Chapter 8
HIGH-PRESSURE ENVIRONMENTS
Tony L. Alleman and Joseph R. Serio*
Humans function well only within a narrow range of barometric pressures. Outside this range, they are subject to major physiologic stresses that occasionally result in disease. On land, workers are exposed to hyperbaric environments (i.e., increased barometric pressure) during tunneling projects that require the use of compressed air or when caissons are used to work in ground saturated with water. In addition, hyperbaric chamber support staff members are routinely exposed when they treat patients in hyperbaric medical treatment facilities. In the water, occupational exposures are diverse. Examples of exposures include breath-hold divers, such as the ama pearl divers of Japan, and compressed gas divers, ranging from instructors of recreational SCUBA students, who breathe compressed air, to saturation divers supporting offshore oil exploration, who dive in excess of up to 1000 ft of seawater (fsw), depending on the scope of the project, while breathing artificial gas mixtures. Divers can also be found inland. These divers are involved in such jobs as inspecting dams and reservoirs, cleaning filters, maintaining fish farms, placing underwater demolitions, and conducting police searches.
The first practical method developed for conducting useful work underwater was the diving bell, which is essentially an upside-down cone. Invented by Smeaton in 1778, the diving bell was the forerunner of the modern caisson (caisse, in French, means “box”).1 This method is still used today in the construction of tunnels and bridge footings. In 1819 in England, Augustus Siebe invented the first practical diving dress; it consisted of a copper helmet bolted to a leather coverall. This apparatus gave humans the ability to walk and function relatively unencumbered underwater without holding their breath. In 1837, Siebe introduced an improved version of this gear, which served as the basic diving dress for deep-sea diving until the early 1980s and is still used by some divers today.2 Modern surface-supplied equipment has incorporated various advances in technology, such as helmets made of lightweight composites with increased fields of vision and improved gas delivery systems that reduce breathing resistance.
Along with these advances, which have enabled divers and caisson workers to work at greater pressures or depths for longer periods of time, have come associated medical problems. The first descriptions of decompression-related disorders were made by Triger in 1841 among pressurized tunnel workers3 and by Alfonse Galin in 1872 with Greek sponge divers.4 By the early 1900s, it was recognized that decompression-related symptoms were due to inert gas and could be relieved by returning the individual to pressure. However, there was still no method for controlling the exposure to prevent the disease. The Royal Navy enlisted the help of the eminent physiologist J. S. Haldane to develop such a method. Haldane published his first set of decompression tables in 1908, based on experiments using sheep.3 The tables incorporated delays on ascent (called stops) to allow time for excess inert gas dissolved in body tissues to be eliminated (off-gassing). Haldanian principles form the basis for most decompression tables used today.
OCCUPATIONAL SETTING
Diving
Commercial divers are often involved in construction, inspection, and repair of pipelines, vessels, aquariums, tunnels, and marine platforms and typically spend a significant part of their time in diving operations. Scientific divers are devoted to the study of the marine environment and may dive only a few days a month as part of their job. Public safety divers become involved primarily with search and rescue operations and may only dive a few days a year. Engineers that design and oversee construction and maintenance of bridges often dive to inspect the underwater structures.
Diving operations can be classified into three basic categories: air, mixed gas, and saturation. Air diving is restricted to relatively shallow depths, due to the increasing narcotic effect of the nitrogen component of air as depth increases (the Occupational Safety and Health Administration (OSHA) restricts exposures on air to <190 fsw but will permit surface-supplied air for dives with bottom times of 30 minutes or less for depths of 220 ft). Mixed-gas diving uses a breathing mixture other than air; it is employed principally when deeper working depths are required. It generally specifies the use of helium as the inert gas constituent; however, nitrogen–oxygen mixtures (nitrox) are becoming more common for relatively shallow applications, and hydrogen has been tested for deep commercial applications. Although greater depths are permitted in standard mixed-gas diving, they are also limited by the amount of decompression required. For a constant bottom time, the required decompression time increases significantly with depth, prolonging exposure to the environment and limiting the useful work period. To overcome this problem, saturation diving methods have been developed.
Saturation diving, a specialized extension of standard decompression diving, is based on the principle that at a constant depth tissues will on-gas until the tissue partial pressure reaches equilibrium with the ambient pressure (see Henry’s law, p. 116). Once tissue equilibrium is reached, no net uptake will occur (unless the pressure is increased), resulting in no further increase in decompression time. At this point, the bottom time may be increased without additional decompression penalty. The divers are housed at “depth” for up to a month in a dry, pressurized chamber on the surface and transported under pressure to and from the work site via a diver transport capsule (Figure 8.1). Saturation diving techniques represent a cost-effective alternative to standard diving when deep or prolonged bottom times are required, since the need for repetitive, long in-water decompressions is avoided. The other advantage of saturation diving is that only one decompression is required, thereby reducing the risk of decompression-related incidents.
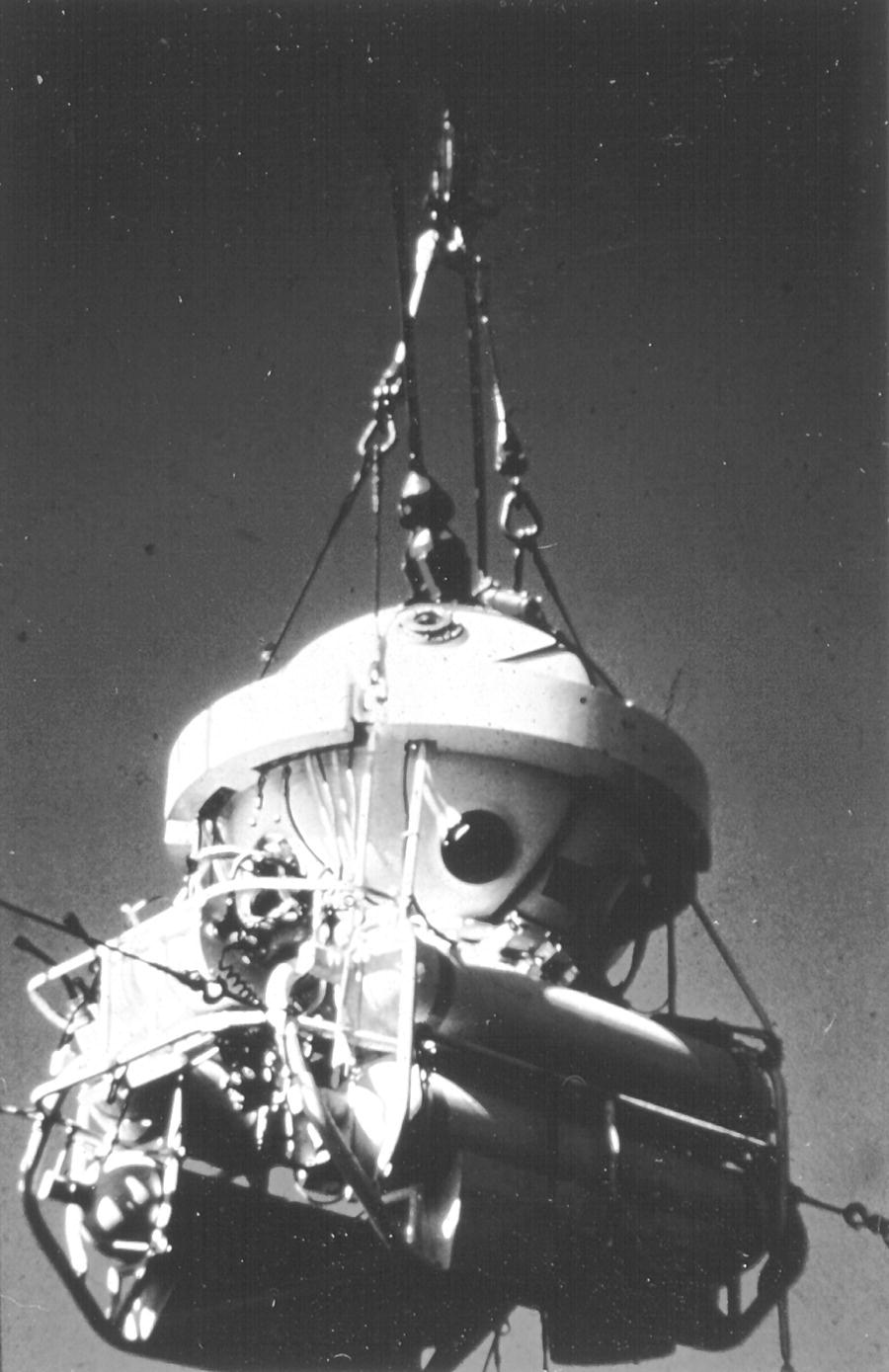
FIGURE 8.1 A saturation system diving bell.
(Courtesy of D. R. Chandler)
Equipment
There are two basic classes of equipment employed by divers today: self-contained underwater breathing apparatus (SCUBA) and surface-supplied equipment. Choice of equipment depends primarily on the job requirements and the employer’s capabilities. Equipment generally used in high-pressure and diving environments is summarized in Table 8.1.
TABLE 8.1 High-pressure and diving environments.
| Class | Equipment type | Type of air supplied |
| SCUBA | Open circuit | Air |
| Closed circuit | ||
| Semiclosed circuit | ||
| Surface supplied | Lightweight | Air |
| Deep sea | Mixed gas | |
| Saturation | Mixed gas | |
| Caisson | Shirt sleeve | Pressurized air |
Modern SCUBA has evolved from equipment originally developed during World War II by Cousteau and Gagnon.2 The advent of SCUBA ushered in a new era of underwater mobility. SCUBA equipment is designed either to be open circuit, closed circuit, or semiclosed (a hybrid between open circuit and closed circuit). Modern open-circuit SCUBA (Figure 8.2) is the principal equipment used in recreational diving; it is also used for many commercial applications. Closed-circuit SCUBA (rebreathers) (Figure 8.3) removes the exhaled carbon dioxide from the breathing gas prior to returning the “scrubbed” gas to the diver. Oxygen is added as needed. Some closed-circuit rigs maintain a constant partial pressure of oxygen in the breathing gas, increasing depth capabilities. Closed-circuit equipment is generally more complex and expensive, but it has the advantage of less gas consumption without the generation of bubbles. The absence of bubbles is useful in marine research, marine photography, and various military applications.5

FIGURE 8.2 SCUBA equipment.
(Courtesy of US Navy)

FIGURE 8.3 Diver using Draeger LAR V closed-circuit SCUBA equipment.
(Courtesy of T.J. Doubt)
Many advances have been made in surface-supplied diving since the 1960s, including specialized materials for helmets and suits, hot water heating for suits, and advanced communication systems. Surface-supplied diving equipment can generally be divided into two types: lightweight and deep sea. Deep-sea equipment can be further subdivided into air, mixed gas, and saturation capable. Lightweight equipment, such as a “band mask,” is customarily used for work where surface communications are required, but significant diver protection is not necessary. Deep-sea diving equipment (Figure 8.4) affords increased protection, generally consisting of a “hard hat” offering maximum head/neck protection along with surface-to-diver communication video capability, protective gloves and boots, weights, an emergency gas supply, and varying levels of thermal protection, as complex as suits that circulate hot water supplied from the surface.

