Chapter 2
ERGONOMICS and UPPER EXTREMITY MUSCULOSKELETAL DISORDERS
Thomas R. Hales
Ergonomics has been defined as the science of fitting the job to the worker1 or the art of matching job demands with worker capabilities. Upper extremity (UE) musculoskeletal disorders (MSDs) are soft tissue disorders of the muscles, tendons, ligaments, peripheral nerves, joints, cartilage, or supporting blood vessels in the neck, shoulder, arm, elbow, forearm, hand, or wrist. Examples of specific disorders include tension neck syndrome, rotator cuff tendinitis, epicondylitis, peritendinitis, and carpal tunnel syndrome (CTS).2 When job demands overwhelm an employee’s mental and/or physical capacity, employee health, comfort, and productivity can be adversely affected.3 While comfort and productivity levels are important outcomes to consider, this chapter will focus upon the effect of workplace physical stressors (repetition, force, posture, and vibration) on the musculoskeletal system of the upper extremities. This chapter reviews the epidemiologic association between UE MSDs and work, and provides practical tools for healthcare providers to (i) assess physical stressors in the workplace and (ii) recognize, treat, and prevent UE MSDs.
OCCUPATIONAL SETTING
Magnitude of the problem
BUREAU OF LABOR STATISTICS DATA
An injury or illness is work related if an event or exposure in the work environment either caused or contributed to the resulting condition or significantly aggravated a preexisting condition.4 The Bureau of Labor Statistics (BLS) annually reports on the number of workplace injuries, illnesses, and fatal injuries in the United States. In addition to collecting private sector data, since 2008 the BLS began reporting injury and illness data on public sector workers in state and local governments (e.g., police and fire fighters).
MSDs are the most common type of occupational condition reported on the BLS survey, typically representing almost a third of all BLS-reported injuries and illnesses.5 In 2014, the BLS estimated that 365 580 cases of MSDs occurred for an incidence rate of 33.8 cases per 10 000 full-time workers, a rate that is trending downward since 2011 (Figure 2.1). In 2014, workers who sustained an MSD required a median of 13 days to recuperate before returning to work, compared to 10 days in previous years.5 This finding suggests that while MSDs are trending down, the cases may be becoming more severe. Carpal tunnel syndrome is probably the most well-known MSD, but sprains, strains, and tears are the most common diagnosis.6 The BLS reports the MSD rate is higher among males. In 2014 the MSD incident rate was 37.5 per 10 000 full-time workers, compared to 29.7 per 10 000 among female workers, a trend that has persisted over the past decade.5 The 45–54-year-old age group has the highest reported rate (40.4 per 10 000), followed by the 35–44-year-old age group (36.2 per 10 000) in 2014, again a trend that has persisted over the past decade5 (Figure 2.2). It should be noted that the BLS data significantly underestimates the true number of these conditions.7,8

FIGURE 2.1 Number and incident rate of musculoskeletal disorders involving days away from work, 2008–2014.5
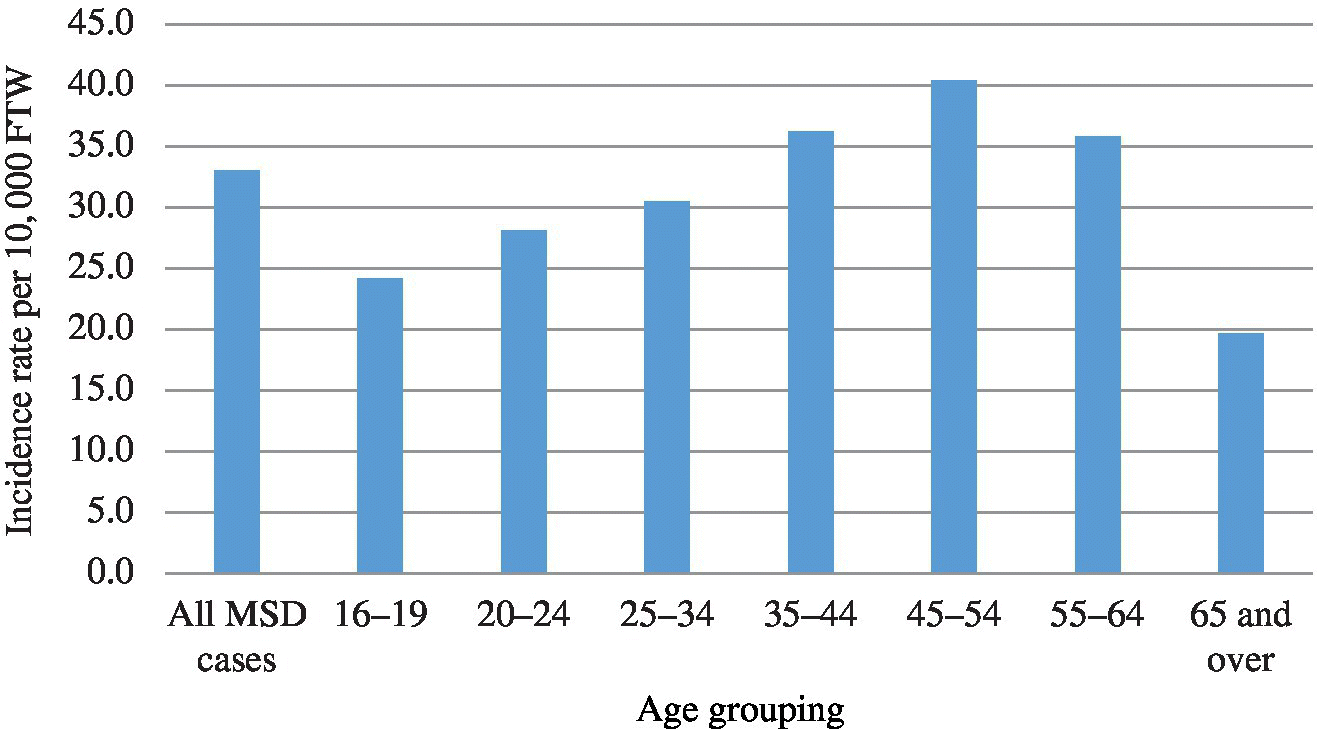
FIGURE 2.2 Incidence rate of musculoskeletal disorders involving days away from work by age group, 2014.5 FTW, full-time workers.
WORKERS’ COMPENSATION DATA
A number of studies have described the magnitude of the problem of MSDs in terms of workers’ compensation costs. In 1989, workers’ compensation claims for policy holders in 45 states reported a mean cost of $8070 per UE cumulative trauma disorder claim.9 They estimated the total direct US workers’ compensation costs for UE cumulative trauma disorders to be $563 million in 1989.9 For 1987–1995, the state of Washington adjudicated over 160 000 UE MSD claims with a mean direct (medical and indemnity) cost of a claim ranging from $6 593 to 15 790.10 For all MSD claims (neck and back in addition to UE), Washington state accepted 392 925 claims resulting in $2.6 billion in direct costs and 20.5 million lost workdays.11 These estimates do not include the indirect costs, such as administrative costs for claims processing, lost productivity, and the cost of recruiting and training replacements. It has been suggested that indirect costs are two to three times the direct compensation costs.12
While these numbers highlight the costs of UE MSD to society, they do not take into account those workers who suffer a UE MSD but are never recorded onto the Occupational Safety and Health Administration (OSHA) 300 Log or workers who choose not to file a claim.13–15 For the state of Michigan in 1996, only 25% of workers with work-related MSD filed for workers’ compensation.13,14 Factors associated with filing a claim included increased length of employment, lower annual income, dissatisfaction with coworkers, physician restriction on activities, type of physician providing treatment, being off work for at least 7 days, decreased current health status, and increased severity of illness.15 Other factors workers consider when deciding whether to file a claim include: Will the claim be contested, will there be employer retribution, and are there alternatives available for payment of medical costs?16,17
Occupations at risk
Case reports have given rise to a number of disorders named for the patient’s occupation (Table 2.1).
TABLE 2.1 Work-related MSD named by occupation.
| Bricklayer’s shoulder |
| Carpenter’s elbow |
| Golfer’s elbow |
| Tennis elbow |
| Janitor’s elbow |
| Stitcher’s wrist |
| Cotton twister’s hand |
| Telegraphist’s cramp |
| Writer’s cramp |
| Bowler’s thumb |
| Jeweler’s thumb |
| Cherry pitter’s thumb |
| Gamekeeper’s thumb |
| Carpetlayer’s knee |
These disorders are not unique to their occupations. In 2014, the BLS reported nursing assistants had the highest MSD rates, followed by emergency medical technicians/paramedics, fire fighters, and refuse/recyclable material collectors.5 The National Health Interview Survey described cases of self-reported carpal tunnel syndrome to be highest among mail/message distributors (prevalence 3.2%), health assessment and treatment occupations (2.7%), and construction trades (2.5%).18 The Wisconsin workers’ compensation program reported wrist injury to be highest among dental hygienists, data entry keyers, and hand-grinding and polishing occupations.19 Although the various occupations have different rates of MSD, the BLS and workers’ compensation data point to almost all occupations reporting at least one case of work-related MSD.
Industries at risk
According to the BLS data, the transportation and warehousing industry had the highest number and rate of MSDs followed by the healthcare and social assistance industry (Figure 2.3). Work-place evaluations have also identified a high prevalence of UE MSD in the animal-slaughtering and processing industries (beef, pork, poultry, and fish).20–23 Workers in the poultry industry with an astonishingly high prevalence (34%) were found to have carpal tunnel syndrome using self-reported hand and wrist symptoms, hand diagrams, and nerve conduction studies (median mononeuropathy) to define carpal tunnel syndrome.20

FIGURE 2.3 Musculoskeletal disorder incidence rates for selected private sector industries, 2013–2014.6
In summary, despite their numerous limitations, BLS and workers’ compensation data are sufficient to confirm that the UE MSD problem is large and that rates significantly differ between industries and occupations signifying that workplace factors are important risk factors.
Epidemiology
One of the main purposes of epidemiologic studies is to identify factors that are associated (positively or negatively) with the development or recurrence of adverse medical conditions. No single epidemiologic study determines causality. Rather, results from epidemiologic studies can contribute to the evidence of causality. Over the past decade, several publications have reviewed the medical and ergonomic literature to determine whether scientific evidence supports a relationship between workplace physical factors and MSDs. The most comprehensive review was completed by Bernard et al. at the National Institute for Occupational Safety and Health (NIOSH).24 This review focused on disorders that affected the neck (tension neck syndrome), upper extremities (shoulder tendinitis, epicondylitis, CTS, hand–wrist tendinitis, and hand–arm vibration syndrome), and the lower back (work-related low back pain). A database search strategy initially identified 2000 studies, but laboratory, biomechanical, clinical treatment, and other nonepidemiologic studies were excluded, leaving 600 for systematic review. The review process consisted of three steps.
