a written program, training, and record keeping.28 Additionally, OSHA will look to the CDC’s Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Settings for enforcement under the General Duty Clause.
The updated CDC guidelines include an expanded scope by incorporating healthcare-associated settings.18 These include:
- Inpatient settings (patient rooms, emergency departments (EDs), intensive care units (ICUs), surgical suites, laboratories, laboratory procedure areas, bronchoscopy suites, sputum induction or inhalation therapy rooms, autopsy suites, and embalming rooms)
- Outpatient settings (medical offices, ambulatory-care settings, dialysis units, and dental-care settings)
- TB clinics
- Settings in correctional facilities in which healthcare is delivered
- Settings in which home-based healthcare is delivered
- Long-term care settings (hospices, skilled nursing facilities)
- Settings in which emergency medical services are provided
- Laboratories handling clinical specimens that might contain M. tuberculosis
- Other settings in which suspected and confirmed TB patients might be encountered (cafeterias, kitchens, laundry areas, maintenance shops, pharmacies, and law enforcement settings)
The CDC guidelines recommend that every healthcare setting should have a written TB infection-control plan that is part of an overall infection-control program. The plan should ensure prompt detection, use of environmental and respiratory precautions, and prompt treatment. The first steps in developing the plan are assigning responsibility and performing a risk assessment. The results of the TB risk assessment determine which administrative, environmental, and respiratory protection controls are needed. The risk assessment should begin with a review of past experience with TB, past occupational exposure to tuberculosis, and the frequency with which active tuberculosis occurs in the surrounding community.18
The following preventive practices are important for worker protection and regulatory compliance: a written Exposure Control Plan, training for employees, a medical surveillance program, procedures for prompt identification of individuals with suspected or confirmed infectious tuberculosis, use of isolation rooms employing engineering controls for such individuals, placement of warning signs at the entrance to high-risk areas, use of specific work practices including Respiratory Protection, procedures to evaluate employee exposures, medical removal protection for workers who contract tuberculosis occupationally, and a record-keeping system that protects confidential medical information appropriately.
Employee training should be provided on hire and annually for those workers with occupational exposure. The training should include the signs and symptoms of tuberculosis, hazards of transmission, the purpose and nature of medical surveillance, and site-specific controls. The primary method of transmission of tuberculosis through aerosols should be reviewed. Methods to ensure early identification of suspected cases of tuberculosis should be in place at the facility and should be emphasized during training. Medical surveillance should include pre-placement evaluation, periodic Mantoux or IGRA testing, and management of persons with positive test results. The CDC guidelines recommend the use of risk classification to determine the need for and frequency of screening18(Table 23.3).
TABLE 23.3 Risk classifications for various health-care settings and recommended frequency of screening for Mycobacterium tuberculosis infection among health-care workers (HCWs).*
Source: Centers for Disease Control and Prevention. Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Settings, MMWR 2005; 54:RR-17 Appendix C.
| Setting | Risk classification† | ||
| Low risk | Medium risk | Potential ongoing transmission§ | |
| Inpatient <200 beds | <3 TB patients/year | ≥3 TB patients/year | Evidence of ongoing M. tuberculosis infection, regardless of setting |
| Inpatient ≥200 beds | <6 TB patients/year | ≥6 TB patients/year | |
| Outpatient; and nontraditional facility-based | <3 TB patients/year | ≥3 TB patients/year | |
| TB treatment facilities | Settings in which | Settings in which | |
|
|
||
| Laboratories | Laboratories in which clinical specimens that might contain M. tuberculosis are not manipulated | Laboratories in which clinical specimens that might contain M. tuberculosis might be manipulated | |
| Recommendations for Screening Frequency | |||
| Baseline two-step TST or one BAMT¶ | Yes, for all HCWs upon hire | Yes, for all HCWs upon hire | Yes, for all HCWs upon hire |
| Serial TST or BAMT screening of HCWs | No** | At least every 12 months†† | As needed in the investigation of potential ongoing transmission§§ |
| TST or BAMT for HCWs upon unprotected exposure to M. tuberculosis | Perform a contact investigation (i.e., administer one TST or BAMT as soon as possible at the time of exposure, and, if the result is negative, give a second test [TST or BAMT, whichever was used for the first test] 8–10 weeks after the end of exposure to M. tuberculosis)¶¶ | ||
* The term Health-care workers (HCWs) refers to all paid and unpaid persons working in health-care settings who have the potential for exposure to M. tuberculosis through air space shared with persons with TB disease.