FIGURE 8.4 Deep sea diving equipment
As in most occupational settings, the choice of equipment used in diving generally depends on the characteristics of the environment and the work to be completed, along with the personal preferences and the capabilities of the individual diver. The principal advantages of SCUBA diving are its ease of use, transportability, and freedom of movement. Its disadvantages include limited gas supply, depth restrictions, minimal head protection, lack of communication, and inability to function safely in strong currents. Surface-supplied diving overcomes many of the disadvantages of SCUBA by providing increased head protection, thus reducing the chance of drowning if the diver becomes unconscious; “unlimited” gas supply; better buoyancy control, thus enabling the use of heavy construction techniques; hardwired communications; and greater depth capabilities (Figures 8.5, 8.6, 8.7, and 8.8). However, surface-supplied diving is more complex and costly, because it requires significantly more surface support in both equipment and personnel. In addition, it decreases the diver’s comfort on the surface due to weight, decreases mobility in the water, carries a risk of entanglement, and can present a significant noise hazard. Essentially all of the commercial diving in the offshore environment is done with surface-supplied air or gas or in a saturation environment.
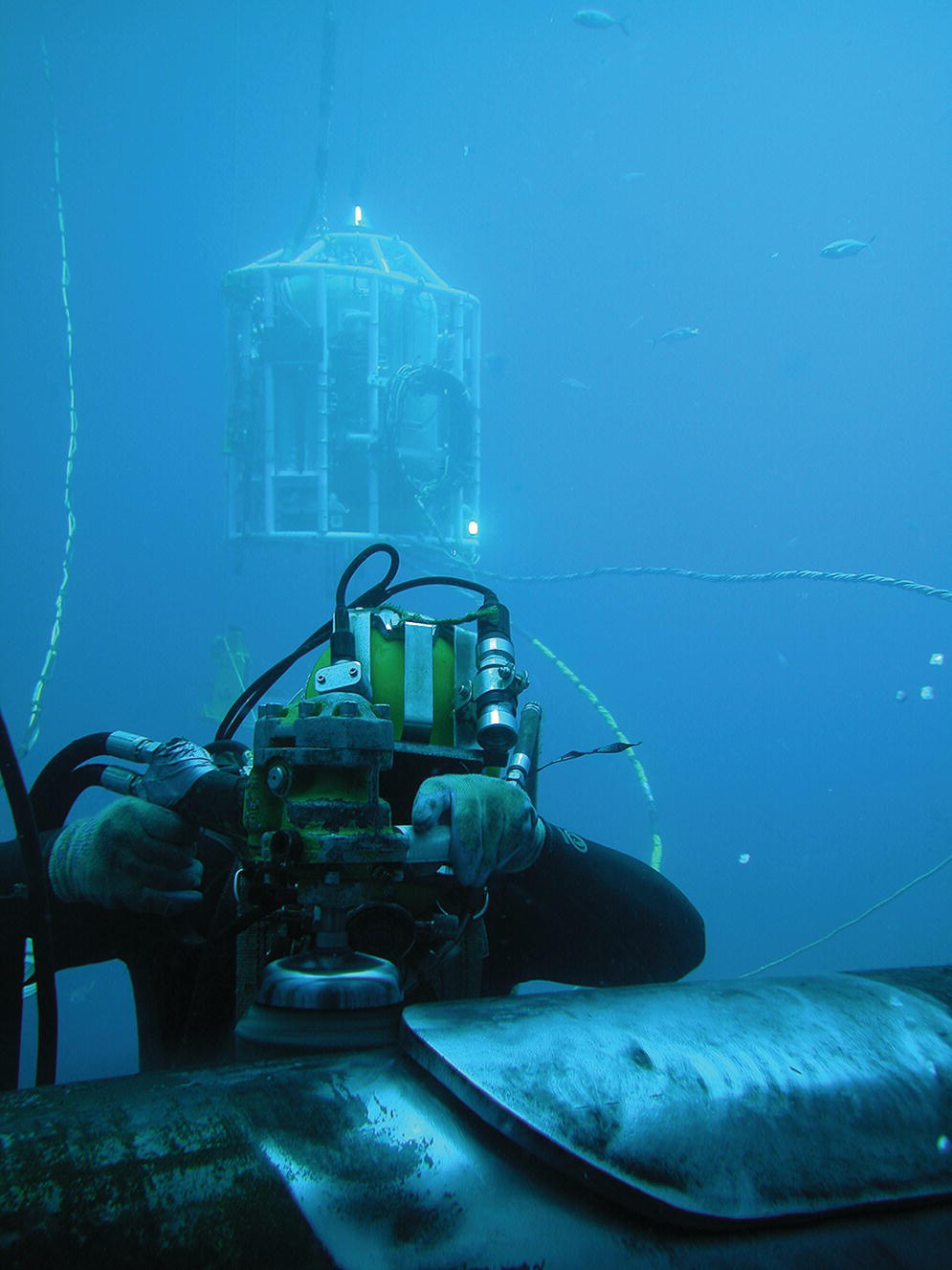
FIGURE 8.5 Surface-supplied diving: Diver using a grinding tool.
Copyright 2016 Oceaneering International, Inc. Used with permission of Oceaneering International, Inc.

FIGURE 8.6 Surface-supplied diving: Diver water gouging.
Copyright 2016 Oceaneering International, Inc. Used with permission of Oceaneering International, Inc.

FIGURE 8.7 Surface-supplied diving: Diver welding.
Copyright 2016 Oceaneering International, Inc. Used with permission of Oceaneering International, Inc.

FIGURE 8.8 Surface-supplied diving: Diver working on Habitat with Bell.
Copyright 2016 Oceaneering International, Inc. Used with permission of Oceaneering International, Inc.
Caissons
A caisson is a watertight retaining structure that is used for engineering projects where water or waterlogged soil precludes standard construction techniques. It can be envisioned as an inverted cone or diving bell that rests on the bottom. It is pressurized to exclude water and to allow a relatively dry working environment. Similar principles are also applied in the construction of tunnels through waterlogged or unstable “mucky” earth. Workers enter and exit the workplace through a pressurized lock system where decompression may be carried out on exit. At depth, the workers may labor for up to 8 hours at pressure. Decompression is dependent on the exposure time and the pressure. The length of the decompression is determined from tables developed specifically for this purpose. Fire safety is of concern in pressurized environments, due to the higher partial pressures of oxygen; however, modern systems are constructed from steel, minimizing flammable materials.6 OSHA provides guidelines for safety in caisson work (29 CFR 1926.801).
The need for caisson techniques has been gradually declining as other engineering methods, such as pressure-balanced shields and unmanned excavating systems, have been developed to avoid the cost and complexity of caisson work. Currently, there is limited caisson work being done in the United States. Noncaisson construction is less costly since it eliminates both high-pressure equipment and the extra labor costs related to high-pressure work. However, with automated equipment, men occasionally need to enter a high-pressure compressed air environment to repair or maintain the equipment.7
The majority of hyperbaric exposures today occur in the undersea environment. Since caisson work is very limited, the remainder of this chapter will focus on undersea hyperbaric exposures. The pathophysiology, diagnosis, and treatment of hyperbaric exposures and their sequelae are similar in caisson and undersea workers.
MEASUREMENT ISSUES AND PHYSICS OF PRESSURE
Pressure is a measurement of force per unit area. Air pressure is the force exerted by the column of atmosphere above a particular point. The height of the column determines the pressure. Water pressure, measured by a gauge, is defined as the force exerted by the column of water above the submerged object. Pressure increases linearly with depth. Absolute pressure measures the force per unit area of the combined air and water column. The absolute pressure at 33 fsw is 2 ata (atmospheres absolute) since 1 atm of pressure is contributed by both the air column (weight of atmosphere) and the water column (weight of water). Table 8.2 defines some useful measurements of pressure in diving.
TABLE 8.2 Units of pressure used in hyperbaric environments.
| 10.00 m of seawater (msw)a = 32.646 ft of seawater (fsw)b |
| 10.00 msw = 1 bar = 100 kilopascals (kPa)c |
| 1 atmosphere (atm) = 1.013 bar |
| 1 atm = 760 Torr (mm of mercury) |
| = 1033 cm of water |
| = 14.69 lb per in.2 |
| = 33.08 fsw |
a The definition of fsw (feet seawater) assumes a density (weight per unit volume) for seawater of 1.025 at 40°C.
b The definition of msw (meters seawater) assumes a density for seawater of 1.020 at 40°C.
c The unit for the pascal is defined as a Newton per meter squared.
An understanding of the physiologic effects of pressure requires knowledge of basic physics. The relationship between pressure, volume, and temperature is defined by the ideal gas law, which states
where P is absolute pressure, V is volume, n is the number of moles of the gas, R is the universal gas constant, and T is the absolute temperature.
Boyle’s law states that at constant temperature, the volume is inversely proportional to the absolute pressure—that is, no gas enters or exits the system (P1V1 = P2V2).8 For example, if a balloon is filled with 2.0 L of compressed gas at a depth of 99 ft (4.0 ata) and brought to 33 ft (2.0 ata), the gas volume will have expanded to 4.0 L (V2 = P1V1/P2 = 4 × 2/2 = 4). If taken to the surface (1.0 atm), the volume will have expanded to 8.0 L (VS = P1V1/PS = 4 × 2/1 = 8). It is important to note that the proportional change in volume per depth change increases toward the surface.
The law of partial pressures (Dalton’s law) states that, in a mixture of gases, the total gas pressure is the same as the sum of the partial pressures of the individual gases in the mixture (P = p1 + p2⋯pn). As a result, the partial pressure of the gas can be calculated by knowing the total pressure of the mixture and the percentage makeup of a particular component. As the total pressure increases, the volume of the individual gases becomes more significant, and eventually Dalton’s law no longer applies.8
Henry’s law states that the amount of gas that will dissolve into a solution is directly proportional to the partial pressure of that gas and inversely proportional to the absolute temperature.8 Therefore, as the pressure increases (depth increases), the amount of gas dissolved in tissues will increase. Once at a constant depth, gas will continue to increase in the tissues until the equilibrium or saturation is attained (i.e., when the partial pressure of the gas in tissue equals the ambient pressure). Gases such as oxygen may be metabolized, whereas inert gases will not be. Subsequently, when the ambient pressure is decreased, inert gases will come out of solution. This law explains why nitrogen bubbles may form when you surface from an air dive and why a can of carbonated beverage fizzes when it is opened.
Archimedes’ principle, which describes buoyancy, states that an object immersed in a fluid is buoyed up by a force equal to the weight of the volume of fluid that the object displaces. The effect of this principle is seen throughout diving. For example, inhaling a large breath will increase the diver’s buoyancy (due to greater displacement of fluid).