The first step gave the increased emphasis, or weight, to studies that had high participation rates (>70%), physical examinations, blinded assessment of health and exposure, and objective exposure assessment. The second step assessed for any other selection bias and any uncontrolled potential confounders. The final step summarized studies with regard to strength of the associations, consistency in the associations, temporal associations, and exposure–response (dose–response) relationships.
Bernard et al. concluded that a substantial body of credible epidemiologic research provides strong evidence of an association between MSDs and work-related physical factors. This is particularly true when there are high levels of exposure or exposure to more than one physical factor (e.g., repetition and forceful exertions). The strength of the associations for specific physical stressors varies from insufficient to strong (Table 2.2). The consistently positive findings from a large number of cross-sectional studies, strengthened by the available prospective studies, provide strong evidence for an increased risk of work-related MSDs for the neck, elbow, and hand–wrist for jobs that require high repetition, high force, awkward postures, and vibration. This conclusion was supported by subsequent prospective studies and reviews by the National Academy of Sciences and the Institute of Medicine.16,25–27
TABLE 2.2 Evidence for causal relationship between physical work factors and upper extremity musculoskeletal disorders.24
Source: Adapted from Bernard.24
| Body part and risk factor | Strong evidence (+++) | Evidence (++) | Insufficient evidence (+/0) | Evidence of no effect (−) |
| Neck and neck/shoulder | ||||
| Repetition | ✓ | |||
| Force | ✓ | |||
| Posture | ✓ | |||
| Vibration | ✓ | |||
| Shoulder | ||||
| Posture | ✓ | |||
| Force | ✓ | |||
| Repetition | ✓ | |||
| Vibration | ✓ | |||
| Elbow | ||||
| Repetition | ✓ | |||
| Force | ✓ | |||
| Posture | ✓ | |||
| Combination | ✓ | |||
| Hand/wrist | ||||
| Carpal tunnel syndrome | ||||
| Repetition | ✓ | |||
| Force | ✓ | |||
| Posture | ✓ | |||
| Vibration | ✓ | |||
| Combination | ✓ | |||
| Tendinitis | ||||
| Repetition | ✓ | |||
| Force | ✓ | |||
| Posture | ✓ | |||
| Combination | ✓ | |||
| Hand–arm vibration syndrome | ||||
| Vibration | ✓ | |||
MEASUREMENT—ASSESSMENT
Physical stressors can be grouped into the following categories: repetition, force, posture, and vibration. They arise from excessive job demands, improperly designed workstations, tools, equipment, or inappropriate work techniques. A number of methods are available to measure/estimate these stressors. The method selected should be based on the purpose of the evaluation. The following grouping provides several options.
Survey methods
Employee or supervisor interviews, employee diaries, and employee-completed questionnaires are useful because of their low cost, rapid availability, and, for some, the ability to obtain historical data about previous exposures.28–32 One of the most commonly used survey tools is the so-called Borg, or rating of perceived exertion, scale.33,34 A 15-point scale (6–20) was created to reflect the linear relationship between physical workload and heart rate divided by 10 (e.g., a heart rate of 60 beats/minute corresponds to 6 on the scale). The scale is presented to the subject before the start of a job or job task with anchors of “no exertion at all = (6)” to “maximal exertion = (20).”34 The subject is then asked to rate his or her exertion level after completing the job and/or job task. A 10-point Borg scale was also created to account for large muscle group exertion, rather than heart rate or total body exertion.34,35
The accuracy of self-assessment surveys has been questioned because of the potential for the worker to either underreport or overreport exposures. For example, highly motivated subjects might underestimate their exertion, while unmotivated subjects might overestimate their exertion.36 This potential problem has led many to utilize observational checklists (described below).
Observational methods
Observational methods, such as observational checklists, are commonly employed to objectively assess the workplace for physical stressors. Some checklists can be used by healthcare providers with limited expertise,37–41 others require some training,29,42,43 while others require a considerable amount of experience and training.44–47Table 2.3 provides the reader with a simple checklist for healthcare providers with limited expertise. For healthcare providers or others with some training, the hand activity level (HAL) could be utilized. It is described in more detail in the Exposure Guidelines section”
TABLE 2.3 Physical stressors checklist.
| Risk Factors | Yes | No |
| Hand force | ||
| 1.1 Gripping with the fingertips with something that weighs ≥2 pounds? | ||
| 1.2 Gripping with the whole hand with something that weighs ≥10 pounds? | ||
| 1.3 Lifting >10 pounds, more than twice a minute for >2 hours per day? | ||
| 1.4 Lifting >25 pounds above the shoulders more than 25 times per day? | ||
| Posture | ||
| 2.1 Does the worker have to stand while performing the job? | ||
| 2.2 Are the hands working above the head (or the elbows above the shoulders) >2 hours per day? | ||
| 2.3 Are the wrists bent >30° in any direction for >2 hours per day? | ||
| 2.4 Does the worker use a twisting (“clothes-wringing”) hand motion or use a pinch grip? | ||
| Repetitiveness | ||
| 3.1 Is the cycle time longer than 30 seconds? | ||
| 3.2 Is the same motion with little change occurring every few seconds for >2 hours per day? | ||
| Hand–arm vibration | ||
| 4.1 Does the worker use tools with high vibration levels (e.g., chain saws, chipping hammers, etc.) for >30 minutes per day? | ||
| 4.2 Does the worker use tools with moderate vibration levels (e.g., grinders, sanders, jigsaws, etc.) for >2 hours per day? | ||
| Workstation hardware | ||
| 5.1 The workstation’s orientation cannot be adjusted? | ||
| 5.2 The height of the work surface cannot be adjusted? | ||
| Physical stress | ||
| 6.1 The worker’s arms/hands/wrists come into contact with sharp edges? | ||
| 6.2 Is the worker using heavy gloves? | ||
| Tool design | ||
| 7.1 Is the span of the tool’s handle between 5 and 7 cm? | ||
| 7.2 Is the handle of the tool made from metal? | ||
| 7.3 The tool is not suspended. | ||
| 7.4 Is the tool handle slippery? |
Measuring workers
If the above checklist suggests that physical stressors exist in the workplace, quantitative measurement of those risk factors should be considered. However, quantitative measurement of ergonomic hazards can require the use of specialized equipment and training and expertise in its use and interpretation of the results.29
Methods used to generate quantitative information on physical stressors include electrogoniometers (dynamic measurements of posture), accelerometers, and imaging techniques (electronic and laser optical recordings). Two devices that may be useful outside of research settings are spring scales or gauges to estimate force requirements and simple goniometers to measure static postures. Both of these tools have been used successfully in workplaces due to their simplicity.
Internal forces can be measured using surface electromyography (EMG), but currently available equipment is expensive, and its use requires training and expertise to perform and interpret. Video and imaging systems as a means to measure posture have been used primarily in the laboratory setting where the camera’s line of sight is perpendicular to the planes of the measured body segments. But given the dynamic nature of most job activities, their use in the workplace seems limited unless multiple cameras can be used from a variety of viewing angles. Goniometer use for measuring static postures is well established, but few jobs require continuous static postures. Electrogoniometers can measure dynamic postures, but their accuracy and associated analytic methods are not well established.
EXPOSURE GUIDELINES
American Conference of Governmental Industrial Hygienists (ACGIH)
The ACGIH provides guidelines for industrial hygienists to use while making decisions regarding safe levels of exposure to various hazards in the workplace. The organization issued a guideline known as the “hand activity level” (HAL) based on the hand, wrist, and forearm exposure to repetition and peak normal force for mono-task jobs.48 Mono-task jobs are defined as jobs that repeatedly perform a similar set of motions or exertions for ≥4 hours per day.
The first step is selecting a job period that represents an average activity. Then observe (or videotape) the activity for several job cycles. The second step rates the HAL. This can be accomplished by two methods: (i) a trained observer using a validated rating scale based on exertion frequency, rest pauses, and speed of motion (Figure 2.4)42,48 or (ii) calculated using information on the frequency of exertion and the work/recovery ratio (Table 2.4).48 The third step identifies forceful exertions and postures by (i) observer ratings, (ii) worker ratings, (iii) biomechanical analysis, or (iv) instrumentation. Since the latter two methods (biomechanical analysis or instrumentation) require considerable expertise and equipment, the following discussion will focus on observer and worker ratings. Observer ratings of force utilize the same Latko et al. scale described earlier (Figure 2.4).42 Factors that the observer should consider include the weight, shape, and friction of the work object, posture, glove fit and friction, mechanical assists, torque specification of power tools, quality control, and equipment maintenance. Worker ratings utilize the same Borg scale (1–10) described earlier.34,35 Suppose, for example, a male worker rates his job’s grip strength requirements as four (somewhat strong). To normalize this force, we measure the worker’s grip strength (300 newtons (N)) and compare this to the average male strength (500 N). Therefore, the normalized peak force = 4 × 300 N/500 N = 2.4. The precision of both the observer and worker ratings is improved by having multiple observers/workers rate the same job.

FIGURE 2.4 Visual analog scale for rating hand activity level (0–10) with verbal anchors.42
TABLE 2.4 Hand activity level (0–10) is related to exertion, frequency, and duty cycle (% of work cycle where force is greater than 5% of maximum).
Source: From American Conference of Governmental Industrial Hygienists (ACGIH®) TLV® Hand Activity Level Document.48 From ACGIH®, 2015 TLVs® and BEIs® Book. Copyright 2015. Reprinted with permission.
| Frequency (exertion/s) | Period (s/exertion) | Duty cycle (%) | ||||
| 0–20 | 20–40 | 40–60 | 60–80 | 80–100 | ||
| 0.125 | 8.0 | 1 | 1 | – | – | – |
| 0.25 | 4.0 | 2 | 2 | 3 | – | – |
| 0.5 | 2.0 | 3 | 4 | 5 | 5 | 6 |
| 1 | 1.0 | 4 | 5 | 5 | 6 | 7 |
| 2 | 0.5 | – | 5 | 6 | 7 | 8 |
The HAL and the normalized force estimates can now be plotted and compared to the TLV® (Figure 2.5). Employees performing job tasks above the solid top line will be at significant risk of acquiring a UE MSD, and specific control measures should be utilized so that the force/repetition for a given level of hand activity is below this line. The dotted lower line represents an “action limit,” the point at which general controls, including surveillance (discussed below), are recommended. The TLV® does not specifically account for awkward or extreme postures, contact stresses, low temperatures, and vibration; therefore, professional judgment is needed to account for these additional stressors. If any of these stressors are present on jobs, the TLV® and the action limit will be lower.