† Settings that serve communities with a high incidence of TB disease or that treat populations at high risk (e.g., those with human immunodeficiency virus infection or other immunocompromising conditions) or that treat patients with drug-resistant TB disease might need to be classified as medium risk, even if they meet the low-risk criteria.
§ A classification of potential ongoing transmission should be applied to a specific group of HCWs or to a specific area of the health-care setting in which evidence of ongoing transmission is apparent, if such a group or area can be identified. Otherwise, a classification of potential ongoing transmission should be applied to the entire setting. This classification should be temporary and warrants immediate investigation and corrective steps after a determination has been made that ongoing transmission has ceased. The setting should be reclassified as medium risk, and the recommended timeframe for this medium risk classification is at least 1 year.
¶ All HCWs upon hire should have a documented baseline two-step tuberculin skin test (TST) or one blood assay for M. tuberculosis (BAMT) result at each new health-care setting, even if the setting is determined to be low risk. In certain settings, a choice might be made to not perform baseline TB screening or serial TB screening for HCWs who 1) will never be in contact with or have shared air space with patients who have TB disease (e.g., telephone operators who work in a separate building from patients) or 2) will never be in contact with clinical specimens that might contain M. tuberculosis. Establishment of a reliable baseline result can be beneficial if subsequent screening is needed after an unexpected exposure to M. tuberculosis.
** HCWs in settings classified as low risk do not need to be included in the serial TB screening program.
†† The frequency of screening for infection with M. tuberculosis will be determined by the risk assessment for the setting and determined by the Infection Control team.
§§ During an investigation of potential ongoing transmission of M. tuberculosis, testing for M. tuberculosis infection should be performed every 8–10 weeks until a determination has been made that ongoing transmission has ceased. Then the setting should be reclassified as medium risk for at least 1 year.
¶¶ Procedures for contact investigations should not be confused with two-step TSTs, which are used for baseline TST results for newly hired HCWs.
Along with efforts to control exposure at the source, isolation rooms with a negative pressure gradient and preferably a single-pass air system should be available. This will prevent tubercle bacilli from being blown into halls, neighboring patient care rooms, and other work areas. Contaminated air from isolation rooms should not be exhausted near air intake vents or public walkways. An appropriate air exchange rate should be maintained and monitored. Ultraviolet light may be used to supplement the engineering controls in a facility, but ultraviolet light is not a substitute for negative pressure, exhaust ventilation, or HEPA filtration. Isolation rooms that are engineered correctly need regular maintenance to ensure that clogged filters do not prevent negative pressure from being achieved. Maintenance and construction employees who are potentially exposed to tuberculosis require training and need to use personal protective equipment. The CDC guidelines provide detailed guidance on environmental controls.18
Respirator use must comply with the provisions of the OSHA Respiratory Protection standard as well as specific requirements for prevention of occupational tuberculosis. Surgical masks are not respirators, as they are designed to prevent the healthcare provider from contaminating the patient, but they do not prevent the wearer from being exposed to ambient organisms.6,7,28 An N95 respirator approved by the National Institute for Occupational Safety and Health (NIOSH) is acceptable in most exposure settings. Combination products incorporating both a surgical mask and an N95 disposable respirator (respirator portion certified by CDC/NIOSH and surgical mask portion listed by FDA) are available that provide both respiratory protection and bloodborne pathogen protection.18
Workers must receive medical clearance prior to using the N95 respirator or other respirators. Training should include instructions on the correct way to wear the respirator, how to self-fit test each time a disposable respirator is worn, and how to store a respirator properly. A worker with a full beard cannot achieve an adequate fact-to-respirator seal and will need to use an alternative respirator, such as powered air-purifying respirators (PAPRs), to achieve protection.