EXPOSURE GUIDELINES
Commercial diving in the United States is covered by various regulations, including OSHA Standard Title 29 Code of Federal Regulations 1910 Subpart T, Commercial Diving Operations, and Title 46 Code of Federal Regulations, Subchapter V, Marine Occupational Safety and Health Standards, Subpart B, Commercial Diving Operations. Diving contractors and operators generally have developed local standard operating procedures that define procedures and safety guidelines in more detail.
Caisson work, and other high-pressure construction work, is covered by OSHA Standard Title 29 CFR 1926 Subpart S—Underground Construction, Caisson, Cofferdams, and Compressed Air.
SPECIAL UNDERWATER STRESSORS
The worker in the undersea environment is exposed to a number of unique environmental stressors that may be enhanced by pressure. These environmental factors include sensory input modifications (sound, visual, proprioceptive), thermal challenges, and gas effects. Professionals generally do not have the luxury of waiting for ideal environmental conditions. As a result, most operations are conducted when at least one element is not ideal. Medical planning for diving operations should take this into consideration.
Sensory changes
Divers must tolerate seriously reduced sensory input while working, forcing increased alertness and vigilance. As water temperature decreases, there can be an associated decrease in sensory perception. If the temperature drops significantly enough, there will be difficulty in motor control as well as sensory perception.9
Sound
Sound travels approximately four times faster in water than in air; as a result, it travels further. Because of this increased speed, the normal delay cues required to place sound in three dimensions are in practice lost, making the localization of sound extremely difficult. Moreover, the increased density of air and increased middle-ear impedance inhibit air conduction, causing a ≤40 dB loss.10 Sound does not transmit across an air–water interface efficiently. As a result, vocal communications through the water are effectively ruled out, thus adding to the sensory deprivation already present secondary to other effects.10 In addition, many surface-supplied helmets are noisy secondary to their design, which compounds hearing difficulties. Divers may sustain aural trauma from underwater blasts or activation of sonar devices while submerged.
Vision
The underwater environment seriously affects vision in a number of ways. First, water transmits light poorly. This is caused principally by turbidity, that is, suspended particles that obstruct light. At 10 m in clear water, ~60% of the light is filtered out, leaving only 40% visible light.11 However, in highly turbid waters, like those found in many ports and inland waters, light may be reduced substantially more than this.
In addition, color vision is lost. As depth increases, long wavelengths (red end of the spectrum) are filtered initially, followed by the blues. In the absence of artificial light, perception of red is generally eliminated by 10 m, followed by yellow by ~20 m.
As the diver descends, the eyes must adapt from day to night light levels. However, the working diver generally descends faster than the retina can compensate, further compounding the visual adjustment.8 Objects appear 25% larger and closer underwater because the refractive index of light is increased ~1.3 times over that in air. This causes problems with determination of distances and eye–hand coordination that improve somewhat, although not completely, with experience. Finally, if the cornea comes into direct contact with water—for example, in the absence of the air interface provided by a mask or helmet—a substantial hyperoptic refractive error of 40–50 diopters (D) results.12
Proprioception
Proprioceptive inputs for orientation to the diver are reduced by buoyancy and the diver’s protective clothing. Additionally, divers receive fewer visual cues. On land, workers rely on visual, proprioceptive, and vestibular inputs to maintain balance and orientation in space. This capacity is significantly impaired by the underwater environment.11
Thermal transfer
Maintaining a constant body temperature while submerged is a challenge, even in relatively warm water. Heat is lost during water immersion, primarily through convection and conductance from the skin and through the respiratory tract. Water has a high coefficient of thermal conductivity (~25 times greater than air), and submersion effectively eliminates the normal protective air insulation layer. Water cools four to five times faster than an unprotected individual at the same temperature in air.13 As a result, an unprotected diver in 27°C (80°F) water loses heat at the same rate as an unprotected subject in 6°C (42°F) air. A water temperature of ~35°C (95°F) is required to keep a resting, unprotected immersed diver thermoneutral.14 To protect from this loss, passive insulation, such as neoprene, is used for mild to moderate cold shallow applications. Wet suits are generally made of neoprene, a material that is compressible and as a result loses insulation capacity with depth. For deeper, colder dives, dry suits (passive) and hot water suits (active) are used. Dry suits consist of an impermeable outer barrier and an insulating inner garment made of a variety of different materials. Dry suits can be inflated with pressurized gas as depth increases to maintain the insulation barrier. Active heating garments, such as hot water suits, which bathe the diver in warm water, are employed in very cold and deep diving, but they require more technical support.
At greater depths (>100 m), heat loss from the respiratory tract becomes significant secondary to the high heat capacity of the dense gas. This respiratory heat loss may not be sensed and therefore can cause asymptomatic hypothermia. Breathing gas is heated to prevent this phenomenon. Occasionally, in unique circumstances, the diver may be exposed to increased water temperatures. Such dives require special planning and measures to protect against hyperthermia, since the diver will be unable to self-regulate body heat by the evaporation of sweat. Hypothermia and cold exposure are discussed in detail in Chapter 7.
Gas effects
Table 8.3 summarizes the toxic effects of gases on divers.
TABLE 8.3 Toxic effects of gases on divers.
| Gas | Causes/sources | Symptoms |
| Inert gas narcosis | Increased partial pressure of inert gas | 100–200 fsw |
| Lightheaded | ||
| Decreased complex reasoning | ||
| Loss of fine discrimination | ||
| Euphoria | ||
| 200–300 fsw | ||
| Poor judgment/reasoning | ||
| Slowed reflexes | ||
| Paresthesia | ||
| Dangerous marine life | ||
| 300–400 fsw | ||
| Progressive depression | ||
| Auditory/visual hallucinations | ||
| Loss of memory | ||
| >400 fsw | ||
| Unconsciousness | ||
| Oxygen toxicity | >1.3 atm abs partial pressure of oxygen | Visual disturbance |
| Central nervous system (acute) | Risks increase with increase in partial pressure |
Tinnitus/decreased visual acuity Nausea/vomiting Twitching Irritability/restlessness Vertigo Convulsions (may occur with no prodromal symptoms from above; no long-term consequences) |
| Pulmonary (chronic) | Prolonged exposure to >0.5 atm abs oxygen |
Chest pain with inspiration Inspiratory irritation Coughing Progressive shortness of breath |
| Hypoxia | Hyperventilation prior to breath-hold diving | Unconsciousness (without symptoms) |
| Improper gas mixture | ||
| Carbon dioxide toxicity | Equipment design | Increased breathing rate |
| Increased gas density | Shortness of breath | |
| Increased partial pressure of oxygen blunts response to CO2 | Headache | |
| CO2 contamination | Unconsciousness | |
| Carbon monoxide toxicity | Use of improper compressor lubricants (flashable) | Headache |
| Compressor failure | Nausea/vomiting | |
| Improper intake placement (e.g., next to combustion engine) | Unconsciousness |
Inert gas narcosis
Inert gas narcosis is defined as the progressive development of symptoms of intoxication/anesthesia with increasing partial pressures of the gas. Inert gases vary substantially in their ability to induce narcosis at a given partial pressure of exposure. Xenon is anesthetic at 1 atm, whereas helium has little narcotic effect even at very great depths.15 The narcotic effect of a specific inert gas is related to its lipid solubility; however, the precise pathophysiologic mechanism of narcosis is not well understood.15
As a practical point, the depth or pressure to which a diver may descend while breathing air is restricted principally by nitrogen narcosis. Significant individual variability in response to the effect exists; but on average, at 100–200 ft (30–60 m), an individual feels lightheaded and euphoric (similar to alcohol intoxication) and experiences decreased reasoning capability, reaction time, and manual dexterity.16 At 200–300 ft, reflexes slow, paresthesias may develop, and decrements in judgment and reasoning may produce dangerous overconfidence.11 At 300–400 ft, marked impairment of judgment,15 anesthesia, progressive depression of the sensorium with auditory and visual hallucinations, and amnesia precede syncope. Finally, at depths >400 ft on air, the diver becomes unconscious.11
Primary prevention involves limiting the depth of exposure and substituting a less narcotic gas, such as helium. Individuals do not acclimatize to narcosis; however, repeated exposures help a diver adjust to this effect.
Oxygen toxicity
Because oxygen is a mandatory constituent of breathing gas, it would be logical to postulate that diving on 100% oxygen (O2) would solve the problems associated with inert gas during hyperbaric exposures. Applications of 100% O2 breathing are restricted, however, because oxygen in the hyperbaric environment becomes increasingly toxic to the central nervous system (CNS) as the partial pressure of oxygen increases. Breathing oxygen with a partial pressure as low as ~1.3 atm abs (equivalent on the surface to 130%) may cause acute CNS manifestations. The risk of CNS oxygen toxicity increases as the oxygen partial pressure increases. The most serious CNS manifestation secondary to oxygen is a tonic–clonic seizure, which may occur without prodromal symptoms. If a seizure occurs underwater, it may cause drowning. Prodromal manifestations include11:
- V = Visual disturbances, such as tunnel vision
- E = Ear problems, including tinnitus or decreased acuity
- N = Nausea and vomiting
- T = Twitching
- I = Irritability and restlessness
- D = Dizziness and vertigo
Manifestations of CNS O2 toxicity are treated by decreasing the partial pressure of oxygen. Divers try to prevent toxicity by limiting their exposure time and the partial pressure of oxygen breathed. Factors that are thought to predispose to oxygen convulsions include exercise, carbon dioxide retention, some medications, and water immersion itself. Long-term health consequences from CNS oxygen toxicity have been demonstrated. Oxygen seizures per se are of less clinical importance than other seizures because the recipient is well oxygenated prior to their occurrence.
Prolonged exposures to partial pressures of oxygen >0.5 atm abs may cause clinically apparent pulmonary toxicity. Usually, only saturation diving scenarios and hyperbaric oxygen therapy, for example, a prolonged therapy associated with decompression illness (DCI), are sufficiently prolonged to cause clinically apparent pulmonary toxicity. However, in rare cases, pulmonary oxygen toxicity may become a limiting factor in repetitive deep bounce dives, which require prolonged decompressions. Manifestations of pulmonary oxygen toxicity are the same as those of patients on ventilators exposed for prolonged periods to increased partial pressures of oxygen. These include (i) substernal chest pain, which begins as irritation on inspiration and progresses with continued exposure to severe burning chest pain during both inspiration and expiration; (ii) coughing, which gradually increases in frequency and duration with exposure; and (iii) progressive shortness of breath.11
Physical examination is generally unremarkable and chest radiographs are clear except in severe cases. In practice, within a few hours after removal from exposure, symptoms begin to resolve, and they generally resolve completely within 24–48 hours (although if severe, the forced vital capacity may take substantially longer to recover). Limiting exposures is the only preventive measure available.
Inspiration of high partial pressures of oxygen can also cause ocular toxicity. Conditions associated with ocular oxygen toxicity include myopia, cataracts, keratoconus, and retinopathy.17 Myopia caused by hyperbaric oxygen requires multiple exposures and is generally reversible. Cataract formation may be dependent upon the partial pressure of oxygen exposure. The retina is susceptible to oxidative stress, the cause for development of retinopathy in hyperbaric conditions.