FIGURE 2.5 The TLV® for reduction of work related musculo-skeletal disorders based on “hand activity” or “HAL” and peak hand force. The top line depicts the TLV®. The bottom line is an Action Limit for which general controls are recommended. .
Source: Adapted from American Conference of Governmental Industrial Hygienist (ACGIH®) TLV® Hand Activity Draft Document. From ACGIH®, 2015 TLVs® and BEIs® Book. Copyright 2015. Reprinted with permission
Others
Since ACGIH TLV® does not account for all potential physical stressors, the reader is encouraged to review other proposed UE exposure guidelines, such as the strain index proposed by Moore and Garg45,49 and the Rapid Upper Limb Assessment (RULA) proposed by McAtamney and Corlett.46
Posture is an important potential physical stressor.29 Posture can be defined as the position of a part of the body relative to an adjacent part, as measured by the angle of the connecting joint. Standard posture definitions (neutral and nonneutral) and normal ranges of motion have been developed by the American Academy of Orthopaedic Surgeons.50 Postural stress develops as a joint reaches its maximal deviation; therefore, postures should be maintained as close to neutral as possible. In addition to postures at the extreme end of a joint’s range, tasks that require finger-pinching postures have been associated with UE musculoskeletal disorders. Kodak has proposed the following posture guidelines51:
- Keep the work surface height low enough to permit employees to work with their elbows at their sides and wrists near their neutral position.
- Keep reaches within 20 inches in front of the work surface so that the elbow is not fully extended when forces are applied.
- Keep motions within 20–30° of the wrist’s neutral point.
- Avoid operations that require more than 90° of rotation around the wrist.
- Avoid gripping requirements in repetitive operations that spread the fingers and thumb apart more than 2.5 inches. Cylindrical grips should not exceed 2 inches in diameter, with 1.5 inches being the preferable size.
Federal Ergonomic Standard
In 2000, OSHA developed and issued an ergonomic standard. In 2001, Congress, under the Congressional Review Act, passed a resolution of disapproval, thereby eliminating the standard. In its place, OSHA has issued industry-specific guidelines.52 OSHA has developed ergonomic guidelines to prevent MSDs for meatpacking plants (1993), retail grocery stores (2004), shipyards (2008), nursing homes (2009), foundries (2012), and poultry processing (2013).52 In addition, OSHA continues to inspect and, if appropriate, cite companies for ergonomic hazards under its general duty clause.
California Ergonomic Standard
In 1993, the California State Legislature required its Occupational Safety and Health Standards Board to develop “standards for ergonomics in the workplace designed to minimize instances of injury from repetitive motion.”53 Subsequent legal challenges shaped its content, coverage, and start date. The standard, adopted in 1999, applies to a job, process, or operation where a repetitive motion injury (RMI) has occurred to more than one employee under the following conditions:
- A licensed physician objectively identified and diagnosed the RMI.
- The RMI was work related (≥50% caused by a repetitive job, process, or operation).
- The employees with RMIs were performing a job process or operation of identical work activity (performing same repetitive motion task).
- The employee reported the RMI to the employer in the last 12 months.
If the above conditions are met, the employer is required to develop an ergonomic program with the following three components: worksite evaluation, control of workplace exposures, and employee training. The worksite evaluation requires that each job, process, or operation of identical work activities be evaluated for exposures causing RMIs. If these exposures are found, they must be corrected in a timely manner or, if not capable of being corrected, have the exposures minimized to the extent feasible. In addition, employees must receive training on the following:
- The employer’s ergonomic program
- The exposures which have been associated with RMIs
- The symptoms and consequences of injuries caused by repetitive motion
- The importance of reporting symptoms and injuries to the employer
- Methods used by the employer to minimize RMIs
NORMAL PHYSIOLOGY AND ANATOMY
Muscles
Muscle consists of muscle fibers (muscle cells), nerve elements (motor neurons, afferent neurons, receptors of different types), connective tissue, and blood vessels. Muscle fibers are classified into two types: Type I fibers, also known as slow-twitch or red muscle fibers, and Type II fibers, also known as fast-twitch or white muscle fibers. In muscle fibers, the smallest morphological contractile unit is the sarcomere, built of actin and myosin filaments. The smallest functional unit is the motor unit, which consists of a motor neuron cell and the muscle fibers that its branches supply. The muscles of the body are the generators of internal force that convert chemically stored energy into mechanical work. A muscle contracts its threadlike fibers, which shortens the length of the muscle, thereby generating a contractile force.
Myalgia is the medical term for the symptom of muscle pain. The most common type of myalgia, delayed-onset muscle soreness (DOMS), is a contraction-induced injury after vigorous or unaccustomed exercise. DOMS is a self-limiting condition that typically appears within the first 24 hours after exercise, peaks at 48–72 hours, and resolves within 1 week. Histologic and chemical changes are found in affected muscles, but these changes are not permanent and lead to a conditioning effect when occurring in a graduated manner.54–56 Armstrong proposed the following theory for the pathogenesis of DOMS54–56:
- High mechanical forces, particularly those associated with eccentric exertions, cause structural damage of the muscle fibers and associated connective tissue structures.
- This structural damage alters the sarcolemma’s permeability, producing a net influx of calcium into the cell. This calcium inhibits mitochondrial production of ATP and activates proteolytic enzymes that degrade Z-disks, troponin, and tropomyosin.
- The progressive degeneration of the sarcolemma is accompanied by diffusion of intracellular products into the interstitium and plasma; this attracts inflammatory cells that release lysosomal proteases, which further degrade the muscle proteins.
- Active phagocytosis and cellular necrosis lead to accumulation of histamine, kinin, and potassium, which stimulate regional nociceptors, resulting in the sensation of DOMS.
Eccentric contractions (muscle activation while the muscle is stretched), rather than isometric contractions, are felt to lead to DOMS.57 Eccentric contractions can occur when muscles are exposed to either a single rapid stretch or a series of repetitive contractions.54 Both models are consistent with DOMS requiring a temporary reduction in physical loading because of pain or discomfort. This is followed by a gradual increase in physical loading to stimulate healing and subsequent tissue-remodeling processes.
Muscle also undergoes a number of age-related changes, such as a 20% decrease in muscle mass, a 20% reduction in maximal isometric force, and a 35% decrease in the maximal rate of developing force and power.58 This latter reduction is not due to differences in muscle recruitment strategies, but rather due to a change in the contractility of the muscle itself.59 This translates into a marked decrease in the ability to sustain power over repeated contractions in older individuals. In addition, animal experiments have also demonstrated that older muscle damages more easily and heals more slowly.60,61 These effects may help explain why older athletes seem to require greater rest intervals between training sessions and why workers in physically demanding jobs tend to change to less demanding jobs with age.62
Tendons
As a general rule, tendons transmit the contractile force generated by muscles to bone. Tendons are composed of collagen fibrils grouped into fibers that are collected together into fiber bundles that are united into fascicles.63 A large number of fascicles form the tendon. The fiber bundles and fascicles are enclosed in thin films of loose connective tissue called the endotenon. This connective tissue contains blood vessels, lymphatic vessels, nerves, and elastic fibers and allows the fascicles to slide relative to one another. The whole tendon is wrapped in connective tissue called the epitenon. In some tendons, a further sheath, the paratenon, surrounds the tendon. The paratenon is merely a specialization of the areolar connective tissue through which many tendons run. A number of structures associated with tendons control and facilitate their movement. Where tendons wrap around bony pulleys or pass over joints, they are held in place by retaining ligaments (retinacula or fibrous sheaths that prevent bowstringing). Tendons glide beneath these retaining structures due to the lubrication provided by the synovial sheath.64 In some regions, tendons are prevented from rubbing against adjacent structures by bursae. Although tendons generally have a good blood and nerve supply, regions of tendon subjected to friction, compression, or torsion are hypovascular or avascular. The general structure of tendons is modified in two regions: the sites where they attach to bone (enthesis) and the region where they are compressed against neighboring structures (around bony pulleys).65 Fibrocartilage formation at the site of this compression loading is considered a normal/adaptive response. In summary, tendons have the capacity to change their structure and composition in response to mechanical stimulation. In most cases, this mechanical stress is beneficial and adaptive for maintaining cell activity and tissue function.
Peripheral nerves
Peripheral nerves carry signals to and from the central nervous system. A nerve fiber (neuron) consists of the nerve body, which is located in the anterior horn of the spinal cord (motor neuron) or in the dorsal root ganglia (sensory neuron), and a process extending into the periphery—the axon.66 The axon is surrounded by Schwann cells. In myelinated fibers, a Schwann cell is wrapped around only one axon, in contrast to nonmyelinated fibers, where the Schwann cell wraps around several axons. Myelinated and nonmyelinated nerve fibers are organized in bundles, called fascicles, which are bound by supportive connective tissue, the perineurium. The bundles are usually organized in groups, held together by loose connective tissue called the epineurium. In between the nerve fibers and their basal membrane is the intrafascicular connective tissue—the endoneurium. The amount of connective tissue components varies between nerves and between various levels along the same nerve. The myelin insulation divides the axon into short, uninsulated regions (nodes of Ranvier) and longer, insulated regions (internodes). Conduction of nerve impulses proceeds by sequential activation of successive nodes without depolarization of the intervening internode (saltatory conduction).
PATHOPHYSIOLOGY AND PATHOGENESIS
Muscles
Myopathy is the medical term for measurable pathologic changes in a muscle, with or without symptoms. Myopathies can be due to a variety of congenital (e.g., muscular dystrophy) or acquired (e.g., inflammatory, metabolic, endocrine, or toxic) disorders. These diseases are not typically work related and will not be discussed further.
Muscle pain syndromes of unknown etiology can be classified into two categories: general and regional. General muscle pain involving all four quadrants of the body is called primary fibromyalgia. Primary fibromyalgia is not work related because, by definition, trauma-induced myalgia is excluded by the specific diagnostic criteria set by the American College of Rheumatology.67 Regional muscle pain syndromes, not involving the whole body, often fall under the term myofascial syndrome. This has been defined as a painful condition of skeletal muscle characterized by the presence of one or more discrete areas (trigger points) that are tender when pressure is applied.68 These muscle-related syndromes, a common example of which is tension neck syndrome, could be associated with work exposures. A variety of mechanisms have been proposed to account for this syndrome. A few are listed below.