Individuals with active pulmonary tuberculosis whose sputum contains acid-fast bacilli should wear a fitted mask (a surgical mask is acceptable) when they leave the isolation room to be transported. They should be instructed to cover their mouth and nose with a tissue when coughing. When these measures are not possible to achieve, for instance, when a patient is combative, the worker should wear a respirator. Attendants should also wear a respirator when transporting a person with active pulmonary tuberculosis in an enclosed vehicle, or when working in the home of such a person, even if the infected individual is wearing a mask.
Sputum induction, administration of aerosolized medications, bronchoscopy, and other procedures likely to produce airborne bacilli should be performed in a properly ventilated setting, such as a sputum induction booth, and attendants must wear proper respiratory protection. In the proposed standard, posting a specific sign at the entrance to high-risk areas will be required. The proposed sign will be red, in the shape of a stop sign, and will contain this text: “No admittance without wearing a Type N95 or more protective respirator.”
In work settings where tissues or cultures are the source of infectious organisms, the use of engineering controls such as biologic safety cabinets, instead of respirators, is the preferred method to prevent exposure. To ensure that the correct air balance occurs in a biologic safety cabinet, the HEPA-filtered exhaust air must be discharged either directly to the outside, or by means of proper connections with the building exhaust system. Equipment such as continuous-flow centrifuges used to process specimens containing tubercle bacilli may produce aerosols, and so these should be exhausted in such a way as to prevent contamination of the laboratory.
In all work settings where there is potential exposure to tuberculosis, workers need to follow standard infection control practices, especially since infectious agents other than tuberculosis may be present. These practices include handwashing and use of protective clothing. Specimen containers should be transported in a plastic bag using gloves. Biohazardous waste should be stored in labeled containers and disposed of properly. Work areas should be cleaned with a disinfectant. Although extensive activities to prevent exposure to fomites are not necessary, the manipulation of biological materials containing high concentrations of infectious tubercle bacilli can create hazardous aerosols. Therefore, trained individuals who use a respirator and other appropriate personal protective equipment should clean up sputum, body fluids, or tissues containing tubercle bacilli.
Engineering controls, administrative controls, patient care procedures, and other work practices should be evaluated periodically for areas that need improvement, with attention to results from medical surveillance such as skin test conversion rates. In the event that an employee contracts tuberculosis at work, procedures should be in place to address medical removal. The now-withdrawn OSHA standard stipulated that medical removal procedures should include protection of the person’s job with full medical earnings and all other rights and benefits until the worker becomes noninfectious and can return to work, or for 18 months, whichever comes first. It is not known whether a future OSHA infectious disease standard will have similar worker protections. However, a workplace-acquired infection would likely be covered under workers’ compensation laws.
References
- 1. American Thoracic Society and CDC. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 2000; 161(4 Pt 1):1376–95.
- 2. Raviglione MC, O’Brien RJ. Tuberculosis. In: Kasper DL, Fauci AS, Longo DL, et al., eds. Harrison’s Principles of Internal Medicine, 19th ed. New York: McGraw-Hill, 2015.
- 3. Centers for Disease Control, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of Tuberculosis Elimination. Core Curriculum on Tuberculosis: What the Clinician Should Know, 6th ed., 2013. Available at: http://www.cdc.gov/tb/education/corecurr/pdf/corecurr_all.pdf (accessed on June 16, 2016).
- 4. Centers for Disease Control and Prevention. Tuberculosis Trends—United States, 2014. MMWR 2014; 64(101):265–9.
- 5. Centers for Disease Control. Outbreak of multidrug-resistant tuberculosis at a hospital—New York City, 1991. MMWR 1993; 42:427, 433–4.
- 6. Mahmoudi A, Iseman MD. Pitfalls in the care of patients with tuberculosis: common errors and their association with the acquisition of drug resistance. JAMA 1993;270:65–8.
- 7. Dooley SW, Villarino ME, Lawrence M, et al. Nosocomial transmission of tuberculosis in a hospital unit for HIV-infected patients. JAMA 1992; 267:2632–5.
- 8. Manangan LP, Simonds DN, Pugliese G, et al. Are US hospitals making progress in implementing guidelines for prevention of Mycobacterium tuberculosis transmission? Arch Intern Med 1998; 158:1440–4.