Hypoxia
Hypoxia is particularly hazardous, because it can cause unconsciousness without warning. Although hypoxia rarely occurs in air diving, it may occur in mixed-gas diving secondary to procedural errors, such as improper gas mix or mechanical failures. Individuals are also prone to hypoxia during breath-hold diving, which is preceded by hyperventilation. This is a result of decreased predive carbon dioxide (CO2) levels secondary to hyperventilation. Since elevated CO2 levels drive the need to breathe, low initial CO2 levels permit divers to comfortably overstay their permissible dive time at depth (secondary to elevated partial pressure of oxygen at depth). On ascent, the individual becomes hypoxic as the partial pressure of oxygen decreases below safe levels.
Carbon dioxide toxicity
Carbon dioxide buildup or retention can occur commonly in the hyperbaric environment. Causes include:
- Increased work of breathing caused by increased gas density (Boyle’s law), increased breathing resistance, and dead space as a result of the use of breathing equipment. Added dead space and breathing resistance are dependent on the design of the breathing equipment.
- Increased partial pressure of oxygen, which blunts the body’s response to elevated partial pressures of CO2.
- CO2 in the breathing gas secondary to failure of CO2 absorbent, poor gas analysis, or contaminated breathing gas.
- Deliberate reduction of ventilation. With some breathing equipment designs, ventilation to the helmet causes a great deal of noise, which interferes with communications. Divers sometimes reduce helmet ventilation to reduce noise levels and facilitate communications, thus predisposing to CO2 buildup.
The early manifestations of CO2 toxicity are shortness of breath, anxiety, and increased heart rate. These symptoms may go unnoticed when a diver is performing hard work. As the exposure increases, the worker may experience a headache (which presents as a mild to moderate throbbing during exposure but may increase in severity post–post exposure). With higher levels, the diver becomes progressively confused, eventually losing consciousness. Eliminating the CO2 buildup through ventilation or surfacing from the dive (i.e., removal from the work site) is the treatment of choice. Symptoms resolve rapidly after removal of exposure, with the possible exception of the headache.
CO2 retention reduces the divers’ exercise tolerance, increases the manifestations of N2 narcosis,18 and may increase the risk of oxygen toxicity and decompression illness (since cerebral vasodilation facilitates on-gassing).
Carbon monoxide toxicity
The principle source of carbon monoxide (CO) in diving is contaminated breathing gas. For example, CO can be drawn into the compressor intake (e.g., from an idling truck in the vicinity of an intake) or via improper compressor maintenance (e.g., use of flashable lubricants or a failure of compressor rings). OSHA requires that breathing gas be tested every 6 months and as needed to prevent unrecognized elevations of CO, oil mist, and CO2.19 The effects of CO may be masked in the diver while breathing contaminated gas because of the high partial pressure of oxygen in the breathing media. Unless the CO concentration is high, the symptoms of CO exposure will be delayed until the partial pressure of oxygen decreases—that is, during ascent/decompression or after surfacing. However, if there is not a high degree of suspicion, manifestations may be confused with other diving-related disorders. In addition, the symptoms associated with carbon monoxide poisoning such as headaches, dizziness, confusion, disorientation, nausea, vomiting, weakness, visual disturbances, loss of consciousness, cardiac arrhythmia, and infarction may be attributed to other diseases rather than effects of carbon monoxide.13
OTHER HAZARDS IN THE DIVING ENVIRONMENT
The elements of the professional diving environment are varied and diverse, but its hazards are similar to the occupational hazards commonly encountered on land. These hazards are summarized in Table 8.4, and most of them are covered elsewhere in the book.
TABLE 8.4 General industrial hazards that may be encountered in the underwater work environment.
|
In addition, it should be remembered that many divers do not dive full time. They often have primary jobs that expose them to more traditional hazards, such as welding, degreasing, paint removal, or other chemical, physical, or biological hazards.
PATHOPHYSIOLOGY OF DIRECT PRESSURE INJURY
The health effects of direct pressure injury can be divided into two major groups: barotrauma and decompression illness. Barotrauma results from the expansion or contraction of gases in anatomic spaces, causing trauma. Decompression illness results when bubbles of inert gas form in body tissues. Body fluids become supersaturated with inert gases at high pressures, and this gas comes out of solution when the ambient pressure is decreased. A summary of direct pressure effects can be found in Table 8.5.
TABLE 8.5 Human health effects of environmental pressure change.
| Barotrauma site |
| Ear |
| Sinus |
| Lung |
| Skin |
| Teeth |
| Gastrointestinal tract |
| Manifestations of decompression illness |
| Pain |
| Neurologic effects |
| Pulmonary effects |
| Cutaneous effects |
| Lymphatic effects |
| Constitutional effects |
Barotrauma
Barotrauma may occur in any gas-filled space in the body. To sustain barotrauma, commonly referred to as a “squeeze,” a pressure change must occur in an enclosed gas-filled space in that consequences of this pressure change will depend on the location of the space, the magnitude of the pressure change, and the physiologic response mounted. Common locations for barotrauma include the ear (middle most commonly, outer and inner depending on circumstances), sinuses, teeth, gastrointestinal tract, and the lung. In addition, the skin may be traumatized by pressure changes in gas pockets found in a suit or under a face mask.
Middle-ear squeeze is the most common form of barotrauma seen in diving. It occurs when the middle-ear space is not vented properly by the Eustachian tube. When an individual descends in a pressure column, whether to a dive site or in an airplane, the gas within the middle ear compresses in accordance with Boyle’s law. To counterbalance this effect, the individual must introduce air into this space via the Eustachian tube, which joins the middle ear to the pharynx. This process, known as clearing the ears, may be accomplished by various methods such as yawning, swallowing, moving the jaw around, or a Valsalva maneuver. The ears will not clear if the Eustachian tube is blocked.
If descent continues without clearing, the diver will initially experience a sense of fullness and pressure, followed by sharp pain.20 If descent is not stopped, the eardrum may rupture, allowing immediate equalization of pressure difference, or the middle-ear space may fill with blood, a noncompressible fluid to equalize the pressure. Middle-ear barotrauma occurs most frequently within the first 10–20 ft of descent, which is the period of greatest proportional pressure change. Following the dive, residual symptoms may include pain, a sensation of fullness in the ear, or a temporary, mild conductive hearing loss across all frequencies, generally <20 dB (or greater if an ossicular rupture has occurred).11 In a small proportion of cases, blood may be visible in the mouth or nose. Treatment depends on the amount of damage sustained. It may range from a mild squeeze, requiring no diving for 48–72 hours, to severe barotrauma, requiring diving restrictions up to 6 weeks or longer. To prevent this form of barotrauma, an individual should not dive when the ears do not clear properly, such as during periods of significant upper respiratory congestion. The absence of predive symptoms does not guarantee adequate Eustachian tube function. In addition, descent should be stopped at the first sign of difficulty in equalizing (clearing ears).
Sinus barotrauma may occur during descent when the openings that vent the sinuses into the nasal cavity are obstructed. It presents as increasing pain over the effected sinus(es). On descent, if the sinus does not properly equalize, pressure in the sinuses decreases relative to ambient pressure. As a result, edema and hemorrhage of the mucosal lining of the sinus may occur. If a sinus opening subsequently becomes blocked (secondary to edema/hemorrhage) during a dive, sinus barotrauma may present during the ascent phase of a dive. In such cases, the sinus pain is caused by a relative increase in pressure within the sinus. This pain may continue for a number of hours after the dive. Relief may be accompanied by a discharge and often a high-pitched sound as gas leaves the sinus.
Pulmonary barotrauma is a very serious form of this disorder. Gas present in the lung expands during ascent. If the lung is allowed to overpressurize by as little as 90–110 cm H2O (1 m of seawater (msw), or 3 ft), the lung may rupture.21,22 This displaced gas causes a variety of sequelae that present individually or in combination. These include mediastinal/subcutaneous emphysema, pneumothorax, and arterial gas embolism.
After rupture, gas may migrate along the bronchial tree to the mediastinum. The result is mediastinal emphysema, which may remain asymptomatic or present as substernal burning chest pain. Mediastinal emphysema is thought to be the most common manifestation of pulmonary barotrauma. From the mediastinum, extrapulmonary gas may track into the neck, presenting as subcutaneous emphysema or occasionally as a voice change secondary to pressure directly on the larynx or the recurrent laryngeal nerve. Alternatively, the gas may be driven into the retroperitoneal region. The rupture may expel gas into the intrapleural space, causing a pneumothorax.23 If this occurs at depth, the damage will be further exacerbated by ascent. Finally, the overpressurization may force gas into the pulmonary veins, causing an arterial gas embolism that presents as neurologic sequelae. Any type of cerebral neurologic manifestation is possible secondary to arterial embolization; symptoms range from subtle neurologic findings to hemiplegia, convulsions, coma, and death.
Any or all of these sequelae may occur simultaneously. Therefore, it is critical to perform a complete neurologic examination to rule out arterial embolization whenever pulmonary overinflation is suspected or detected. Individuals are at an increased risk of lung rupture during (i) diver training courses, particularly during underwater removal and donning of gear (ditch and don); (ii) buoyant ascent training (diver may not exhale completely due to loss of buoyancy); (iii) diving with predisposing lung pathology that impedes gas flow; and (iv) panic/emergency/uncontrolled ascents. It is important to remember that lung rupture can occur after surfacing from a compressed gas dive from as little as 3–4 ft of water (1 msw), even when normal procedures are followed. This should not happen in breath-hold diving, since the volume of gas present on ascent will not exceed the original breath taken on the surface.
Barotrauma may also occur in a tooth, causing implosion or expulsion of amalgam/dental material. Gas can also expand in the gastrointestinal tract; however, this rarely causes more than mild discomfort, unless there is a hernia present. Trapped gas within an intestinal loop may cause incarceration.