WORK AND ECCENTRIC CONTRACTIONS
DOMS is a result of eccentric contractions that could occur on or off the job. DOMS has objective histologic and chemical changes, but these changes are part of the normal physiologic response. The pain or discomfort associated with DOMS, however, typically results in a temporary reduction in physical loading due to pain or discomfort. This is followed by a gradual increase in physical loading to stimulate healing and subsequent tissue remodeling. But if workers with physically demanding jobs have little control over the magnitude and duration of loading, the work can aggravate and hinder the healing process, thereby increasing the risk of developing a more chronic condition. Work-hardening programs are specifically designed to minimize this risk by prescribing graduated physical training regimens.
WORK AND GAMMA MOTOR NEURONS
This theory starts with evidence that muscle pain, inflammation, ischemia, or sustained static muscle contractions are known to lead to the release of potassium chloride, lactic acid, arachidonic acid, bradykinin, serotonin, and histamine in the affected muscle.69 These substances, in turn, are known to excite chemosensitive group II and IV afferents, which have a potent effect on gamma-muscle spindle systems and heighten the response of those spindles to stretch. Increased activity in the primary muscle spindle afferents may cause muscle stiffness, leading to further production of metabolites, more stiffness, and repetition of the cycle.
WORK AND THE OVERLOAD OF TYPE I FIBERS
Another hypothesis for the pathogenesis of tension neck syndrome is that prolonged static contractions of the trapezius muscle result in an overload of Type I muscle fibers. Type I muscle fibers are used for low static contractions. Support for this hypothesis comes from findings on biopsy. When compared to healthy controls, Type I fibers in patients with chronic trapezius muscle pain (i) were larger, (ii) had a lower capillary-to-fiber ratio, (iii) had a more “ragged” appearance, and (iv) had reduced ATD and ADP levels.70–72 Whether these findings are due to inadequate muscle recruitment73 or inadequate tissue oxygenation is unknown.74
WORK AND MUSCLE FATIGUE
Finally, much work has been done on the mechanisms of fatigue relating to muscle disorders. A complete review of these mechanisms can be found in Gandevai et al.75
Tendons
Physicians in sports medicine have suggested that tendon disorders fall into four main categories: paratendonitis, paratendonitis with tendinosis, tendinosis, and tendinitis.76 These categories are based on clinical and histologic findings. It can be difficult to distinguish between these specific conditions on clinical evaluation alone. Because most conditions can be treated conservatively, histologic changes have been documented in only a subset of patients whose cases proceeded to surgery. Thus, many cases are defined simply as “tendinitis” based on history, examination, and impaired function.
Proposed mechanisms for work-related tendon disorders include77(i) ischemia in hypovascular tissues, (ii) microinjuries incurred at a rate that exceeds repair potential, (iii) thermal denaturation, (iv) dysregulation of paratendon–tendon function, and (v) inflammatory processes secondary to some, or all, of these other factors.
Shoulder disorders provide evidence for the ischemic theory. Work above one’s head can have two effects: compression (impingement) and reduced local blood flow. Impingement comes from the narrow space between the humeral head and the tight coracoacromial arch. As the arm is raised in abduction, the rotator cuff tendons and the insertions on the greater tuberosity are forced under the coracoacromial arch.78 Reduced local blood flow occurs when the supraspinatus muscle is statically contracted, increasing the intramuscular pressure higher than the arterial pressure of the vessel traversing the supraspinatus muscle belly and supplying it with oxygen.79 These work factors, in combination with the fact that the entheses of the three tendons comprising the rotator cuff are hypovascular, lead to tendon degeneration manifested by microruptures and calcium deposits. Once the tendons are degenerated, exertion may trigger an inflammatory response resulting in active tendinitis.80
The pathogenesis and pathophysiology of lateral epicondylitis are less clear. Histologic evaluation of tennis elbow tendinosis identifies a noninflammatory response in the tendon. This histopathology reveals disorganized immature collagen formation in association with immature fibroblastic and vascular elements. This has been named angiofibroblastic tendinosis and is thought to be the result of an avascular degenerative process.81 This pathology is located at the enthesis of the extensor carpi radialis brevis (ECRB) tendon. Factors associated with its development include age, systemic factors, direct trauma, and repetitive overuse from sports, occupation, and performing arts. It is theorized that multiple repetitive eccentric loading of the ECRB results in tension loading, microruptures of the peritendon, and secondary anoxia and degenerative consequences.
Tendon sheaths
Tendons can become trapped in their synovial sheaths due to a narrowing of their fibro-osseous canal. Tendons passing through stenotic canals frequently have a nodular or fusiform swelling and can be covered with granulation tissue.82 Whether these tendon changes are a cause or an effect of the narrowing is unclear. If the narrowing occurs in the first dorsal compartment, the disorder is known as De Quervain’s tenosynovitis.82 If it occurs in the flexor digits or thumb (A1 pulley), it is known as trigger finger or trigger thumb.83
While the term tenosynovitis implies inflammation of the tendon sheath, inflammatory cells are rarely found on histology. The lack of inflammatory cells could represent a sampling bias, since typically only chronic severe cases are biopsied, when the inflammatory process could have already run its course.84 The fundamental pathologic change is hypertrophy and/or fibrocartilaginous metaplasia; however, recent studies suggest that the fibrocartilaginous metaplasia represents an adaptive response to compressive forces.65
The pathogenesis of tendon entrapment disorders involves static compression, repeated compression, and acute trauma. The static compression model is based on clinicians’ observations that De Quervain’s tenosynovitis is related to repeated, prolonged, or unaccustomed exertions that involve the thumb in combination with nonneutral wrist or thumb postures. Tensile loading of the abductor pollicis longus or extensor pollicis brevis, in combination with their turning a corner at the extensor retinaculum, creates a compressive force. The retinaculum responds with functional hypertrophy or fibrocartilaginous metaplasia. The duration of compression is more important than the number (repetition) of compressions. The repeated compression theory relies on the same biomechanical argument, except that the number of episodes of loading (repetition) is more critical than the accumulated duration of loading.
Peripheral nerves
Although a number of peripheral nerve entrapment disorders exist, CTS is the most common and most studied and will be the only nerve entrapment disorder discussed in this chapter.
CTS is the entrapment of the median nerve within the carpal canal at the wrist. Three mechanisms have been suggested85: (i) friction associated with repetitive tendon motions, leading to flexor tendon sheath irritation and swelling, (ii) repeated direct mechanical trauma to the median nerve by structures within the carpal tunnel, and (iii) prolonged elevated pressure within the carpal tunnel, leading to ischemia, tissue swelling, and epineural fibrosis. The last mechanism is supported by the following: (i) carpal tunnel pressure (CTP) is almost always higher in patients with CTS than in normal subjects86; (ii) surgical decompression (carpal tunnel release surgery) seems to be effective at reducing the elevated CTP and improving symptoms87; (iii) histologic studies of the flexor tendon sheaths biopsied during carpal tunnel release show edema and vascular changes consistent with long-standing ischemia88; (iv) animal models of acute and chronic nerve compression show physiologic and histologic findings consistent with nerve ischemia86; (v) in human studies, acute elevation of CTP results in acute nerve dysfunction, with the critical threshold varying according to the subject’s diastolic blood pressure89; and (vi) human studies have found a dose–response relationship between CTP and wrist posture,90 fingertip loading,91 and repetitive hand activity.25–27,92 These findings are consistent with the static and dynamic biomechanical models of CTS.93
Psychosocial
Numerous studies have reported an association between psychosocial factors and UE MSD. Several models have been developed to explain these associations including the following:
- The balance theory of job design and stress94
- The biopsychosocial model95
- The ecological model96
- The workstyle model97
Figure 2.6 summarizes the salient features of these models.98

FIGURE 2.6 Explanatory model of the impact of psychosocial work environmental factors on the onset of musculoskeletal disorders.
Source: Hauke A, Flintrop J, Brun E, et al. The impact of work-related psychosocial stressors on the onset of musculoskeletal disorders in specific body regions: a review and meta-analysis of 54 longitudinal studies. Work & Stress: An International Journal of Work, Health & Organizations 2011; 25(3):243–56. Reprinted with permission from Taylor and Francis, Copyright 2011.
The biologic mechanism by which psychosocial stress and psychological strain lead to UE MSD has not been fully elucidated, but research suggests that it may involve neuroendocrine, vascular, and immunological pathways. For example, perceived stress can trigger the “fight-or-flight” response stimulating the endocrine system (e.g., cortisol, adrenalin, and noradrenalin). Stress can also lead to increased muscle tension,99 decreased blood supply in the extremities,100 breakdown of muscle protein and its repair,101 and alteration of the inflammatory or immune response.102 All these short-term responses might increase the risk of UE MSDs.
Skeptics of the possible role between psychosocial factors/stress and UE MSD point out that support for the association comes from a plethora of cross-sectional studies. Due to inherent properties of their study design, cross-sectional studies have difficulty establishing the direction of the association (i.e., cause vs. effect). Longitudinal studies, on the other hand, can overcome this limitation. A review and meta-analysis of 54 longitudinal studies concluded that psychosocial factors are independent predictors for the onset of MSDs98 and should play an important role in prevention and intervention programs.103,104
DIAGNOSIS AND TREATMENT
The successful treatment of UE MSDs relies on early reporting of symptoms, prompt evaluation, early diagnosis, and appropriate interventions. The interventions should include both the medical management of the disorder and the workplace (discussed in the Prevention section).
Clinical evaluation
Like all conditions with a potential occupational etiology, the health history is a fundamental component of the evaluation.105 The history should (i) characterize the symptoms, (ii) provide an employee description of work activities, and (iii) identify predisposing conditions or factors. In addition, the history should inquire about previous occupations and job tasks, symptoms during previous jobs, home responsibilities, home use of power tools, hobbies, similar symptoms among coworkers, and broader changes in the workplace (e.g., changes in equipment, bonus or incentive programs, layoffs, and training). This information should be documented in the employee’s medical record. If the employee description of work activities is unclear, or if further information is needed to understand employee job tasks and workplace conditions, this can be ascertained by visiting the workplace or viewing job tasks recorded on videotape. Simply reviewing a written description of job tasks may not provide an adequate understanding of the ergonomic stresses involved in the job.