- 9. Sutton PM, Nicas M, and Harrison RJ. Tuberculosis isolation: comparison of written procedures and actual practices in three California hospitals. Infect Control Hosp Epidemiol 2000; 21:28–32.
- 10. Porteous NB and Brown JP. Tuberculin skin test conversion rate in dental healthcare workers—results of a prospective study. Am J Infect Control 1999; 27:385–7.
- 11. Steenland K, Levine J, Sieber K, et al. Incidence of tuberculosis infection among New York State prison employees. Am J Public Health 1997; 87:2012–7.
- 12. Templeton GL, Illing LA, Young L, et al. The risk for transmission of Mycobacterium tuberculosis at the bedside and during autopsy. Ann Intern Med 1995; 122:922–5.
- 13. Flavin RJ, Gibbons N, and O’Briain DS. Mycobacterium tuberculosis at autopsy—exposure and protection: an old adversary revisited. J Clin Pathol 2007; 60:487–91.
- 14. Collins CH and Grange JM. Tuberculosis acquired in laboratories and necropsy rooms. Commun Dis Public Health 1999; 2:161–7.
- 15. McNerney R, Maeurer M, Abubakar I, et al. Tuberculosis diagnostics and biomarkers: needs, challenges, recent advances, and opportunities. J Infect Dis 2012; 205(Suppl 2):S147–58.
- 16. Bauer J, Kok-Jensen A, Faurschou P, et al. A prospective evaluation of the clinical value of nation-wide DNA fingerprinting of tuberculosis isolates in Denmark. Int J Tuberc Lung Dis 2000; 4:295–9.
- 17. Centers for Disease Control and Prevention. Guidelines for the investigation of contacts of persons with infectious tuberculosis: recommendations from the National Tuberculosis Controllers Association and CDC. MMWR 2005; 54(RR-15): 1–47. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr5415.pdf (accessed July 1, 2016).
- 18. Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in healthcare settings, MMWR 2005; 54(RR-17): 1–141. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr5417.pdf (accessed July 1, 2016).
- 19. Slater ML, Welland G, Pai M, et al. Challenges with QuantiFERON-TB gold assay for large-scale, routine screening of U.S. healthcare workers. Am J Respir Crit Care Med 2013; 188:1005–10.
- 20. Centers for Disease Control and Prevention. Guidelines for using the QuantiFERON®-TB gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR 2005; 54(RR-15): 49–55. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr5415.pdf (accessed July 1, 2016).
- 21. Centers for Disease Control. Initial therapy for tuberculosis in the era of multidrug resistance: recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR 1993; 42(No. RR-7):l–8.
- 22. Centers for Disease Control. Tuberculosis among pregnant women–New York City, 1985–1992. MMWR 1993; 605:611–2.
- 23. Centers for Disease Control. Severe isoniazid-associated hepatitis—New York, 1991–1993. MMWR 1993; 42:545–7.
- 24. Bloch NB, Cauthen GM, Onorato IM, et al. Nationwide survey of drug-resistant tuberculosis in the United States. JAMA 1994; 271:665–71.
- 25. Centers for Disease and Prevention. The role of BCG vaccine in the prevention and control of tuberculosis in the United States: a joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. MMWR 1996; 45(RR4):1–18.
- 26. Marcus AM, Rose DN, Sacks HS, et al. BCG vaccination to prevent tuberculosis in healthcare workers: a decision analysis. Prev Med 1997; 26:201–7.
- 27. Occupational Safety and Health Administration. Occupational Exposure to Tuberculosis; Proposed Rule – 62-54159-54309. Available at: http://www.osha-slc.gov/FedReg_osha_data/FED19971017.html (accessed on June 16, 2016).
- 28. Occupational Safety and Health Administration. Tuberculosis and Respiratory Protection Enforcement. 29 CFR 1910.134; 1910.134(f)(2). Washington, DC: Department of Labor, 2008. Available at: https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=Interpretations&p_id=26013 (accessed on June 16, 2016).
MYCOBACTERIA OTHER THAN MYCOBACTERIUM TUBERCULOSIS
Occupational setting
Several mycobacteria other than Mycobacterium tuberculosis can cause disease in humans. Significant occupational exposure is much less common for these bacilli than for M. tuberculosis.