Decompression illness
In accordance with Henry’s law, hyperbaric workers will take up or onload gas while breathing compressed gas at elevated pressures. Subsequently, when the diver ascends in the water column or the caisson worker leaves the work site, inert gas already present in the body expands and must be off-gassed. Decompression tables have been developed that provide rules for both ascent rates and stops in the pressure water column, thus allowing time to asymptomatically off-gas the inert components on ascent. Rigorous adherence to these tables is a critical component to prevent decompression illness. The tables and diving computers used today are based principally on perfusion-limited theories (Haldanian principles), which have been modified as needed to reflect human experience. However, even when the tables are rigorously adhered to, decompression illness (DCI) can still occur. Decompression illness is obviously more common if the tables are disregarded. Examples of commonly used tables in the United States today include the US Navy standard air tables24 and the DCIEM air tables.25 These tables are widely available in the sports and commercial diving community. There is an average predicted range of incidence of DCI on commonly used tables of <1 to 5%, but specific dive profiles within each table vary greatly.26,27
The overall incidence of DCI is virtually impossible to measure,28 since records of the total numbers of divers (the denominator) are not routinely maintained. It has been estimated that the operational incidence in US Navy divers is <5 cases per 10 000 dives (0.05%) and the incidence within the US sports diving community has been estimated at <1 per 10 000.29–31 US Navy analysis of dives not requiring decompression completed between 21 and 55 fsw from 1991 to 1994 showed an incidence of 2.9 cases/10 000 dives (0.029%), with the incidence increasing with depth.32 Shields et al. have documented an overall incidence of decompression sickness in the commercial UK sector of the North Sea over the period 1982–1986 of 0.31% and an incidence of 0.10% after the implementation of depth–time restrictions.33Luby reported an incidence of approximately 4.0 cases/10 000 dives (0.04%) shallower than 100 ft and 10 cases/10 000 dives (0.1%) deeper than 100 ft in commercial diving in the Middle East.34
During and after ascent, bubbles may form in tissues. If bubbles form, they may either remain asymptomatic or cause clinically apparent damage. It is generally believed that bubbles form in the tissues, causing damage locally (autochthonous bubbles),35 or that they are distributed widely by the venous system, principally filtered at the lungs. However, this filter may become overloaded with large amounts of venous gas, causing the filter to leak, particularly with an increased load. Alternatively, venous bubbles may traverse a patent foramen ovale, atrial septal defect, or even an extracardiac shunt. Once in the arterial system, the bubbles probably distribute commensurate with the organ’s blood flow, as with the bubbles associated with pulmonary barotrauma.36
Generally, any new pain or neurologic manifestation presenting shortly after a hyperbaric exposure must be considered DCI until ruled out. The range of manifestations of bubble disease is seemingly infinite, but the manifestations may be grouped into the following categories: pain, neurologic, pulmonary, cutaneous, lymphatic, and constitutional.37 Any combination of manifestations from these categories may be present. Limb pain is believed to be the most common manifestation.38–40
DCI pain generally presents as a deep, toothache-like periarticular pain that is not affected by movement. Girdle pain, a distinct DCI pain syndrome, is characterized by a poorly localized, constricting sensation radiating from the back and often heralds the onset of severe neurologic manifestations. A wide variety of neurologic manifestations may be seen, including alterations in consciousness, higher-function abnormalities, derangements in sensory modalities, strength deficits, problems with special senses (audiovestibular particularly, including vertigo, tinnitus, and hearing loss), and loss of sphincter control (particularly bladder function).37 Cutaneous presentations (cutis marmorata) generally start with intense itching, most commonly on the torso, which progresses to an erythematous rash and may continue on to cyanotic marbling (mottling). Lymphatic disease presents as a painful swelling of an individual lymph node or group of lymph nodes, which on rare occasions may be accompanied by swelling/edema, presumably due to obstruction. Pulmonary manifestations (chokes) include shortness of breath, cough, chest pain, or cyanosis. Most of these pulmonary manifestations are quite rare unless substantial decompression has been omitted. Constitutional symptoms, including fatigue, nausea, and anorexia, may accompany any of these manifestations.
A descriptive system of nomenclature for DCI is presented in Table 8.6. The evolutionary and clinical manifestation terms are used to form the label—for example, acute progressive neurologic DCI or acute static neurologic and limb pain DCI. “Acute” is used to discriminate the case from potential long-term health effects related to decompression.37
TABLE 8.6 A matrix for describing decompression illness.
Source: Adapted from Francis TJR, Smith DJ, Sykes JJW. The prevention and management of diving accidents. INM Technical Report R92004.20 Reprinted with permission.
| Acute decompression illness |
| The five following terms are used to describe a case of DCI adequately: |
|
| Format for decompression illness “label” |
| Since lengthy descriptions are unwieldy for communication purposes, a specific abbreviated “label” is needed for each case. |
| The general form of the proposed label is as follows: |
| Acute [Evolution term], [Manifestation term(s)], decompression illness (see text). |
Traditionally, manifestations of arterial gas embolism have been distinguished from those of DCI, despite probable overlapping pathophysiology. As a result of experience in caring for the caisson workers digging the Dartford tunnel below the Thames River in London, Golding et al. further divided DCI into two categories based on presumed severity and disease location.41 Pain, cutaneous effects, and lymphatic manifestations were designated as Type I decompression sickness, whereas neurologic and pulmonary manifestations alone or with any other combination of manifestations were called type Type II decompression sickness. Type II was believed to represent serious disease. This classification quickly became the standard, based on 35 cases of Type II disease. It is still in frequent use today. However, this system has been demonstrated to give inconsistent diagnoses.42–44 Furthermore, as can be seen from their clinical description and our present concepts of the pathophysiology, cerebral arterial gas emboli (CAGE) and DCI probably cannot be distinguished except in a few isolated circumstances.37
DCI can begin on ascent or after surfacing. When Francis et al. reviewed 1070 well-documented cases of neurologic DCI, excluding all cases with histories thought to predispose to arterial gas embolism, they found that 50% presented within 10 minutes of surfacing, >85% of cases presented within 1 hour of surfacing, and >95% presented within 6 hours of surfacing.45
However, symptoms attributable to DCI may present at 24–48 hours or more after a dive.43 The classical arterial gas embolic mechanism secondary to barotrauma may occur as stated above when compressed gas is inhaled deeper than 3 ft and generally presents on surfacing or quickly thereafter. The inert gas mechanism, on the other hand, requires the diver to stay for a minimum time at depth to acquire an adequate gas burden. With normal diving, depths >33 fsw are generally required.46
A number of proposed predisposing factors for decompression illness have been identified in addition to the dive profile. They include individual susceptibility26(which is most likely multifactorial), patent foramen ovale,47–49 atrial septal defect, obesity,50–52 exercise at depth and during decompression,26,53 dehydration,54 and low ambient air temperature/wind chill,55 hot water suits, hot showers in cold divers, oral contraceptives, rapid ascent, heavy work and exertion, residual deficit from previous DCI, and obstructive lung disease.56 Data are lacking to translate these associations, such as obesity and patent foramen ovale, into specific individual recommendations. Patent foramen ovale, for example, has a high prevalence in normal populations, including divers, despite a low incidence of resulting DCI. Therefore, most experts do not recommend screening divers for a patent foramen ovale unless they have experienced recurrent episodes of “unexplained” neurologic DCI.57 In addition, age, poor physical fitness, recent tissue injury, previous DCI, and dehydration have been suggested as predisposing factors; however, little epidemiologic evidence exists to support these suggestions, and the studies are conflicting.
Diving while pregnant is a controversial issue, due to the paucity of available data, which are also conflicting. However, most hyperbaric authorities agree that a woman should not dive while pregnant.58–61 Safe depth–time profiles have not been established.62 The fetus may be susceptible to intravascular bubble formation, and these bubbles may have a deleterious effect on the nervous system of the fetus with a patent foramen ovale and ductus arteriosus. Moreover, there is concern about the potential effects of hypoxia during an unanticipated emergency while diving. The military and most commercial diving operations prohibit women from diving while pregnant.
Unique problems of saturation diving
Saturation diving techniques represent a cost-effective alternative to standard diving when deep or prolonged bottom times are required, since the need for frequent and long in-water decompressions is avoided. The worker essentially completes one decompression after a prolonged exposure. However, at greater depths, unique medical problems arise.
Rapid compression at deep depths (>150 m) is associated with high-pressure nervous syndrome (HPNS) and compression arthralgia. The symptoms of HPNS frequently include a 5–8-Hz tremor, dizziness, nausea and associated vomiting, decreased mental alertness, and microsleep.63 The manifestations are related to depth and rate of compression. Compression arthralgia comprises pains or ill-defined discomforts in joints on moving during and immediately after compression. The knees, hips, and wrists are most commonly involved.11 To avoid both HPNS and compression arthralgia, the compression rates are slowed in comparison to conventional diving, and nitrogen is added to the helium and oxygen mixture (heliox) to produce trimix (helium, oxygen, and nitrogen).63
Maintenance of thermal balance is a significant problem at depth. At 300 m, the comfort range varies by less than 2°C. Additionally, while the diver is lying down, the exposed surfaces may become cold secondary to thermal conduction, and the surfaces in contact with the mattress may become too warm. While the diver is actually working in the water, thermal balance is maintained with hot water suits, and the breathing media are heated.
Because the saturation environment is humid and warm, pathogens grow well. Meticulous housekeeping is required to keep all divers within this closed community healthy. Otitis externa has been a particular problem, forcing the early completion of some dives. A preventive regimen using 2% acetic acid in aluminum acetate eardrops has been developed; this regimen has effectively controlled the problem in most situations.64
Communication is extremely difficult in a helium environment. Electronic “unscramblers” are mandatory for adequate communications between the diver and support personnel. All materials are transferred to depth and back via “medical locks,” which are small pressure-transfer chambers with doors on the outside and inside of the chamber. These locks can be pressurized to allow transfer of food, medical supplies, mail, and any important personal objects. Greater depths degrade the taste of food, requiring increased flavoring and spices. The heat of compression must be anticipated, because the additional heat generated will further cook food on descent.
Once they enter the chamber, the physician no longer has ready access to the divers; therefore, medical screening for deep saturation diving must be rigorous. The lack of access is due to the slow compression times required and subsequent decompression obligations incurred by the medical attendant, making access unfeasible. Depending on the depth of storage of the saturation system, decompression obligations for saturation divers may take up to a week or more. Predive physicals should pay particular attention to the ears, skin, and respiratory tract. To be better prepared for emergencies, divers should be trained in various first aid and life-support techniques. It is standard practice in commercial diving for one or more of the saturation dive team members to be trained as a diving medical technician (DMT).65
LONG-TERM HEALTH EFFECTS
Dysbaric osteonecrosis, or aseptic bone necrosis, is a well-recognized, relatively uncommon long-term occupational hazard associated with compressed air work. The bone necrosis lesions occur principally in the femur, tibia, and humerus.66 As a rule, shaft lesions and lesions that occur away from articular joints do not produce clinical symptoms. Juxta-articular lesions, on the other hand, can progress and produce debilitating disease. Juxta-articular lesions are more commonly found in compressed air workers than in divers. Necrosis is associated with increasing depth and duration of exposure, increasing age (although age may only be a surrogate measure of exposure), and a history of DCI, though not related to the site of DCI.66 The lesions of dysbaric osteonecrosis are indistinguishable from other causes of aseptic necrosis. However, as McCallum and Harrison note, “in men with a history of work in compressed air or diving, the probability of bone necrosis being due to compressed air is very high”.66 The pathogenesis of the dysbaric form is not well understood. An excellent review of dysbaric osteonecrosis was completed by Jones and Neuman.66
Divers are at risk for sensorineural hearing loss secondary due to barotrauma, DCI, and potentially long-term noise exposure. Conductive hearing loss is also a possibility with middle-ear barotrauma and exposure to underwater explosions. Although the hearing threshold is increased underwater, a number of studies have shown that measured sound intensity levels of some diving helmets when pressurized are as high as 90–120 dB(A), depending on the application.67,68 In addition, hyperbaric chambers during compression and some chambers during ventilation have high noise levels. Some investigators have documented standard threshold shifts with “normal” dive profiles.68–71 The principal cause of the high noise level is the volume of gas flow required to sufficiently ventilate helmets to prevent CO2 buildup. The frequency range of noise is 800–3000 Hz, which is within normal communication ranges. The redesign has significantly improved the noise levels of modern helmets.69 Some epidemiologic studies have shown little difference in hearing acuity between professional divers and matched controls when corrected for age,70 whereas others71–74 have noted significant differences in divers. One recent age-adjusted prospective study in SCUBA divers demonstrated increased hearing loss in low frequencies only, suggesting a compression/decompression effect.74 Although some of the high-frequency hearing loss seen in divers is probably due to other exposures, the studies imply that some of the losses may be due to workplace noise exposure.75 Therefore, engineering controls and hearing protection should be instituted wherever possible.