After the history is taken and information obtained about workplace conditions and job tasks, the neck and UE should be examined.106 A comprehensive examination would include inspection, palpation, assessment of the ranges of motion, evaluation of sensory and motor function, and applicable provocative maneuvers. Employees with underlying systemic disease (e.g., diabetes mellitus) may require a more complete examination involving other organ systems. Results of the examination findings, both positive and negative, should be documented in the employee’s medical record.
Clinical diagnosis
Using the information from the clinical evaluation, an assessment or diagnosis should be made. Diagnoses should be consistent with the International Classification of Diseases, Tenth Revision (ICD-10) (Table 2.5). Terms such as repetitive motion disorder (RMD), repetitive strain injury (RSI), overuse syndrome, and cumulative trauma disorder (CTD) may be useful for surveillance purposes or epidemiologic investigations, but they are not ICD-10 diagnoses and should not be used as individual medical diagnoses.
TABLE 2.5 Common upper extremity musculoskeletal disorders by ICD-10 codes.
Source: Keyserling et al.37 Reproduced with permission of Taylor and Francis.
| ICD-10 Code | Diagnosis |
| Nerve and Nerve root disorders | |
| G540 | Brachial plexus lesions |
| G549 | Nerve root and plexus disorder, unspecified |
| G5600 | Carpal tunnel syndrome |
| G5610 | Other lesions of median nerve |
| G5620 | Lesion of ulnar nerve |
| G5630 | Lesion of radial nerve |
| G5640 | Causalgia of the upper limb |
| G5690 | Unspecified mononeuropathy of the upper limb |
| Tendon or Tendon Sheath Disorders | |
| M66239 | Spontaneous rupture of extensor tendons, forearm |
| M66249 | Spontaneous rupture of extensor tendons, hand |
| M66339 | Spontaneous rupture of flexor tendons, forearm |
| M66349 | Spontaneous rupture of flexor tendons, hand |
| M6688 | Spontaneous rupture of other tendons |
| M7520 | Bicipital tendinitis of the shoulder |
| M7530 | Calcific tendinitis of the shoulder |
| M7540 | Impingement syndrome of the shoulder |
| M7580 | Other shoulder lesions, unspecified |
| M7700 | Medial epicondylitis |
| M7710 | Lateral epicondylitis |
| M6530 | Trigger Finger |
| M654 | Radial styloid tenosynovitis [de Quervain] |
| M65849 | Other synovitis and tenosynovitis, hand |
| M6790 | Unspecified disorder of synovium and tendon |
| M70039 | Crepitant synovitis (acute or chronic), wrist |
| M6740 | Ganglion cysts |
| M67419 | Ganglion, shoulder |
| M67429 | Ganglion, elbow |
| M67439 | Ganglion, wrist |
| M67449 | Ganglion, hand |
| Joint Disorders | |
| M7010 | Bursitis, hand |
| M7020 | Bursitis, elbow (olecranon) |
| M7550 | Bursitis, shoulder |
| M7720 | Periarthritis, unspecified wrist |
| Muscle Disorders | |
| M609 | Myositis, unspecified |
| M629 | Disorder of muscle, unspecified |
| M791 | Myalgia |
| M79609 | Pain in limb |
| Soft Tissue Disorders | |
| R252 | Cramp and spasm |
| R29898 | Other symptoms and signs involving the musculoskeletal system |
| M7098 | Unspecified soft tissue disorder related to use, overuse and pressure other |
| M7981 | Nontraumatic hematoma of soft tissue |
| M7989 | Other specified soft tissue disorders |
| Vascular | |
| I742 | Embolism and thrombosis of arteries of the upper extremities |
Once a diagnosis is made, an opinion is usually rendered regarding whether occupational factors caused, aggravated, or contributed to the condition. This tends to be the most difficult portion of the assessment. Unlike the classic occupational diseases, such as asbestosis or silicosis, most occupational MSDs do not have a pathognomonic finding or test specific to the exposure. Therefore, the importance of a thorough exposure assessment cannot be overemphasized.107 In 1979, NIOSH published a guide for state agencies and physicians on the process of determining work relatedness of disease.108 The process outlined in this guide, modified for MSD, is still relevant today:
- Has a disease condition been established by accepted clinical criteria?
- Does the literature support that the disease can result from the suspected agent?
- Has exposure to the agent been demonstrated?
- Has the exposure been of sufficient degree and duration to result in the diseased condition?
- Have nonoccupational factors been considered?
- Have other special circumstances been considered?
Clinical interventions
The goals for treatment are the elimination or reduction of symptoms and impairment and return to work under conditions that will not exacerbate the disorder. The expected duration of treatment, dates for follow-up evaluations, and time frames for improvement or resolution of symptoms should be specified at the initial evaluation and updated on subsequent evaluations.
RESTING THE SYMPTOMATIC AREA
Resting the symptomatic area is a mainstay of conservative treatment. Reducing or eliminating employee exposure to musculoskeletal risk factors by changing the job conditions (forceful exertions, repetitive activities, extreme or prolonged static postures, vibration, direct trauma) is the most effective way to rest the symptomatic area. This allows employees to remain productive members of the workforce and is best accomplished by engineering and work practice controls in the workplace (see Prevention section).
Until effective controls are installed, employee exposure to biomechanical stressors can be reduced through restricted duty and/or temporary job transfer. The principle of restricted duty and temporary job transfer is to reduce or eliminate the total amount of time spent exposed to the same or similar musculoskeletal risk factors.109,110 A variety of factors (e.g., symptom type, duration, and severity, response to treatment, and biomechanical stressors associated with work) must be considered when determining the length of time for which an employee is assigned to restricted duty. When trying to determine the length of time assigned to restricted work, the following principle applies: the degree of restriction should be proportional to symptom severity and intensity of the job’s biomechanical stressors. In addition, caution must be used in deciding which jobs are suitable for job transfer, because different jobs may pose similar biomechanical demands on the same muscles and tendons.
Complete removal from the work environment should be reserved for severe disorders, workplaces where the only available jobs involve significant biomechanical stressors for the symptomatic area, or workplaces where significant modifications to the current or available jobs are not feasible. For purposes of removal from the work environment, severe disorders can be defined as those that negatively affect that employee’s activities of daily living (e.g., difficulty in buttoning clothes, opening jars, and brushing hair). In addition, employees with MSDs should be advised about the potential risk posed by hobbies, recreational activities, and other personal habits that involve certain biomechanical stressors. Employees should modify their behaviors to reduce such stress.
TREATMENT
Clinical interventions should be tailored to the specific diagnosis. In general, involving a physical or occupational therapist has been shown to improve outcomes.111–115 However, the effectiveness of various treatment modalities (traditional, complementary, and alternative) used by physiotherapists is less clear.116–120Table 2.6 lists a number of these treatment modalities. According to Crawford et al., there is evidence of the efficacy of conservative treatments for CTS, epicondylitis, rotator cuff tendonitis, bicipital tendonitis, and tension neck syndrome, but the evidence is less clear for tenosynovitis, tendonitis, de Quervain’s disease, or diffuse nonspecific UE MSDs.115
TABLE 2.6 Interventions used by physiotherapists to treat upper extremity musculoskeletal disorders.
| Stretching and strengthening exercises (passive and active) |
| Nonsteroidal anti-inflammatory drugs (oral or topical) |
| Orthotic devices (e.g., brace and splint) |
| Therapeutic ultrasound (thermal and mechanical) |
| Heat and cold therapy |
| General exercise |
| Massage |
| Various injections |
| Glucocorticoid (e.g., steroids) |
| Glycosaminoglycan polysulfate |
| Sodium hyaluronate |
| Autologous blood |
| Botulinum toxin |
| Platelet-rich plasma |
| Prolotherapy (proliferative injection therapy) |
| Acupuncture |
| Iontophoresis |
| Low-level lasers |
| Pulsed electromagnetic field |
| Extracorporeal shock wave therapy |
Most (approximately 80%) UE MSDs improve with conservative measures, but about 20% of patients continue to have symptoms for a year or more.121 Symptomatic employees should be followed until their symptoms improve. Employees who do not improve within the expected time frames should be reevaluated, or a second opinion should be obtained.122 Surgical options should be reserved for severe, chronic cases that, after an adequate trial of conservative therapy (described above), prevent return to work or show objective signs of disease progression.123 The length of time needed for an “adequate” trial of conservative therapy depends on many variables, including an employee’s ability to remain productive without jeopardizing his or her long-term health.
SURVEILLANCE
Surveillance is “the ongoing systematic collection, analysis, and interpretation of health and exposure data in the process of describing and monitoring a health event.”124 The goal of both hazard and health surveillance systems is the identification of hazardous exposures. Once hazardous exposures are recognized, intervention efforts can be targeted at those exposures with the purpose of preventing future health problems in (as yet) unaffected individuals.
Surveillance should not be confused with screening. Screening is the application of a clinical test to asymptomatic individuals at increased risk for a particular disease.125 The goal of a screening test is the identification of individuals who need further medical evaluation or other interventions. Although clinical tests can identify individuals with MSDs early in their development, there are currently no known screening tests to predict which asymptomatic individuals will develop symptoms and disease. Exposure and health surveillance can be divided into two types: passive and active.
Passive surveillance
Passive health surveillance systems utilize existing databases to identify high-risk industries, occupations, or tasks that are associated with disease or injury. Examples of these databases in occupational medicine include the OSHA 300 Logs, workers’ compensation records, medical department logs, clinical laboratory data, hospital discharge records, and accident reports. Jobs or tasks with an increased UE MSD injury rate can be targeted for further ergonomic and/or medical evaluation. In addition, jobs or departments can be followed over time, to monitor trends in these rates.
Although attractive because of their low cost, passive surveillance databases are sometimes developed for purposes other than surveillance and may have significant limitations.126 These limitations include underreporting and exposure misclassification. For example, underreporting in the OSHA 300 Logs can occur for any of the following reasons: symptomatic employees not seeking medical care (“macho” attitude, ignorance that the condition could be work related, or fear of employer retaliation), restricted or no access to employee health facilities, or misunderstanding about when a case is to be recorded on the OSHA 300 Log.7 Exposure misclassification can occur when employees use a general term to describe their job title; for example, an employee in the poultry industry may report his or her job title as “cutter” in a plant with five distinct cutting positions. Each one of these cutting jobs may be associated with a different ergonomic hazard, and the identification of high-risk jobs requires specific knowledge of the employees’ cutting position.