Potential contact with M. kansasii and M. avium intracellulare could occur in patient care settings, especially during care of immunosuppressed patients with pulmonary infections from these organisms. However, person-to-person transmission may not be the only route of exposure.1 The organisms are also found in the environment and in animal reservoirs. M. kansasii outbreaks have occurred among miners, particularly when the miners are in poor health, water used during mining is contaminated with the organism and the miners have evidence of dust-related pulmonary disease or silicosis.2,3M. avium intracellulare infections and hypersensitivity pneumonitis have been described in spa workers, referred to as “hot tub lung,” as well as in a swim instructor and lifeguards.4–9 Water aerosolization during filter cleaning and inadequate ventilation may have contributed to these cases.7,10
Occupational contact with M. bovis may occur from working with dairy cattle. Infected beef cattle and game animals such as deer can also transmit the disease.11,12 Tuberculin testing of cattle and pasteurization of milk have greatly reduced the incidence of M. bovis infection, but in some developed and developing countries, infection rates remain high in cattle herds, so that farm and slaughterhouse workers may be at risk.13–17 A case of animal-to-human transmission has also occurred in a veterinarian.18
M. marinum and M. abscessus can be acquired via skin exposure to infected fish or to freshwater or saltwater aquatic environments. Individuals who are occupationally infected often give a history of minor skin trauma to the hands or other skin immersed in water.19–21
M. immunogen and other Mycobacterium species have been identified as a contaminant in metal-working fluid and a possible cause or contributor to hypersensitivity pneumonitis.10,22
Occupational contact with M. leprae is rare in the United States. In the 1980s, more than 900 immigrants and refugees from Southeast Asia had leprosy, but there was no evidence that these individuals transmitted the disease to others after their arrival.23 The incidence among immigrants has declined significantly and the incidence in U.S. borns has remained low (Figure 23.3).24 The organism is difficult to transmit, although nasal colonization can occur occupationally. In one study, a polymerase chain reaction test revealed M. leprae genetic material in nasal swab specimens among 55% of untreated patients, 19% of occupational contacts, and 12% of controls from an endemic region.25 Less than 10% of close family members of affected patients contract the disease.1,26
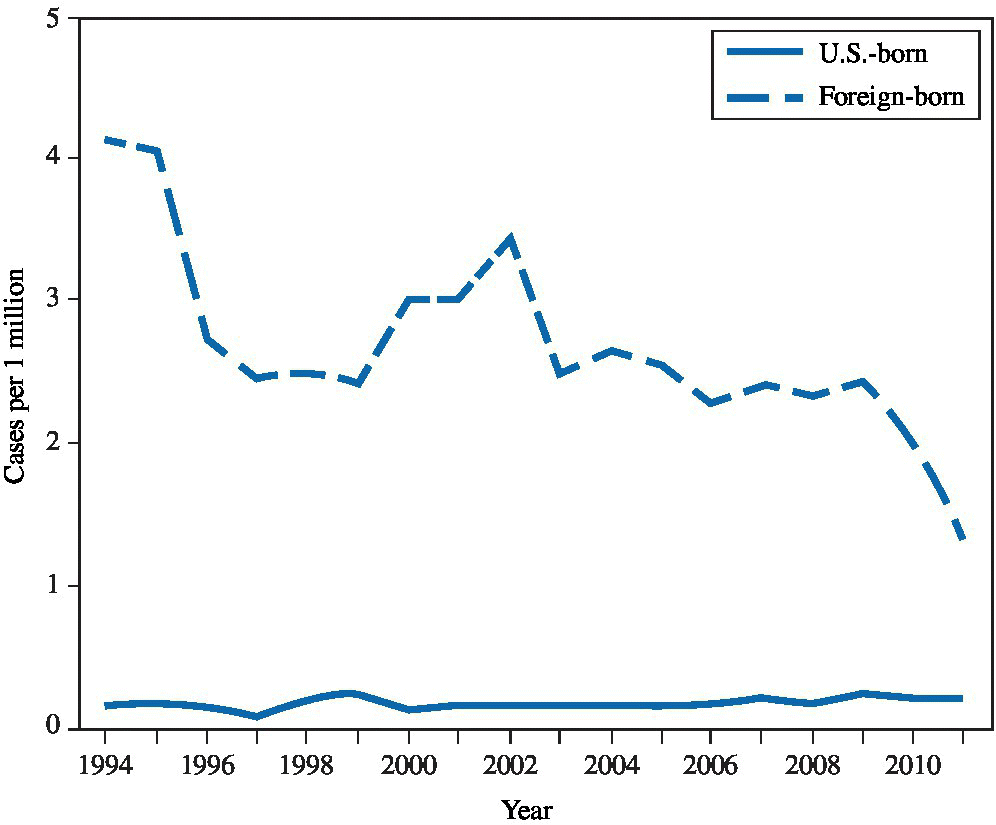
FIGURE 23.3 Rate of new diagnoses of Hansen’s disease, by U.S. birth status — United States, 1994–2011.