Sequelae from episodes of acute DCI, particularly neurologic ones, are well recognized. However, there may be less obvious but potentially serious long-term health effects from diving, based primarily on descriptive and anecdotal evidence. This possibility has generated a number of hypotheses, which currently lack good epidemiologic support. Subsequent studies have frequently not supported the hypothesized findings. These effects include neuropsychiatric deficits, such as short-term memory deficits and emotional lability,76–79 electroencephalographic abnormalities80,81(principally slow waves and spikes), and retinoangiography aberrations, including pigment and capillary changes in the retina.82 Pulmonary function changes have been documented in groups of divers; these include increased vital capacities, which may be adaptive.83 The clinical relevance and validity of these findings are still being investigated. An excellent review of the relevant literature can be found in Elliott and Moon.84 This review concludes that “in the absence of a history of acute decompression illness, the possibility of a clinical syndrome among divers or ex-divers remains unproven. If it exists, the prevalence is unknown, and probably low.”
TREATMENT OF BUBBLE-RELATED DISEASE
Because symptoms and signs can progress, a diagnosis of DCI should be treated urgently. The patient should be placed on oxygen at as high a partial pressure as reasonably feasible. Additionally, fluids (generally oral, but intravenous or intraosseous fluids may be administered as appropriate for the condition of the patient) are pushed, and the individual is placed in a recumbent position. A hyperbaric chamber should be found and the patient transferred for recompression therapy (Figure 8.9). An excellent source of emergency information and referral is the Divers Alert Network.a
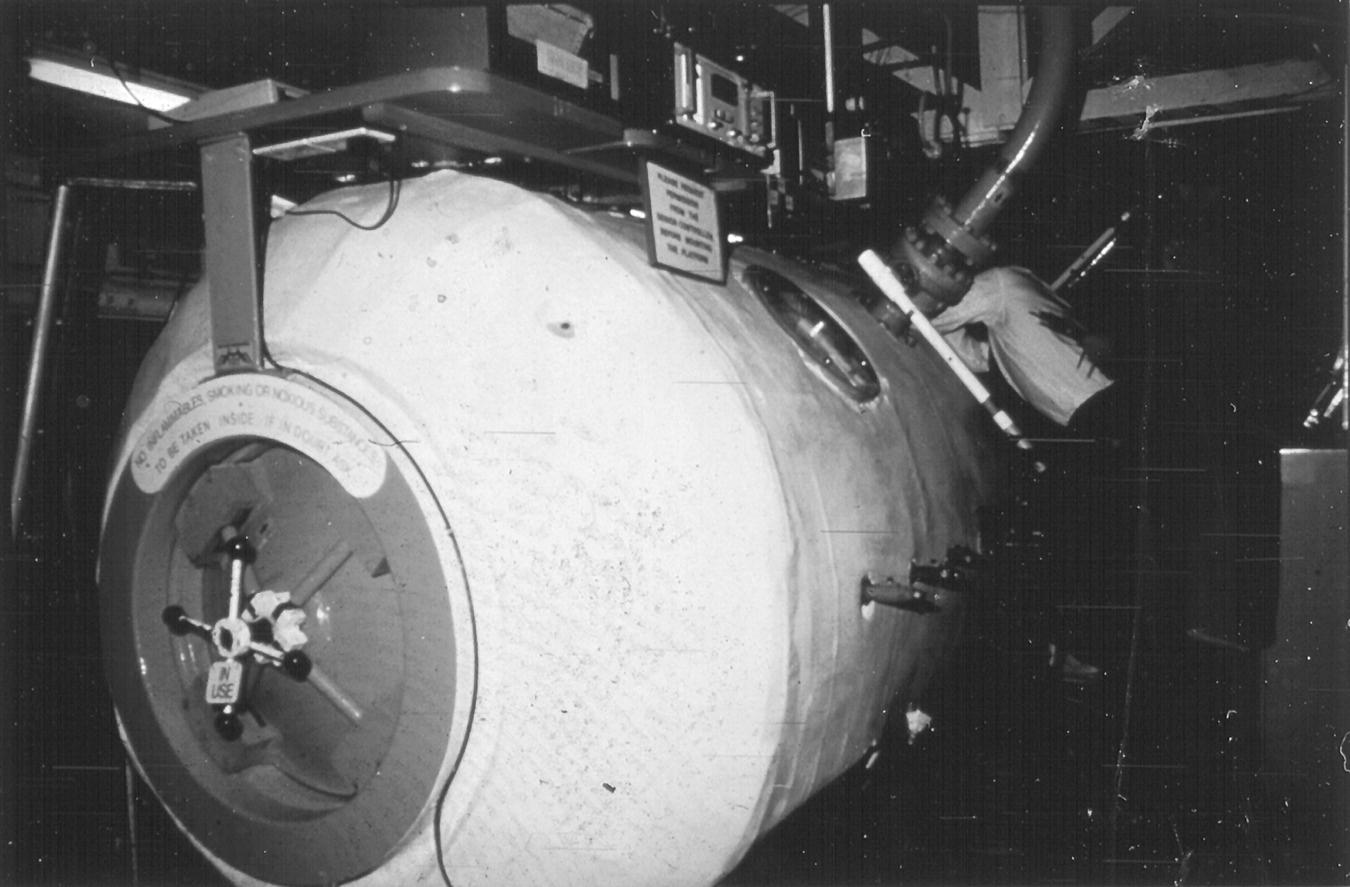
FIGURE 8.9 Hyperbaric recompression chamber.
(Courtesy of U.S. Navy)
Many recompression tables exist; however, the US Navy Treatment Table 6 (Figure 8.10 and Table 8.7) is the principal therapeutic table used for the treatment of decompression illness. The patient is given oxygen at depth (60 fsw) initially, with intermittent air breaks to reduce the incidence of oxygen toxicity. Response to therapy is generally excellent if recompression therapy is instituted promptly. Depending on the response to therapy, table modifications and follow-on hyperbaric treatments may be required if manifestations do not resolve or recur. Deeper tables include the Comex 30 Treatment Table using 50% oxygen and nitrogen mixture (100 fsw), US Navy Treatment Table 6A using 50% oxygen and nitrogen mixture (165 fsw), and the Lamberston/SOSI Treatment Table 7A to depths greater than 165 fsw. Rarely saturation tables such as US Navy Treatment Table 7 will be used if the diver does not respond to treatment based on the other treatment tables.
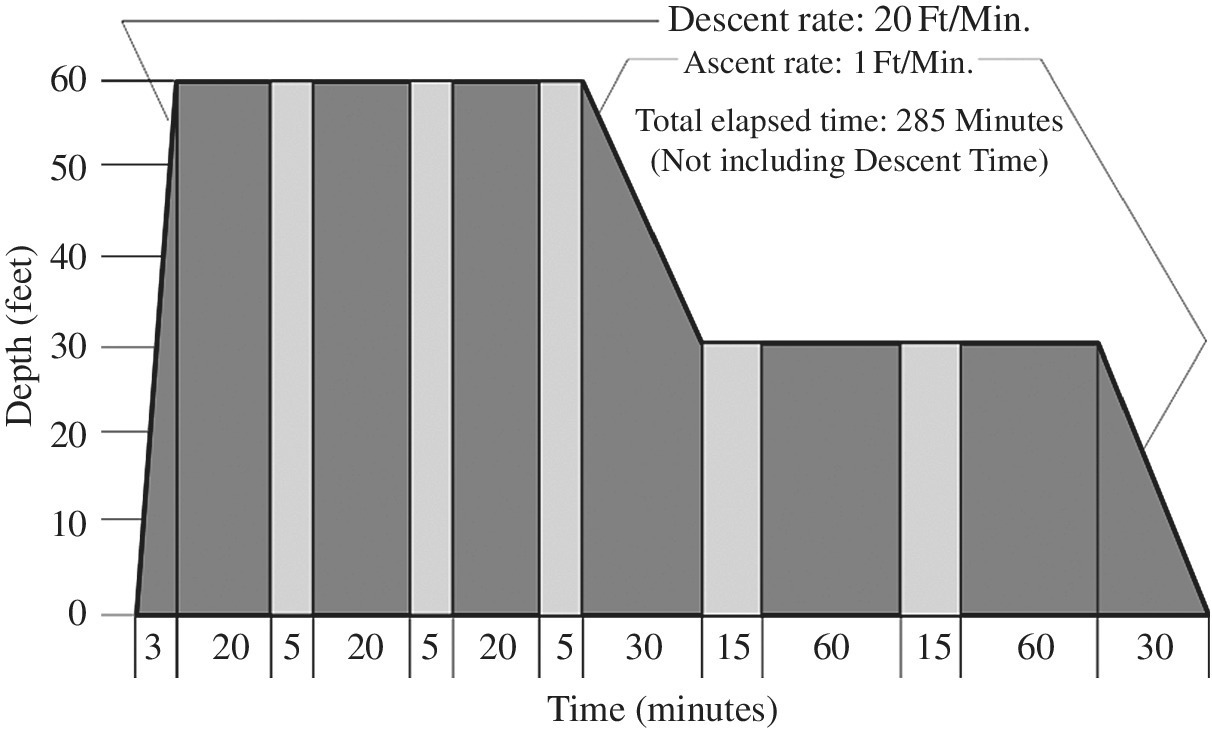
FIGURE 8.10 U.S. Navy Treatment Table 6.
Source: U.S. Navy Diving Manual, Chapter 21. NAVSEA 0910-LP-0708-8000, Washington, DC: U.S. Government Printing Office, 1999.
TABLE 8.7 US Navy Treatment Table 6: Oxygen Treatment of Type II Decompression Sicknessa,b.