Active surveillance
Active surveillance systems generate more accurate databases to identify high-risk positions. Direct symptom surveys are good examples of active disease surveillance tools in occupational medicine127 and have been developed for musculoskeletal disorders.128 Symptom surveys collect more accurate information and can serve a triage function if performed in a confidential manner. Active surveillance systems can also collect information on exposures. This information is typically established by plant personnel or nonmedical consultants; however, healthcare providers may be called upon to participate in a comprehensive ergonomic program. If exposure surveillance is a component of that program, the survey instrument in Tables 2.4 and Figures 2.4 and 2.5 could be used to establish an exposure surveillance database.
PREVENTION
The control of identified ergonomic hazards is the most effective means of preventing work-related UE MSDs and is the primary focus of any ergonomic program. Intervention strategies should follow a three-tiered approach: engineering controls, administrative controls, and medical treatment.16,129
Engineering controls
Studies have shown that engineering interventions can reduce ergonomic hazards and can lead to a reduction in MSDs.16,130,131 In some situations, the interventions are obvious and represent common sense solutions. On the other hand, worksites frequently require a more comprehensive approach to the control of ergonomic hazards. This comprehensive approach should address the following risk factors: repetition, force, posture, and vibration.
To reduce repetitiveness, the following interventions could be used: (i) enlarged work content, (ii) automation of some job tasks, (iii) uniform spreading of work across the work shift, and (iv) job restructuring. To reduce force or mechanical stressors, the following interventions should be considered: (i) decreasing the weight of tools, containers, and parts; (ii) optimizing the size, shape, and friction of handles; and (iii) using torque control devices. Reduction of awkward or extreme postures could be achieved by (i) locating the work more appropriately and (ii) selecting tool design and location based upon workstation characteristics. Engineering controls for the reduction of vibration are reviewed in Chapter 4.
In some instances, however, engineering controls are not currently available. Until engineering controls become available, other aspects of an ergonomic program—administrative and medical treatment controls—can be implemented.
Administrative controls
Administrative controls can be defined as work practices or training used to reduce employee exposure to ergonomic stressors. Examples of work practice controls include (i) more frequent and longer rest breaks,132(ii) limiting overtime, (iii) varying work tasks or broadening job responsibilities, and (iv) periodic rotation of workers between stressful and less stressful jobs. Since job rotation exposes more workers to the more stressful job, it is suitable only where short-term performance of the stressful job poses no appreciable ergonomic hazard. Otherwise, other control methods must be utilized. Training programs range from fundamental instruction on the proper use of tools and materials to instruction on the use of protective devices. Improper work technique has been associated with the development of UE MSDs.133,134 Finally, efforts to improve the workplace climate and culture with programs like participatory ergonomics are also considered a type of administrative control.135,136
Medical treatment
The goal of medical treatment as a component of an ergonomic program is to provide prompt evaluation and treatment to limit the severity, disability, and costs associated with these disorders. It should also serve to initiate a reevaluation of the ergonomic stresses associated with the affected worker’s job and institution of appropriate control measures. Medical treatment in this sense is a secondary and tertiary prevention mechanism. Medical management programs are an important component of any successful ergonomic program.137–139 However, they should always be used with engineering and administrative controls during the implementation of a complete ergonomic program.129
References
- 1. Chaffin DB, Andersson GBJ. Occupational biomechanics. New York: John Wiley & Sons, Inc., 1984.
- 2. Boocock MG, Collier JMK, McNair PJ, et al. A framework for the classification and diagnosis of work-related upper extremity conditions: systematic review. Semin Arthritis Rheum 2009; 38(4):296–311.
- 3. Eastman Kodak Company. Ergonomic design for people at work, Vol. 1. New York: Van Nostrand Reinhold Company, 1983:3.
- 4. BLS 2012. Occupational Safety and Health Definitions. U.S. Department of Labor, Bureau of Labor Statistics. Available at: http://www.bls.gov/iif/oshdef.htm. Accessed on October 18, 2015.
- 5. BLS 2015. Nonfatal Occupational Injuries and Illnesses Requiring Days Away from Work, 2014. U.S. Department of Labor, Bureau of Labor Statistics, BSDL 15-2205. Available at: http://www.bls.gov/news.release/archives/osh2_11192015.pdf. Accessed on December 22, 2015.
- 6. NIOSH 2009. Worker Health eChartbook. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health. Available at: http://wwwn.cdc.gov/niosh-survapps/echartbook/Category.aspx?id=563. Accessed on October 20, 2015.
- 7. Rosenman KD, Kalush A, Reilly MJ, et al. How much work-related injury and illness is missed by the current national surveillance system? J Occup Environ Med 2006; 48(4):357–65.
- 8. Leigh JP, Marcin JP, Miller TR. An estimate of the U.S. Government’s undercount of nonfatal occupational injuries. J Occup Environ Med 2004; 46(1):10–8.
- 9. Webster BS, Snook SH. The cost of compensable upper extremity cumulative trauma disorders. J Occup Med 1994; 36:713–27.
- 10. Silverstein B, Welp E, Nelson N, et al. Claims incidence of work-related disorders of the upper extremities: Washington State, 1987 through 1995. Am J Public Health 1998; 88:1827–33.
- 11. Silverstein B, Viikari-Juntura E, Kalat J. Use of a prevention index to identify industries at high risk for work-related musculoskeletal disorders of the neck, back, and upper extremity in Washington State, 1990–1998. Am J Ind Med 2002; 41:149–69.
- 12. Hagberg M, Silverstein B, Wells R, et al. Introduction. In: Kuorinka I, Forcier L, eds. Work related musculoskeletal disorders (WMSDs): a reference book for prevention. London: Taylor & Francis, 1995:1.
- 13. Pansky G, Synder T, Dembe A, et al. Under-reporting of work-related disorders in the workplace: a case study and review of the literature. Ergonomics 1999; 42:171–82.
- 14. Biddle J, Roberts K, Rosenman KD, et al. What percentage of workers with work-related illnesses receive workers’ compensation benefits? J Occup Environ Med 1998; 40:325–31.
- 15. Rosenman KD, Gardiner JC, Wang J, et al. Why most workers with occupational repetitive trauma do not file workers’ compensation. J Occup Environ Med 2000; 42:25–34.
- 16. National Research Council, Panel on Musculoskeletal Disorders and the Workplace. Commission on Behavioral and Social Sciences and Education, and Institute of Medicine. Musculoskeletal disorders and the workplace: low back and upper extremities. Washington, DC: National Academy Press, 2001. Available at: http://www.nap.edu/read/10032/chapter/1. Accessed on June 15, 2016.
- 17. Fan ZJ, Bonauto DK, Foley MP, et al. Underreporting of work-related injury or illness to workers’ compensation: individual and industry factors. J Occup Environ Med 2006; 48(9):914–22.
- 18. Tanaka S, Wild DK, Seligman P, et al. Prevalence and work-relatedness of self-reported carpal tunnel syndrome among US workers: analysis of the Occupational Health Supplement Data of the 1988 National Health Interview Survey. Am J Ind Med 1995; 27:451–70.
- 19. Hanrahan LP, Moll MB. Injury surveillance. Am J Public Health 1989; 9(Suppl):38–45.
- 20. Ramsey J, Musolin K, Mueller C, and NIOSH 2015. Health Hazard Evaluation Report: Evaluation of Carpal Tunnel Syndrome and Other Musculoskeletal Disorders among Employees at a Poultry Processing Plant. NIOSH HHE Report No. 2014-0040-3232. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health. Available at: https://www.cdc.gov/niosh/hhe/reports/pdfs/2014-0040-3232.pdf. Accessed on June 15, 2016.
- 21. Hales T, Habes D, Fine L, et al. 1989. Health Hazard Evaluation Report: John Morrell & Co. Sioux Falls, South Dakota, NIOSH HHE Report No. 88-180-1958. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health.
- 22. Kim JY, Kim JI, Son JE, et al. Prevalence of carpal tunnel syndrome in meat and fish processing plants. J Occup Health 2004; 46(3):230–4.
- 23. Lipscomb H, Kucera K, Epling C, et al. Upper extremity musculoskeletal symptoms and disorders among a cohort of women employed in poultry processing. Am J Ind Med 2008; 51(1):24–36.
- 24. Bernard B, ed. Musculoskeletal disorders and workplace factors: a critical review of epidemiologic evidence for work-related musculoskeletal disorders of the neck, upper extremity, and low back. DHHS (NIOSH) Publication No. 97-141. Cincinnati, OH: National Institute for Occupational Safety and Health, 1997.
- 25. Fan ZJ, Harris-Adamson C, Gerr F, et al. Associations between workplace factors and carpal tunnel syndrome: a multi-site cross sectional study. Am J Ind Med 2015; 58(5):509–18.
- 26. Rempel D, Gerr F, Harris-Adamson C, et al. Personal and workplace factors and median nerve function in a pooled study of 2396 US workers. J Occup Environ Med 2015; 57(1):98–104.
- 27. Bonfiglioli R, Mattioli S, Armstrong T, et al. Validation of the ACGIH TLV for hand activity level in the OCTOPUS cohort: a two-year longitudinal study of carpal tunnel syndrome. Scand J Work Environ Health 2013; 39(2):155–63.
- 28. Radwin RG, Lavender SA. Work factors, personal factors, and internal loads: biomechanics of work stressors. In: National Resource Council, eds. Work-related musculoskeletal disorders. Washington, DC: National Academy Press, 1999: 116–51.
- 29. Lowe BD, Weir PL, Andrews DM, NIOSH 2014. Observation-Based Posture Assessment: Review of Current Practice and Recommendations for Improvement, DHHS (NIOSH) Publication No. 2014-131. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health.
- 30. Wiktorin C, Karlqvist PT, Winkel J, et al. Validity of self-reported exposures to work postures and manual materials handling. Scand J Work Environ Health 1993; 19:208–14.
- 31. Viikari-Juntura E, Rauas S, Martikainen R, et al. Validity of self-report physical work load in epidemiologic studies on musculoskeletal disorders. Scand J Work Environ Health 1996; 22:251–9.
- 32. Buchholz B, Park J-S, Gold JE, et al. Subjective ratings of upper extremity exposures: inter-method agreement with direct measurement of exposures. Ergonomics 2008; 51(7):1064–77.
- 33. Borg GAV. An introduction to Borg’s RPE-Scale. Ithaca, NY: Movement Publications, 1985.
- 34. Borg GAV. Psychological bases of perceived exertion. Med Sci Sports Exerc 1982; 4:377–81.