Source: MMWR 2014:63(43):970.
If infected specimens are handled improperly, laboratory workers can be exposed. Contact during travel to underdeveloped nations, where the incidence of mycobacterial disease may be high, is a theoretical possibility, but it is unlikely unless the traveler has prolonged close physical contact with infected, untreated individuals.
Exposure (route)
Routes of exposure have not been entirely elucidated; however, person-to-person contact is not believed to be as high a risk as with M. tuberculosis.1 Most pathogenic Mycobacterium species are thought to be absorbed from the upper respiratory tract. Infection with some species can occur after skin contact (M. marinum, M. abscessus, M. ulcerans, M. fortuitum, and M. leprae). The gastroenterologic route of exposure is important for M. bovis (contaminated milk), as is respiratory exposure to aerosols produced during the slaughtering process.1,4,5,26 Some armadillos in the southern United States are naturally infected with Hansen’s disease.27 While the risk of transmission is considered low, infections can occur following contact.27 There is also evidence that M. leprae can also survive in amebal cysts representing another potential source for exposure.28
Pathobiology
M. avium intracellulare, M. kansasii, and other species can cause a potentially serious pulmonary infection similar to that caused by M. tuberculosis. Disseminated disease is more common among immunosuppressed individuals, especially those with AIDS or organ transplants. M. bovis can cause pulmonary disease. Localized skin infection or lymphadenitis can occur after a percutaneous exposure from M. fortuitum, M. marinum, or M. ulcerans. Lymphadenopathy, primarily cervical, appears after oral exposure to M. scrofulaceum.1,3
M. leprae affects the skin, soft tissues, and peripheral nerves. The average incubation period is 2–4 years. It is common in some underdeveloped nations but rare in the United States. Anesthetic or paresthetic skin lesions with a typical appearance are usually the first clinical sign of infection with M. leprae. In advanced cases, destruction of the soft tissues of the nose and face, fingers, toes, and other structures leads to cosmetic deformities. The granulomatous reaction of the host, rather than a neurotoxic effect of the bacilli themselves, is thought to cause nerve damage.25
Diagnosis
Pulmonary infection is diagnosed in the same manner as M. tuberculosis-using microscopic examination of sputum (Ziehl-Nielsen staining), sputum cultures, and chest radiographs. Sputum cultures should be sent to a qualified laboratory. Biopsy material from skin or lymph node lesions suspected of harboring Mycobacterium species should be evaluated similarly with staining and culture. Skin tests with PPD can be positive, but tissue diagnosis is more definitive, especially since many infected patients are immunosuppressed.1,3
Hansen’s disease (leprosy) is diagnosed among individuals with suggestive symptoms by demonstrating M. leprae organisms on biopsy of skin or peripheral nerves. Skin testing is of no use, but a serodiagnostic test to a specific phenolic glycolipid from the surface of the bacillus has a high diagnostic specificity. The organism can be cultured in footpads of mice and in the armadillo. In the United States, the National Hansen’s Disease Program is responsible for Hansen’s disease (leprosy) care and research. Care is provided for patients at its facility at the Ochsner Medical Center in Baton Rouge, LA, and the program also oversees an ambulatory care network with 11 clinics in seven states and Puerto Rico. It also conducts biomedical research.29
Treatment
With the exception of Hansen’s disease, infections with the most common species are treated with the many of the same drugs used with M. tuberculosis. M. avium intracellulare is commonly multidrug-resistant; therefore, two to six drugs are usually chosen from among isoniazid, ethambutol, rifampin, ethionamide, pyrazinamide, cycloserine, amikacin, and clofazimine. Surgical excision is sometimes used for lymphadenitis and some other localized infections. A physician who is experienced with infectious diseases should direct the patient’s care.1,30
Three drugs have been traditionally used to treat the more severe forms of Hansen’s disease—dapsone, rifampin, and clofazimine. At least 2 years of drug therapy are required, and indefinite treatment may be necessary. Cosmetic deformities can often be surgically corrected. For less-severe cases, dapsone and rifampin are given for at least 6 months.26 In 1998, the US Food and Drug Administration approved the use of thalidomide to treat skin lesions in leprosy known as erythema nodosum leprosum.