Source: Adapted from the US Navy Diving Manual, Revision 4, chapter 21.
| Depth (ft)c | Time (min) | Breathing mediad | Total elapsed time (h:min) |
| 60 | 20 | O2e | 0:20 f |
| 60 | 5 | Air | 0:25 |
| 60 | 20 | O2 | 0:45 |
| 60 | 5 | Air | 0:50 |
| 60 | 20 | O2 | 1:10 |
| 60 | 5 | Air | 1:15 |
| 60 to 30 | 30 | O2 | 1:45 |
| 0 | 15 | Air | 2:00 |
| 30 | 60 | O2 | 3:00 |
| 30 | 15 | Air | 3:15 |
| 30 | 60 | O2 | 4:15 |
| 30 to 0 | 30 | O2 | 4:45 |
a Treatment of Type II or Type I DCI when symptoms are not relieved within 10 minutes at 60 ft.
b Extensions to Table 6: Table 6 can be lengthened up to two additional 25-minute oxygen-breathing periods at 60 ft (20 minutes on oxygen and 5 minutes on air) or up to two additional 75 minutes oxygen-breathing periods at 30 ft (15 minutes on air and 60 minutes on oxygen) or both. If Table 6 is extended only once at either 60 or 30 ft, the tender breathes oxygen during the ascent from 30 ft to the surface. If more than one extension is done, the care-giver begins oxygen breathing for the last hour at 30 ft during ascent to the surface.
c Descent rate—25 ft/min. Ascent rate—1 ft/min. Do not compensate for slower ascent rates. Compensate for faster rates by halting the ascent.
d Caregiver breathes air throughout unless he has had a hyperbaric exposure within the past 12 hours, in which case he breathes oxygen at 30 ft.
e If oxygen must be interrupted because of adverse reaction, allow 15 minutes after the reaction has entirely subsided and resume schedule at point of interruption.
f Time at 60 ft begins on arrival at 60 ft.
MEDICAL SURVEILLANCE
Healthcare personnel must understand that few other workers experience the magnitude of physiologic stresses imposed routinely on divers and caisson workers. Medical surveillance requires a thorough understanding of the physiologic aspects of diving, along with the specific workplace hazards that may be encountered by the worker. In general, different classes of workers are exposed to varying levels or types of hazards, requiring a tailored approach. Most professional divers, including military, commercial, and scientific divers, as well as caisson workers and hyperbaric chamber attendants are provided with specific guidance and standards by their employer. For example, the Association of Diving Contractors International periodically publishes medical requirements for their members. Title 29 CFR 1910 Subpart T Appendix A provides examples of conditions that restrict or limit exposure to hyperbaric conditions.19 Other sources provide more detailed guidance.57,85–89 Examination frequency varies depending on type of exposure and the regulations being followed. Reexamination must be completed after any significant illness or injury, particularly exposure-related injuries, diving with any neurological involvement, gas embolus, or pulmonary or ear barotrauma.
Healthcare providers who conduct medical surveillance on divers should have formal instruction in diving/hyperbaric medicine. This training enables them to correlate various medical conditions to the unique hyperbaric environment. One source for information on diving medicine courses, scientific meetings, general information on diving or hyperbaric medicine, or addresses of practitioners with an interest in diving or hyperbaric medicine is the Undersea and Hyperbaric Medical Society.b
Medical history
The medical history is of primary importance in hyperbaric medical surveillance. It must be remembered that in the underwater environment, any condition that incapacitates an individual, even temporarily (e.g., seizure, fainting), may cause drowning in addition to the standard sequelae. Moreover, divers for the most part rely on the buddy system, which means that the divers must be able to help their buddies when they are in distress and must not endanger their buddies secondary to their own medical condition. In addition, a careful occupational history—including type of dives, number of dives, maximum depth obtained, and any untoward events—is uniquely important.
Physical examination
A complete physical examination should be completed, with an emphasis on ear, nose, and throat, pulmonary, cardiovascular, skeletal, and neurologic systems. Caisson and diving workers require a significant amount of cardiovascular reserve and aerobic work capacity, along with adequate dexterity and strength. During each examination, a neurologic examination should be completed to, at a minimum, document preexisting deficits. A well-documented neurologic examination may prevent confusion during evaluation of symptoms and signs postdiving.
PREVENTION
The only certain method to prevent hyperbaric injuries is to simply avoid all high-pressure and diving work. Thanks to modern engineering techniques, the need for caisson work has been significantly decreased. However, preventing diving injuries by avoiding diving altogether is generally regarded as unfeasible. Therefore, the principal methods used to prevent diving injuries are (i) extensive training, including both an academic understanding of principles and job-specific practical training; (ii) dive planning, to include emergency procedures and appropriate use of tables; (iii) maintenance of a high level of fitness; (iv) meticulous care of equipment, along with ongoing improvement in equipment design; and (v) a healthy respect for the indigenous hazards of the profession. These same principles also apply to caisson work. Current research is directed toward the refinement of decompression models and tables, improving equipment design, enhancing treatment methods, and understanding the pathophysiology and associated risk factors of diving disorders.
References
- 1. Elliott DH. Raised barometric pressure. In: Baxter PJ, Adams PH, Aw T-C, Cockcroft A, Harrington JM, eds. Hunter’s diseases of the occupations 9th ed. London: Arnold, 2000:343–60.
- 2. Bachrach AJ. A short history of man in the sea. In: Bennett PB, Elliott DH, eds. The physiology and medicine of diving, 3rd edn. San Pedro, CA: Best Publishing, 1982:1–14.
- 3. Kindwall EP. A short history of diving and diving medicine. In: Bove AA ed. Bove and Davis’ diving medicine, 4th edn. Philadelphia, PA: Elsevier, 2004:1–9.
- 4. Gal A. Des dangers du travail dans l’air comprimé et des moyens de les prévenir. In: Bert P, ed. Barometric pressure––researches in experimental physiology. Columbus, OH: College Book Company, 1943:398.
- 5. Butler FK Jr., Smith DJ. U.S. Navy diving equipment and techniques. In: Bove AA ed. Bove and Davis’ diving medicine, 4th edn. Philadelphia, PA: Elsevier, 2004:547–71.
- 6. Kindwall EP. Compressed air work. In: Brubakk AO, Neuman TS, eds. Bennett and Elliott’s physiology and medicine of diving, 5th edn. Philadelphia, PA: WB Saunders, 2003:17–28.
- 7. Kindwall EP. Compressed air tunneling and caisson work decompression procedures: development, problems, and solutions. Undersea Hyperb Med 1997; 24(4):337–45.
- 8. Taylor LH. Diving physics. In: Bove AA ed. Bove and Davis’ diving medicine, 4th edn. Philadelphia, PA: Elsevier, 2004:11–35.
- 9. Bookspan J. Diving in cold and heat. In: Bookspan J ed. Diving physiology in plain English. Dunkirk, MD: Underwater and Hyperbaric Medical Society, 2006.
- 10. Farmer JC. Vestibular and auditory function. In: Shilling CXV, Carlston CB, Mathias RA, eds. The physician’s guide to diving medicine. New York: Plenum, 1984:192–8.
- 11. Flynn ET, Catron PW, Bayne CG. Diving medical officer’s student guide. Memphis, TN: Naval Technical Training Command, 1981.
- 12. Kinney JAS. Physical factors in underwater seeing. In: Shilling CXV, Carlston CB, Mathias RA, eds. The physician’s guide to diving medicine. New York: Plenum, 1984:199–205.
- 13. Tipton MJ, Mekjavic IB, Golden FSC. Hypothermia. In: Bove AA ed. Bove and Davis’ diving medicine, 4th edn. Philadelphia, PA: Elsevier, 2004:261–73.
- 14. Craig AB, Dvorak M. Thermal regulation during water immersion. J Appl Physiol 1966; 21:1577–85.
- 15. Bennett PB, Rostain JC. Inert gas narcosis. In: Brubakk AO, Neuman TS, eds. Bennett and Elliott’s physiology and medicine of diving, 5th edn. Philadelphia, PA: WB Saunders, 2003:300–22.
- 16. Kiessling RJ, Maag CH. Performance impairment as a function of nitrogen narcosis. J Appl Psychol 1962; 46:91–5.
- 17. McMonnies CW. Hyperbaric oxygen therapy and the possibility of ocular complications or contraindications. Clin Exp Optom 2015; 98:122–5.
- 18. Hesser CM, Fagraeus L, Adolfson J. Roles of nitrogen, oxygen and carbon dioxide in compressed air narcosis. Undersea Biomed Res 1978; 5:391–400.
- 19. Code of Federal Regulations 29, part 1910, subpart T—Commercial diving operations. 1, Washington: US Government Printing Offices, 1992 July.
- 20. Francis TJR, Smith DJ, Sykes JJW. The prevention and management of diving accidents. INM Technical Report R92004. Alverstoke: Institute of Naval Medicine, 1992.
- 21. Malhotra MC, Wright HC. The effects of a raised intrapulmonary pressure on the lungs of fresh unchilled cadavers. J Pathol Bacteriol 1961; 82:198–202.
- 22. Vann RD, Butler FK, Mitchell SJ, et al. Decompression illness. Lancet 2011; 377:153–64.
- 23. Broome JR, Smith DJ. Pneumothorax as a complication of recompression therapy for cerebral arterial gas embolism. Undersea Biomed Res 1992; 19:447–55.
- 24. US Navy Diving Manual; rev. 6. U. S. Department of the Navy, Naval Sea Systems Command 2008. Available at: http://www.navsea.navy.mil/Portals/103/Documents/SUPSALV/Diving/Dive%20Manual%20Rev%206%20with%20Chg%20A.pdf?ver=2016-02-26-123349-523 (accessed on September 3, 2016).
- 25. Lauckner GR, Nishi RY. Decompression tables and procedures for compressed air diving based on the DCIEM 1983 decompression model. No. 84-R-74. Toronto: DCIEM, 1984.
- 26. Vann RD, Thalmann ED. Decompression physiology and practice. In: Bennett PB, Elliott DH, eds. The physiology and medicine of diving, 4th edn. Philadelphia, PA: WB Saunders, 1993:376–432.
- 27. Weathersby PK, Survanshi SS, Homer LD, et al. Statistically based decompression tables. I. Analysis of standard air dives: 1950–1970. NMRI Report 85-16. Bethesda, MD: Naval Medical Research Institute, 1985.
- 28. Sykes JJW. Is the pattern of acute decompression sickness changing? J R Nav Med Serv 1989; 75:69–73.
- 29. Dembert ML. Individual factors affecting decompression sickness. In: Vann RD, ed. The physiological basis of decompression. Proceedings of the 38th Undersea and Hyperbaric Medical Society Workshop, Duke University Medical Center, Durham, NC, June. Bethesda, MD: Undersea and Hyperbaric Medical Society, 1989:355–67.
- 30. Wilmshurst P, Allen C, Parish T. Incidence of decompression illness in amateur SCUBA divers. Health Trends 1994–1995; 26(4):116–8.
- 31. Arness MK. Scuba decompression illness and diving fatalities in an overseas military community. Aviat Space Environ Med 1997; 68:325–33.
- 32. Flynn ET, Parker EC, Ball R. Risk of decompression sickness in shallow no-stop air diving: an analysis of US Navy experience 1990–94. In: Proceedings of the 14th meeting of United States–Japan Cooperative Program in Natural Resources (UJNR), Panel on Diving Physiology, Panama City, FL, USA, September 16–17, 1997. Spring, MD: U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Undersea Research Program, 1998:23–38. Available at: https://searchworks.stanford.ed (accessed on June 28, 2016).
- 33. Shields TG, Duff PM, Wilcock SE, et al. Decompression sickness from commercial offshore air-diving operations on the UK continental shelf during 1982 to 1988. In: Subtech’89. Fitness for Purpose, Vol. 23. Amsterdam: Society for Underwater Technology, 1990:259–77. Available at: https://www.onepetro.org/conference-paper/SUT-AUTOE-v23-259?sort=&start=0&q=isbn%3A%28%220-7923-0742-9%22%29&fromSearchResults=true&rows=50# (accessed on June 28, 2016).