- 35. Borg GAV. Borg’s perceived exertion and pain scale. Champaign, IL: Human Kinetics, 1998.
- 36. Spielholz P, Silverstein B, Morgan M, et al. Comparison of self-report, video observation and direct measurement methods for upper extremity musculoskeletal disorder physical risk factors. Ergonomics 2001; 44(6):588–613.
- 37. Keyserling WM, Stetson DS, Silverstein BA, et al. A checklist for evaluating ergonomic risk factors associated with upper extremity cumulative trauma disorders. Ergonomics 1993; 36:807–31.
- 38. Lifshitz Y, Armstrong TJ. A design checklist for control and prediction of cumulative trauma disorders in hand intensive manual jobs. In: Proceedings of the 30th Annual Meeting of Human Factors Society, September 29–October 3, Dayton, OH: Human Factors and Ergonomics Society. 1986, pp. 837–841.
- 39. Kemmert K. A method assigned for the identification of ergonomic hazards: PLIBEL. Appl Ergon 1995; 26:199–201.
- 40. International Labour Office. Ergonomic checkpoints: practical and easy-to-implement solutions for improving safety, heath and working conditions. Geneva: International Labour Office, 1996.
- 41. Washington Labor and Industries. 2014. Ergonomics Checklist. Available at: http://www.lni.wa.gov/Safety/SprainsStrains/AwkwardPostures/ErgonomicsChecklist.pdf. Accessed on June 15, 2016.
- 42. Latko WA, Armstrong TJ, Foulke JA, et al. Development and evaluation of an observational method for assessing repetition in hand tasks. Am Ind Hyg Assoc J 1997; 58:278–85.
- 43. Rodgers S. Job evaluation in worker fitness determination. In: Himmelstein JS, Pransky GS, eds. Occupational medicine, state of the art reviews: worker fitness and risk evaluations. Philadelphia, PA: Handley & Belfus, Inc., 1988:219–40.
- 44. Bao S, Howard N, Spielholz P, et al. Two posture analysis approaches and their application in a modified rapid upper limb assessment evaluation. Ergonomics 2007; 50(12):2118–36.
- 45. Moore JS, Garg A. The strain index: a proposed method to analyze jobs for risk of distal upper extremity disorders. Am Ind Hyg Assoc J 1995; 56:443–58.
- 46. McAtamney L, Corlett EN. RULA: a survey method for the investigation of work-related upper limb disorders. Appl Ergon 1993; 24:91–9.
- 47. Louhevaara V, Suurnakki T. OWAS: a method for the evaluation of postural load during work. Training Publication No. 11. Helsinki: Institute of Occupational Health, 1992.
- 48. American Conference of Governmental Industrial Hygienists (ACGIH). 2015 TLVs® and BEIs®: threshold limit values for chemical substances and physical agents and biological exposure indices. Cincinnati, OH: ACGIH Signature Publications, 2015: 179–81.
- 49. Kapellusch JM, Garg A, Hegmann KT, et al. The Strain Index and ACGIH TLV for HAL: risk of trigger digit in the WISTAH prospective cohort. Hum Factors 2014; 56(1):98–111.
- 50. American Academy of Orthopedic Surgeons. Joint motion: method of measuring and recording. Edinburgh: Churchill Livingstone, 1965.
- 51. Eastman Kodak Company. Ergonomic design for people at work, Vol. 2. New York: Van Nostrand Reinhold Company, 1983: 255.
- 52. Occupational Safety and Health Administration. Ergonomics. Available at: https://www.osha.gov/SLTC/ergonomics/. Accessed on June 15, 2016.
- 53. California Department of Industrial Relations. General Industry Safety Orders: Ergonomics. Available at: http://www.dir.ca.gov/title8/5110.html. Accessed on June 15, 2016.
- 54. Armstrong RB. Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Med Sci Sports Exerc 1984; 16:529–36.
- 55. Armstrong RB. Initial events in exercise-induced muscular injury. Med Sci Sports Exerc 1990; 22:429–35.
- 56. Armstrong RB, Warren GL, Warren JA. Mechanisms of exercise-induced muscle fiber injury. J Sports Med 1991; 12:184–207.
- 57. Ashton-Miller JA. Soft tissue responses to physical stressors: muscles, tendons, and ligaments. In: National Resource Council, eds. Work-related musculoskeletal disorders. Washington, DC: National Academy Press, 1999:39–41.
- 58. Faulkner JA, Brooks SV. Muscle fatigue in old animals: unique aspects of fatigue in elderly humans. In: Gandevai S, Enoka R, McComas A, et al., eds. Fatigue, neural and muscular mechanisms. New York: Plenum Press, 1995:471–80.
- 59. Thelen DG, Ashton-Miller JA, Schultz AB, et al. Do neural factors underlie age differences in rapid ankle torque development? J Am Geriatr Soc 1996; 44:804–8.
- 60. Brooks SV, Faulkner JA. The magnitude of the initial injury induced by stretches of maximally activated muscle fibers of mice and rats increases in old age. J Physiol 1996; 497:573–80.
- 61. Brooks SV, Faulkner JA. Contraction-induced injury: recovery of skeletal muscles in young and old mice. Am J Physiol 1990; 258:C436–42.
- 62. Ashton-Miller JA. Response of muscle and tendon to injury and overuse. In: National Resource Council, eds. Work-related musculoskeletal disorders. Washington, DC: National Academy Press, 1999:73–97.
- 63. Gelberman R, Goldberg V, An K-N, et al. Tendon. In: Woo SL-Y, Buckwater JA, eds. Injury and repair of the musculoskeletal soft tissues. Park Ridge, IL: American Academy of Orthopedic Surgeons, 1988:5–40.
- 64. Schumacher HR Jr. Morphology and physiology of normal synovium and the effects of mechanical stimulation. In: Gordon SL, Blair SJ, Fine LJ, eds. Repetitive motion disorders of the upper extremity. Rosemont, IL: American Academy of Orthopedic Surgeons, 1995:263–76.
- 65. Vogel KG. Fibrocartilage in tendon: a response to compressive load. In: Gordon SL, Blair SJ, Fine LJ, eds. Repetitive motion disorders of the upper extremity. Rosemont, IL: American Academy of Orthopedic Surgeons, 1995:205–15.
- 66. Terzis JK, Smith KL, eds. The peripheral nerve: structure, function, and reconstruction. New York: Raven Press, 1990.
- 67. Wolfe F, Smythe HA, Yunus MB et al. The American College of Rheumatology 1990: criteria for the classification of fibromyalgia. Arthritis Rheum 1990; 33:160–72.
- 68. Grosshandler S, Burney R. The myofascial syndrome. N C Med J 1979; 40:562–5.
- 69. Johansson H, Sojka P. Pathophysiological mechanisms involved in genesis and spread of muscle tension in occupational muscle pain and in chronic musculoskeletal pain syndromes: a hypothesis. Med Hypothesis 1991; 35:196–203.
- 70. Lindman R, Eriksson A, Thornell LE. Fiber type composition of the human female trapezius muscle. Am J Anat 1991; 190:385–92.
- 71. Lindman R, Hagberg M, Angquist KA, et al. Changes in the muscle morphology in chronic trapezius myalgia. Scand J Work Environ Health 1991; 17:347–55.
- 72. Larsson SE, Bengtsson A, Bodegard L, et al. Muscle changes in work related chronic myalgia. Acta Orthop Scand 1988; 59:552–6.
- 73. Hagberg M, Angquist KA, Eriksson HE, et al. EMG-relationship in patients with occupational shoulder–neck myofascial pain. In: deGroot G, Hollander AP, Huijing PA, et al., eds. Biomechanics XI-A. Amsterdam: Free University Press, 1988:450–4.
- 74. Murthy G, Kahan NH, Hargens AR, et al. Forearm muscle oxygenation decreases with low levels of voluntary contraction. J Orthop Res 1997; 15:507–11.
- 75. Gandevai S, Enoka R, McComas AJ, et al., eds. Fatigue, neural and muscular mechanisms. New York: Plenum Press, 1995.
- 76. Clancy WG Jr. Tendon trauma and overuse injuries. In: Leadbetter W, Buckwater JA, Gordon SL, eds. Sports-induced inflammation: clinical and basic science concepts. Park Ridge, IL: American Academy of Orthopedic Surgeons, 1990:609–18.
- 77. Hart DA, Frank CB, Bray RC. Inflammatory processes in repetitive motion and overuse syndromes: potential role of neurogenic mechanisms in tendon and ligaments. In: Gordon SL, Blair SJ, Fine LJ, eds. Repetitive motion disorders of the upper extremity. Rosemont, IL: American Academy of Orthopedic Surgeons, 1995:249.
- 78. Fu FH, Harner CD, Klein AH. Shoulder impingement syndrome: a critical review. Clin Orthop 1991; 269:162–73.
- 79. Jarvholm U, Palmerud G, Styf J, et al. Intramuscular pressure in the supraspinatus muscle. J Orthop Res 1988; 6:230–8.
- 80. Hagberg M, Silverstein B, Wells R, et al. Evidence of the association between work and selected tendon disorders: shoulder tendinitis, epicondylitis, de Quervain’s tendinitis, Dupuytren’s contracture, Achilles tendinitis. In: Kuorinka I, Forcier L, eds. Work related musculoskeletal disorders (WMSDs): a reference book for prevention. London: Taylor & Francis, 1995:55–6.
- 81. Nirschl RP, Ashman ES. Elbow tendinopathy: tennis elbow. Clin Sports Med 2003; 22:813–36.
- 82. Moore JS. De Quervain’s tenosynovitis. Stenosing tenosynovitis of the first dorsal compartment. J Occup Environ Med 1997; 39:990–1002.
- 83. Moore JS. Flexor tendon entrapment of the digits (trigger finger and trigger thumb). J Occup Environ Med 2000; 42:526–45.
- 84. Leadbetter WB. Cell–matrix response in tendon injury. Clin Sports Med 1992; 11:533–78.
- 85. Rempel D. Musculoskeletal loading and carpal tunnel syndrome. In: Gordon SL, Blair SJ, Fine LJ, eds. Repetitive motion disorders of the upper extremity. Rosemont, IL: American Academy of Orthopedic Surgeons, 1995:123–32.
- 86. Rempel D, Dahlin L, Lundborg G. Biological response of peripheral nerves to loading: pathophysiology of nerve compression syndromes and vibration induced neuropathy. In: National Resource Council, eds. Work-related musculoskeletal disorders. Washington, DC: National Academy Press, 1999:98–115.