Medical surveillance
Many workers at risk for exposure to these Mycobacterium species will already be in a medical surveillance program for M. tuberculosis, which should be adequate. CDC can be used as a resource for program planning. Recommended medical surveillance for the rare worker who may have significant occupational contact with M. leprae should consist at least of a clinical examination focusing on detection of typical skin lesions. Serologic testing may be a useful adjunct.26
Prevention
A prudent strategy for prevention of possible exposure to bioaerosols of Mycobacterium species that cause pulmonary infection follows the same principles as the program for M. tuberculosis. Because of concomitant risk of exposure to M. tuberculosis, control measures should already be in place in most targeted workplaces. Control measures include rapid diagnosis of infectious patients, respiratory isolation, negative pressure room ventilation, and maintenance of ventilation systems. Worker training should focus on the use of gloves, protective clothing, and an approved respirator. Biological wastes should be disposed of properly. Patient care procedures likely to produce bioaerosols should be conducted in a properly ventilated area, such as a sputum induction booth. Laboratory workers should use biologic safety cabinets. Workers in the fishing industry and in agriculture should use protective clothing and gloves.
To prevent exposure to M. leprae, standard infection control measures should be followed. Prophylactic postexposure chemotherapy under the direction of a physician experienced in caring for patients with Hansen’s disease should be considered for individuals who have had a significant occupational exposure.26
References
- 1. Holland SM. Nontuberculous mycobacterial infections. In: Kasper DL, Fauci AS, Longo DL, et al., eds.Harrison’s principles of internal medicine, 19th ed. New York: McGraw-Hill, 2015.
- 2. Corbett EL, Blumberg L, Churchyard GJ, et al. Nontuberculous mycobacteria: defining disease in a prospective cohort of South African miners. Am J Respir Crit Care Med 1999; 160:15–21.
- 3. Chobot S, Malis J, Sebakova H, et al. Endemic incidence of infections caused by Mycobacterium kansasii in the Karvina district in 1968–1995 (analysis of epidemiological data & review). Cent Eur J Public Health 1997; 5:164–73.
- 4. Moraga-McHaley SA, Landen M, Krapfl H, et al. Hypersensitivity pneumonitis with Mycobacterium avium complex among spa workers. Int J Occup Environ Health 2013; 19(1):55–61.
- 5. Rose CS, Martyny J, Huitt J, et al. Hot tub associated granulomatous lung disease from mycobacterial aerosols. Am J Respir Crit Care Med 2000; 161:A730.
- 6. Agarwal R and Nath A. Hot-tub lung: hypersensitivity to Mycobacterium avium but not hypersensitivity pneumonitis. Respir Med 2006; 100:1478.
- 7. Fjallbrant H, Akerstrom M, Svensson E, et al. Hot tub lung: an occupational hazard. Eur Respir Rev 2013; 22(127):88–95.
- 8. Yu TC, Ahmed R, Yap E, et al. Dyspnoea in a 17-year-old swim instructor: a diagnosis of hot tub lung. NZ Med J 2008; 121:78–80.
- 9. Rose CS, Martyny JW, Newman LS, et al. “Lifeguard lung”: endemic granulomatous pneumonitis in an indoor swimming pool. Am J Public Health 1998; 88:1795–1800.
- 10. Falkinham III JO. Mycobacterial aerosols and respiratory disease. Emerg Infect Dis 2003; 9(7):763–7.