- 34. Luby J. A study of decompression sickness after commercial air diving in the northern Arabian gulf: 1993–95. Occup Med 1999; 49(5):279–83.
- 35. Francis TJR, Dutka AJ, Flynn ET. Experimental determination of latency, severity, and outcome in CNS decompression sickness. Undersea Biomed Res 1988; 15:419–27.
- 36. Francis TJR. A current view of the pathogenesis of spinal cord decompression sickness in a historical perspective. In: Vann RD, ed. The physiological basis of decompression. Proceedings of the 38th Undersea and Hyperbaric Medical Society Workshop, Duke University Medical Center, Durham, NC, June. Bethesda, MD: Undersea and Hyperbaric Medical Society, 1989:241–79.
- 37. Francis TJR, Smith DJ, eds. Describing decompression illness. Proceedings of the 42nd Undersea and Hyperbaric Medical Society Workshop, Institute of Naval Medicine, Alverstoke, Gosport, Hampshire, UK, October 9–10, 1990. Bethesda, MD: Undersea and Hyperbaric Medical Society, 1991.
- 38. Rivera JC. Decompression sickness among divers: an analysis of 935 cases. Mil Med 1964; 129:314–34.
- 39. Kelleher PC, Francis TJR, Smith DJ, et al. INM diving accident database: analysis of cases reported in 1991 and 1992. Undersea Biomed Res 1993; 20(suppl):13 (abstract).
- 40. Denoble P, Vann RD, de L Dear G. Describing decompression illness in recreational diving. Undersea Biomed Res 1993; 20(suppl):14 (abstract).
- 41. Golding FC, Griffiths P, Hemplemen HV, et al. Decompression sickness during the construction of the Dartford tunnel. Br J Ind Med 1960; 17:167–80.
- 42. Kemper GB, Stegmann BJ, Pilmanis AA. Inconsistent classification and treatment of type I/type II decompression sickness. Aviat Space Environ Med 1992; 63:153 (abstract).
- 43. Smith DJ, Francis TJR, Pethybridge RJ, et al. Concordance: a problem with the current classification of diving disorders. Undersea Biomed Res 1992; 19(suppl):47 (abstract).
- 44. Smith DJ, Francis TJR, Pethybridge RJ, et al. An evaluation of the classification of decompression disorders. Undersea Hyperbaric Med 1993; 20(suppl):11 (abstract).
- 45. Francis TJR, Pearson RR, Robertson AG, et al. Central nervous system decompression sickness: latency of 1070 human cases. Undersea Biomed Res 1988; 15:403–17.
- 46. Elliott DH, Moon RE. Manifestations of the decompression disorders. In: Bennett PB, Elliott DH, eds. The physiology and medicine of diving, 4th edn. Philadelphia, PA: WB Saunders, 1993:492.
- 47. Moon RE, Camporesi EM, Kisslo JA. Patent foramen ovale and decompression sickness in divers. Lancet 1989; I:513–4.
- 48. Wilmshurst P, Byrne JC, Webb-Peploe MM. Relation between interatrial shunts and decompression sickness in divers. Lancet 1989; II:1302–6.
- 49. Gernompré P, Dendale P, Unger P, et al. Patent foramen ovale and decompression sickness in sports divers. J Appl Physiol 1998; 84(5):1622–6.
- 50. Medical Research Council Decompression Central Registry, University of Newcastle-upon-Tyne. Decompression sickness and aseptic necrosis of bone. Investigations carried out during and after the construction of the Tyne Road Tunnel (1962–66). Br J Ind Med 1971; 28:1–21.
- 51. Lam TH, Yau KP. Analysis of some individual risk factors for decompression sickness in Hong Kong. Undersea Biomed Res 1989; 16:283–92.
- 52. Dembert ML, Jekel JF, Mooney LW. Health risk factors for DCS. Undersea Biomed Res 1984; 11:395–406.
- 53. Van der Aue OE, Kellar RJ, Brinton ES. The effect of exercise during decompression from increased barometric pressures on the incidence of decompression sickness on man. Report no. 8–49. Panama City, FL: United States Navy Experimental Diving Unit, 1949.
- 54. Suzuki N, Yagishita K, Togawa S, et al. Risk factors for decompression sickness. Undersea Hyperb Med 2014; 41(6):521–30.
- 55. Broome JR. Climatic and environmental factors in the aetiology of DCI in divers. Undersea Biomed Res 1992; 19(suppl):17 (abstract).
- 56. Moon RE. Treatment of decompression illness. In: Bove AA ed. Bove and Davis’ diving medicine, 4th edn. Philadelphia, PA: Elsevier, 2004:195–223.
- 57. Elliott DH. Medical evaluation of working divers. In: Bove AA ed. Bove and Davis’ diving medicine, 4th edn. Philadelphia, PA: Elsevier, 2004:533–45.
- 58. Fife WP, ed. Effects of diving on pregnancy. Proceedings of the 19th Undersea Medical Society Workshop. Bethesda, MD: Undersea Medical Society, 1978:15–9.
- 59. Fife WP, ed. Women in diving. Proceedings of the 35th Undersea and Hyperbaric Medical Society Workshop, Bethesda, MD, May 21–22. Bethesda, MD: Undersea and Hyperbaric Medical Society, 1986:3–10.
- 60. Vorosmarti J, ed. Fitness to dive. Proceedings of the 34th Undersea and Hyperbaric Medical Society Workshop, Bethesda, MD, May 15–16. Bethesda, MD: Undersea and Hyperbaric Medical Society, 1987:101–2. Available at: http://rubicon-foundation.org/uhms-workshops/ (accessed on June 28, 2016).
- 61. Hill RK. Pregnancy and travel. JAMA 1989; 262:498.
- 62. Cresswell JE, St Leger-Dowse M. Women and scuba diving. Br Med J 1991; 302:1590–1.
- 63. Bennett PB, Rostain JC. The high pressure nervous syndrome. In: Brubakk AO, Neuman TS, eds. Bennett and Elliott’s physiology and medicine of diving, 5th edn. Philadelphia, PA: WB Saunders, 2003:323–57.
- 64. Thalmann ED. A prophylactic program for the prevention of otitis externa in saturation divers. Report no. 10-74. Washington, DC: Navy Experimental Diving Unit, 1974.
- 65. International Marine Contractors Association, International Code of Practice for Offshore Diving, 2007. International Consensus Standards for Commercial Diving and Underwater Operations, 6.1 edn. London: Association of Diving Contractors International, 2014.
- 66. Jones JP Jr., Neuman TS. Dysbaric osteonecrosis: aseptic necrosis of the bone. In: Brubakk AO, Neuman TS, eds. Bennett and Elliott’s physiology and medicine of diving, 5th edn. Philadelphia, PA: WB Saunders, 2003:659–99.
- 67. Summitt JK, Reimers SD. Noise: a hazard to divers and hyperbaric chamber personnel. Aerosp. Med 1971; 42:1173–7.
- 68. Curley MD, Knafelc ME. Evaluation of noise within the MKl2 SSDS helmet and its effect on divers’ hearing. Undersea Biomed Res 1987; 14:187–204.
- 69. Molvaer OI, Gjestland T. Hearing damage to divers operating noisy tools under water. Scand J Work Environ Health 1981; 7:263–70.
- 70. Brady JI, Summitt JK, Berghage TE. An audiometric survey of navy divers. Undersea Biomed Res 1976; 3:41–7.
- 71. Edmonds C. Hearing loss with frequent diving (deaf divers). Undersea Biomed Res 1985; 12:315–9.
- 72. Molvær OI, Lehmann EH. Hearing acuity in professional divers. Undersea Biomed Res 1985; 12:333–49.
- 73. Molvaer OI, Albrektsen G. Hearing deterioration in professional divers: an epidemiologic study. Undersea Biomed Res 1990; 17(23):1–46.
- 74. Haraguchi H, Ohgaki T, Okubo J, et al. Progressive sensorineural hearing impairment in professional fishery divers. Ann Otol Rhinol Laryngol 1999; 108:1165–9.
- 75. Molvær O. Otorhinolaryngological aspects of diving. In: Brubakk AO, Neuman TS, eds. Bennett and Elliott’s physiology and medicine of diving, 5th edn. Philadelphia, PA: WB Saunders, 2003:227–64.
- 76. Rózsahegyi I. Late consequences of the neurological forms of decompression sickness. Br J Ind Med 1959; 16:311–7.
- 77. Edmonds C, Boughton J. Intellectual deterioration with excessive diving. Undersea Biomed Res 1985; 12:321–6.
- 78. Edmonds C, Hayward C. Intellectual impairment in diving: a review. In: Bove AA, Bachrach AJ, Greenbaum U, eds. Proceedings of the 9th International Symposium on Underwater and Hyperbaric Physiology, September 16–20, 1986. Kobe, Japan. Bethesda, MD: Undersea and Hyperbaric Medical Society, 1987:877–86.
- 79. Curley MD. US Navy saturation diving and diver neuropsychologic status. Undersea Biomed Res 1988; 15:39–50.
- 80. Todnem K, Nyland H, Skiedsvoll H, et al. Neurological long term consequences of deep diving. Br J Ind Med 1991; 48:258–66.
- 81. Murrison AW. The contribution of neurophysiologic techniques to the investigation of diving-related illness. Undersea Biomed Res 1993; 20(4):347–73.
- 82. Polkinghome PJ, Sebmi K, Cross MR, et al. Ocular fundus lesions in divers. Lancet 1988; 2:1381–3.
- 83. Crosbie WA, Reed JW, Clarke MC. Function characteristics of the large lungs found in commercial divers. J Appl Physiol 1979; 46:639–45.
- 84. Elliott DH, Moon RE. Long-term health effects of diving. In: Bennett PB, Elliott DH, eds. The physiology and medicine of diving, 4th edn. Philadelphia, PA: WB Saunders, 1993:585–604.
- 85. Davis JC, ed. Medical examination of sports scuba divers, 2nd edn. San Antonio, TX: Medical Seminars, 1986.
- 86. Bove AA. Fitness to dive. In: Brubakk AO, Neuman TS, eds. Bennett and Elliott’s physiology and medicine of diving, 5th edn. Philadelphia, PA: WB Saunders, 2003:700–17.
- 87. Health and Safety Executive. The medical examination of divers (MA 1), revision 4. London: Health and Safety Executive, 2015:1–27. Available at: http://www.hse.gov.uk/pubns/ma1.pdf (accessed on May 2, 2016).
- 88. Elliott DH, ed. Medical assessment of fitness to dive. Proceedings of an International Conference, Edinburgh Conference Center, Edinburgh, UK, March 8–11, 1994. Ewell: Biomedical Seminars, 1995. Available at: https://catalog.hathitrust.org/Record/008331599 (accessed on June 28, 2016).
- 89. Wendling J, Elliott DH, Nome T, eds. Medical assessment of working divers. Hyperbaric Editions, CH 2501. Biel-Bienne, Switzerland, 2004.