- 87. Gelbermann RH, Rydevik BL, Pess GM, et al. Carpal tunnel syndrome: a scientific basis for clinical care. Orthop Clin North Am 1988; 19:115–24.
- 88. Fuchs PC, Nathan PA, Meyers LD. Synovial histology in carpal tunnel syndrome. J Hand Surg 1991; 16A:753–8.
- 89. Gelbermann RH, Szabo RM, Williamson RV, et al. Tissue pressure threshold for peripheral nerve viability. Clin Orthop 1983; 178:285–91.
- 90. Weiss N, Gordon L, Bloom T, et al. Wrist position of lowest carpal tunnel pressure: implication for splint design. J Bone Joint Surg 1995; 77:1695–9.
- 91. Rempel D, Smutz WP, So Y, et al. Effect of fingertip loading on carpal tunnel pressure. Trans Orthop Res Soc 1994; 19:698.
- 92. Rempel D, Manojlovic R, Levinsohn D, et al. The effect of wearing a flexible wrist splint on carpal tunnel pressure during repetitive hand activity. J Hand Surg 1994; 19A:106–10.
- 93. Keyserling WM. Workplace risk factors and occupational musculoskeletal disorders. Part 2: A review of biomechanical and psychophysical research on risk factors associated with upper extremity disorders. Am Ind Hyg Assoc J 2000; 61:31–43.
- 94. Smith MJ, Carayon P. Work organization, stress, and cumulative trauma disorders. In: Moon SL, Sauter SD, eds. Beyond biomechanics: psychosocial aspects of musculoskeletal disorders in office work. New York: Taylor & Francis, 1996.
- 95. Melin B, Lundberg U. A biopsychosocial approach to work-stress and musculoskeletal disorders. J Psychophysiol 1997; 11:238–47.
- 96. Sauter SL, Swanson NG. An ecological model of musculoskeletal disorders in office work. In: Moon SL, Sauter SD, eds. Beyond biomechanics: psychosocial aspects of musculoskeletal disorders in office work. New York: Taylor & Francis, 1996.
- 97. Feuerstein M. Workstyle: definition, empirical support, and implications for prevention, evaluation and rehabilitation of occupational upper-extremity disorders. In: Moon SL, Sauter SD, eds. Beyond biomechanics: psychosocial aspects of musculoskeletal disorders in office work. New York: Taylor & Francis, 1996.
- 98. Hauke A, Flintrop J, Brun E, et al. The impact of work-related psychosocial stressors on the onset of musculoskeletal disorders in specific body regions: a review and meta-analysis of 54 longitudinal studies. Work Stress 2011; 25(3):243–56.
- 99. Bongers PM, Ijmker S, van den Heuvel S, et al. Epidemiology of work related neck and upper limb problems: psychosocial and personal risk factors (Part I) and effective interventions from a bio behavioural perspective (Part II). J Occup Rehabil 2006; 16:272–95.
- 100. Visser B, van Dieën JH. Pathophysiology of upper extremity muscle disorders. J of Electromyogr Kinesiol, 2006; 16(1):1–16.
- 101. Theorell T, Hasselhorn HM. Endocrinological and immunological variables sensitive to psychosocial factors of possible relevance to work-related musculoskeletal disorders. Work Stress 2002; 16:154–65.
- 102. Chrousos GP, Gold PW. The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. J Am Med Assoc 1992; 267(9):1244–52.
- 103. Bailey TS, Dollard MF, McLinton SS, et al. Psychosocial safety climate, psychosocial and physical factors in the aetiology of musculoskeletal disorder symptoms and workplace injury compensation claims. Work Stress 2015; 29(2):190–211.
- 104. Moshe S, Izhaki R, Chodick G, et al. Predictors of return to work with upper limb disorders. Occup Med 2015; 65:564–9.
- 105. Goldman RH, Peters JM. The occupational and environmental health history. J Am Med Assoc 1981; 246:2831–6.
- 106. Piligian G, Herbert R, Hearns M, et al. Evaluation and management of chronic work-related musculoskeletal disorders of the distal upper extremity. Am J Ind Med 2000; 37:75–93.
- 107. Moore JS. Clinical determination of work-relatedness in carpal tunnel syndrome. J Occup Rehabil 1991; 1:145–58.
- 108. Kusnetz S, Hutchison MK, eds. A guide to the work-relatedness of disease. DHHS (NIOSH) Publication No. 79-116. Cincinnati, OH: Department of Health and Human Services, National Institute for Occupational Safety and Health, 1979.
- 109. McKenzie F, Storment J, Van Hook P, et al. A program for control of repetitive trauma disorders associated with hand tool operations in a telecommunications manufacturing facility. Am Ind Hyg Assoc J 1985; 46(11):674–8.
- 110. Lederman RJ, Calabrese LH. Overuse syndromes in instrumentalists. Med Probl Perform Art 1986; 1:7–11.
- 111. Kuhn JE, Dunn WR, Sanders R, et al. Effectiveness of physical therapy in treating atraumatic full-thickness rotator cuff tears: a multicenter prospective cohort study. J Shoulder Elbow Surg 2013; 22(10):1371–9.
- 112. Kohia M, Brackle J, Byrd K, et al. Effectiveness of physical therapy treatments on lateral epicondylitis. J Sport Rehabil 2008; 17:119–36.
- 113. Piper S, Shearer HM, Côté P, et al. The effectiveness of soft-tissue therapy for the management of musculoskeletal disorders and injuries of the upper and lower extremities: a systematic review by the Ontario Protocol for Traffic Injury management (OPTIMa) collaboration. Man Ther 2016; 21:18–34.
- 114. Raman J, MacDermid JC, Grewal R. Effectiveness of different methods of resistance exercises in lateral epicondylosis: a systematic review. J Hand Ther 2012; 25:5–25.
- 115. Crawford JO, Laiou E. Conservative treatment of work-related upper limb disorders: a review. Occup Med 2007; 57(1):4–17.
- 116. Verhagen AP, Bierma-Zeinstra SM, Burdorf A, et al. Conservative interventions for treating work-related complaints of the arm, neck or shoulder in adults. Cochrane Database Syst Rev 2013;12:CD008742.
- 117. Long L, Briscoe S, Cooper C, et al. What is the clinical effectiveness and cost-effectiveness of conservative interventions for tendinopathy? An overview of systematic reviews of clinical effectiveness and systematic review of economic evaluations. Health Technol Assess 2015; 19(8):1–134.
- 118. National Institute for Health and Care Excellence (NICE). Conservative interventions for elbow tendinopathy (HTA No 12/73). London: NICE, 2012.
- 119. Page MJ, O’Connor D, Pitt V, et al. Exercise and mobilisation interventions for carpal tunnel syndrome. Cochrane Database Syst Rev 2012; 13(6):CD009899.
- 120. Page MJ, O’Connor D, Pitt V, et al. Therapeutic ultrasound for carpal tunnel syndrome. Cochrane Database Syst Rev 2013; 28(3):CD009601.
- 121. Bisset L, Coombes B, Vicenzino B. Tennis elbow. BMJ Clin Evid 2011; 2011:1117.
- 122. Hales TR, Bertsche PA. Management of upper extremity cumulative trauma disorders. Am Assoc Occup Health Nurses J 1992; 40:118–28.
- 123. Verdugo RJ, Salinas RA, Castillo JL, et al. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev 2008; 8(4):CD001552.
- 124. Klauke DN, Buehler JW, Thacker SB, et al. Guidelines for evaluation of surveillance systems. MMWR 1988; 17(Suppl 5):1–18.
- 125. Halperin WE, Ratcliffe J, Frazier TM, et al. Medical screening in the workplace: proposed principles. J Occup Med 1986; 28:547–52.
- 126. Baker EL, Melius JM, Millar JD. Surveillance of occupational illness and injury in the United States: current perspectives and future directions. J Public Health Policy 1988; 9:188–221.
- 127. Ehrenberg RL. Use of direct survey in the surveillance of occupational illness and injury. Am J Public Health 1989; 79(Suppl):11–4.
- 128. Kuorinka I, Jonsson B, Kilbom A, et al. Standardized Nordic questionnaires for the analysis of musculoskeletal symptoms. Appl Ergon 1987; 18:233–7.
- 129. National Institute for Occupational Safety and Health (NIOSH). Elements of ergonomics programs: a primer based on workplace evaluations of musculoskeletal disorders. DHHS (NIOSH) Publication No. 97-117. Cincinnati, OH: NIOSH, 1997.
- 130. Burton AK, Kendall NAS, Pearce BG, et al. Management of work-relevant upper limb disorders: a review. Occup Med 2009; 59(1):44–52.
- 131. Van Eerd D, Munhall C, Irvin E, et al. Effectiveness of workplace interventions in the prevention of upper extremity musculoskeletal disorders and symptoms: an update of the evidence. Occup Environ Med 2016; 73:62–70. doi:10.1136/oemed-2015-102992
- 132. Galinsky TL, Swanson NG, Sauter SL, et al. A field study of supplementary rest breaks for data-entry operators. Ergonomics 2000; 43:622–38.
- 133. Feuerstein M, Fitzgerald TE. Biomechanical factors affecting upper extremity cumulative trauma disorders in sign language interpreters. J Occup Med 1992; 34:257–64.
- 134. Kilbom A, Persson J. Work technique and its consequences for task. Ergonomics 1987; 30:273–9.
- 135. Cole DC, Theberge N, Dixon SM, et al. Reflecting on a program of participatory ergonomics interventions: a multiple case study. Work 2009; 34(2):161–78.
- 136. Hoffmeister K, Gibbons A, Schwatka N, et al. Ergonomics climate assessment: a measure of operational performance and employee well-being. Appl Ergon 2015; 50:160–9.
- 137. Mehorn MJ, Wilkinson L, Gardner P, et al. An outcomes study of an occupational medicine intervention program for the reduction of musculoskeletal disorders and cumulative trauma disorders in the workplace. J Occup Environ Med 1999; 41:833–46.
- 138. Government Accounting Office 1997. Report to Congressional Requestors: Worker Protection, Private Sector, Ergonomics Programs Yield Positive Results, Report No. GAO/HEHS-97-163. Washington, DC: United States Government Accounting Office/Health, Education, and Human Services Division.
- 139. Lutz G, Hansford T. Cumulative trauma disorder controls: the ergonomics program at Ethicon; Inc. Part 2. J Hand Surg 1987; 12A(5):863–6.