- 11. Cousins DV, Williams SN, and Dawson DJ. Tuberculosis due to Mycobacterium bovis in the Australian population: DNA typing of isolates, 1970–1994. Int J Tuberc Lung Dis 1999; 3:722–31.
- 12. Liss, GM, Wong L, Kittle DC, et al. Occupational exposure to Mycobacterium bovis infection in deer and elk in Ontario. Can J Public Health 1994; 85:326–9.
- 13. de Kantor IN and Ritacco V. Bovine tuberculosis in Latin American and the Caribbean: current status, control and eradication programs. Vet Microbiol 1994; 40:5–14.
- 14. de la Rua-Domenech, R. Human Mycobacterium bovis infection in the United Kingdom: incidence, risks, control measures and review of the zoonotic aspects of bovine tuberculosis. Tuberculosis 2006; 86:77–109.
- 15. Torres-Gonzalez P, Soberanis-Ramos O, Martinez-Gamboa A, et al. Prevalence of latent and active tuberculosis in dairy farm workers exposed to cattle infected by Mycobacterium bovis. PLoS Negl Trop Dis 2013; 7(4):e2177.
- 16. Evans JT, Smith EG, Banarjee A, et al. Cluster of human tuberculosis caused by Mycobacterium bovis: evidence for person-to-person transmission in the UK. Lancet 2007; 369: 1270–76.
- 17. Thoen C, LoBue P, and de Kantor I. The importance of Mycobacterium bovis as a zoonosis. Vet Microbiol 2006;112:339–45.
- 18. Corcoran JP, Hallifax RJ, Bettinson HV, et al. Tuberculous pleuritis secondary to Mycobacterium bovis in a veterinarian. Clin Respir J 2014; Oct 22. 10.1111/crj.12231. [Epub ahead of print]
- 19. Iredell J, Whitby M, and Blacklock Z. Mycobacterium marinum infection: epidemiology and presentation in Queensland 1971–1990. Med J Aust 1992; 157:596–8.
- 20. Cheung JP, Fung B, Wong SS, et al. Review article: Mycobacterium marinum infection of the hand and wrist. J Orthopaedic Surg 2010; 18(1):98–103.
- 21. Kang GC, Gan AW, Yam A, et al. Mycobacterium abscessus hand infections in immunocompetent fish handlers: case report. J Hand Surg Am 2010; 35(7):1142–5.
- 22. Shelton BG, Flanders WD, and Morris GK Mycobacterium sp. as a possible cause of hypersensitivity pneumonitis in machine workers. Emerg Infect Dis 1999; 5:270–3.
- 23. Mastro TD, Redd SC, and Breiman RF. Imported leprosy in the United States, 1978 through 1988: an epidemic without secondary transmission. Am J Public Health 1992; 82:1127–80.
- 24. Nolen L, Haberling D, Scollard D, et al. Incidence of Hansen’s disease—United States, 1994–2011. MMWR 2014; 63(43):969–72.
- 25. de Wit MY, Douglas JT, McFadden J, et al. Polymerase chain reaction for detection of Mycobacterium leprae in nasal swab specimens. J Clin Microbiol 1993; 31:502–6.
- 26. Gelber RH. Leprosy. In: Kasper DL, Fauci AS, Longo DL, et al, eds. Harrison’s Principles of Internal Medicine, 19th ed. New York: McGraw-Hill, 2015.
- 27. Centers for Disease Control and Prevention. Hansens Disease (Leprosy): Risk of Exposure. Available at: http://www.cdc.gov/leprosy/exposure/armadillos.html (accessed on June 16, 2016).
- 28. Wheat WH, Casali AL, Thomas V, et al. (2014) Long-term survival and virulence of Mycobacterium leprae in amoebal cysts. PLoS Negl Trop Dis; 8(12):e3405. 10.1371/journal.pntd.0003405.
- 29. Health Resources and Services Administration. National Hansen’s Disease (Leprosy) Program. Available at: http://www.hrsa.gov/hansensdisease/ (accessed on July 1, 2016).
- 30. Griffith DE, Aksamit T, Brown-Elliott BA, et al. (2007). An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Resp Critic Care Med, 175(4):367–416.
